Supplemental Digital Content is available in the text
Keywords: depression, meta-analysis, myocardial infarction, prevalence
Abstract
Background:
Depression is common in the aftermath of myocardial infarction (MI) and may not only lead to impaired long-term quality of life, but also cause increased mortality among patients with MI. The reported prevalence of depression among patients with MI varied considerably across studies, for which a pooled prevalence was obtained in the only 1 meta-analysis conducted in March 2004. Subsequently, numerous relevant studies have been published, indicating the need for an update on the pooled prevalence. Therefore, this study was aimed at updating the pooled prevalence of depression among patients with MI.
Methods:
A comprehensive literature search in 3 electronic databases, PubMed, Embase, and PsycINFO, was performed in April 2018. The heterogeneity across studies was examined by the Cochran's Q test and quantified by the I2 statistic. If significant heterogeneity was observed, meta-regression analyses and subgroup analyses were performed to identify the source of heterogeneity. Publication bias was assessed by a funnel plot and verified by the Egger's and Begg's tests.
Results:
Nineteen eligible studies conducted in 10 countries were included, which consisted of 12,315 patients with MI, among whom 3818 were identified with depression. High heterogeneity was observed across the eligible studies (I2 = 98.4%), with the reported prevalence of depression ranging from 9.17% to 65.88%. The pooled prevalence of depression among patients with MI was 28.70% (95% CI: 22.39–35.46%) by a random effects model. Subgroup analyses showed that the pooled prevalence differed significantly by region, tool used to identify depression, study quality, sex, race, anterior MI, and diabetes status (P < .05). Meta-regression analyses did not identify any moderators of heterogeneity, and the heterogeneity was high within most subgroups. Nonetheless, for unmarried subjects, the heterogeneity was low (I2 = 19.5). The Egger's test and the Begg's test indicated no evidence of publication bias (P > .05).
Conclusions:
Given the high pooled prevalence of depression found in this study and the association between depression and adverse health outcomes among patients with MI, more psychological resources including early assessment and effective treatment of depression should be allocated to patients with MI.
1. Introduction
Cardiovascular disease (CVD) is the 1st leading cause of death globally contributing 31% of all mortality.[1] According to the latest statistics released by the World Health Organization (WHO), an estimated number of people who died of CVD in 2015 was 17.7 million, among whom 7.4 million died of coronary heart disease (CHD).[1] Myocardial infarction (MI), characterized by the myocardial cell necrosis due to significant and sustained ischaemia, is the main manifestation of CHD and has been a significant burden of both high-income countries and low-income countries.[2] The MI triggers not only physiological responses such as severe pain, but also psychological responses such as depression.[3–5]
Accumulated evidence has consistently shown that depression is one of the most common psychological reactions in the aftermath of MI which may not only lead to impaired long-term quality of life, but also cause increased mortality among patients with MI.[6–8] For example, Hosseini et al conducted a 5-year follow-up study among 196 hospitalized patients with MI and found that baseline depression was strongly significantly associated with reduced long-term quality of life in both the mental and physical domains.[6] Additionally, Meijer et al conducted a meta-analysis exploring the effects of post-MI depression on cardiovascular outcomes and found that post-MI depression could put patients with MI at 2.25 times higher risk of all-cause mortality, 2.71 times higher risk of cardiac mortality, and 1.59 times higher risk of cardiac events within 24 months.[7] Furthermore, Bush et al found that even minimal depressive symptom could lead to an increased mortality risk following MI.[8] Based on these findings, early assessment of depression among patients with MI is imperative, and the implementation of effective psychological interventions for those with depression is necessary.
The reported prevalence of depression among patients with MI in the past 2 decades varied considerably across studies, ranging from 13.5% to 41.6%,[9–13] which may be explained by the differences in socio-demographic characteristics, such as sex, race, and marital status; and the tool used to identify depression.[9,13–17] The differences in MI characteristics, such as a history of previous MI, anterior MI, and Killip class; and the differences in the exposure to cardiovascular risk factors, such as current smoking, diabetes, hypertension and hyperlipidemia, may also explain the variation in the reported prevalence of depression across the previous studies.[9,13–15] Furthermore, social support may affect the occurrence of depression following MI.[18] The inconsistent findings of the prevalence of depression among patients with MI, reported in the previous studies, may result in uncertainty for the service providers to allocate psychological intervention resources. Therefore, an estimate of the pooled prevalence of depression among patients with MI was needed, as it would not only accelerate the efforts to determine the accurate number of subjects who may develop depression following MI, but also facilitate the task of balancing the cost of prevention and treatment of depression.
