Abstract
Background
Anemia, the world’s most common micro-nutrient deficiency disorder, can affect a person at any time and at all stages of life. Though all members of the community may face the problem, children aged 6–23 months are particularly at higher risk. If left untreated, it adversely affects the health, cognitive development, school achievement, and work performance. However, little was investigated among young children in Sub-Saharan countries including Ethiopia. This research aimed to investigate its magnitude and correlates to address the gap and guide design of evidence based intervention.
Methods
A community-based cross-sectional study was conducted from May -June 2016 in rural districts of Wolaita Zone. Multi-stage sampling technique was applied and 990 mother-child pairs were selected. Socio-demography, health and nutritional characteristics were collected by administering interview questionnaire to mothers/care-givers. Blood samples were taken to diagnose anemia by using HemoCue device, and the status was determined using cut-offs used for children aged 6–59 months. Hemoglobin concentration below 11.0 g/dl was considered anemic. Data were analyzed with Stata V14. Bivariate and multivariable logistic regressions were applied to identify candidates and predictor variables respectively. Statistical significance was determined at p-value < 0.05 at 95% confidence interval.
Results
The mean hemoglobin level of children was 10.44±1.3g/dl, and 65.7% of them were anemic. Among anemic children, 0.4% were severely anemic (<7.0g/dl), while 28.1% and 37.2% were mildly (10.0–10.9g/dl) and moderately (7.0–9.9g/dl) anemic, respectively. In the multivariable analysis, having maternal age of 35 years and above (AOR = 1.96), being government employee (AOR = 0.29), being merchant (AOR = 0.43) and ‘other’ occupation (AOR = 3.17) were correlated with anemia in children in rural Wolaita. Similarly, receiving anti-helminthic drugs (AOR = 0.39), being female child (AOR = 1.76), consuming poor dietary diversity (AOR = 1.40), and having moderate household food insecurity (AOR = 1.72) were associated with anemia in rural Wolaita.
Conclusion
A large majority of children in the rural Wolaita were anemic and the need for proven public health interventions such as food diversification, provision of anti-helminthic drugs and ensuring household food security is crucial. In addition, educating women on nutrition and diet diversification, as well as engaging them with alternative sources of income might be interventions in the study area.
Introduction
Anemia is the world’s most common micro-nutrient deficiency disorder that affects more than 2 billion people globally [1]. It can affect a person at any time and at all stages of life. However, in most parts of the world, children aged 6–23 months are at particularly higher risk. It primarily affects infants and young children because of their higher iron requirements related to growth, and women of childbearing age due to menstrual loss and pregnancy [2].
If left untreated, anemia can adversely affect the health, cognitive development, school achievement, and work performance of individuals. Low oxygenation of brain tissues, a consequence of anemia, may lead to impaired cognitive function, growth and psychomotor development in children. Children under 5-year-old and pregnant women have greater susceptibility to anemia because of their increased iron requirements for rapid body growth and expansion of red blood cells [3]. During childhood period, anemia is strongly associated with poor health and physical development [4, 5], mild and moderate mental retardation [6], and poor motor development and its control [7]. Iron deficiency anemia leads to reduced academic achievement and work capacity which intern reduces the earning potential of individuals and hence damages national economic growth at large [5]. It also increases the risks of mortality and morbidity which come from other diseases [4, 8].
Childhood anemia is mostly caused by dietary iron deficiency, infectious and genetic diseases, and other nutrient deficiencies [9]. While more than half of the anemia burden in children is attributed to iron deficiency, only very small fraction is due to genetic causes [10]. In early childhood, bad feeding habits, especially during the weaning period as breast milk is replaced by foods that are poor in iron, vitamin B12 and folic acid, are main contributors for anemia [11].
In Ethiopia, evidence showed varying magnitudes of anemia ranging from 41% in Amhara region to 83% in Somali region [12]. Many studies showed that factors contributing to anemia in children varied from one geographical area to another [4, 6, 7, 10]. However, most of those studies were done using small sample size, conducted on urban dwellers, facility-based, and lacked representativeness. Besides, there was no study reported the problem in a wider context at district level in Woita Zone. Hence, the present study was conducted to fill the gap in a rural setting in Wolaita Zone with an aim of determining the magnitude of anemia and associated factors among children aged 6–23 months.
Materials and methods
Study design and study area
A community based cross-sectional study was conducted among children aged 6–23 months residing in rural districts of Wolaita Zone, Southern Ethiopia, from May to June 2016. Wolaita Zone is one of the 13 administrative zones of southern region (SNNPR) which has 12 rural and 3 urban districts. The Zone was inhabited by over 1.8 million people in 2016 [13]. Wolaita Sodo, the capital of the Zone, is located at 6° 49' N latitude and 39° 47' E longitude, at an altitude of about 1900 meters above sea level. It is located at 330 km south-west of Addis Ababa, Ethiopia. The zone is characterized by its dense population. The majority (88.3%) of its population reside in rural districts whose major livelihood is agriculture. The major food crops cultivated in the zone are maize, sweet potato, enset (false banana), teff (Eragrostis tef), haricot bean, taro, sorghum, Irish potato, yam and cassava [14].
