Abstract
Recent studies reported that high doses of short-acting loop diuretics are associated with poor outcomes in patients with heart failure (HF). Short-acting loop diuretics have been shown to activate the renin-angiotensin system (RAS) and have no favorable effects on cardiac sympathetic nervous system (SNS) activity. The goal of this study is to investigate the relationship between daily doses of furosemide and the outcomes of patients with left ventricular dysfunction (LVD) from the viewpoint of cardiac SNS abnormalities using iodine-123-labeled metaiodobenzylguanidine (123l-MIBG) myocardial scintigraphy.
We enrolled 137 hospitalized patients (62.5 ± 14.2 years old, 103 men) with LVEF < 45% who underwent 123l-MIBG myocardial scintigraphy. A delayed heart-to-mediastinum ratio (delayed HMR) was assessed using 123l-MIBG scintigraphy. Cardiac events were defined as cardiac death or re-hospitalization due to the deterioration of HF. Cox proportional hazard analysis was used to identify predictors of cardiac events.
Cardiac events occurred in 57 patients in a follow-up period of 33.1 ± 30 months. In a multivariate Cox proportional hazard analysis, delayed HMR and furosemide doses were identified as independent predictors of cardiac events (P = .0042, P = .033, respectively). Inverse probability of treatment weighting Cox modeling showed that the use of furosemide (≥40 mg /day) was associated with cardiac events with a hazard ratio of 1.96 (P = .003). In the Kaplan-Mayer analysis, the cardiac event-free survival rate was significantly lower in patients treated with high doses of furosemide (≥60 mg/day vs 40–60 mg/day vs <40 mg/day, the Log-rank test P < .0001). In a receiver-operating characteristic (ROC) analysis, the cut-off value for cardiac events was 40 mg/day of furosemide. The cardiac event-free rate was significantly lower in patients with delayed HMR <1.8 (median value) and receiving furosemide ≥40 mg/day than in other patients (the Log-rank test P < .0001). Significant differences in cardiac event rates according to furosemide doses among patients with delayed HMR <1.8 were observed among patients without β-blocker therapy (P = .001), but not among those with β-blocker therapy (P = .127).
The present results indicate that a relationship exists between higher doses of furosemide and poor outcomes. The prognosis of HF patients with severe cardiac SNS abnormalities receiving high-dose short-acting loop diuretics is poor.
Keywords: cardiac sympathetic nerve activity, heart failure, outcome, short-acting loop diuretics
1. Introduction
Loop diuretics are widely used as basic therapy to improve volume overload in patients with heart failure (HF). However, the long-term influence of short-acting loop diuretics on the prognosis of patients with HF has not yet been established. Previous studies showed that furosemide, a short-acting loop diuretic, activated the renin-angiotensin system (RAS) and did not have any favorable effects on cardiac sympathetic nervous system (SNS) activity over long-acting loop diuretics.[1,2] An independent, dose-dependent relationship has been reported between short-acting loop diuretic use and an impaired prognosis in patients with advanced HF.[3–6] Eshaghian et al previously showed that the highest diuretic doses (>160 mg per day) had a more negative impact on outcomes than the lowest loop diuretic doses (0–40 mg per day).[3]
Iodine-123-labeled metaiodobenzylguanidine (123I-MIBG) scintigraphy is now an established modality for evaluating cardiac SNS activity.[7–9]123I-MIBG imaging is widely used for risk stratification and predicting the outcomes of patients with HF.[10–13] The aim of the present study is to evaluate the relationship between furosemide doses and patient prognoses from the viewpoint of cardiac SNS activity using 123I-MIBG imaging.
2. Materials and methods
2.1. Study population
A total of 203 patients admitted to Miyazaki University Hospital between January 2005 and December 2010 had left ventricular dysfunction (LVD) with a left ventricular ejection fraction (LVEF) less than 45% on echocardiography, including hospitalization due to acute HF. All patients were hospitalized to treat HF or evaluate its causes. A flow diagram was shown in Figure 1. Patients on hemodialysis, with inflammatory disease, malignant disease, hemorrhagic disease, acute coronary syndrome, psychiatric disease, or autonomic failure related to neurological diseases, such as Parkinson's disease, were excluded. Patients with ischemic heart disease scheduled for revascularization and with severe valvular diseases scheduled for valve replacement were also excluded. No medications that directly affected the uptake or secretion of MIBG had been administered to any patient. We examined 137 patients. All patients were in New York Heart Association (NYHA) functional classes I–III at entry and underwent cardiac 123I-MIBG imaging. This study was approved by the Human Investigation Review Committee of the University of Miyazaki (No. 826) and confirmed to the principles outlined in the Declaration of Helsinki. Informed consent was obtained from all patients.
Figure 1.
