Supplemental Digital Content is available in the text
Keywords: calcium, hypocalcaemia, routine, thyroidectomy, vitamin D
Abstract
Background:
Thyroidectomy is the main intervention for thyroid malignancies and some benign thyroid diseases. Its most common complication is hypocalcaemia, which requires oral or intravenous calcium therapy. The aim of this meta-analysis was to assess the efficacy of routine calcium supplementation with or without vitamin D in preventing hypocalcaemia post-thyroidectomy.
Methods:
Systematic searches of the PubMed, EMBASE, and Cochrane Library databases were performed. The qualities of the included articles were assessed using the Cochrane risk of bias tool. The studies’ qualities of outcomes and strengths of evidence were evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) tool. Data analysis was performed using Review Manager 5.3, and odds ratio (ORs) with their 95% confidence intervals (CIs) were computed for dichotomous data.
Results:
Ten randomized controlled trials (RCTs) were included. The combined study recruited 1620 patients (343 men and 1277 women) who underwent total thyroidectomy alone or with neck dissection. Calcium supplementation decreased the risk of transient postoperative hypocalcaemia (OR 0.48 [95% CI, 0.31–0.74]; P < .001) but did not decrease the demand for intravenous supplementation or the rate of permanent hypocalcaemia compared to no treatment. Calcium and vitamin D supplementation significantly reduced the rate of transient hypocalcaemia and the demand for intravenous supplementation compared to either no treatment (OR 0.21 [95% CI, 0.11–0.40]; P < .001 and OR 0.26 [95% CI, 0.10–0.69]; P = .007, respectively) or calcium alone (OR 0.39 [95% CI, 0.18–0.84]; P = .02 and OR 0.18 [95% CI, 0.07–0.47]; P < .001, respectively), but did not decrease the rate of permanent hypocalcaemia. GRADE-based confidence was moderate.
Conclusion:
Postoperative calcium supplementation is effective for preventing post-thyroidectomy hypocalcaemia. Calcium plus vitamin D was more effective than calcium alone in preventing postoperative hypocalcaemia and decreasing the demand for intravenous calcium supplementation. Further, well-designed RCTs with larger sample sizes are required to validate our findings.
1. Introduction
Hypocalcaemia is one of the most common complications of bilateral thyroid surgery.[1–4] The reported incidence of post-thyroidectomy hypocalcaemia ranges widely from 13% to 49%.[4,5] Transient hypocalcaemia can normalize spontaneously after the recovery of parathyroid function, while permanent hypocalcaemia can last for more than 1 year and often requires lifelong therapy or parathyroid transplantation.[6]
Symptoms of mildly hypocalcaemia such as perioral or acral paraesthesia, tingling, and carpopedal tetany can impose different degrees of pressure on patients’ emotions.[7] Moreover, severe hypocalcaemia can cause life-threatening complications such as laryngospasm and cardiac arrhythmias.[8,9] Many patients who experience symptoms of mild hypocalcaemia can be treated with oral calcium/vitamin D (VD).[10] However, serious hypocalcaemic symptoms should be relieved by intravenous calcium supplementation as quickly as possible.[11–16] Moreover, intravenous calcium administration to patients with refractory hypocalcaemia is a major reason for prolonged hospitalization after thyroid surgery.[17]
Serum calcium levels reportedly drop to their lowest levels 24 to 48 hours after thyroid surgery, during which concomitant symptoms are also present. There are no reliable markers for identifying patients who are likely to develop symptomatic hypocalcaemia.[18,19] Thus, patients who undergo thyroidectomy always require close observation in case they require calcium and VD supplementation. This results in prolonged in-hospital stay and repeated biochemical testing.[20,21] In order to reduce the risk of post-thyroidectomy hypocalcaemia and expedite early discharge, some centers have proposed routine calcium/VD supplementation.[22,23] However, there is no solid evidence of any benefits provided by such management. To that end, the aim of this meta-analysis was to assess the effectiveness of administering routine oral calcium or calcium plus VD supplements in preventing postoperative hypocalcaemia.
2. Materials and methods
As this is a meta-analysis of previously published data, ethics committee approval was not required.
2.1. Search strategy and study selection
The search strategy was in accordance with a recent publication that described optimal literature search methods for surgical reviews.[24] The PubMed, Embase, and Cochrane Library databases were searched without date constraints to identify studies that evaluated the effectiveness of calcium/VD supplementation in preventing postoperative hypocalcaemia; the final search was performed on June 1, 2018. The following free words and Medical Subject Headings terms were used in the search: “Vitamin D”, “Calcium”, “Calcitriol”, “calciferol”, “Caltrate”, “ergocalciferol”, “hypocalcemia”, “low calcium”, “thyroidectomy”, “thyroid surgery”, and “thyroidectomies”. The review was limited to published articles that were written in English. References within the extracted articles were explored for additional publications.
2.2. Population, interventions, and outcomes
Inclusion criteria: All randomized controlled trials that were designed to investigate the effectiveness of postoperative oral calcium/VD supplementation in preventing post-thyroidectomy hypocalcaemia were included.