Sampling
Multi-stage sampling technique was applied to select mother-child paired study population. Children aged 6–23 months were the source population for the study. Initially, four districts were randomly selected from the 12 rural districts. Damot Gale, Boloso Bombe, Humbo and Sodo Zuria districts were selected as study districts. Then, three kebeles (the lowest administrative unit of Ethiopia consisting of nearly 5000 population) were randomly selected from each of the selected districts, making the total number of kebeles included in the study 12. Finally, the study participants were selected through systematic sampling technique from each of the selected kebeles by probability proportional to size i.e., allocating the sample size with regard to the respective kebeles’ population.
The total 993 sample size needed for this study was determined by the formula to estimate a single population proportion based on the following assumptions: 71.2% prevalence of anemia in Sub-Saharan Africa [15], 95% confidence interval, 5% margin of error, and 5% non-response rate and design effect of 3.
Data collection tools and procedures
Data were collected through interviewer administered questionnaire prepared in English and translated into Amharic language). The questionnaire was developed by reviewing guidelines and related literatures [12, 15, 16, 17]. It consisted of demographic characteristics, household wealth indicators and anemia risk factors such as health service utilization, recent illnesses, and dietary practices of both mother and child.
Anemia diagnosis
Hemoglobin concentration was used to determine anemia status of the study participants by taking finger-prick blood sample. Hemoglobin level was analyzed onsite by using HemoCue device (HemoCueHb 301), and values were adjusted for altitude using the UNICEF/WHO guideline. Anemia status was determined using cut-offs used for children aged 6–59 months. Hemoglobin concentration below 11.0 g/dl was considered anemic, whereas, hemoglobin concentrations of 11.0 g/dl and above were considered normal. Severity of anemia was categorized based on the UNICEF, UNU, WHO guideline as follows: children were categorized as mildly, moderately and severely anemic if their blood hemoglobin concentrations are between 10.0–10.9 g/dl, 7.0–9.9 g/dl and < 7.0 g/dl, respectively. Maternal anemia (hemoglobin concentration below 12 g/dl) was diagnosed with the same procedure and device used for child anemia, followed by adjustment for pregnancy and altitude [16].
Dietary assessment
A 24-hour dietary recall method was used to assess the dietary practice. The Dietary Diversity Score of children was calculated by asking mothers/caregivers about the food items their children consumed in the past 24 hours preceding the survey. All food items consumed by the children in the last 24 hours preceding the survey were categorized into seven food groups such as (1) grains, roots, and tubers, (2) legumes and nuts, (3) milk and milk products, (4) flesh foods, (5) eggs, (6) vitamin-A rich fruits and vegetables, and (7) other fruits and vegetables. Finally, the food groups consumed by the child were added together to obtain dietary diversity score [17].
Food insecurity
Food insecurity was measured by HFIAS (Household Food Insecurity Access Scale) tool developed by FANTA (Food and Nutrition Technical Assistant) project. The tool has nine questions asking household’s about the three domains of food insecurity: feeling uncertainty of food supply, insufficient quality of food, and insufficient food intake and its physical consequences in the last month. The households participating in the study were categorized into four levels of food-security (food secure, mildly food insecure, moderately food insecure and severely food insecure) based on the guideline’s recommendation [18].
Wealth index
Household wealth index was constructed using household asset data through PCA (Principal Component Analysis) based on interview responses adopted from Ethiopian Demographic and Health Survey. The presence or absence of each household items such as plow oxen, table, animal-drawn cart, chair, etc. were asked and their responses were coded as ‘0’ for No and ‘1’ for Yes. Finally, the common factor score for each household was produced for grouping households as lower, middle and higher wealth quantile households [19].
Under-nutrition
Chronic energy deficiency (malnutrition) was assessed using WHO guideline. The WHO Anthro 2005 software was used to calculate Z-score. MUACZ cut-off- point of negative two (−2) was used to define under-nutrition [20].
Data management and analysis
Data were entered into Epi-Data software version 1.4.4.0 and analyzed with Stata software version 14 (College Station, Texas). Proportions, means and standard deviations (SD) were used to describe the study population by independent variables and anemia. Bivariate logistic regression was done using thirty independent variables to identify the candidate variables (p-value < 0.25) for multivariable regression. Finally, predictors of anemia were determined using multivariable logistic regression model among the selected eleven candidate variables. Multi co-linearity, interaction and mediation among independent variables were checked based on the assumptions such as tolerance, variance inflation factors (VIF), correlation coefficient of interaction, and others to assure the fitness of logistic regression model. The independent variables used for checking multi co-linearity, interaction and mediation were; age of child, age of mother, religion, occupation, family size, meal frequency of mother, meal frequency of child, initiation of complementary feeding, receiving anti-helminthic drug, sex of child, dietary diversity score of child (DDS) and household food insecurity access scale (HFIAS). The statistical significance was determined at p-value < 0.05 at 95% confidence interval.
Quality control
Interviewers and laboratory technicians were trained for two days prior to data collection. A pilot study was done among 50 children who were selected from areas outside the actual study area. The data collection was regularly supervised by trained supervisors and the investigators.
