Abstract
Objective:
This systematic review and meta-analysis aims to compare surgical and endoscopic treatment for pancreatic pseudocyst (PP).
Methods:
The researchers did a search in Medline, EMBASE, Scielo/Lilacs, and Cochrane electronic databases for studies comparing surgical and endoscopic drainage of PP s in adult patients. Then, the extracted data were used to perform a meta-analysis. The outcomes were therapeutic success, drainage-related adverse events, general adverse events, recurrence rate, cost, and time of hospitalization.
Results:
There was no significant difference between treatment success rate (risk difference [RD] −0.09; 95% confidence interval [CI] [0.20,0.01]; P = .07), drainage-related adverse events (RD −0.02; 95% CI [−0.04,0.08]; P = .48), general adverse events (RD −0.05; 95% CI [−0.12, 0.02]; P = .13) and recurrence (RD: 0.02; 95% CI [−0.04,0.07]; P = .58) between surgical and endoscopic treatment.
Regarding time of hospitalization, the endoscopic group had better results (RD: −4.23; 95% CI [−5.18, −3.29]; P < .00001). When it comes to treatment cost, the endoscopic arm also had better outcomes (RD: −4.68; 95% CI [−5.43,−3.94]; P < .00001).
Conclusion:
There is no significant difference between surgical and endoscopic treatment success rates, adverse events and recurrence for PP. However, time of hospitalization and treatment costs were lower in the endoscopic group.
Keywords: digestive system, drainage, endoscopy, pancreas, pancreatic pseudocysts
1. Introduction
Pancreatic fluid collections are complications associated with some pancreatic diseases, especially after episodes of acute biliary pancreatitis, chronic pancreatitis, trauma, or surgical procedures. Most acute collections are asymptomatic and resolve spontaneously. However, some persist for >4 weeks and are considered to be pseudocysts.[1] Spontaneous resolution is dependent on size and time of evolution.[2] The first widespread classification system was developed in 1993 and was named as Atlanta Criteria.[3] This criteria classified pancreatic fluid collections as acute or chronic collections, with chronic collections being further divided into pancreatic necrosis, pseudocysts, and pancreatic abscesses. According to the revised Atlanta criteria,[4] these inflammatory collections are: acute peripancreatic fluid collections, pseudocysts, acute necrotic collections, and walled-off necrosis (WON). The pancreatic pseudocyst (PP) is abundant with amylase, has a nonepithelialized wall, and has no internal debris or solid component. PP is a complication that develops in approximately 7% of cases of acute pancreatitis and 10% to 30% of chronic pancreatitis.[1,5] It can progress to bleeding, obstructive symptoms, or infection in 10% to 20% of cases.[6] In chronic pancreatitis, many of these pseudocysts are small, cause mild symptoms, and therefore do not require treatment. PP has a mortality rate of around 10%.[7,8]
In most cases, PPs are asymptomatic and resolve spontaneously without any intervention. Indications to drain PP are limited to symptomatic patients, size (>6 cm), and rapid growth and/or associated with complications, such as infection and bleeding.[9,10,11,12] Drainage can be performed by endoscopic, surgical, or percutaneous procedure. Percutaneous drainage is associated with high rates of recurrence. Therefore, it is reserved for immature pseudocysts, no definitive treatment, infected cysts, and in patients with co morbidities that do not allow for definitive surgical treatment.[13] For several years, open surgical approach was considered the criterion standard treatment, but with evolvement of less invasive techniques, such as laparoscopic and endoscopic drainage, these new techniques have gained increased usage in recent years. Depending on topography and anatomical relationships, PP drainage can be accomplished in several ways: safely through the stomach, duodenum, or small intestine.[14] Endoscopic approach has the benefit of being less invasive than surgery.
To date, few studies in the literature have compared surgical and endoscopic approach for treatment of PPs; thus, it is necessary to study these methods through systematic review and meta-analysis, including recent comparative studies, to compare these therapeutic options.
2. Objective
The aim of this study was to compare surgical and endoscopic approaches for treatment of PPs. The outcomes were therapeutic success, drainage-related adverse events, general adverse events, recurrence rate, cost and time of hospitalization.