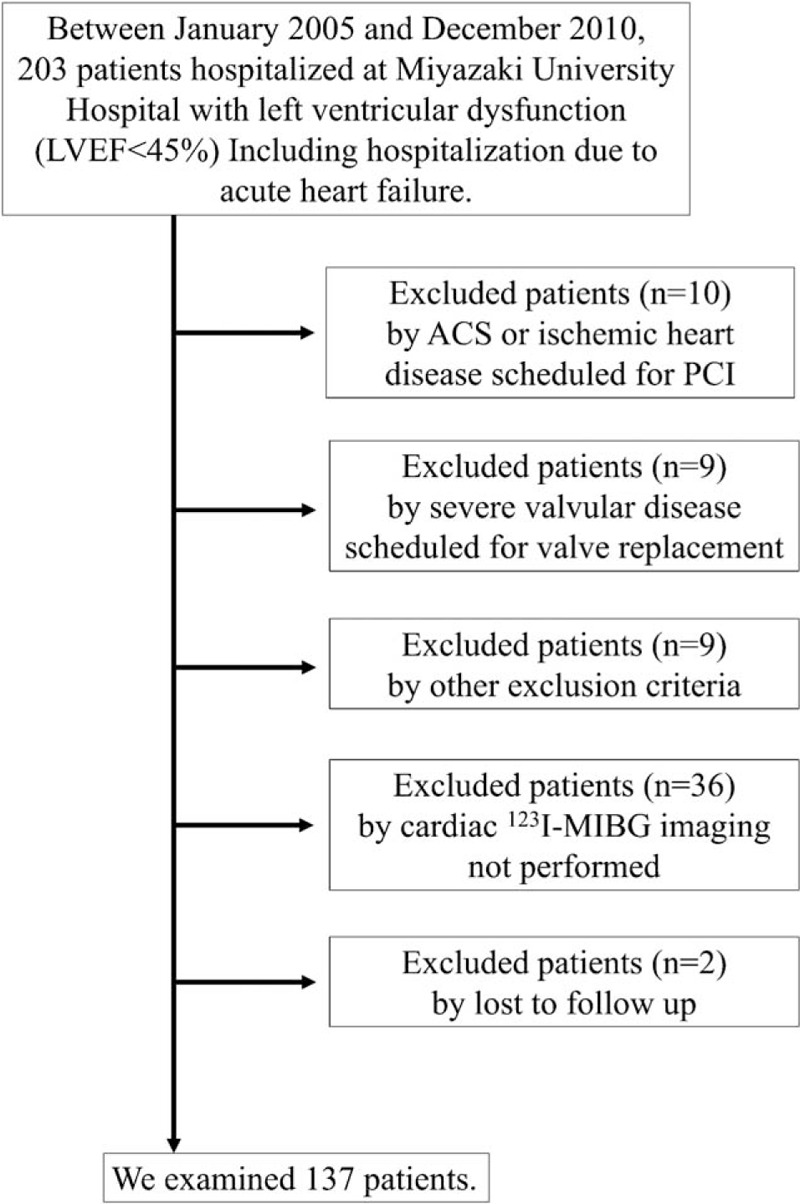
Flow diagram of participants in the present study. ACS = acute coronary syndrome, PCI = percutaneous coronary intervention, LVEF = left ventricular ejection fraction, 123l-MIBG = iodine-123-labeled metaiodobenzylguanidine.
2.2. Data collection and study design
Baseline demographic data, including age, sex, height, weight, history of acute HF at admission, the NYHA functional class, furosemide dose, other medications (e.g., angiotensin-converting enzyme inhibitor: ACE-I, angiotensin receptor blockers: ARB, β-blockers, calcium channel blockers: CCB, spironolactone, statins), comorbid diseases (hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation), underlying diseases (ischemic cardiomyopathy: ICM or non-ICM), heart rate on resting ECG, plasma B-type natriuretic peptide (BNP), blood urea nitrogen (BUN), serum creatinine, total bilirubin, serum uric acid, hemoglobin (Hb), echocardiography data, and parameters of 123I-MIBG imaging, were retrospectively collected from medical records. Estimated GFR (eGFR) was calculated using the Japanese GFR estimated equation [14]: eGFR (in ml/min/1.73 m2) = 194 × (serum creatinine in mg/dl)−1.094 × (age)−0.287 × (0.739 if female). The echocardiographic study, including blood sampling, and MIBG imaging study were performed within 2 weeks of the participants becoming clinically stable. Blood samples were collected to assess plasma human BNP after resting in the supine position for at least 1 week in the morning of the day of the MIBG imaging study. In patients with acute HF at admission, data collection was performed in a stable state after intensive care.
2.3. Echocardiography
Echocardiography was performed using the Sonos 5500 or ATL Philips IE33 Ultrasound machine (Garnerville, NY) with the measurement of standard and Doppler parameters. Echocardiographic data were obtained in the stable phase during hospitalization in patients with acute HF. The left ventricular diastolic dimension (LVDd), left ventricular systolic dimension (LVDs), intraventricular septal diastolic wall thickness (IVSTd), and posterior diastolic wall thickness (LVPWTd) were assessed from the two-dimensional parasternal long-axis view. LVEF was calculated using the modified Simpson method in apical 4-chamber and 2-chamber views. Left ventricular mass (LVM) was estimated by the left ventricular cavity dimension and wall thickness at end-diastole, according to the formulae of a previous study.[15] The left ventricular mass index (LVMI) was then calculated as the ratio of LVM to body surface area.
2.4. 123I-MIBG imaging
All patients underwent resting 123I-MIBG scintigraphy under pain-free conditions after fasting for more than 3 hours. Each dose of 111 MBq of 123l-MIBG (FUJIFILM RI Pharma, Co., Ltd., Tokyo, Japan) was injected intravenously with patients seated. At 30 minutes and 4 hours after the injection, static data were acquired in the anterior view with a double-head commercially available gamma camera (E.CAM; SIEMENS, Wittelsbacherplatz, Munich, Germany) with a low-energy, general purpose collimator. A region of interest (ROI) was selected to encompass the whole heart, and a square ROI was selected to include the upper mediastinum on early and delayed anterior planner projection images for a quantitative analysis of the myocardial accumulation of 123l-MIBG. Counts per pixel were measured for each ROI, and the heart-to-mediastinum (H/M) activity ratio was calculated. The activity of 123l-MIBG was corrected by the time interval and background, and the myocardial washout rate of 123l-MIBG was assessed as the percentage of the change in activity from the early image (early HMR) to delayed image (delayed HMR). The clearance rate from the myocardium (washout rate; WR) was calculated as follows: [early myocardial 123I-MIBG uptake - delayed myocardial 123I-MIBG uptake] / [initial 123I-MIBG uptake] × 100. The normal ranges of delayed HMR and WR in our institution were 2.8 ± 0.43 (SD) and 20.9 ± 7.39 (%) (SD), respectively.