Ethical approval and consent to participate
The actual data collection was started after Wolaita Sodo University College of Health Sciences and Medicine approved the study. Local administrative bodies were also communicated about the study and permission was obtained ahead of the study. Finally, informed written consent was obtained from the mothers/caregivers. Children diagnosed with anemia were counseled, and those with hemoglobin concentration below 9.0 g/dl were referred to health facility for further treatment and follow up.
Results
Socio-demography
A total of 990 children were included in the survey making the response rate 99.7%. The mean age of children was 14.96 months with a standard deviation of 5.37 months. About 434 (43.8%) of the children were females; 293 (29.6%) were born with short birth interval (less than 2 years interval); 191 (19.3%) were born from young mothers (mothers aged 15–24 years); and 60 (6.1%) were raised by single parents (Table 1).
Table 1. Socio-demographic characteristics of children aged 6–23 months residing in rural districts of Wolaita Zone, 2016 (N = 990).
| Variable | Category | Number | Percent |
|---|---|---|---|
| Child age (in months) | 6–11 | 336 | 33.9 |
| 12–17 | 289 | 29.2 | |
| 18–23 | 365 | 36.9 | |
| Sex of child | Male | 556 | 56.2 |
| Female | 434 | 43.8 | |
| Birth interval | First born child | 221 | 22.3 |
| below 24 month | 293 | 29.6 | |
| 25–48 month | 330 | 33.3 | |
| above 48 | 146 | 14.8 | |
| Age of mother (in years) | 15–24 | 191 | 19.3 |
| 25–34 | 685 | 69.2 | |
| 35 and above | 114 | 11.5 | |
| Marital status of mother | In a union | 930 | 93.9 |
| Not in a union | 60 | 6.1 | |
| Ethnicity of mother | Wolaita | 963 | 97.3 |
| Others | 27 | 2.7 | |
| Religion of mother | Protestant | 857 | 86.6 |
| Orthodox | 121 | 12.2 | |
| Others1 | 12 | 1.2 | |
| Occupation of mother | Housewife | 881 | 89.0 |
| Government employee | 36 | 3.6 | |
| Merchant | 33 | 3.3 | |
| Others2 | 40 | 4.0 | |
| Occupation of father | Farmer | 624 | 63.0 |
| Government employee | 111 | 11.2 | |
| Merchant | 175 | 17.7 | |
| Others2 | 80 | 8.1 | |
| Home distance from health facility (foot walk) | Below 30 min | 423 | 42.7 |
| 30–45 min | 443 | 44.8 | |
| Above 45 min | 124 | 12.5 | |
| Family size | Below 5 | 307 | 31.0 |
| 5–7 | 451 | 45.6 | |
| Above 7 | 232 | 23.4 |
Others1– Catholic, Muslim, Adventist
Others2 –Daily laborers, potters, housemaid
Health and nutrition characteristics of children
The majority 922 (93.1%) of children were fully immunized and only 109 (11%) of them were given anti-helminthic drugs to treat intestinal parasites. A total of 409 (41.3%) children had at least one of the following symptoms within the past two weeks preceding the survey: cough, difficulty of breathing, fever, diarrhea (with/without blood), or visible parasite on stool. Based on the Mid-Upper Arm Circumference (MUAC) measurement, 688 (69.5%) of the children were well-nourished, whereas the rest 302 (30.5%) had chronic energy deficiency.
The children had mean dietary diversity score of 3.4 with 1.5 standard deviation, and only 422 (42.6%) of them consumed more than half of the recommended seven food groups. Furthermore, more than one-third (36.3%) drank either tea or coffee in their usual meal (that contained tannins which reduce iron absorption). Nearly one out of ten children stopped breastfeeding at least one week before the survey (Table 2).
Table 2. Health and nutrition characteristics of children in rural districts of Wolaita Zone, 2016 (N = 990).
| Variable | Response Category | Number | Percent |
|---|---|---|---|
| Fully vaccinated | No | 68 | 6.9 |
| Yes | 922 | 93.1 | |
| Received anti-helminthes drug | No | 881 | 89.0 |
| Yes | 109 | 11.0 | |
| Sick in the past two weeks | No | 581 | 58.7 |
| Yes | 409 | 41.3 | |
| Ever caught malaria | No | 642 | 64.8 |
| Yes | 348 | 35.2 | |
| Usually drinks tea or coffee | No | 631 | 63.7 |
| Yes | 359 | 36.3 | |
| Bottle-feed | No | 884 | 89.3 |
| Yes | 106 | 10.7 | |
| Breast-feeding(currently) | No | 110 | 11.1 |
| Yes | 880 | 88.9 | |
| Complementary feeding frequency | 1–3 | 507 | 51.2 |
| (Within 24 hours) | 4–5 | 440 | 44.4 |
| 6–8 | 43 | 4.3 | |
| Breastfeeding frequency | 1–6 | 194 | 19.6 |
| 7–8 | 343 | 34.6 | |
| Above 8 | 453 | 45.8 | |
| Initiation of complementary feeding | Before 6 month | 90 | 9.1 |
| At 6 month | 614 | 62.0 | |
| After 6 month | 286 | 28.9 | |
| Dietary diversity score | Below 4 | 568 | 57.4 |
| 4 and above | 422 | 42.6 | |
| Nutritional status (MUACZ) | ≤-3 | 80 | 8.1 |
| Between -2 and -3 | 222 | 22.4 | |
| Normal | 688 | 69.5 |
Based on the household food insecurity access scale measurement (HFIAS), 628 (63.4%) of the children were born and lived in food-secure households; whereas the rest were born and lived in a household suffering from food insecurity. Four hundred and four (40.8%) mothers fully attended the four WHO recommended ANC visits, while only 86 (8.7%) of them had no ANC visit. The prevalence of maternal anemia was 13.4%, and three-fourth (734) of them usually consumed fewer than four meals per day (Table 3).