Exclusion criteria: Studies designed for animals, and those that were in languages other than English or were non-randomized controlled trial (RCTs) were excluded. Studies that included patients with prior neck surgery, routine intravenous calcium supplementation, those that examined preoperative supplementation or those with underlying diseases or treatments like the use of corticosteroids, furosemide, or bisphosphates that could influence serum calcium levels were also excluded.
Interventions included calcium, calcium plus VD, or no treatment. Examined outcomes were the incidences of transient or permanent hypocalcaemia as well as the need for intravenous supplementation. Hypocalcaemia was defined as a calcium level below lower limit of normal or hypocalcaemic symptoms such as muscle spasms or cramps, numbness.[25,26] Transient hypocalcemia was usually defined as the need for replacement therapy for less than 6 months or 1 year after thyroidectomy; permanent hypocalcaemia was usually defined as the need for replacement therapy for more than 6 months or 1 year after thyroidectomy.[27,28] Intravenous supplementation was most used when symptoms persisted despite oral therapy.[13–15,29,30]
2.3. Data collection and analysis
Two investigators (Tengfei Xing and Yiyi Hu) extracted data independently. Any discrepancy was resolved through discussion. The extracted data included the name of the first author, year of publication, study location, number of cases and controls, number of patients with hypocalcaemia, and any intravenous supplementation requirements.
The Cochrane Collaboration's risk of bias tool was used by 2 independent reviewers (Yiyi Hu and Bin Wang) to assess the quality of RCTs. The quality of key outcomes and the overall strength of the supporting evidence from the included studies were evaluated by using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) tool.[31]
The random effects model was used owing to heterogeneity across the included studies, as this can provide a more conservative result than a fixed effects model.[32] Pooled odds ratios (ORs) with their 95% confidence intervals (CIs) were calculated to evaluate the association between calcium with or without VD supplementation as well as the rate of post-thyroidectomy hypocalcaemia and the demand for intravenous supplementation. Cochrane's Q test and I2 test were used to evaluate heterogeneity and its magnitude across studies; a P value <.1 or I2 > 50% was defined as significant heterogeneity.[33] Funnel plots were used to evaluate publication bias and identify the potential outlier studies that might cause heterogeneity.[34] All analyses were performed using the Revman 5.3 software. This meta-analysis is reported in accordance with the Cochrane Collaboration guidelines and the PRISMA statement.[35]
3. Results
3.1. Search results
Flow-chart from PRISMA shows the flow diagram of the selection process in which 260 references were retrieved by the electronic database. After screening and removal of duplicate publications, 15 references were considered to be potentially eligible. After further evaluation, 10 studies[13–16,25,29,30,36–38] finally met our inclusion criteria. Of the 5 excluded studies, 3 were omitted because the treatment groups were routinely administered preoperative VD supplements[6,39] or hydrochlorothiazide.[21] One article was excluded because patients in that study received prophylactic infusion of calcium,[40] while another was excluded because the treatment group was compared to a historical control.[23] All included studies were RCTs that were published in English.
The combined study comprised 1620 patients (343 men and 1277 women). The mean age was 49 ± 13 years and the overall incidence of post-thyroidectomy hypocalcaemia was 37.1%. Table 1 summarizes the key characteristics of the included studies. Patients in 5 studies[13,16,30,36,38] underwent total thyroidectomy, while those in the remaining studies[14,15,25,29,37] underwent total thyroidectomy and neck dissection. The calcium supplements prescribed in the studies included calcium carbonate,[16,25,30,37] effervescent calcium,[36] calcium,[13,14,29] calcium salts,[38] and Calcimax D3.[15] The types of VD supplements were Calcitriol,[13,16,25,38] Cholecalciferol,[16,25,30] VD,[14,29] and Calcimax D3.[15] The most common biochemical cut-off value used to define hypocalcaemia was a total calcium <8.0 mg/dl or ionized calcium <1.0 mmol/L. Sun et al defined laboratory hypocalcaemia as serum calcium <2.1 mmol/L[37] while Langner et al defined biochemical hypocalcaemia as ionized calcium <1.1 mmol/L.[36] The most common indication for intravenous supplementation was the persistence of hypocalcaemic symptoms despite oral supplementation. Choe et al reported that intravenous supplementation was needed only when patients had severe or worsening hypocalcaemic symptoms combined with Ca < 7.5 mg/dl or ionized calcium <0.8 mmol/L.[25] Permanent hypocalcaemia was defined as that which lasted for 6 months or more. Risk of bias assessments are summarized in Figure 1. One study was rated as being at high risk of selection bias because the allocation was performed sequentially.[38] Two studies did not interpret how allocation sequences were generated.[36,37] Letters were sent to the corresponding authors, and only Langner[38] replied that patients were allocated in a sequential form. Ten studies did not report whether allocation concealment was performed, while 8 did not offer any information on blinding procedures and 1 clearly indicated that it was not blinded. Nine studies explicitly reported the details of the hypocalcaemia assessment and indications for intravenous supplementation. One study[16] did not specify the postoperative serum calcium detection method used for the diagnosis of hypocalcaemia. For the assessment of attrition bias, 3 studies were graded as high-risk because complete data of the incidence of biochemical hypocalcaemia was lacking. Therefore, we could only extract data on symptomatic hypocalcaemia, which may not be as objective as biochemical data. Reporting and other biases were low in all the studies.