2.5. Outcomes
Cardiac events were defined as death from HF, lethal arrhythmia and sudden cardiac death (SCD), the need for cardiac transplantation, and unexpected hospitalization due to the deterioration of HF. SCD was defined as witnessed cardiac arrest and death within 1 hour after onset of acute symptoms or unexpected death in patients known to have been well within the previous 24 hours. Information on cardiac events was collected from medical records or contact with cardiologists. All patients were followed up until October 2013 unless a major end-point terminated the follow-up.
2.6. Statistical analyses
Values are expressed as the mean ± standard deviation. Mean values for continuous variables were compared using the Wilcoxon rank-sum test and the frequencies of categorical variables were compared using the chi-squared test or χ2 test. Variables that predicted outcomes in univariate analyses (P < .05) were entered into multivariate analyses, and their significance as independent predictors of cardiac events was assessed using Cox proportional hazards regression model. In addition, to reduce the impact of treatment selection bias and potential confounders in an observational study, we performed weighted Cox proportional hazards regression models using an inverse probability of treatment weighting (IPTW). Kaplan-Meier survival curves were compared using the Log-rank test. A receiver-operating characteristic (ROC) analysis was performed to obtain cut-off values for parameters predicting cardiac events. Significance was defined as P < .05. All data were statistically analyzed using JMP version 9.1 (SAS Institute Inc.) and software R version 3.5.0 (http://www.r-project.org/).
The datasets analyzed during the present study are available from the corresponding author on reasonable request.
3. Results
3.1. Clinical characteristics of patients with and without cardiac events
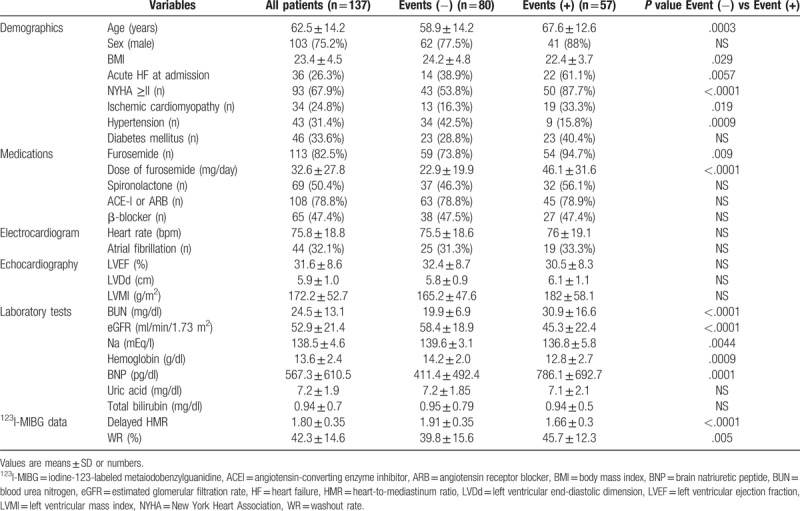
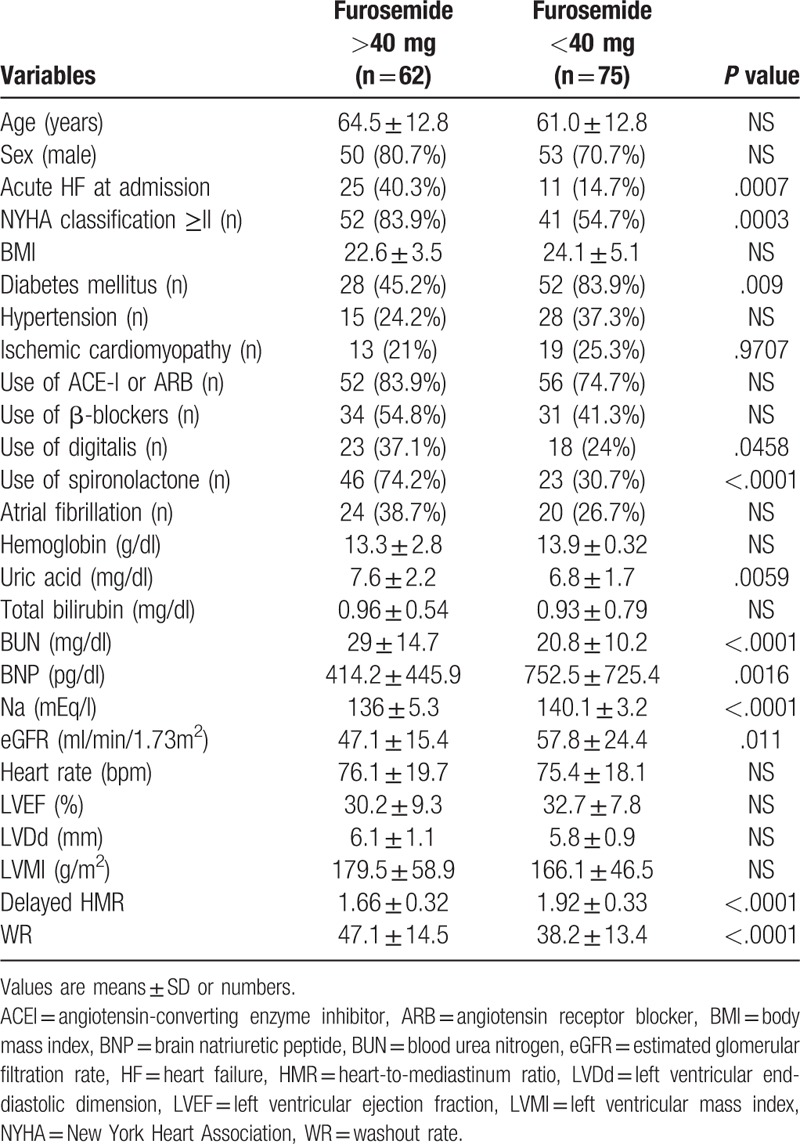
The baseline characteristics of patients in this study are shown in Table 1. The median age of patients was 64 years (27–89 years) and there were 103 males (75.2%). The number of patients with acute HF at admission was 36 (26.3%). Ninety-three patients (67.9%) had NYHA functional class ≥II. The number of patients with ischemic cardiomyopathy, chronic atrial fibrillation, hypertension, and diabetes mellitus were 34 (24.8%), 44 (32.1%), 43 (31.4%), and 46 (33.6%), respectively. The number of patients receiving furosemide, spironolactone, ACE-I, or ARB and β-blockers were 113 (82.5%), 69 (50.4%), 108 (78.8%), and 65 (47.4%), respectively. The median values of LVEF, eGFR, Hb, and BNP were 32% (10–44%), 51.3 ml/min/1.73 m2 (10.8–158.1), 13.9 g/dl (6.5–20.5), and 355.8 pg/ml (14.8–3480), respectively. The median dose of furosemide administered was 20 mg/day (0–160). Cardiac death occurred in 36 patients (26.3%), while 21 patients (15.3%) had unexpected hospitalization in the follow-up period of 33.1 ± 30 months. 