3. Methods
This systematic review was performed according to the Cochrane Handbook for Systematic Reviews of Interventions and Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).[15] The study was recorded in the International Prospective Register of Systematic Reviews database (PROSPERO)—available at https://www.crd.york.ac.uk/prospero/, from the Center for Reviews and Dissemination of the University of York (England) under CRD42017068477 code.
This study was approved by ethics committee of the Faculty of Medicine of the University of São Paulo with number 294/17.
4. Eligibility criteria
Types of study: Comparative studies between surgical and endoscopic treatment for PPs. Exclusion criteria were: non-explicit method, studies that did not provide sufficient data to analyze outcomes, no full text available, or studies that included WON
Types of participants: adult patients (>18 years) with PPs requiring interventional treatment
Types of intervention and control: endoscopic treatment (intervention) versus surgical treatment (control)
Outcomes assessed: primary outcomes are therapeutic success, adverse events related to drainage, general adverse events, and recurrence, whereas secondary outcomes are cost and time of hospitalization.
4.1. Data extraction
Two independent investigators extracted data according to pre-defined data extraction form. Disagreements were resolved by consultation with a third researcher.
The following data were collected: study model, total number of patients included, therapeutic success rate, general and drainage-related adverse events, recurrence, cost, and time of hospitalization.
4.2. Search
Research for available articles carried out with these searches in databases. Databases (all years) were PubMed / Medline, Embase, Scopus, Cochrane Central Register of Randomized Controlled Trials / CENTRAL, LILACS, and Cinahl. Last search was performed on April 30, 2018. Search utilized presented in annex 1. Research in the gray literature was also performed through a review of references on the topic.
Medline: (pancreatic pseudocyst∗ OR pancreatic collections OR pancreatic fluid collections) AND (surgery OR cystogastrostomy OR cystojejunostomy OR pseudocyst drainage laparoscopic OR percutaneous drainage OR endosonographic OR surgical drainage OR endoscopic drainage OR endoscop∗ OR EUS OR endoscopic ultrasound)
Embase: (pancreas pseudocyst) AND (endoscopic) AND (surgical)
Web of Science, Scopus, Cochrane, EBsCo/CINAHL, LILACS/Bireme, OVID, CAPES(Brazil): (pancreatic pseudocyst OR pancreas pseudocyst) AND (endoscopic) AND (surgical)
4.3. Statistical analysis
In regard to the meta-analysis, the difference in risk difference (RD) was calculated with Mantel Haenszel Cochran method with a 95% confidence interval (CI), and in mean difference, using a random effect with inverse variance and a 95% CI for continuous variables.
Semiquantitative values were described as a weighted arithmetic average using the number of patients from each study, reported with standard deviation and the use of Student t test. All data were analyzed as intention-to-treat analysis.
4.4. Synthesis of results
RevMan 5 software (Review Manager version 5.3.5 - Cochrane Collaboration, 2014) was used for the meta-analysis. Student test was used to compare weighted arithmetic values for items that did not have prerequisites for meta-analysis (SD for continuous variables and absolute individual data for dichotomous variables). Heterogeneity was assessed with the χ2 test and maintained up to 50% with sensitivity analysis when possible and necessary.
4.5. Risk of bias
Randomized clinical trial (RCT) bias was assessed by the Cochrane Collaboration's tool for assessing risk of bias[16] (Table 1A). Biases of retrospective studies were evaluated by the Modified Newcastle-Ottawa Quality Assessment Scale for Cohort Studies[13,17–21](Table 1B).
Table 1.
A. Cochrane Collaboration's tool for assessing risk of bias; Modified New Castle Ottawa Quality Assessment Scale for Cohort Studies.

4.6. Definitions
Therapeutic success was defined as complete resolution or decrease in pseudocyst size to ≤2 cm in imaging method with total improvement of symptoms after the first intervention. Adverse events consisted of those related to drainage such as bleeding, infection, perforation, and migration of stents and those not related to drainage such as incisional hernia, abdominal wall infection, deep venous thrombosis, and cardiopulmonary dysfunctions. Recurrence was defined as a new pseudocyst observed by imaging methods at follow-up after previously reported resolution. Time of hospitalization was length of stay from day of surgical or endoscopic approach to discharge. Cost was determined by all costs related to drainage and follow-up.