Table 1.
Characteristics of included studies.
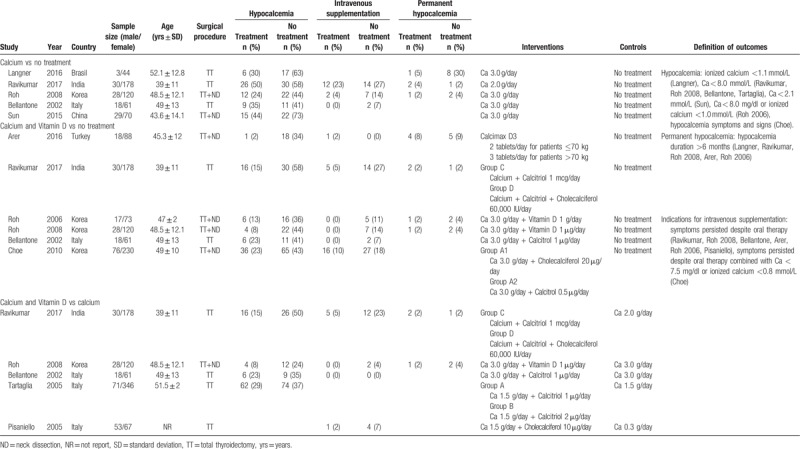
Figure 1.
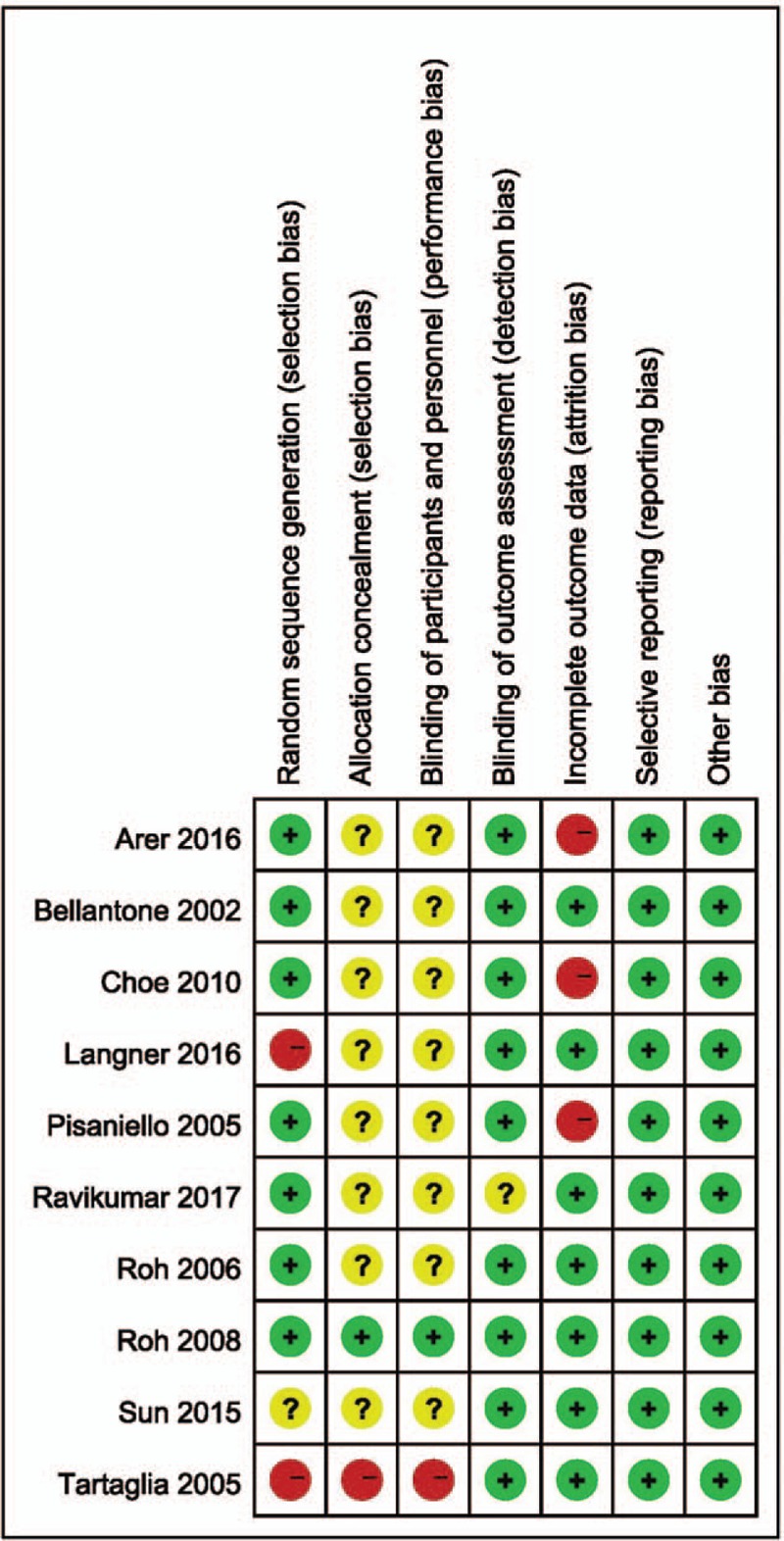
Risk of bias summary.