Thus far, the latest quantitative systematic research regarding depression among patients with MI was conducted by Thombs et al and published in March 2004.[19] They found that the pooled prevalence of depression identified by structured interview and Beck Depression Inventory (BDI), with a cutoff value of 10, was 19.8% (95% confidence interval [CI]: 19.1%–20.6%), and 31.1% (95%CI: 29.2%–33.0%), respectively.[19] However, numerous factors, including socio-demographic characteristics and cardiovascular factors that may be associated with the prevalence of depression among patients with MI were not taken into consideration, which significantly limited the generalizability of their findings. Also, the statistical analyses performed in that study were not adequate in the sense that there was no attempt to examine heterogeneity, publication bias, as well as sensitivity. Moreover, that study was published in March 2004 and subsequently, numerous relevant studies have been published. In this regard, a comprehensive update on the pooled prevalence of depression among patients with MI was warranted. Therefore, this study aimed to update the pooled prevalence of depression among patients with MI by synthesizing relevant evidence. The pooled prevalence of depression among patients with MI stratified by the socio-demographic characteristics, tool used to identify depression, MI characteristics, and cardiovascular factors was also explored.
2. Methods
2.1. Ethical approval
Ethical approval was not necessary for this study since this study utilized published data which were already ethically approved.
2.2. Search strategy
This meta-analysis was in accordance with the checklist of Preferred Reporting Items for Systematic Review and Meta-Analyses (additional file 1). A comprehensive literature search in 3 electronic databases (PubMed, Embase, and PsycINFO) was performed from database inception to April 2018. Subject headings related to MI and depression were used to develop a search strategy which was customized across databases. Full search strategies were listed in the additional file 2. The eligible studies of previous relevant reviews,[7,19–22] and the reference lists of full-text articles were also examined for more relevant articles.
2.3. Study selection
Two investigators independently identified the eligibility of studies for this meta-analysis, and any disagreement between them was resolved via consensus. Articles were included if they: Firstly, were cross-sectional studies, or baseline data of longitudinal studies, or baseline data of randomized controlled trials (RCTs) before group allocation; secondly, focused on patients with MI confirmed by medical records; thirdly, assessed depression among patients with MI using validated tools, including structured interviews and self-report questionnaires with established cutoff values. Fourth, reported the prevalence of depression among patients with MI and the sample size. Fifth, recruited a sample of no less than 200 subjects. Sixth, were published in peer-reviewed journals in English. Additionally, only the 1st publication was included if multiple publications from the same cohort were observed. Studies were excluded if they: First, were case reports, comments, or review articles. Second, reported exclusively the prevalence of depression among specific subgroups of patients with MI, such as those with heart failure, due to the absence of representativeness. Third, aimed to explore the psychometric properties of assessment tools.
2.4. Data collection
The primary outcome for this meta-analysis was the prevalence of depression among patients with MI. For the purpose of this study, 2 investigators independently assessed the quality of the eligible studies and extracted the following data: 1st author, publication year, region, sample source, mean age of participants, percentage of male participants, percentage of participants with 1st-time MI, timing of depression assessment, tool used to identify depression, number of subjects with depression, sample size, and the prevalence of depression among patients with MI. Wherever possible, data on sex, race, marital status, a history of previous MI, anterior MI, Killip class, current smoking, diabetes, hypertension, and hyperlipidemia were also extracted to perform subgroup analyses. Any discrepancies between the foregoing reviewers were resolved via discussion with a 3rd reviewer.