5. Results
5.1. Study selection
Initially, 4291 studies were retrieved and, after title screening and abstracts, 14 articles were assessed for eligibility. Subsequently, we excluded 8 articles, 7 because they were not comparative studies and 1 because it did not present enough data to calculate the outcomes, thereafter including 6 studies for meta-analysis (Fig. 1).
Figure 1.

Selection of studies for inclusion in the meta-analysis.
5.2. Characteristics
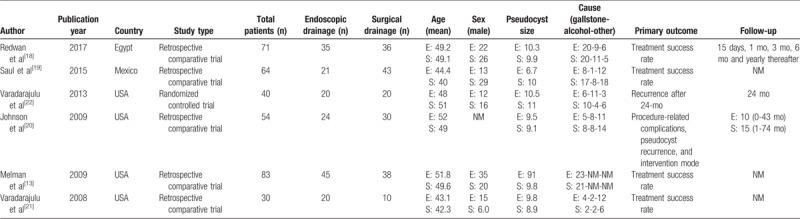
Selected studies were published between 2008 and 2017. Six comparative studies were selected[13,18–22] with one RCT and five retrospective cohort studies. Study characteristics summarized in Table 2. Melman et al and Redwan et al[13,18] defined pseudocysts as encapsulated fluid collections, not true cysts because they lacked an epithelial lining. Johnson et al and Varadarajulu et al[20,21] defined pseudocysts as collections of pancreatic fluid contained by a wall of fibrous tissue and pancreatic fluid collection was categorized according to the Atlanta classification, respectively.
Table 2.
Characteristics of the studies.
5.3. Descriptive results
A total of 342 patients were included of whom 177 were treated by surgery (open drainage or videolaparoscopic and resection) and 165 treated by endoscopy. Male sex was most common (2: 1 ratio). Main causative etiology was biliary pancreatitis (40%) followed by alcoholic pancreatitis (Table 2).
In the selected studies, several endoscopic and surgical techniques for PP drainage were performed. In both groups (surgical and endoscopic), cystogastrostomy was most common. In the surgical treatment group, cystogastrostomy was performed in 119 of 177 patients (67%). In the endoscopic group, cystogastrostomy was performed in 143 of 165 patients (86%). All drainage characteristics in the studies are described in Table 3.
Table 3.
Drainage characteristics.

5.4. Therapeutic success
All studies[13,18–22] reported successful outcomes and there was no statistical difference (P = .07), as denoted by method equivalence (RD: 0.09; 95% CI [−0.20, 0.01]). Therapeutic success rates ranged from 51% to 95% in the endoscopic group and 81.2% to 100% in the surgery group (Fig. 2).
Figure 2.

Forest plot for the outcome of therapeutic success (n), using a random-effects model, with the M–H method. CI = confidence interval, M–H = Mantel–Haenszel.
5.5. Adverse events:
Adverse events were related to drainage and to general adverse events.
In the endoscopic group, there were 19 adverse events among the 165 procedures (11.5%), whereas in the surgical group, there were 35 among 177 procedures (19.7%).
The most common adverse event in both groups was bleeding that occurred in 9 of 165 cases (5.4%) in the endoscopy group, and in 6 of 177 cases (3.3%) in the surgery group.
General adverse events not related to drainage occurred mainly in patients treated by surgery. Most common complications were incisional hernia and abdominal wall infection (2.8%). Adverse events are listed in Table 4.
Table 4.
Adverse events.

5.6. Adverse events related to drainage:
All studies reported[13,18–22] rates of adverse events. There was no statistical difference between the 2 groups and there was an equivalence between methods (RD 0.02; 95% CI [−0.04 to 0.08], P = .48).
Adverse events rates related to drainage ranged from 0 to 23.8% in the endoscopic group and 0 to 18.6% in the surgery group (Fig. 3).
Figure 3.
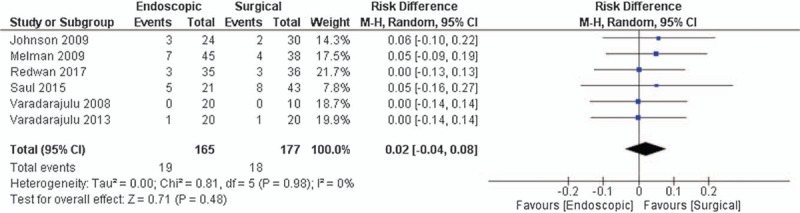
Forest plot for the outcome of adverse events related to drainage (n), using a random-effects model, with the M–H method. CI = confidence interval, M–H = Mantel–Haenszel.