3.2. Results of meta-analysis
Three comparisons were performed in our review: calcium vs no treatment, calcium plus VD vs no treatment, and calcium plus VD vs calcium alone.
3.2.1. Calcium vs no treatment
Routine supplementation of calcium significantly decreased the rate of postoperative hypocalcaemia but had no effect on the demand for intravenous supplementation or the rate of permanent hypocalcaemia compared with no prophylaxis (Fig. 2; OR 0.48 [95% CI, 0.31–0.74]; P < .001). Utilizing the GRADE tool, this evidence was considered of moderate quality (see Table S1, Supplemental Content, which illustrates the quality of this outcome and the strength of the evidence).
Figure 2.
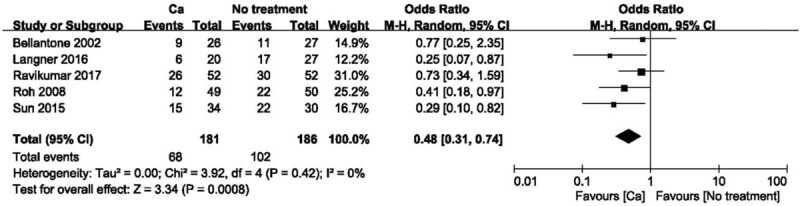
Forest plot of comparing calcium vs no treatment on the incidence of hypocalcemia. Ca = calcium, CI = confidence interval, OR = odds ratio.
3.2.2. Calcium plus VD vs no treatment
The use of calcium plus VD or its metabolites significantly reduced the rate of transient hypocalcaemia (Fig. 3; OR 0.21 [95% CI, 0.11–0.40]; P < .001) and the demand for intravenous supplementation (Fig. 4; OR 0.26 [95% CI, 0.10–0.69]; P = .007), but did not affect the rate of permanent hypocalcaemia compared to no treatment. There was considerable heterogeneity in hypocalcaemia rates when comparing calcium plus VD vs no treatment (P = .03; I2 = 60%) based on the funnel plot constructed to determine the extent of heterogeneity (see Fig. S1, Supplemental Content, which shows the studies that contributed to the heterogeneity). After 1 study that had extraordinary heterogeneity was excluded, the results were unchanged although the 95% CI was narrower (Fig. 5; OR 0.17 [95% CI, 0.09–0.32]; P < .001). Using the GRADE tool, the evidence for comparison between calcium plus VD vs no treatment was considered of moderate quality (see Table S2, Supplemental Content, which illustrates the quality of this outcome and the strength of the evidence).
Figure 3.
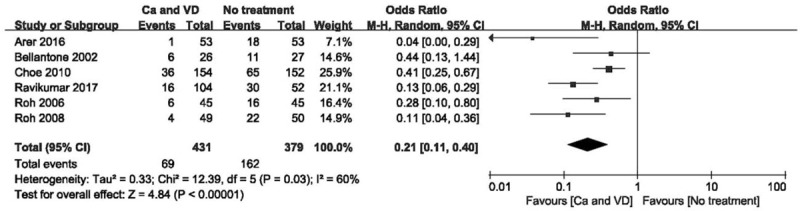
Forest plot of comparing calcium plus VD vs no treatment on the incidence of hypocalcemia. Ca = calcium, CI = confidence interval, OR = odds ratio, VD = vitamin D.
Figure 4.
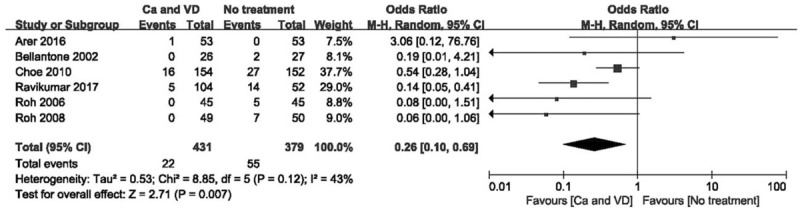
Forest plot of comparing calcium plus VD vs no treatment on the demand for intravenous supplementation. Ca = calcium, CI = confidence interval, OR = odds ratio, VD = vitamin D.
Figure 5.
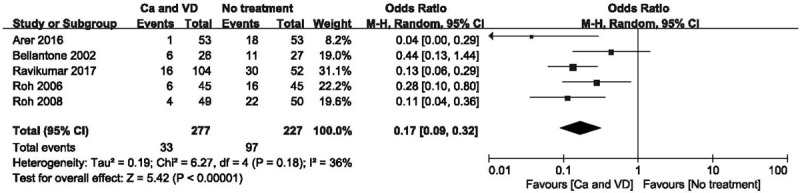
Forest plot of comparing calcium plus VD vs no treatment on the incidence of hypocalcemia after the study with high heterogeneity was excluded. Ca = calcium, CI = confidence interval, OR = odds ratio, VD = vitamin D.