26 patients died of HF and 10 patients had arrhythmic death or SCD. No significant differences were observed in gender, uric acid, total bilirubin, LVEF, LVDd, LVMI, or the prevalence of atrial fibrillation. Patients with cardiac events were significantly older (P = .0003) and had a higher proportion of acute HF at admission (P = .0057) and a higher prevalence of ICM (P = .019). The NYHA class was significantly worse in patients with cardiac events (P < .0001). The dose of furosemide administered was significantly higher in patients with cardiac events (P < .0001). No significant differences were observed in the administration of other medications such as ACE-I or ARB, aldosterone blockers, and β-blockers between patients with and without cardiac events. Serum sodium (P = .0044), eGFR (P < .0001), and Hb (P = .0009) were significantly lower, while BUN (P < .0001) and plasma BNP (P = .0001) were significantly higher in patients with cardiac events. In parameters of 123I-MIBG scintigraphy, delayed HMR was significantly lower (P < .0001) and WR was significantly higher (P = .005) in patients with cardiac events (Table 1). The percentage of patients treated with β-blocker therapy was low at baseline (47.4%), but increased at discharge (73.7%). The ratio of patients treated with β-blocker therapy was not significantly different between patients with and without cardiac events at discharge (66.7% vs 78.8%).
Table 1.
Clinical characteristics of with events and without events groups.
3.2. Evaluation of factors predicting outcomes
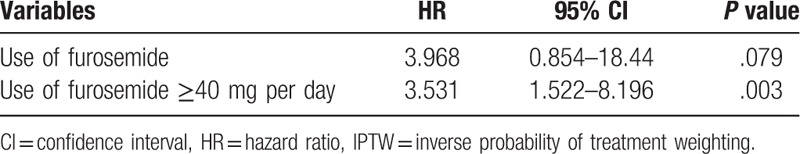
Table 2 shows the determinants of cardiac events identified in univariate and multivariate Cox hazard regression analyses. Hypertension (HR: 0.435, P = .026), ICM (HR: 2.51, P = .008), the dose of furosemide (HR: 1.016, P = .0042), and delayed HMR (HR: 0.212, P = .033) were identified as independent predictors of cardiac events. In the ROC analysis, the cut-off value for the dose of furosemide to predict cardiac events was 40 mg per day. IPTW Cox regression hazard analyses revealed that the use of high-dose furosemide (≥40 mg per day) correlated with a poor prognosis (adjusted HR, 3.531; 95% CI: 1.522–8.196, P = .003, Table 3).
Table 2.
Univariate and multivariate Cox hazard regression analysis of characteristics for cardiac events.
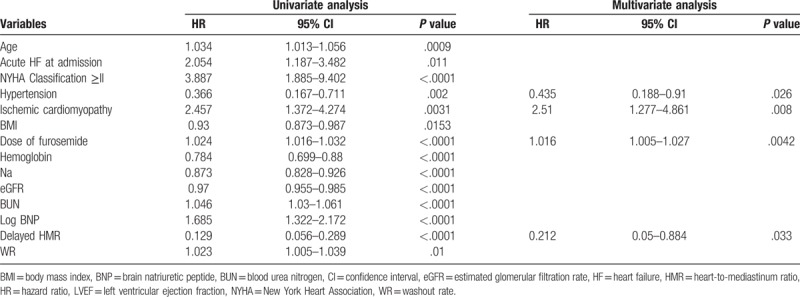
Table 3.
IPTW Cox hazard multivariate regression analysis of characteristics for cardiac events.
3.3. Relationship between furosemide doses and cardiac events
In the Kaplan-Mayer analysis, the cardiac event-free survival rate was significantly lower in patients treated with high doses of furosemide (≥60 mg per day vs 40–60 mg per day vs <40 mg per day, the Log-rank test P < .0001, Fig. 2).
Figure 2.