5.7. General adverse events:
All studies[13,18–22] reported general adverse events related to therapy. There was no statistical difference between the 2 groups. An equivalence between the methods (RD −0.05; 95% CI [−0.12 to 0.02], P = .13) was observed (Fig. 4).
Figure 4.

Forest plot for the outcome of general adverse events (n), using a random-effects model, with the M–H method. CI = confidence interval, M–H = Mantel–Haenszel.
5.8. Recurrence
Four studies[18,19,22,20] reported recurrence related to applied therapy. There was no statistical difference between the 2 groups. An equivalence between methods (RD: 0.04; 95% CI [−0.04 to 0.07], P = .58) was observed (Fig. 5).
Figure 5.

Forest plot for the outcome of recurrence (n), using a random-effects model, with the M–H method. CI = confidence interval, M–H = Mantel–Haenszel.
5.9. Cost
Three studies[19,22,20] reported costs related to therapy. Of these, 1[20] did not provide standard deviation. This study was excluded from analysis.
Comparison between groups demonstrated that endoscopy was associated with lower costs than surgical treatment in drainage of pancreas pseudocysts (RD: −4.68; 95% CI [−5.43, −3.94]; P < .00001) (Fig. 6).
Figure 6.

Forest plot for the outcome of cost (dollars), using a random-effects model, with the Mantel–Haenszel method. CI = confidence interval, SD = standard deviation.
5.10. Hospitalization period
Five studies[13,18,19,21,22] reported time of hospitalization related to each drainage method. Of these, 1 study[19] did not provide variation and was excluded from analysis. Endoscopic drainage had shorter hospitalization time compared to the surgical group (RD: −4.23; 95% CI [−5.18 to −3.29]; P < .00001).
Hospitalization period varied from 3 to 82 days in the surgery group and 0 to 25 days in the endoscopy group (Fig. 7).
Figure 7.

Forest plot for the outcome of Hospitalization period (days), using a random-effects model, with the Mantel–Haenszel method. CI = confidence interval, SD = standard deviation.
6. Discussion
We performed a systematic review and meta-analysis, including 6 comparative studies,[13,18–22] 1 randomized clinical trial, and 5 retrospective cohort studies that compared surgical and endoscopic treatment for PPs. Total number of patients was 342: 177 in the surgical and 165 in the endoscopic group.
In the literature, a similar review was performed by Zhao et al,[23] which involved 5 comparative studies with 255 patients.[13,19–22] This study demonstrated higher therapeutic success rates with surgical treatment for PP (91.2%, ranging from 81.2% to 100%) with no statistical difference between adverse events and recurrence. However, they used odds ratio (OR) to perform the meta-analysis, increasing effect. To avoid such a bias, we employed RD in our study. In our analysis, we included 1 study,[18] in addition to the cases treated by videolaparoscopic surgery in the study by Melman et al,[13] which were not included by Zhao et al. Another systematic review by Teoh et al[24] compared surgical, percutaneous, and endoscopic treatment for PP. The authors concluded that endoscopic and surgical drainage are equally effective with reduction in hospitalization time, lower costs, and better quality of life in the endoscopic group. However, surgical or percutaneous drainage should be considered in patients with an unfavorable anatomy.
In our systematic review, the main adverse events related to drainage were bleeding and infection, similar to that reported in Baron et al[25] that reported bleeding, perforations, infections, pancreatitis, migration of the prosthesis, and lesion of the pancreatic duct as the main complications related to drainage in endoscopic procedures. Adams and Anderson[26] related bleeding and infection as major adverse events related to surgical drainage. The main complication in our study related to surgical drainage was bleeding, whereas incisional hernia and abdominal wall infection were the most common complications unrelated to drainage. There was no statistical difference in the incidence of general adverse events and adverse events related to drainage between the 2 approaches in our study.