3.2.3. Calcium plus VD vs calcium alone
When compared with calcium supplementation alone, calcium plus VD supplementation significantly reduced the risk of transient hypocalcaemia (Fig. 6; OR 0.39 [95% CI, 0.18–0.84]; P = .02) and the demand for intravenous supplementation (Fig. 7; OR 0.18 [95% CI, 0.07–0.47]; P < .001) but did not decrease the rate of permanent hypocalcaemia. The source of heterogeneity when comparing the demand for intravenous supplementation was determined by funnel plot (see Fig. S2, Supplemental Content, which shows the studies that caused heterogeneity). After the study with extreme heterogeneity was excluded, the results remained significant (Fig. 8; OR 0.62 [95% CI, 0.41–0.93]; P = .02). Using the GRADE tool, this evidence was deemed to be of moderate quality (see Table S3, Supplemental Content, which illustrates the quality of this outcome and the strength of the evidence).
Figure 6.
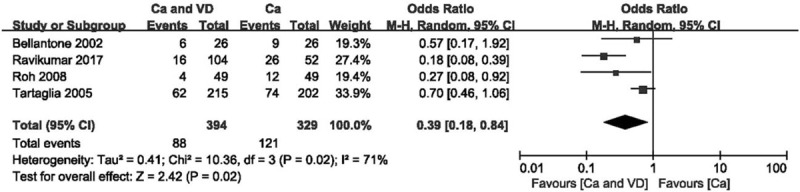
Forest plot of comparing calcium plus VD vs calcium alone on the incidence of hypocalcemia. Ca = calcium, CI = confidence interval, OR = odds ratio, VD = vitamin D.
Figure 7.
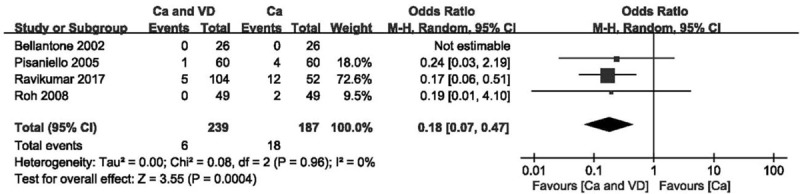
Forest plot of comparing calcium plus VD vs calcium alone on the demand for intravenous supplementation. Ca = calcium, CI = confidence interval, OR = odds ratio, VD = vitamin D.
Figure 8.
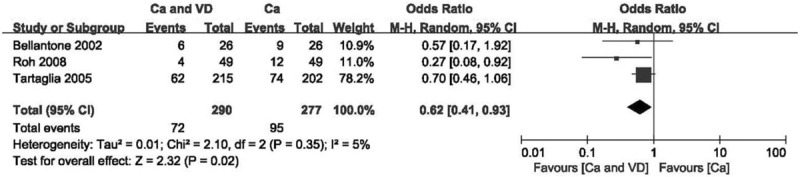
Forest plot of comparing calcium plus VD vs calcium alone on the incidence of hypocalcemia after the study with high heterogeneity was excluded. Ca = calcium, CI = confidence interval, OR = odds ratio, VD = vitamin D.
4. Discussion
Thyroid cancer, especially papillary thyroid cancer (PTC), is one of the most rapidly growing cancers in the world.[41] One of the most common characteristics of PTC is susceptibility to central lymphoid node metastasis; therefore, thyroidectomy plus routine central neck dissection is recommended for the treatment of PTC in many countries.[42,43] Although central neck dissection reduces the risk of locoregional recurrence, this procedure has been shown to significantly increase the rate of postoperative temporary hypocalcaemia.[44]
Symptomatic hypocalcaemia exposes patients to physical and mental suffering, and several measures to reduce the risk of postoperative hypocalcaemia have been suggested. These mainly include modifying surgical procedures such as reducing the extent of thyroid surgery,[45] application of alternative surgical instruments,[46,47] and routine or selected calcium and VD supplementation after thyroidectomy.[48] Various predictors of hypocalcaemia following thyroid surgery have been investigated to direct the use of calcium with or without VD supplementation; however, reproducible, reliable, and stable predictors have not yet been established.[48–51] Therefore, routine supplementation of calcium and VD is advocated in many clinical centers.[52–54] Our meta-analysis found that prophylactic calcium supplementation can significantly reduce the risk of postoperative hypocalcaemia, and that this effect is reinforced if calcium and VD are administered together. Moreover, our analysis is the first to reveal that combined calcium plus VD therapy can significantly decrease the demand for intravenous calcium supplementation. Thus, calcium can be administered routinely after thyroid surgery to reduce the risk of postoperative hypocalcaemia. Calcium plus VD administration should be reserved for patients who are more likely to develop hypocalcaemia, such as those who undergo parathyroid glands implantation, central lymph node dissection, or extensive thyroid surgery, for patients with recurrent neck surgery, patients who are known to have vitamin D deficiency, patients who have postoperative PTH below lower limit of normal, as calcium supplementation alone may not decrease the risk of requiring intravenous supplementation for such high-risk individuals. Routine calcium plus VD administration for high-risk patients may also reduce the incidence of readmission, especially as prolonged hospitalization increases medical costs. Results of your study support ACCE/ACE Disease State Clinical Review and American Thyroid Association Statement on Postoperative Hypoparathyroidism regarding the recommendations for an empirical prophylactic approach for managing potential post-thyroidectomy hypocalcemia.[55,56] However, the variability in cut-off values used to define hypocalcaemia, the dose and form of administered supplements, and the indications for intravenous supplementation limit the strength of evidence. Besides, this analysis had some limitations. First, inclusion criteria that only English articles were included might contribute to bias. Second, the number of patients that treated with intravenous supplementation and patients diagnosed as permanent hypocalcemia were relatively small. Only 1 publication reported an adverse effect of supplementation in the form of mild gastric pyrosis, mostly in patients undergoing long-lasting therapies.[30] Therefore, adverse effects were not considered in our analysis.