Table 3. Maternal health and nutrition in rural districts of Wolaita Zone, 2016 (N = 990).
| Variable | Category | Number | Percent |
|---|---|---|---|
| Household food insecurity | Food secure | 628 | 63.4 |
| Mildly food insecure | 78 | 7.9 | |
| Moderately food insecure | 208 | 21.0 | |
| Severely food insecure | 76 | 7.7 | |
| Number of pregnancies | 1–2 | 397 | 40.1 |
| 3–4 | 298 | 30.1 | |
| Above 4 | 295 | 29.8 | |
| Antenatal care follow-up | No visit | 86 | 8.7 |
| 1–3 visit | 500 | 50.5 | |
| 4 and above | 404 | 40.8 | |
| Place of delivery | Home | 446 | 45.1 |
| Health facility | 544 | 54.9 | |
| Maternal anemia | Yes | 133 | 13.4 |
| No | 857 | 86.6 | |
| Frequency of meals/day | Less than or equal to 3 | 734 | 74.1 |
| Above 3 | 256 | 25.9 |
Magnitude and severity of anemia
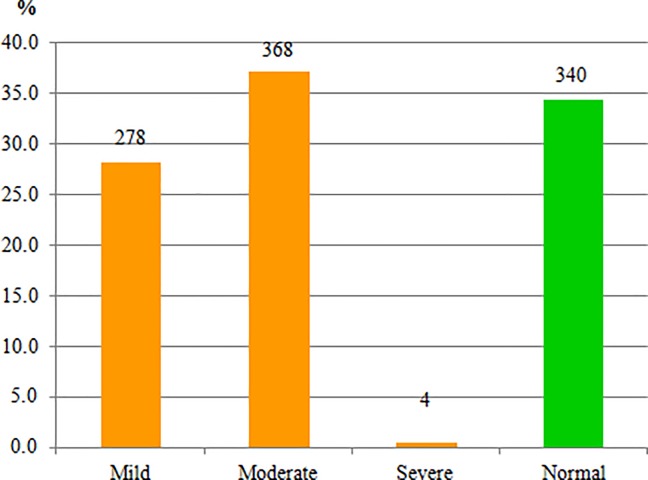
The mean hemoglobin concentration of the children was 10.44 g/dl (95% C.I: 10.36, 10.52 g/dl) with standard deviation of 1.30 g/dl, and with a minimum and maximum hemoglobin concentration of 6.64 to 17.31g/dl. Overall, 650 (65.7%) of the children had anemia with hemoglobin level below 11g/dl (95% C.I: 62.6% - 68.6%). Most of them were mildly (10–10.9 g/dl) and moderately (7–9.9 g/dl) anemic accounting for 278 (28.1%) and 368 (37.2%), respectively; whereas only 4 (0.4%) of children were severely anemic with hemoglobin level below 7 g/dl. The prevalence of anemia decreased as age increased i.e. from as high as 72.6% among infants aged 6–11 months to as low as 61.4% among young children aged 18–23 months “Fig 1”.
Fig 1. Magnitude and severity of anemia among children aged 6–23 months in rural districts of Wolaita Zone, 2016 (N = 990).
Factors associated with anemia
Bivariate logistic regression showed that age of child, age of mother, religion, occupation of mother, family size, child’s meal frequency, initiation of complementary feeding, receiving anti-helminthic drugs, sex of child and food insecurity were associated with anemia in children (P < 0.05).
However, in the multivariable analysis, age of child, religion, family size and initiation of complementary feeding time were not associated with child anemia (p > 0.05); whereas maternal age and occupation, child’s anti-helminthic drug intake, sex, dietary diversity score and household food insecurity were identified as predictors of child anemia in the multivariable analysis (p < 0.05).
The multivariable logistic regression showed that children of mothers whose age was 35 years and above were two times more likely to be anemic as compared to children of mothers whose age was between 15–24 years, AOR = 1.96 (95% C.I: 1.01, 3.85), p = 0.049. Children of government employee mothers had 79% lower chance of being anemic as compared to children of housewife mothers, AOR = 0.21 (95% C.I: 0.09, 0.47), p<0.001. Similarly, children of merchant mothers had 57% lower chance of getting anemia than children of housewife mothers, AOR = 0.43 (95%C.I: 0.20, 0.92), p = 0.029. In contrary, children of mothers whose occupation was classified as ‘other occupation’ (daily laborers, potters and housemaid) had greater chance of being anemic than children of housewives, AOR = 3.17 (95%C.I: 1.35, 7.43), p = 0.008.