2.5. Quality assessment
The methodological quality of eligible studies was assessed by the checklist of Prevalence Study Quality.[23] This checklist has been widely used to evaluate the methodological quality of studies on the prevalence of health-related outcomes.[24,25] It consists of 11 items each of which with response options of “Yes”, “No”, or “Unclear”. If the response for an item is “Yes”, it is scored “1”. Otherwise, it is scored “0”. Therefore, the total score for this instrument ranges from 0 to 11, and studies are categorized as low quality, moderate quality, and high quality with a total score of 0 to 3 points, 4 to 7 points, and 8 to 11 points, respectively.
2.6. Statistical analysis
The heterogeneity across studies was examined by the Cochran's Q test and quantified by the I2 statistic, which describes the percentage of total variation across studies resulting from heterogeneity rather than chance, with its values of 25%, 50%, and 75% indicating low, moderate, and high heterogeneity, respectively.[26] The pooled prevalence of depression among patients with MI was calculated using a random effects model by Freeman-Tukey double arcsine method when significant heterogeneity (P value for Cochran's Q test <.05) was observed. Otherwise, a fixed effects model was applied.[27] For each pooled estimate, its corresponding 95%CI was calculated.
If significant heterogeneity was observed across the eligible studies, meta-regression analyses were performed, using the restricted maximum-likelihood estimator method, to identify the source of heterogeneity according to the following continuous study-level characteristics: mean age of participants, percentage of male participants, percentage of participants with 1st-time MI, and quality assessment score. Furthermore, subgroup analyses were performed to explore the pooled prevalence of depression among patients with MI according to each of the following categorical study-level characteristics: region, study quality, tool used to identify depression, sex, race, marital status, a history of previous MI, anterior MI, Killip class, current smoking, diabetes, hypertension, and hyperlipidemia. Differences in the pooled prevalence of depression within each subgroup were compared using the chi-square test, and a P value of less than .05 was considered significant.
Sensitivity analysis was done to examine the robustness of the pooled prevalence of depression not only by excluding the eligible studies one-by-one but also removing the studies with relatively low quality. Publication bias was assessed by the funnel plot and verified by the Egger's and Begg's tests. All statistical analyses were performed using the R statistical software version 3.4.1.
3. Results
3.1. Literature search and study selection
Initially, a total of 3936 records were identified by the search strategy. After removing duplicates and reviewing titles and abstracts, 65 full-text articles were shortlisted for assessing the eligibility. Among these articles, 1 was excluded for being an RCT which reported results only after group allocation, 1 was excluded for providing no confirmation of MI by medical records, 2 were excluded for not using validated tools to identify depression, 4 were excluded for not reporting the prevalence of depression among patients with MI, 27 were excluded for recruiting a sample size of less than 200, 8 were excluded for repeated data, 2 were excluded for reporting exclusively the prevalence of depression among specific subgroups of patients with MI, and 1 was excluded for aiming to explore the psychometric properties of an assessment tool. Therefore, a total of 19 eligible studies were included in this meta-analysis (Fig. 1).
Figure 1.
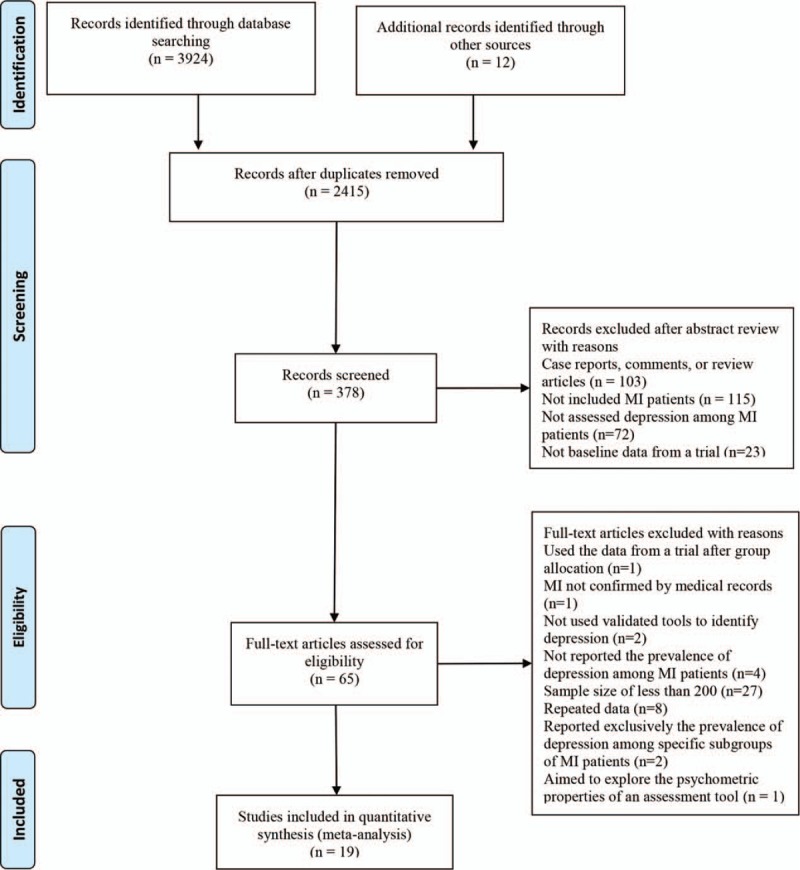
PRISMA flowchart of study selection.