Our results are consistent with those of the meta-analysis conducted by Alhefdhi et al involving 2285 patients enrolled in 9 studies, although they only included a single cohort study.[23] Another meta-analysis assessed various preventative and surgical measures performed to decrease the rate of post-thyroidectomy hypocalcaemia,[57] in which combined calcium and VD supplements were found to decrease the rates of transient postoperative hypocalcaemia significantly more than either calcium alone or no supplements.
5. Conclusion
The results of our meta-analysis verified that the prophylactic administration of calcium is effective for reducing the risk of postoperative hypocalcaemia. Calcium plus VD was more effective than calcium alone in preventing immediate postoperative hypocalcaemia and in decreasing the demand for intravenous calcium supplementation. Routine supplementation may allow earlier and safer patient discharge, but could also lead to overtreatment in some patients with normal serum calcium. Therefore, further investigations should be considered to evaluate the applicability of this management strategy in actual clinical practice.
Author contributions
Data curation: Yiyi Hu.
Formal analysis: Yiyi Hu.
Methodology: Tengfei Xing.
Software: Yiyi Hu, Bin Wang.
Validation: Bin Wang.
Writing – original draft: Tengfei Xing.
Writing – review & editing: Jingqiang Zhu.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, OR = odds ratio, PTC = papillary thyroid cancer, RCT = randomized controlled trial, VD = vitamin D.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Gross ND. Postoperative hypocalcemia-the difference a definition makes. Commentary. Head Neck 2010;32:283–4. [DOI] [PubMed] [Google Scholar]
- [2].Cmilansky P, Mrozova Hypocalcemia - the most common complication after total thyroidectomy. Bratisl Lek Listy 2014;115:175. [DOI] [PubMed] [Google Scholar]
- [3].Prowse SJ, Sethi N, Ghosh A. Temporary hypocalcemia is one of the most common complications of total thyroidectomy. Ann Otol Rhinol Laryngol 2012;121:827. [DOI] [PubMed] [Google Scholar]
- [4].Wingert DJ, Friesen SR, Iliopoulos JI, et al. Post-thyroidectomy hypocalcemia. Incidence and risk factors. Am J Surg 1986;152:606–10. [DOI] [PubMed] [Google Scholar]
- [5].Wiseman JE, Mossanen M, Ituarte PH, et al. An algorithm informed by the parathyroid hormone level reduces hypocalcemic complications of thyroidectomy. World J Surg 2010;34:532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jaan S, Sehgal A, Wani RA, et al. Usefulness of pre- and post-operative calcium and vitamin D supplementation in prevention of hypocalcemia after total thyroidectomy: A randomized controlled trial. Ind J Endocrinol Metab 2017;21:51–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fong J, Khan A. Hypocalcemia: updates in diagnosis and management for primary care. Can Fam Physician 2012;58:158–62. [PMC free article] [PubMed] [Google Scholar]
- [8].Groman RP. Acute management of calcium disorders. Top Compan Anim Med 2012;27:167–71. [DOI] [PubMed] [Google Scholar]
- [9].Bilezikian JP, Brandi ML, Cusano NE, et al. Management of hypoparathyroidism: present and future. J Clin Endocrinol Metab 2016;101:2313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Al-Azem H, Khan AA. Hypoparathyroidism. Best Pract Res Clin Endocrinol Metab 2012;26:517–22. [DOI] [PubMed] [Google Scholar]
- [11].Oikonomou D, Laina A, Xydaki A, et al. Steroid-induced hypocalcaemia with tetany in a patient with hypoparathyroidism. BMJ Case Rep 2014;2014: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].An CM, Tang PZ, Zhang A. Prediction and treatment of the hypocalcemia after total thyroidectomy. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2009;44:698. [PubMed] [Google Scholar]
- [13].Bellantone R, Lombardi CP, Raffaelli M, et al. Is routine supplementation therapy (calcium and vitamin D) useful after total thyroidectomy? Surgery 2002;132:1109–12. discussion 1112-1103. [DOI] [PubMed] [Google Scholar]
- [14].Roh JL, Park JY, Park CI. Prevention of postoperative hypocalcemia with routine oral calcium and vitamin D supplements in patients with differentiated papillary thyroid carcinoma undergoing total thyroidectomy plus central neck dissection. Cancer 2009;115:251–8. [DOI] [PubMed] [Google Scholar]
- [15].Arer IM, Kus M, Akkapulu N, et al. Prophylactic oral calcium supplementation therapy to prevent early post thyroidectomy hypocalcemia and evaluation of postoperative parathyroid hormone levels to detect hypocalcemia: a prospective randomized study. Int J Surg 2017;38:9–14. [DOI] [PubMed] [Google Scholar]