In our review, cystogastrostomy was the main type of drainage, both in endoscopic and surgical treatment. With surgical approach, the technique of choice is based according to pseudocyst location, adjacent structures, surgeon preference, and may be open or laparoscopic. Internal surgical drainage can be performed by communication between the pseudocyst and stomach, jejunum, or duodenum. If resection is used, it will depend on pseudocyst location. A distal pancreatectomy may be performed or even a duodenopancrectomy.[27–29] Khaled et al[30] showed that laparoscopic surgical approach of internal drainage of PPs with cystogastrostomy offered advantages over open surgery in terms of reduced operative time, operative morbidity, and postoperative period of hospitalization.
In the endoscopic approach, drainage routes vary between transmural, transpapillary, or combined therapy. Transmural drainage can be performed if the pseudocyst is directly placed against the stomach or duodenal wall. Transpapillary drainage is made possible when the pseudocyst communicates with the main pancreatic duct (MPD).[31] In our review, approximately 92% of cases were transmural and the remaining 8% were transpapillary or combined.
Most endoscopists agree that ultrasound-guided access is superior and should be used wherever available. Ultrasound guidance allows for precise cavity segmentation and decreases risk of vascular injury and other abdominal structures as well.[32] Traditionally, double-pig-tail prostheses are used for endoscopic drainage. As drainage techniques evolve, fully-covered self-expanding metal prostheses have become tactically important. This development led to metal juxtaposed or lumens apposition, specifically designed for drainage of pancreatic fluid collections.[33] Amin et al[34] showed that prosthesis placement in the pancreatic duct does not provide any additional clinical benefit in patients who underwent transmural drainage (particularly pseudocysts).
Recurrence occurred in approximately 4% of the drainages in our study. The major risk factor for recurrence is primary disconnection from the MPD. Endoscopic retrograde cholangiopancreatography with MPD exploration can be performed simultaneously with drainage. Disconnection from MDP is associated with severe pancreatitis, increased risk of recurrent pancreatitis, long-term complications, and a decrease in resolution rates of pseudocysts after drainage.[35] Varadarajulu et alconcluded that endoscopic insertion of the transpapillary prosthesis is effective and safe for patients with interruption of the MPD. It also has good results in cases of partial rupture of the MPD.[36] Regarding the need for re-intervention, Redwan et al[18] demonstrated that endoscopic treatment has higher rates (8.6% × 0%), whereas Varadarajulu et al[22] showed no statistical difference.
In this systematic review, the therapeutic success rate, general adverse events, adverse events related to drainage, and recurrence did not present statistical difference after meta-analysis.
The main limitation of our systematic review was the presence of only 1 clinical trial among the analyzed studies because of a lack of such studies in the literature, thereby weakening the analysis. Another limitation of this study is that some studies have defined therapeutic success in different ways, reducing the quality of the evidence. To improve the quality of the evidence, it is necessary to standardize the definitions of the outcomes.
The most recent systematic reviews in this field did not perform a meta-analysis of costs and time of hospitalization. In our systematic review, we provided a meta-analysis of these variables. Endoscopic treatment demonstrated lower costs and shorter hospitalization compared to surgical treatment.
7. Conclusion
Endoscopic drainage of PPs demonstrated a therapeutic success rate, drainage-related adverse events, general adverse events, and recurrence similar to that seen in surgical treatment, but with lower costs and reduced time of hospitalization, supporting its use as the preferred modality for drainage of pancreas pseudocysts.
Author contributions
Conceptualization: Galileu Ferreira Ayala Farias, Wanderley Marques Bernardo, Hugo Gonçalo Guedes, Vitor Ottoboni Brunaldi, Christiano Makoto Sakai, Sergio Eiji Matuguma, Marcos Eduardo Lera dos Santos.
Data curation: Galileu Ferreira Ayala Farias.
Formal analysis: Galileu Ferreira Ayala Farias, Thiago Arantes De Carvalho Visconti, Caio Vinicius Gonçalves Tranquillini.
Funding acquisition: Galileu Ferreira Ayala Farias.
Investigation: Galileu Ferreira Ayala Farias.
Methodology: Galileu Ferreira Ayala Farias, Wanderley Marques Bernardo, Hugo Gonçalo Guedes.
Project administration: Vitor Ottoboni Brunaldi, Thiago Arantes De Carvalho Visconti, Sergio Eiji Matuguma, Marcos Eduardo Lera dos Santos.