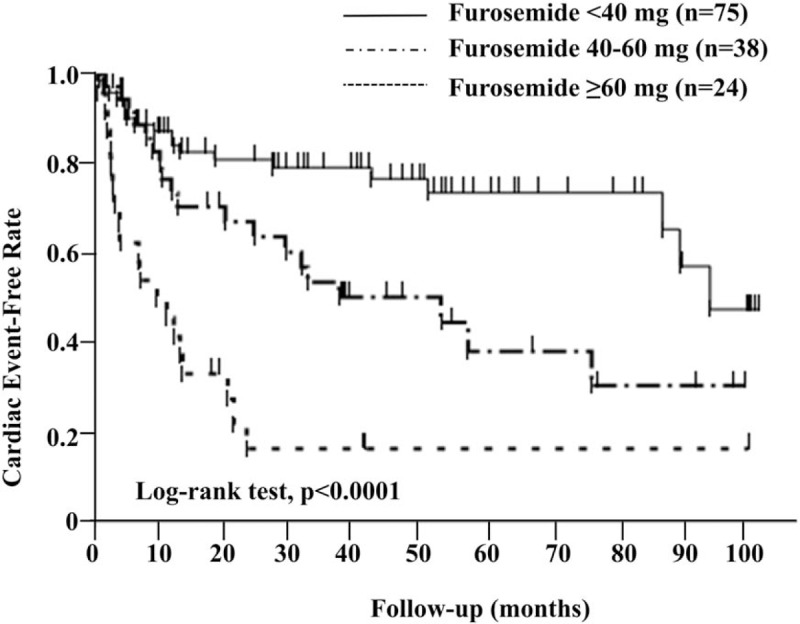
Kaplan-Meier event-free curves according to furosemide doses for cardiac events.
3.4. Relationship between clinical parameters and furosemide doses
The relationships between the dose of furosemide and clinical parameters were shown in Table 4. The proportion of acute HF at admission was significantly higher (P = .0007) and the NYHA functional class was significantly worse in patients receiving furosemide ≥40 mg per day (P = .0003). Serum sodium (P < .0001) and eGFR (P = .011) were also significantly lower. Furthermore, plasma BNP (P = .0016), BUN (P < .0001), and uric acid (P = .0059) were significantly higher than in patients treated with furosemide <40 mg per day. Regarding scintigraphic parameters, delayed HMR was significantly lower (P < .0001) and WR was significantly higher (P < .0001) in patients receiving furosemide ≥40 mg per day than in those receiving <40 mg per day.
Table 4.
Relationships between furosemide doses and clinical parameters.
3.5. Impact of the combination of cardiac SNS abnormalities and furosemide doses on outcomes
When stratified into a higher delayed HMR group (≥1.8) and lower delayed HMR group (<1.8) according to the median value for delayed HMR, the cardiac event-free survival curve was significantly lower in patients with delayed HMR (<1.8) and receiving furosemide ≥40 mg per day than in other patients in the Kaplan-Mayer analysis (the Log-rank test P < .0001, Fig. 3). Furthermore, among patients with delayed HMR <1.8, an increase in the cardiac event rate according to the dose of furosemide was observed in patients without β-blocker therapy (P = .0011), but not in those with β-blocker therapy (Fig. 4).
Figure 3.
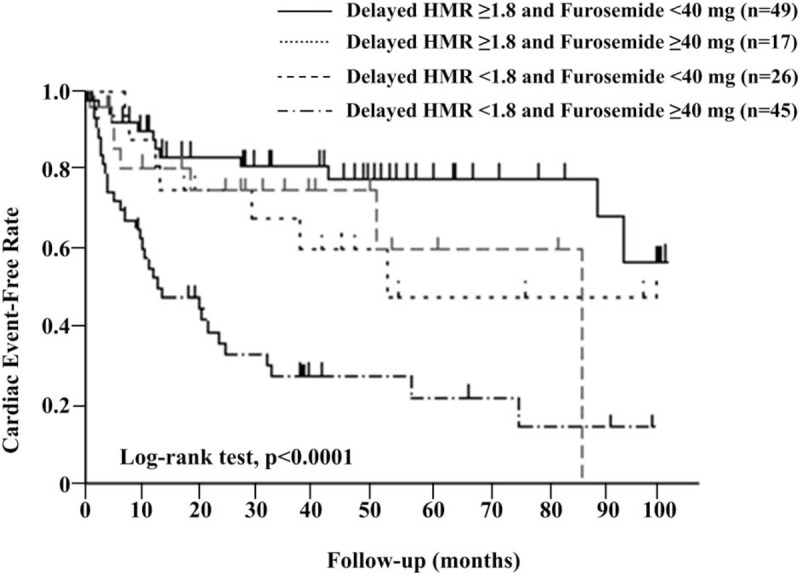
Kaplan-Meier event-free curves according to the combination of furosemide doses and delayed HMR for cardiac events. HMR = heart-to-mediastinum ratio.
Figure 4.
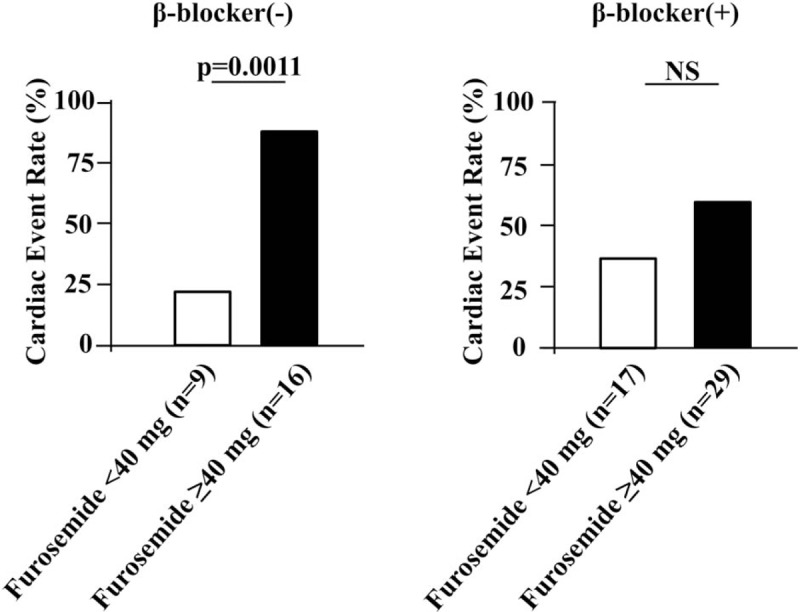
Cardiac event rate according to furosemide doses with and without β-blocker treatments among patients with delayed HMR <1.8. HMR = heart-to-mediastinum ratio.