- [16].Ravikumar K, Sadacharan D, Muthukumar S, et al. A prospective study on role of supplemental oral calcium and vitamin d in prevention of postthyroidectomy hypocalcemia. Indian J Endocrinol Metab 2017;21:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Karatzanis AD, Ierodiakonou DP, Fountakis ES, et al. Postoperative day 1 levels of parathyroid as predictor of occurrence and severity of hypocalcaemia after total thyroidectomy. Head Neck 2018;40:1040–5. [DOI] [PubMed] [Google Scholar]
- [18].Lee GH, Ku YH, Kim HI, et al. Vitamin D level is not a predictor of hypocalcemia after total thyroidectomy. Langenbecks Arch Surg 2015;400:617–22. [DOI] [PubMed] [Google Scholar]
- [19].Pradeep PV, Ramalingam K. Postoperative PTH measurement is not a reliable predictor for hypocalcemia after total thyroidectomy in vitamin D deficiency: prospective study of 203 cases. World J Surg 2014;38:564–7. [DOI] [PubMed] [Google Scholar]
- [20].Grainger J, Ahmed M, Gama R, et al. Post-thyroidectomy hypocalcemia: impact on length of stay. Ear Nose Throat J 2015;94:276–81. [DOI] [PubMed] [Google Scholar]
- [21].Abdollahi A, Nakhjavani M, Alibakhshi A, et al. Is there any relationship between serum level of Vitamin D and postoperative hypocalcemia after total thyroidectomy? Biomed Pharmacol J 2017;10:207–11. [Google Scholar]
- [22].Testa A, Fant V, De Rosa A, et al. Calcitriol plus hydrochlorothiazide prevents transient post-thyroidectomy hypocalcemia. Horm Metab Res 2006;38:821–6. [DOI] [PubMed] [Google Scholar]
- [23].Kurukahvecioglu O, Karamercan A, Akin M, et al. Potential benefit of oral calcium/vitamin D administration for prevention of symptomatic hypocalcemia after total thyroidectomy. Endocr Regul 2007;41:35–9. [PubMed] [Google Scholar]
- [24].Goossen K, Tenckhoff S, Probst P, et al. Optimal literature search for systematic reviews in surgery. Langenbecks Arch Surg 2018;403:119–29. [DOI] [PubMed] [Google Scholar]
- [25].Choe JH, Kim WW, Lee SK, et al. Comparison of calcitriol versus cholecalciferol therapy in addition to oral calcium after total thyroidectomy with central neck lymph node dissection: a prospective randomized study. Head Neck 2011;33:1265–71. [DOI] [PubMed] [Google Scholar]
- [26].Docimo G, Ruggiero R, Casalino G, et al. Risk factors for postoperative hypocalcemia. Updates Surg 2017;69:255–60. [DOI] [PubMed] [Google Scholar]
- [27].Mehanna HM, Jain A, Randeva H, et al. Postoperative hypocalcemia--the difference a definition makes. Head Neck 2010;32:279–83. [DOI] [PubMed] [Google Scholar]
- [28].Lorente-Poch L, Sancho JJ, Munoz-Nova JL, et al. Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surg 2015;4:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Roh JL, Park CI. Routine oral calcium and vitamin D supplements for prevention of hypocalcemia after total thyroidectomy. Am J Surg 2006;192:675–8. [DOI] [PubMed] [Google Scholar]
- [30].Pisaniello D, Parmeggiani D, Piatto A, et al. Which therapy to prevent post-thyroidectomy hypocalcemia? G Chir 2005;26:357–61. [PubMed] [Google Scholar]
- [31].Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. [DOI] [PubMed] [Google Scholar]
- [32].Wang X, Cheng W, Ma Y, et al. Vitamin D receptor gene FokI but not TaqI, ApaI, BsmI polymorphism is associated with Hashimoto's thyroiditis: a meta-analysis. Sci Rep 2017;7:41540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [34].Alhefdhi A, Mazeh H, Chen H. Role of postoperative vitamin D and/or calcium routine supplementation in preventing hypocalcemia after thyroidectomy: a systematic review and meta-analysis. Oncologist 2013;18:533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Langner E, Tincani AJ, Negro AD. Use of prophylactic oral calcium after total thyroidectomy: a prospective study. Arch Endocrinol Metab 2017;61:447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sun R, Zhang J, Zhang F, et al. Selectively predictive calcium supplementation using NCCN risk stratification system after thyroidectomy with differentiated thyroid cancer. Int J Clin Exp Med 2015;8:21939–46. http://cochranelibrary-wiley.com/o/cochrane/clcentral/articles/237/CN-01134237/frame.htmlhttp://cochranelibrary-wiley.com/o/cochrane/clcentral/articles/237/CN-01134237/frame.html. Accessed November 7, 2018. [PMC free article] [PubMed] [Google Scholar]
- [38].Tartaglia F, Giuliani A, Sgueglia M, et al. Randomized study on oral administration of calcitriol to prevent symptomatic hypocalcemia after total thyroidectomy. Am J Surg 2005;190:424–9. [DOI] [PubMed] [Google Scholar]