Receiving anti-helminthic drugs was found to be inversely related with childhood anemia. Accordingly, children who received anti-helminthic drugs had 61% lower chance of getting anemia than their counterparts, AOR = 0.39 (95% C.I: 0.24, 0.63), p <0.001. Female children were also twice more likely to be anemic than male children, AOR = 1.76 (95% C.I: 1.30, 2.38), p < 0.001. Nutritional characteristics were also found to be predictive factors of childhood anemia. Accordingly, children who consumed poor dietary diversity (DDS < 4) were found to be more likely anemic than their counterparts, AOR = 1.40 (95% C.I: 1.03, 1.92), p = 0.034. Last but not least, children from moderately food insecure households were two times more likely to be anemic as compared to those from food secure households, AOR = 1.57 (95% C.I: 1.07, 2.32), p = 0.023 (Table 4).
Table 4. Multivariable analysis of factors associated with anemia among children in rural districts of Wolaita Zone, 2016 (N = 990).
| Variable | Category | Status | COR | 95% CI for COR | AOR | 95% CI for AOR | P value for AOR | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Anemic | Normal | Lower | Upper | Lower | Upper | |||||
| Age of child | 6–11 month | 244 | 92 | |||||||
| 12–17 month | 182 | 107 | 0.64* | 0.46 | 0.90 | 0.77 | 0.52 | 1.12 | 0.175 | |
| 18–23 month | 224 | 141 | 0.60** | 0.44 | 0.82 | 0.76 | 0.52 | 1.11 | 0.154 | |
| Age of mother | 15–24 | 113 | 78 | |||||||
| 25–34 | 447 | 238 | 1.30 | 0.93 | 1.80 | 1.52 | 0.98 | 2.36 | 0.061 | |
| 35 and above | 90 | 24 | 2.59** | 1.52 | 4.42 | 1.96* | 1.01 | 3.85 | 0.049 | |
| Religion | Protestant | 571 | 286 | |||||||
| Orthodox | 67 | 54 | 0.62* | 0.42 | 0.91 | 0.66 | 0.43 | 1.02 | 0.060 | |
| Others1 | 12 | 0 | — | — | — | — | — | — | — | |
| Occupation | Housewife | 592 | 289 | |||||||
| of mother | Government employee | 10 | 26 | 0.19** | 0.09 | 0.39 | 0.21** | 0.09 | 0.47 | 0.000 |
| Merchant | 16 | 17 | 0.46* | 0.23 | 0.92 | 0.43* | 0.20 | 0.92 | 0.029 | |
| Others2 | 32 | 8 | 1.95 | 0.89 | 4.29 | 3.17** | 1.35 | 7.43 | 0.008 | |
| Family size | Below 5 | 195 | 112 | |||||||
| 5–7 | 280 | 171 | 0.94 | 0.70 | 1.27 | 0.81 | 0.55 | 1.21 | 0.311 | |
| 8 and above | 175 | 57 | 1.76** | 1.21 | 2.57 | 1.27 | 0.79 | 2.04 | 0.320 | |
| Child’s | <4 | 394 | 113 | |||||||
| meal | 4–5 | 241 | 199 | 0.35** | 0.26 | 0.46 | 0.38** | 0.27 | 0.52 | 0.000 |
| frequency | 6–8 | 15 | 28 | 0.15** | 0.08 | 0.30 | 0.23** | 0.10 | 0.49 | 0.000 |
| Initiation of | < 6 month | 68 | 22 | |||||||
| complementary | At 6 month | 374 | 240 | 0.50** | 0.30 | 0.84 | 0.98 | 0.55 | 1.73 | 0.947 |
| feeding | > 6 month | 208 | 78 | 0.86 | 0.50 | 1.49 | 0.96 | 0.53 | 1.75 | 0.903 |
| Received anti-helminthic drug | No | 609 | 272 | |||||||
| Yes | 41 | 68 | 0.27** | 0.18 | 0.41 | 0.39** | 0.24 | 0.63 | 0.000 | |
| Sex of child | Male | 343 | 213 | |||||||
| Female | 307 | 127 | 1.50** | 1.15 | 1.96 | 1.76** | 1.30 | 2.38 | 0.000 | |
| Dietary | Below 4 | 376 | 192 | 0.95 | 0.73 | 1.23 | 1.40* | 1.03 | 1.92 | 0.034 |
| diversity score | 4 and above | 274 | 148 | |||||||
| Food insecurity | Food secure | 396 | 232 | |||||||
| Mildly insecure | 48 | 30 | 0.94 | 0.58 | 1.52 | 0.93 | 0.54 | 1.62 | 0.809 | |
| Moderately insecure | 152 | 56 | 1.59** | 1.12 | 2.25 | 1.57* | 1.07 | 2.32 | 0.023 | |
| Severely insecure | 54 | 22 | 1.44 | 0.85 | 2.42 | 1.10 | 0.62 | 1.94 | 0.748 | |
*- P<0.05
**- P<0.01
Others1– Catholic, Muslim, Adventist
Others2 –Daily laborers, potters, housemaid
Discussion
Anemia remains one of the major health problems that result in grave health outcomes in developing countries despite the progresses seen in nutrition interventions. The Global Burden of Diseases Study [21] shows that anemia in children is one of the most common causes of child death in Ethiopia, and it continued to be a major public health problem. Similarly, our study showed that two-third of the young children in rural Wolaita had anemia standing as a severe public health problem (above the WHO cut-off point of 40%) [22]. Studies done elsewhere in Ethiopia [23] also reported similar findings although severe anemia was found to be lower in the present study area (0.4%) as compared to Ethiopia’s national rate among young children (3.5%) [12].