Resources: Galileu Ferreira Ayala Farias.
Software: Wanderley Marques Bernardo, Hugo Gonçalo Guedes, Caio Vinicius Gonçalves Tranquillini.
Supervision: Diogo Turiani Hourneaux De Moura, Paulo Sakai, Eduardo Guimarães Hourneaux De Moura.
Validation: Hugo Gonçalo Guedes.
Visualization: Diogo Turiani Hourneaux De Moura.
Writing – original draft: Galileu Ferreira Ayala Farias.
Writing – review & editing: Diogo Turiani Hourneaux De Moura, Vitor Ottoboni Brunaldi, Christiano Makoto Sakai, Sergio Eiji Matuguma, Marcos Eduardo Lera dos Santos, Eduardo Guimarães Hourneaux De Moura.
Footnotes
Abbreviations: CI = confidence interval, MPD = main pancreatic duct, OR = odds ratio, PP = pancreatic pseudocyst, RCT = randomized clinical trial, RD = risk difference, WON = walled-off necrosis.
The authors report no conflicts of interest
References
- [1].Lenhart DK, Balthazar EJ. MDCT of acute mild (nonnecrotizing) pancreatitis: abdominal complications and fate of fluid collections. AJR Am J Roentgenol 2008;190:643–9. [DOI] [PubMed] [Google Scholar]
- [2].Vargas RD, Sepúlveda-Copete M, Zuleta JE, et al. Case report: Treatment of pancreatic pseudocysts with endoscopic transpapillary drainage (Asociaciones Colombianas de Gastroenterologia, Endoscopia digestiva, Coloproctologia y Hepatologia). Rev Col Gastroenterol 2010;2:203–6. [Google Scholar]
- [3].Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Sym∗∗posium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg 1993;128:586–90. [DOI] [PubMed] [Google Scholar]
- [4].Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013. 62–102. [DOI] [PubMed] [Google Scholar]
- [5].Yang CC, Shin JS, Liu YT, et al. Management of pancreatic pseudocysts by endoscopic cystogastrostomy. J Formos Med Assoc 1999;98:283–6. [PubMed] [Google Scholar]
- [6].Vitas GJ, Sarr MG. Selected management of pancreatic pseudocysts: operative versus expectant management. Surgery 1992;111:123–30. [PubMed] [Google Scholar]
- [7].Bradley EL. The natural and unnatural history of pancreatic fluid collections associated with acute pancreatitis. Dig Dis Sci 2014;59:908–10. [DOI] [PubMed] [Google Scholar]
- [8].Sandberg AA, Dervenis C. Pancreatic pseudocyst in the 21st century. Part II: natural history. J Pancreas 2004;5:64–70. [PubMed] [Google Scholar]
- [9].Lehman GA. Pseudocysts. Gastro intest Endosc 1999;49:S81–4. [DOI] [PubMed] [Google Scholar]
- [10].Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology 2004;126:1330–6. [DOI] [PubMed] [Google Scholar]
- [11].Yeo CJ, Bastidas JA, Lynch-Nyhan A, et al. The natural history of pancreatic pseudocysts documented by computed tomography. Surg Gynecol Obstet 1990;170:411–7. [PubMed] [Google Scholar]
- [12].Bradley EL, Clements JL, Gonzalez AC. The natural history of pancreatic pseudocysts: a unified concept of management. Am J Surg 1979;137:135–41. [DOI] [PubMed] [Google Scholar]
- [13].Melman L, Azar R, Beddow K, et al. Primary and overall success rates for clinical outcomes after laparoscopic, endoscopic, and open pancreatic cystgastrostomy for pancreatic pseudocysts. Surg Endosc 2009;23:267–71. [DOI] [PubMed] [Google Scholar]
- [14].Behrns KE, Ben-David K. J Gastrointest Surg 2008;12:2231–9. [DOI] [PubMed] [Google Scholar]
- [15].Moher D, Liberati A, Tetzlaff J. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [16].Higgins JPTSG. Cochrane Handbook for Systematic Reviews of Interventions: The Cochrane Collaboration 2011. [Google Scholar]
- [17].Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available at: URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- [18].Redwan AA, Hamad MA, Omar MA. Pancreatic pseudocyst dilemma: cumulative multicenter experience in management using endoscopy, laparoscopy, and open surgery. J Laparoendosc Adv Surg Tech A 2017;27:1022–30. [DOI] [PubMed] [Google Scholar]