3.2. Study characteristics
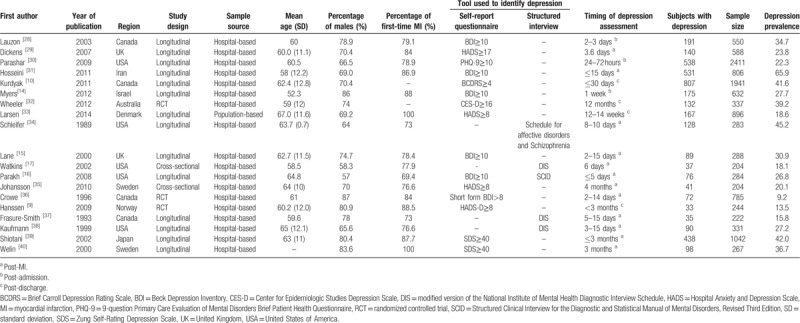
The characteristics of the 19 eligible studies are shown in Table 1. Collectively, 12,315 patients with MI were included, of which 3818 were identified with depression. The 19 eligible studies were conducted in 10 countries including Canada, United Kingdom (UK), United States of America (USA), Iran, Israel, Australia, Denmark, Sweden, Norway, and Japan. Among these 19 eligible studies, 2 were cross-sectional, 3 were RCTs, 14 were longitudinal, 1 was population-based, 18 were hospital-based, 4 used exclusively structured interview to identify depression, 14 used exclusively self-report questionnaire to identify depression, and 1 used both structured interview and self-report questionnaire to identify depression. The mean age of participants ranged from 52 to 67 years, and the percentage of male participants ranged from 57 to 87%. Additionally, the percentage of participants with 1st-time MI ranged from 69 to 100%.
Table 1.
Characteristics of the eligible studies for this meta-analysis.
The results of quality assessment are shown in the additional file 3. The overall quality among the eligible studies was moderate to high. According to the checklist of Prevalence Study Quality, 1 was scored 5 points, 1 was scored 7 points, 2 were scored 8 points, 9 were scored 9 points, and 6 were scored 10 points. Therefore, 2 were categorized as moderate quality and 17 were categorized as high quality.
3.3. Pooled prevalence of depression among patients with MI
The reported prevalence of depression among patients with MI ranged from 9.17 to 65.88% among the eligible studies. The highest prevalence was reported in a hospital-based study in Iran which used BDI with a cutoff value of 10 to identify depression,[31] and the lowest prevalence was reported in a hospital-based study in Canada which used the short-form BDI with a cutoff value of 8 to identify depression.[36] Since the overall heterogeneity across the 19 eligible studies was significantly high (I2 = 98.4%), a random effects model was applied to generate the pooled prevalence of depression among patients with MI and it was 28.70% (95% CI: 22.39–35.46%) (Fig. 2).
Figure 2.