UNICEF and WHO recommend adequate breastfeeding, iron supplementation and fortification, and nutrition education for mothers [24] in order to curve health loss (high morbidity and mortality) due to iron deficiency anemia in children. Ethiopia’s nutrition strategy puts weight on the above nutrition intervention with the vision of ensuring adequate micronutrient intake for all children and its citizens [25]. Implementation of the national nutrition strategy with focus to young children will be vital in preventing and treating anemia in rural Wolaita.
The present study showed that maternal age, type of occupation, child’s anti-helminthic drug intake, sex of child, dietary diversity score and household food insecurity were associated with child anemia. Studies have shown that consuming diverse food prevents anemia although this is a difficult option for households in developing countries where there is recurrent food insecurity problem. In conformity to this study, several studies [23, 26, 27] have shown children who had poor dietary diversity score had a higher chance of having anemia. Similarly, WHO report has shown that feeding children diverse foods increases the bioavailability of micronutrients including iron, and this is one of the recommended practices [28]. For instance, eating iron rich food items of animal source such as flesh meat, organ meat, poultry; and non-animal source such as legumes and green leafy vegetables increase the bioavailability of iron and hence enable micronutrient demands for children. Consumption of fruits, vegetables, and tubers that are good sources of vitamins A and C, and folic acid enhances the absorption and utilization of iron [24].
De-worming intestinal parasites also has a positive impact on prevention of anemia among children [24]. Similarly, in this study, children in rural Wolaita who undergone de-worming had lower odds of having iron deficiency anemia. A report of study conducted in 25 Sub-Saharan countries stated that de-worming and iron intake for more than six months prevented anemia in children [15]. De-worming is an essential public health intervention as intestinal parasites, especially hookworm infection, results in intestinal blood loss which in turn contributes to anemia.
Household food security also plays a significant role in preventing anemia among children [24]. In this study, we found that food insecurity was associated with the development of anemia which was higher among children of moderately food insecure households. Studies elsewhere showed similar finding [29], and it is important to address food insecurity problems in rural areas to prevent anemia in children of such areas. In the absence of such measures, it will be difficult to achieve global and national targets of micronutrient problems. The efforts to ensure and assist local people with food security may need implementation of programs funded by government and non-governmental organizations. This could be an important means of intervention if such programs are implemented according to the standard [24] and prioritize people with severe or moderate food insecurity problems. Nevertheless, this study did not attempt to identify households supported by such programs.
Sex was also one of the factors which affected blood hemoglobin level of the children in the present study. Many studies found the correlation between sex and hemoglobin level [29, 30, 31, 32]. According to those studies, the role of sex varied from place to place which gave different gender value for the sex of child with respect to the culture of the community. In the present study, female children were more likely to have anemia than males. Although the exact cause for increased anemia in female children in the present study is difficult to point out, the possible reasons could be high prevalence of sex bias (negatively affecting the feminine gender), late initiation of complementary feeding for female children and the community’s belief to give better care for male children than females [33]. Based on the finding of the present study and other similar studies, female children are not favored to get iron rich food, which increases the risk of iron deficiency anemia in female children. Therefore, it is important to educate the rural community about the importance of providing iron rich food for all children regardless of sex. Further studies may also be required to validate the role of gender in iron deficiency anemia in Ethiopian context.
This study also showed that maternal age and occupation were correlated with hemoglobin level of children. The risk of anemia in children increased with the age of their mothers. In line with this finding, a study in Northeastern Brazil also showed similar trends of iron deficiency anemia [34]. Studies also found that parental occupation was one of the significant predictors of hemoglobin level in children [27]. In this study, mothers who made relatively better income from government employment and trading had a lower chance of having anemic children as compared to housewives. This indicates that anemia is significantly prevalent among families with less educated parents and low income, and hence shows the role of socio-economic inequality in the prevalence of anemia [35]. Therefore, it is important to engage women in income generating activities to make better income and afford supplemental food for their children.
We mention the following limitations of our study. The level of hemoglobin concentration at one point in time was used to diagnose anemia among children. Though this method is the most reliable indicator of anemia, the present study did not aim to identify the etiology of anemia, rather to determine the prevalence and associate anemia with its risk factors. We tried to assess the most common factors of childhood anemia through the dietary assessments and the household food insecurity measurements; however, we did not see the role of genetic factor to cause anemia in the present study for diagnostic limitation within the existing setup [9, 10].