- [39].Genser L, Tresallet C, Godiris-Petit G, et al. Randomized controlled trial of alfacalcidol supplementation for the reduction of hypocalcemia after total thyroidectomy. Am J Surg 2014;207:39–45. [DOI] [PubMed] [Google Scholar]
- [40].Uruno T, Miyauchi A, Shimizu K, et al. A prophylactic infusion of calcium solution reduces the risk of symptomatic hypocalcemia in patients after total thyroidectomy. World J Surg 2006;30:304–8. [DOI] [PubMed] [Google Scholar]
- [41].Sahli ZT, Smith PW, Umbricht CB, et al. Preoperative molecular markers in thyroid nodules. Front Endocrinol 2018;9:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang YL, Lin P. The diagnosis and treatment of thyroid microcarcinoma. J Clin Otorhinolaryngol Head Neck Surg 2016;30:1251–3. [DOI] [PubMed] [Google Scholar]
- [43].Sancho JJ, Lennard TW, Paunovic I, et al. Prophylactic central neck disection in papillary thyroid cancer: a consensus report of the European Society of Endocrine Surgeons (ESES). Langenbecks Arch Surg 2014;399:155–63. [DOI] [PubMed] [Google Scholar]
- [44].Zhao W, You L, Hou X, et al. The effect of prophylactic central neck dissection on locoregional recurrence in papillary thyroid cancer after total thyroidectomy: a systematic review and meta-analysis: pCND for the locoregional recurrence of papillary thyroid cancer. Ann Surg Oncol 2017;24:2189–98. [DOI] [PubMed] [Google Scholar]
- [45].Lee DY, Cha W, Jeong WJ, et al. Preservation of the inferior thyroidal vein reduces post-thyroidectomy hypocalcemia. Laryngoscope 2014;124:1272–7. [DOI] [PubMed] [Google Scholar]
- [46].Su AP, Wang B, Gong YP, et al. Carbon nanoparticles facilitate lymph nodes dissection and parathyroid glands identification in reoperation of papillary thyroid cancer. Medicine 2017;96:e8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sista F, Schietroma M, Ruscitti C, et al. New ultrasonic dissector versus conventional hemostasis in thyroid surgery: a randomized prospective study. J Laparoendosc Adv Surg Tech Part A 2012;22:220–4. [DOI] [PubMed] [Google Scholar]
- [48].Nahas ZS, Farrag TY, Lin FR, et al. A safe and cost-effective short hospital stay protocol to identify patients at low risk for the development of significant hypocalcemia after total thyroidectomy. Laryngoscope 2006;116:906–10. [DOI] [PubMed] [Google Scholar]
- [49].Payne RJ, Hier MP, Tamilia M, et al. Same-day discharge after total thyroidectomy: the value of 6-hour serum parathyroid hormone and calcium levels. Head Neck 2005;27:1–7. [DOI] [PubMed] [Google Scholar]
- [50].Diez Alonso M, Sanchez Lopez JD, Sanchez-Seco Pena MI, et al. Serum PTH levels as a predictive factor of hypocalcaemia after total thyroidectomy. Cir Esp 2009;85:96–102. [DOI] [PubMed] [Google Scholar]
- [51].Moriyama T, Yamashita H, Noguchi S, et al. Intraoperative parathyroid hormone assay in patients with Graves’ disease for prediction of postoperative tetany. World J Surg 2005;29:1282–7. [DOI] [PubMed] [Google Scholar]
- [52].Docimo G, Tolone S, Ruggiero R, et al. Total thyroidectomy without prophylactic central neck dissection combined with routine oral calcium and vitamin D supplements: is it a good option to achieve a low recurrence rate avoiding hypocalcemia? A retrospective study. Minerva Chir 2013;68:321–8. [PubMed] [Google Scholar]
- [53].Singer MC, Bhakta D, Seybt MW, et al. Calcium management after thyroidectomy: a simple and cost-effective method. Otolaryngol Head Neck Surg 2012;146:362–5. [DOI] [PubMed] [Google Scholar]
- [54].Sanabria A, Dominguez LC, Vega V, et al. Cost-effectiveness analysis regarding postoperative administration of vitamin-D and calcium after thyroidectomy to prevent hypocalcaemia. Rev Salud Publica (Bogota, Colombia) 2011;13:804–13. [DOI] [PubMed] [Google Scholar]
- [55].Orloff LA, Wiseman SM, Bernet VJ, et al. American thyroid association statement on postoperative hypoparathyroidism: diagnosis, prevention, and management in adults. Thyroid 2018;28:830–41. [DOI] [PubMed] [Google Scholar]
- [56].Stack BC, Jr, Bimston DN, Bodenner DL, et al. American association of clinical endocrinologists and American college of endocrinology disease state clinical review: postoperative hypoparathyroidism--definitions and management. Endocr Pract 2015;21:674–85. [DOI] [PubMed] [Google Scholar]
- [57].Antakia R, Edafe O, Uttley L, et al. Effectiveness of preventative and other surgical measures on hypocalcemia following bilateral thyroid surgery: a systematic review and meta-analysis. Thyroid 2015;25:95–106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