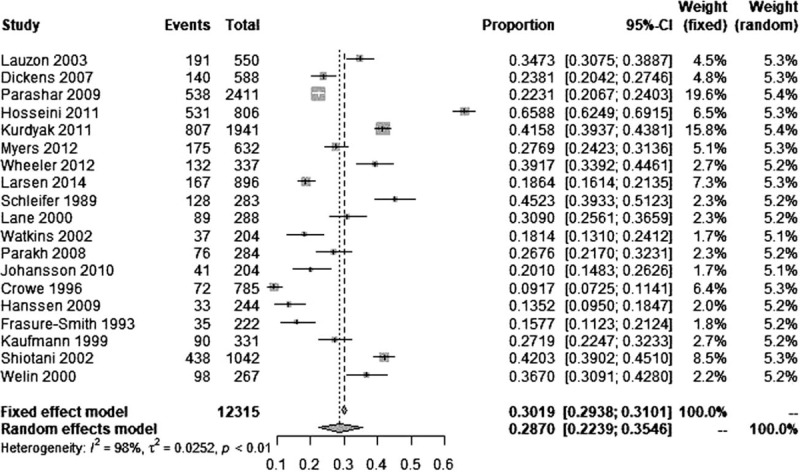
Forest plot of the 19 eligible studies.
3.4. Meta-regression analyses
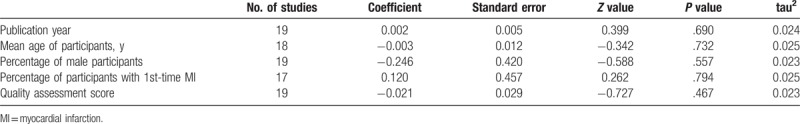
The results of meta-regression analyses indicated that publication year (β=0.002, P = .690), mean age of participants (β=-0.003, P = .732), percentage of male participants (β=-0.246, P = .557), percentage of participants with 1st-time MI (β=0.120, P = .794), and quality assessment score (β=-0.021, P = .467) were not significant moderators of the overall heterogeneity (Table 2).
Table 2.
Meta-regression analyses of the effects of potential moderators on the overall heterogeneity.
3.5. Subgroup analyses
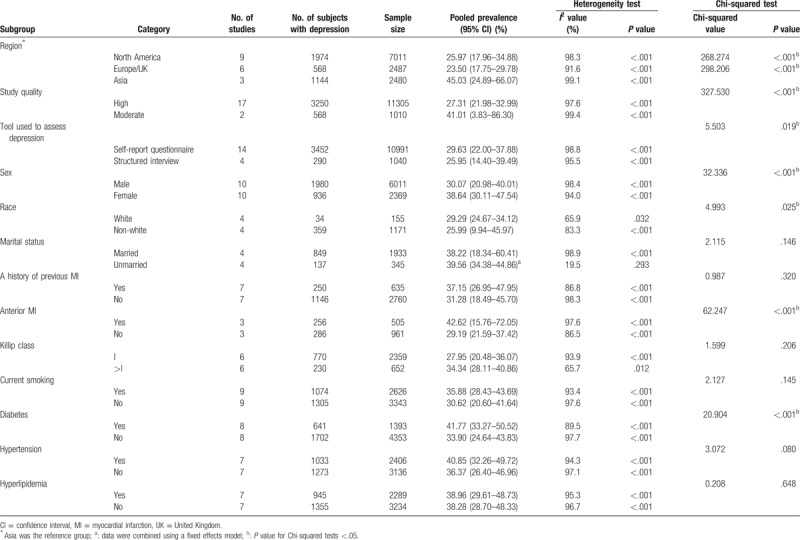
The results of subgroup analyses are shown in Table 3. The pooled prevalence of depression among patients with MI in North America, Europe/UK, and Asia was 25.97% (95% CI: 17.96–34.88%), 23.50% (95% CI: 17.75–29.78%), and 45.03% (95% CI: 24.89–66.07%), respectively; among those assessed by structured interview and self-report questionnaire it was 25.95% (95% CI: 14.40–39.49%) and 29.63% (95% CI: 22.00–37.88%), respectively; among female and male subjects it was 38.64% (95% CI: 30.11–47.54%) and 30.07% (95% CI: 20.98–40.01%), respectively; among those with and without a history of previous MI it was 37.15% (95% CI: 26.95–47.95%) and 31.28% (95% CI: 18.49–45.70%), respectively; among those with and without anterior MI it was 42.62% (95% CI: 15.76–72.05%) and 29.19% (95% CI: 21.59–37.42%), respectively; among those with Killip class equal to I and more than I it was 27.95% (95% CI: 20.48–36.07%) and 34.34% (95% CI: 28.11–40.86%), respectively; among those with and without diabetes it was 41.77% (95% CI: 33.27–50.52%) and 33.90% (95% CI: 24.64–43.83%), respectively; and among those with and without hypertension it was 40.85% (95% CI: 32.26–49.72%) and 36.37% (95% CI: 26.40–46.96%) respectively. Moreover, the pooled prevalence of depression among patients with MI differed significantly in subgroups according to region, tool used to identify depression, study quality, sex, race, anterior MI, and diabetes status (P < .05). Heterogeneity was high within most subgroups. For unmarried subjects, the heterogeneity was low (I2 = 19.5, P = .293, 4 included studies).