4. Discussion
In the present study, the dose of furosemide was identified as an independent predictor of cardiac death or unexpected hospitalization due to the deterioration of CHF in patients with LVD. The cut-off value for the furosemide dose to predict cardiac events was 40 mg per day. Background-adjusted IPTW analyses identified the use of high-dose furosemide (≥40 mg per day) as a significant determinant for a poor prognosis. Furthermore, cardiac SNS activity was more abnormal in patients treated with high doses of furosemide. In addition, the prognosis of patients with cardiac SNS abnormalities and receiving high doses of furosemide was poor.
Diuretic therapy improves clinical symptoms in patients with HF. However, the adverse effects of short-acting loop diuretics need to be considered. Bayliss et al previously reported that furosemide activated plasma renin activity.[31] Recent studies revealed that long-acting loop diuretics had a number of advantages over short-acting loop diuretics. Kasama et al showed that torasemide improved cardiac SNS activity and LV remodeling in patients with CHF, whereas furosemide had no favorable effects.[1] Furthermore, Matsuo et al demonstrated that azosemide produced more favorable changes on 123I-MIBG scintigraphy than furosemide in patients with CHF.[32] Masuyama et al reported that azosemide reduced the risk of cardiovascular death or re-hospitalization due to the deterioration of CHF more than furosemide.[33] They speculated an impact on cardiac SNS activity as a reason for the prognostic difference between azosemide and furosemide, but were unable to provide an explanation from the viewpoint of plasma epinephrine levels.
Experimental studies have suggested neurohumoral and sympathetic activation by short-acting loop diuretics. McCurley et al demonstrated that the acceleration of LVD by furosemide was associated with an increase in plasma aldosterone levels in a tachycardia-induced HF model.[34] Yoshida et al suggested sympathetic activation as a reason for the lack of improvements in mortality for HF treated with furosemide despite reductions in the preload and afterload.[35]
Sympathetic nerve hyperactivity plays an important role in the pathophysiology of HF. SNS hyperactivation also maintains cardiac contractility by releasing norepinephrine (NE) from sympathetic nerve dendrites.[16] In HF patients, the spillover of NE into plasma increases exponentially due to sympathetic nerve hyperactivity.[17]
Furthermore, the cardiac content of NE is markedly reduced.[18] The impaired reuptake of NE by presynaptic neurons may also contribute to decreasing the cardiac content of NE in human HF.[19] In addition, β adrenergic receptors (βARs) are down-regulated in HF. Decreased βAR responsiveness, increased circulating catecholamines, and overall hyposensitivity to adrenergic stress have been observed in patients with failing hearts. Decreased βAR responsiveness is related to changes in G-protein and kinase activities.[16]
SNS activation adversely affects cardiac energy metabolism.[16,20] SNS hyperactivity increases plasma free fatty acid (FFA) levels and modulates the insulin secretion of Langerhans’ pancreatic islets.[16] In an animal model, increased FFA was associated with LVD.[21] Djoussé et al showed that higher plasma FFA was associated with an increased risk of HF.[22] Elevated plasma FFA may increase myocardial FFA uptake. Changes in myocardial fatty acid metabolism reduced glucose utilization. [20] Moreover, a chronic βAR stimulation induced insulin resistance.[23] Cardiac insulin resistance contributes to the development of LVD by reducing cardiac efficiency through a metabolic shift toward fatty acid use.[24] Furthermore, sympathetic overactivation may trigger interstitial matrix remodeling and fibrosis through the induction of matrix metalloproteinase/tissue inhibitor of metalloproteinase activity.[25]
Hence, increased cardiac SNS activity is strongly associated with the deterioration of LV function, the development of lethal ventricular tachyarrhythmia, and a poor prognosis.[26,27]
123I-MIBG is an analog of noradrenaline that reflects cardiac SNS activity.[6,7] Myocardial imaging with MIBG was previously reported to be useful for evaluating disease severity, prognosis, and treatment responses in patients with HF. Delayed HMR and WR are generally used as predictors of the severity and prognosis of HF.[28–30] In the present study, delayed HMR was identified as an independent predictor of cardiac events, which is consistent with previous findings.
Furthermore, the prognosis of patients with cardiac SNS abnormalities (delayed HMR <1.8) and receiving high doses of furosemide (≥40 mg per day) was poor. A difference in cardiac event rates according to the furosemide dose among patients with cardiac SNS abnormalities was noted among patients without β-blocker therapy, but not among those with β-blocker therapy. β-blocker therapy is an established treatment for HF.[36,37] Therefore, patients receiving high doses of short-acting loop diuretics with cardiac SNS abnormalities or without β-blocker therapy need to be carefully monitored.