- [19].Saul A, Luna MA, Chan C, et al. EUS-guided drainage of pancreatic pseudocysts offers similar success and complications compared to surgical treatment but with a lower cost. Surg Endosc 2016;30:1459–65. [DOI] [PubMed] [Google Scholar]
- [20].Johnson MD, Walsh RM, Henderson JM, et al. Surgical versus nonsurgical management of pancreatic pseudocysts. J Clin Gastroenterol 2009;43:586–90. [DOI] [PubMed] [Google Scholar]
- [21].Varadarajulu S, Lopes TL, Wilcox CM, et al. EUS versus surgical cyst-gastrostomy for management of pancreatic pseudocysts. Gastrointest Endosc 2008;68:649–55. [DOI] [PubMed] [Google Scholar]
- [22].Varadarajulu S, Bang JY, Sutton BS, et al. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology 2013;145:583–90. [DOI] [PubMed] [Google Scholar]
- [23].Zhao X, Feng T, Ji W. Endoscopic versus surgical treatment for pancreatic pseudocyst. Dig Endosc 2016;28:83–91. [DOI] [PubMed] [Google Scholar]
- [24].Teoh AY, Dhir V, Jin ZD, et al. Systematic review comparing endoscopic, percutaneous and surgical pancreatic pseudocyst drainage. World J Gastrointest Endosc 2016;8:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Baron Todd H, Kozarek Richard A, Carr-Locke David L, et al. CPRE Drenagem Endoscópica Dos Pseudocistos Pancreáticos, Abscessos e Necrose Loculada. Sao Paulo: Revinter; 2015. [Google Scholar]
- [26].Adams DB, Anderson MC. Percutaneous catheter drainage compared with internal drainage in the management of pancreatic pseudocyst. Ann Surg 1992;215:571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tenner S, Baillie J, DeWitt J, et al. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013;108:1400–15. [DOI] [PubMed] [Google Scholar]
- [28].Park AE, Heniford BT. Therapeutic laparoscopy of the pancreas. Ann Surg 2002;236:149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Morton JM, Brown A, Galanko JA, et al. A national comparison of surgical versus percutaneous drainage of pancreatic pseudocyts:. J Gastrointest Surg 2005;9:15–20. [DOI] [PubMed] [Google Scholar]
- [30].Khaled YS, Malde DJ, Packer J, et al. Laparoscopic versus open cystgastrostomy for pancreatic pseudocysts: a case-matched comparative study. J Hepatobiliary Pancreat Sci 2014;21:818–23. [DOI] [PubMed] [Google Scholar]
- [31].Binmoeller KF, Seifert H, Walter A, et al. Transpapillary and transmural drainage of pancreatic pseudocysts. Gastrointest Endosc 1995;42:219–24. [DOI] [PubMed] [Google Scholar]
- [32].Baron TH, Harewood GC, Morgan DE, et al. Outcome differences after endoscopic drainage of pancreatic necrosis acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc 2002;56:7–17. [DOI] [PubMed] [Google Scholar]
- [33].Bhasin DK, Rana SS, Nanda M, et al. Comparative evaluation of transpapillary drainage with nasopancreatic drain and stent in patients with large pseudocysts located near tail of pancreas. J Gastrointest Surg 2011;15:772–7. [DOI] [PubMed] [Google Scholar]
- [34].Law R, Baron TH. Endoscopic ultrasonography-guided drainage of pancreatic collections, including the role of necrosectomy. Gastrointest Endosc Clin N Am 2017;27:715–26. [DOI] [PubMed] [Google Scholar]
- [35].Alali A, Mosko J, May G, et al. Endoscopic ultrasound-guided management of pancreatic fluid collections: update and review of the literature. Clin Endosc 2017;50:117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Amin S, Yang DJ, Lucas AL, et al. There is no advantage to transpapillary pancreatic duct stenting for the transmural endoscopic drainage of pancreatic fluid collections: a meta-analysis. Clin Endosc 2017;50:388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]