Table 3.
Subgroup analyses of the prevalence of depression in myocardial infarction.
3.6. Sensitivity analysis and publication bias
After serially excluding each study, the pooled prevalence of depression among patients with MI ranged from 26.79% (95% CI: 21.65–32.26%) to 30.04% (95% CI: 23.97–36.48%), and the I2 statistic values ranged from 97.5 to 98.5%. Specifically, after excluding one population-based study, the pooled prevalence was 29.31% (95% CI: 22.71–36.38%), and the I2 statistic value was 98.4%. Furthermore, after excluding studies with moderate quality, the pooled prevalence decreased slightly from 28.70% (95% CI: 22.39–35.46%) to 27.31% (95% CI: 21.98–32.99%).
The results of Egger's test (t = -0.435, P = .669) and Begg's test (z = -0.630, P = .529) indicated no evidence of publication bias, and in accordance with these results, the funnel plot was symmetrical (Fig. 3).
Figure 3.
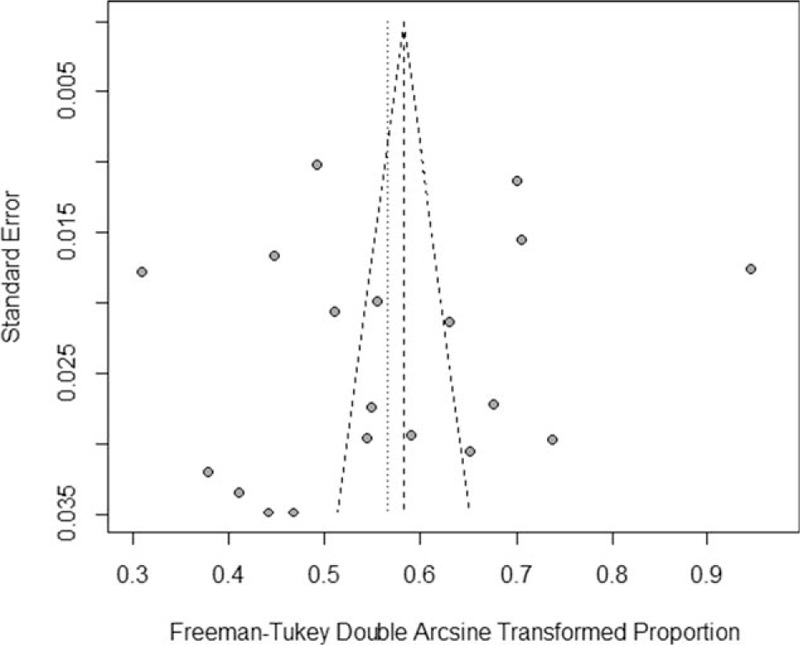
Funnel plot of the 19 eligible studies.
4. Discussion
This meta-analysis synthesized the evidence regarding the prevalence of depression among patients with MI and provided an updated estimate on the pooled prevalence. Nineteen eligible studies conducted in 10 countries with a total of 12,315 patients with MI were included, of which 3818 were identified with depression. The reported prevalence of depression ranged from 9.17% to 65.88% across the eligible studies, and the pooled prevalence of depression among patients with MI was 28.70% (95% CI: 22.39–35.46%) by a random effects model.