There were several notable limitations in the present study. This study was small in size and retrospective. Since we selected patients who underwent 123I-MIBG scintigraphy, we cannot deny that the background of this population was biased. In the present study, high doses of furosemide were associated with the severity of HF (Table 4). Patients with more severe HF generally receive larger doses of loop diuretics to improve volume overload. The use of higher doses of furosemide may be employed to identify patients at high risk of mortality regardless of diuretic therapy. In the present study, IPTW Cox regression hazard analysis showed that the use of furosemide was not associated with outcomes; only high doses of furosemide correlated with a poor prognosis. These results indicate that high doses of furosemide are detrimental to the prognosis of patients with HF. Despite the use of IPTW Cox hazard multivariate regression analysis, we cannot completely exclude the selection bias and other unmeasurable confounders. Recently, the evaluation of left ventricular remodeling and myocardial fibrosis using cardiac magnetic resonance (CMR) imaging is considered to be important in predicting the prognosis of patients with HF.[38,39] But we did not focus on left ventricular remodeling in this study. Therefore we entrust the investigation from viewpoint of CMR imaging to a future research.
5. Conclusion
The results of the present study indicate that a relationship exists between higher doses of furosemide and poor outcomes. The prognosis of HF patients with severe cardiac SNS abnormalities receiving high-dose short-acting loop diuretics is poor. 123I-MIBG scintigraphy may be useful for predicting the outcomes of high-risk patients treated with higher doses of short-acting loop diuretics. The present results indicate that patients receiving high doses of furosemide with the overactivation of cardiac SNS activity need to be carefully monitored.
Author contributions
Conceptualization: Hisamitsu Onitsuka.
Data curation: Hisamitsu Onitsuka.
Formal analysis: Hisamitsu Onitsuka.
Investigation: Hisamitsu Onitsuka, Shouhei Koyama, Takeshi Ideguchi, Tetsunori Ishikawa.
Methodology: Hisamitsu Onitsuka.
Software: Shigeki Nagamachi.
Supervision: Tetsunori Ishikawa, Kazuo Kitamura, Shigeki Nagamachi.
Writing – original draft: Hisamitsu Onitsuka.
Writing – review & editing: Hisamitsu Onitsuka.
Hisamitsu Onitsuka orcid: 0000-0001-7003-3586.
Footnotes
Abbreviations: 123l-MIBG = iodine-123-labeled metaiodobenzylguanidine, ACE-I = angiotensin-converting enzyme inhibitor, ARB = angiotensin receptor blocker, BNP = plasma B-type natriuretic peptide, BUN = blood urea nitrogen, CCB = calcium channel blocker, CMR = cardiac magnetic resonance, eGFR = Estimated glomerular filtration rate, Hb = hemoglobin, HF = heart failure, HMR = heart-to-mediastinum activity ratio, ICM = ischemic cardiomyopathy, IPTW = inverse probability of treatment weighting, IVSTd = intraventricular septum thickness at diastole, LVD = left ventricular dysfunction, LVDd = left ventricular diastolic dimension, LVDs = left ventricular systolic dimension, LVEF = left ventricular ejection fraction, LVM= left ventricular mass, LVMI = left ventricular mass index, LVPWTd = left ventricular posterior wall thickness at diastole, NYHA = New York Heart Association, RAS = renin-angiotensin system, ROC = receiver-operating characteristic, SCD = sudden cardiac death, SNS = sympathetic nervous system.
The authors have no conflicts of interest to disclose.
References
- [1].Kasama S, Toyama T, Hatori T, et al. Effects of torasemide on cardiac sympathetic nerve activity and left ventricular remodelling in patients with congestive heart failure. Heart 2006;92:1434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hisatake S, Nanjo S, Fujimoto S, et al. Comparative analysis of the therapeutic effects of long-acting and short-acting loop diuretics in the treatment of chronic heart failure using 123I-metaiodo-benzylguanidine scintigraphy. Eur J Heart Fail 2011;13:892–8. [DOI] [PubMed] [Google Scholar]
- [3].Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol 2006;97:1759–64. [DOI] [PubMed] [Google Scholar]
- [4].Abdel-Qadir HM, Tu JV, Yun L, et al. Diuretic dose and long-term outcomes in elderly patients with heart failure after hospitalization. Am Heart J 2010;160:264–71. [DOI] [PubMed] [Google Scholar]
- [5].Hasselblad V, Gattis Stough W, Shah MR, et al. Califf RM, et al. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail 2007;10:1064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dini FL, Guglin M, Simioniuc A, et al. Association of furosemide dose with clinical status, left ventricular dysfunction, natriuretic peptides, and outcome in clinically stable patients with chronic systolic heart failure. Congest Heart Fail 2012;18:98–106. [DOI] [PubMed] [Google Scholar]
- [7].Glowniak JV. Cardiac studies with metaiodobenzylguanidine: a critique of methods and interpretation of results. J Nucl Med 1995;36:2133–7. [PubMed] [Google Scholar]
- [8].Sisson JC, Shapiro B, Meyers L, et al. Metaiodobenzylguanidine to map scintigraphically the adrenergic nervous system in man. J Nucl Med 1987;28:1625–36. [PubMed] [Google Scholar]
- [9].Kline RC, Swanson DP, Wieland DM, et al. Myocardial imaging in man with I-123 meta-iodobenzylguanidine. J Nucl Med 1981;22:129–32. [PubMed] [Google Scholar]
- [10].Imamura Y, Fukuyama T, Mochizuki T, et al. Prognostic Value of Iodine-123-metaiodobenzylguanidine imaging and cardiac natriuretic peptide levels in patients with left ventricular dysfunction resulting from cardiomyopathy. Jpn Circ J 2001;65:155–60. [DOI] [PubMed] [Google Scholar]