Conclusion and recommendation
Based on the World Health Organization cut-off point, anemia is found to be a severe public health problem among young children residing in rural districts of Wolaita zone. Nearly two third of children aged 6–23 months were diagnosed to be anemic by having hemoglobin level below 11g/dl. Poor dietary diversity, female sex of child, failure to take anti-helminthic drug, household food insecurity, maternal age and occupation were significantly associated with child anemia.
A large majority of children in the rural Wolaita were anemic, and the need for proven public health interventions such as food diversification, provision of anti-helminthic drugs and ensuring household food security is crucial. In addition, nutrition education and diet diversification through provision of alternative sources of income for women might be useful interventions.
Supporting information
(DOCX)
(DOCX)
(DTA)
Acknowledgments
We would like to acknowledge Wolaita Sodo University for logistics and technical support. We also extend our gratitude to respective district administrative bodies, data collectors, data clerk and study participants for their cooperation and technical assistance.
Abbreviations
- ANC
Antenatal Care
- AOR
Adjusted Odds Ratio
- C.I
Confidence Interval
- COR
Crude Odds Ratio
- EDHS
Ethiopian Demographic and Health Survey
- FANTA
Food and nutrition technical assistant
- Hb
Hemoglobin
- HFIAS
Household Food Insecurity Access Scale
- MUACZ
Mid Upper Arm Circumference Z-score
- PCA
Principal Component Analysis
- SD
Standard Deviation
- SNNPR
Southern Nations, Nationalities and Peoples Region
- UNICEF
United Nations Children's Fund
- WHO
World Health Organization
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the Wolaita Sodo University, R2016-CHSM-05 to MAA. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. Micronutrient deficiency: Battling iron deficiency anemia: the challenge. 2004. Available from: http://www.who.int/nut/ida.htm, accessed on December, 2016.
- 2.Lozoff B. Iron deficiency and child development. Food Nutr Bull. 2007; 28:S560–71. 10.1177/15648265070284S409 [DOI] [PubMed] [Google Scholar]
- 3.Walter T, de Andraca I, Chadud P, Perales CG. Iron deficiency anemia: adverse effects on infant psychomotor development. Pediatrics. 1989; 84(1): 7–17. [PubMed] [Google Scholar]
- 4.McLean E, Cogswell M, Egli I, Wojdyla D, De Benoist B. Worldwide prevalence of anemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutrition. 2009;12(4):444–454. 10.1017/S1368980008002401 [DOI] [PubMed] [Google Scholar]
- 5.Victora CG, Adair L, Fall C, Halla PC, Martorell R, Richter L, et al. Maternal and child undernutrition: consequences for adult health and human capital. The Lancet. 2008;371(9609):340–357. 10.1016/s0140-6736(07)61692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shafir T, Angulo-Barroso R, Su J, Jacobson S W, Lozoff B. Iron deficiency anemia in infancy and reach and grasp development. Infant Behavior and Development. 2009;32(4):366–375. 10.1016/j.infbeh.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardoso M A, Scopel K K G, Muniz P T, Villamor E, Ferreira MU. Underlying factors associated with anemia in amazonian children: a population-based, cross-sectional study. PLoSONE. 2012;7(5) 10.1371/journal.pone.0036341.e36341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollitt E. Early iron deficiency anemia and later mental retardation. The American Journal of Clinical Nutrition. 1999;69(1):4–5. 10.1093/ajcn/69.1.4 [DOI] [PubMed] [Google Scholar]
- 9.Simon B, Willis A, Rachel P, Benson E, Siân EC, et al. Epidemiology of Plasmodium-Helminth Co-Infection in Africa: Populations at risk, potential impact on anemia, and prospects for combining control. Am J Trop Med Hyg. 2007; 77(6): 88–98. [PMC free article] [PubMed] [Google Scholar]
- 10.Stoltzfus R, Mullany L, Black RE. Iron deficiency anemia In: Ezzati M, Lopez A, Rodgers A, Murray CJL, eds. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. Geneva, World Health Organization, 2004, 163–210. [Google Scholar]
- 11.Torres MA, Sato K, Queiroz SS. Anemia in children under 2 years in basic health care units in the State of São Paulo, Brazil. Rev SaúdePública. 1994; 28(4): 290–4. [DOI] [PubMed] [Google Scholar]
- 12.Central Statistical Agency [Ethiopia] and ICF International. Ethiopia Demographic and Health Survey, 2016 Addis Ababa EaC, Maryland, USA: Central Statistical Agency and ICF International. [Google Scholar]
- 13.Summary and statistical report of the 2007 population and housing census, Federal democratic republic of Ethiopia, 2008.
- 14.Prospects for integrating food and food production in Wolaita Sodo, Ethiopia: available at http://www.fao.org/wairdocs/ILRI/x5519B/x5519b19.htmaccessed by Nov. 2016
- 15.Calistus W, Shiro T, Fabian E, Koji K. Prenatal anemia control and anemia in children aged 6–23 months in Sub-Saharan Africa. Matern Child Nutr. 2016; 1–10. 10.1111/mcn12375 [DOI] [Google Scholar]
- 16.Iron deficiency anemia assessment, prevention, and control: A guide for program managers. UNICEF, UNU, WHO, 2001 (WHO/NHD/01.2). Available at http://www.who.int/reproductive-health/docs/anaemia.pdf accessed by Feb 2016.