- [11].Christensen TE, Kjaer A, Hasbak P. The clinical value of cardiac sympathetic imaging in heart failure. Clin Physiol Funct Imaging 2014;34:178–82. [DOI] [PubMed] [Google Scholar]
- [12].Merlet P, Valette H, Dubois-Rande JL, et al. Prognostic value of cardiac metaiodobenzylguanidine imaging in patients with heart failure. J Nucl Med 1992;33:471–7. [PubMed] [Google Scholar]
- [13].Narula J, Gerson M, Thomas GS, et al. 123I-MIBG imaging for prediction of mortality and potentially fatal events in heart failure: the ADMIRE-HFX study. J Nucl Med 2015;56:1011–8. [DOI] [PubMed] [Google Scholar]
- [14].Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009;53:982–92. [DOI] [PubMed] [Google Scholar]
- [15].Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. [DOI] [PubMed] [Google Scholar]
- [16].Satulli G, Iaccarino G. Adrenergic signaling in heart failure and cardiovascular aging. Maturitas 2016;93:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Meredith IT, Eisenhofer G, Lambert GW, et al. Cardiac sympathetic nervous activity in congestive heart failure. Evidence for increased neuronal norepinephrine release and preserved neuronal uptake. Circulation 1993;88:136–45. [DOI] [PubMed] [Google Scholar]
- [18].Chidsey CA, Braunwald E, Morrow AG. Catecholamine excretion and cardiac stores of norepinephrine in congestive heart failure. Am J Med 1965;39:442–51. [DOI] [PubMed] [Google Scholar]
- [19].Himura Y, Felten SY, Kashiki M, et al. Cardiac noradrenergic nerve terminal abnormalities in dogs with experimental congestive heart failure. Circulation 1993;88:1299–309. [DOI] [PubMed] [Google Scholar]
- [20].Ciccarelli M, Santulli G, Pascale V, et al. Adrenergic receptors and metabolism: role in development of cardiovascular disease. Front Physiol 2013;4:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sun X, Pan H, Tan H, et al. High free fatty acids level related with cardiac dysfunction in obese rats. Diabetes Res Clin Pract 2012;95:251–9. [DOI] [PubMed] [Google Scholar]
- [22].Djoussé L, Benkeser D, Arnold A, et al. Plasma free fatty acids and risk of heart failure: the cardiovascular health study. Circ Heart Fail 2013;6:964–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cipolletta E, Campanile A, Santulli G, et al. The G protein coupled receptor kinase 2 plays an essential role in beta-adrenergic receptor-induced insulin resistance. Cardiovasc Res 2009;84:407–15. [DOI] [PubMed] [Google Scholar]
- [24].Peterson LR, Herrero P, Schechtman KB, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 2004;109:2191–6. [DOI] [PubMed] [Google Scholar]
- [25].Seeland U, Selejan S, Engelhardt S, et al. Interstitial remodeling in beta1-adrenergic receptor transgenic mice. Basic Res Cardiol 2007;102:183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Brunner-La Rocca HP, Esler MD, Jennings GL, et al. Effect of cardiac sympathetic nervous activity on mode of death in congestive heart failure. Eur Heart J 2001;22:1136–43. [DOI] [PubMed] [Google Scholar]
- [27].Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995;26:1257–63. [DOI] [PubMed] [Google Scholar]
- [28].Momose M, Okayama D, Nagamatsu H, et al. Long-term prognostic stratification by a combination of (123) I- metaiodo benzylguanidine scintigraphy and ejection fraction in dilated cardiomyopathy. Ann Nucl Med 2011;25:419–24. [DOI] [PubMed] [Google Scholar]
- [29].Nakajima K, Nakata T, Yamada T, et al. A prediction model for 5-year cardiac mortality in patients with chronic heart failure using 123I-metaiodobenzylguanidine imaging. Eur J Nucl Med Mol Imaging 2014;41:1673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Agostini D, Verberne HJ, Burchert W, et al. I-123-mIBG myocardial imaging for assessment of risk for a major cardiac event in heart failure patients: insights from a retrospective European multicenter study. Eur J Nucl Med Mol Imaging 2008;35:535–46. [DOI] [PubMed] [Google Scholar]
- [31].Bayliss J, Norell M, Canepa-Anson R, et al. Untreated heart failure: clinical and neuroendocrine effects of introducing diuretics. Br Heart J 1987;57:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Matsuo Y, Kasama S, Toyama T, et al. Comparative effects of long-acting and short-acting loop diuretics on cardiac sympathetic nerve activity in patients with chronic heart failure. Open Heart 2016;2: 3:e000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Masuyama T, Tsujino T, Origasa H, et al. Superiority of long-acting to short-acting loop diuretics in the treatment of congestive heart failure. Circ J 2012;76:833–42. [DOI] [PubMed] [Google Scholar]
- [34].McCurley JM, Hanlon SU, Wei SK, et al. Furosemide and the progression of left ventriculardysfunction in experimental heart failure. J Am Coll Cardiol 2004;44:1301–7. [DOI] [PubMed] [Google Scholar]
- [35].Yoshida J, Yamamoto K, Mano T, et al. Different effects of long- and short-acting loop diuretics on survival rate in Dahl high-salt heart failure model rats. Cardiovasc Res 2005;68:118–27. [DOI] [PubMed] [Google Scholar]
- [36].Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651–8. [DOI] [PubMed] [Google Scholar]
- [37].The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- [38].Wu KC, Weiss RG, Thiemann DR, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adeverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol 2008;51:2414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xu C, Xu L, Gao Z, et al. Direct delineation of myocardial infarction without contrast agents using a joint motion feature learning architecture. Med Image Anal 2018;50:82–94. [DOI] [PubMed] [Google Scholar]