- 17.World Health Organization, Indicators for Assessing Infant and Young Child Feeding Practices, WHO Press, Geneva, Switzerland, 2010. [Google Scholar]
- 18.Jennifer C, Anne S, Paula B. Household Food Insecurity Access Scale (HFIAS) for Measurement of Household Food Access: Indicator Guide Washington, D.C: Food and Nutrition Technical Assistance Project: 2007. Available at http://www.fao.org/fileadmin/user_upload/eufao-fsi4dm/doc-training/hfias.pdf. Accessed by June 2016. [Google Scholar]
- 19.Central Statistical Agency [Ethiopia] and ICF International. 2012. Ethiopia Demographic and Health Survey 2011 Addis Ababa EaC, Maryland, USA: Central Statistical Agency and ICF International. [Google Scholar]
- 20.World Health Organization, “Physical status: the use and interpretation of anthropometry: report of a WHO expert committee,” WHO Technical Report Series 854, World Health Organization, Geneva, Switzerland, 1995, http://www.who.int/childgrowth/publications/physical_status/en/. [PubMed] [Google Scholar]
- 21.Awoke M, Tilahun NH, Kebede D, Gizachew AT, Amare D. National mortality burden due to communicable, non-communicable, and other diseases in Ethiopia, 1990–2015: findings from the Global Burden of Disease Study. Population Health Metrics: 2015; 15(29): 10.1186/s12963-017-0145-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Iron deficiency anemia; assessment, prevention and control A guide for program managers Geneva: World Health Organization; 2005. Available from: www.who.int/nutrition/publications/en/ida_assessment_prevention_control. [Google Scholar]
- 23.Woldie H, Kebede Y, Tariku A. Factors Associated with Anemia among Children Aged 6–23 Months Attending Growth Monitoring at Tsitsika Health Center, Wag-Himra Zone, Northeast Ethiopia. J NutrMetab. 2015; 2015: 928632 10.1155/2015/928632.0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iron deficiency anemia assessment, prevention, and control: A guide for program managers. UNICEF, UNU, WHO, 2001 (WHO/NHD/01.2). Available at http://www.who.int/reproductive-health/docs/anaemia.pdf accessed by Feb 2016.
- 25.The federal Democratic Republic of Ethiopia. National Nutrition Strategy. Available at http://extranet.who.int/nutrition/gina/sites/default/files/ETH%202008%20National%20Nutrition%20Strategy_1.pdf accessed on June 2017
- 26.Mahama S. Relationship between Dietary Diversity and Haematological Status of Children aged 6–59 months in Northern Ghana: Nessa J Nutritional Health Sciences. 2016;1(1); 1–23 [Google Scholar]
- 27.Desalegn A, Mossie A, Gedefaw L. Nutritional Iron Deficiency Anemia: Magnitude and Its Predictors among School Age Children, Southwest Ethiopia: A Community Based Cross-Sectional Study. PLoS ONE. 2014; 9(12): e114059 10.1371/journal.pone.0114059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization and Working Group on Infant and Young Child Feeding Indicators, Developing and Validating Simple Indicators of Dietary Quality and Energy Intake of Infants and Young Child in Developing Countries, World Health Organization, Washington, DC, USA, 2007.
- 29.Sant R, James B, Sumithra M, Anita S, Vijay B, Savitha N, et al. Determinants of anemia among young children in rural India. Pediatrics. 2010; 126(1):e140 10.1542/peds.2009-3108 [DOI] [PubMed] [Google Scholar]
- 30.Michael OF, Frank OF, Michael OA. Factors Associated with Hemoglobin Prevalence among Ghanaian Children Aged 6–59 months. Journal of Biology, Agriculture and Health care; 2014. 4(2); 132–140. [Google Scholar]
- 31.Monica MO, Pedro IC, Malaquias B, Ann A. Prevalence of anemia in children 6–59 months old in the state of Pernambuco, Brazil. Pan Am J Public Health 2001; 10(2): 101–107. [DOI] [PubMed] [Google Scholar]
- 32.Chitra B, Nisha N, Jesni KJ, Jesina B, Nazar A, Balakrishna. Study on Prevalence of Anemia among School Children in a Rural Community Setup. Sch. Acad. J. Pharm. 2014; 3(6): 423–426. [Google Scholar]
- 33.Agumasie S, Gezahegn T, Alemayehu B. Complementary feeding practice of mothers and associated factors in HiwotFana Hospital, Eastern Ethiopia. Pan Afr Med J. 2014; 18: 143 10.11604/pamj.2014.18.143.3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luciana PL, Malaquias BF, Pedro IC, José NF, Monica MO. Prevalence of anemia and associated factors in children aged 6–59 months in Pernambuco, Northeastern Brazil. Rev. SaúdePública. 2011; 45(3): 457–466 [DOI] [PubMed] [Google Scholar]
- 35.Malkanthi R, Silva K, Jayasinghe-Mudalige U. Risk factors associated with high prevalence of anemia among children under 5 years of age in paddy-farming households in Sri Lanka. Food and Nutrition Bulletin. 2010; 31(4): 475–482. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DTA)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.