The pooled prevalence of depression among patients with MI found in this meta-analysis (28.70%) was comparable with that found in previous meta-analyses on patients with multiple sclerosis (30.5%),[41] hypertension (26.8%),[42] and chronic obstructive pulmonary disease (27.1%).[43] However, it was significantly higher than that among patients with cancer (8%–25%),[44–46] osteoarthritis (19.9%),[47] and spinal cord injury (22.2%).[48] Given the high pooled prevalence of depression found in this study and the association between depression and subsequent adverse health outcomes, such as impaired quality of life and increased risk of mortality among patients with MI, more psychological resources including early assessment and effective treatment of depression should be allocated to patients with MI.
This study found that the pooled prevalence of depression among patients with MI differed significantly based on region and race. Regional differences in the pooled prevalence of depression could be explained by the differences in the socio-economic levels, as well as the differences in the socio-demographic and social-cultural characteristics.[49] Similar finding was observed in a meta-analysis exploring the pooled prevalence of paternal depression in pregnancy and postpartum.[49] Racial differences in the pooled prevalence of depression could be mainly accounted for by the differences in social-cultural characteristics, as well as racial differences in the genetic background.[50]
This study also found that the pooled prevalence of depression identified by self-report questionnaire (29.63%) was significantly higher than that identified by structured interview (26.13%). Numerous studies have consistently shown that compared with structured interview, self-report questionnaire may overestimate the prevalence of depression.[44,51,52] For example, Li et al found that the pooled prevalence of depression among patients with hypertension identified by structured interview was 21.3%, while for studies using self-report questionnaire to identify depression, it was 29.8%.[42] Zhang et al found that the pooled prevalence of depression among patients with systemic lupus erythematosus identified by structured interview was 24%, while for studies that used BDI with a cutoff value of 11, it was 39%, and for Hospital Anxiety and Depression Scale (HADS) with a cutoff value of 8, it was 30%.[51] Therefore, caution should be applied when using self-report tools to identify depression. However, given the fact that even minimal depressive symptom could lead to an increased mortality risk following MI,[8] it is recommended for future studies on MI populations to identify depression using both self-report questionnaire and structured interview.
Significant sex differences in the pooled prevalence of depression among patients with MI were also observed in this study, which may be explained by the differences in the biological factors, such as hormones; and psychosocial factors, such as coping strategies, personality traits, and role overload.[53,54] Poynter et al conducted a systematic review exploring sex differences in the prevalence of depression among stroke patients and found that, among the 56 eligible studies, 35 reported that the prevalence of depression was higher among females than males.[55] In addition, by pooling the results of 8 eligible studies, Shanmugasegaram et al found that female CHD patients were at 1.77 times higher risk of suffering from major depression compared with males.[56] Based on these findings, special attention should be given to female subjects when implementing prevention strategies and psychological intervention of depression among patients with MI.
Some studies found that the MI characteristics, such as a history of previous MI, anterior MI, and Killip class may affect the prevalence of depression following MI,[14,16,17] while others showed contradictory results.[15,28,37] Furthermore, findings on the association of cardiovascular risk factors, such as smoking status, hypertension and diabetes, with the prevalence of depression were controversial.[9,15–17,37,39] This study showed that a history of previous MI, Killip class, current smoking, hypertension and hyperlipidemia did not contribute significantly to the pooled prevalence of depression, whereas anterior MI and diabetes status did. Subjects with anterior MI or diabetes exhibited higher pooled prevalence of depression than their counterparts. Given the high heterogeneity observed across the included studies of these subgroups, future studies are still needed to clarify the associations of MI characteristics and cardiovascular risk factors with depression following MI.
Though the overall quality of eligible studies was moderate to high, this study found that the pooled prevalence of depression varied significantly according to study quality, with moderate quality studies showing higher pooled prevalence than high quality studies. It has been well established that studies with relatively lower quality are more prone to employ biased sampling frames and induce selection bias, as a consequence of which, the effect size may be overestimated.[57,58] Therefore, more studies with high quality are warranted to obtain an accurate and reliable estimate.
Some limitations should be acknowledged. First, the overall heterogeneity across the eligible studies was high. Meta-regression analyses according to publication year, mean age of participants, percentage of male participants, percentage of participants with 1st-time MI, and quality assessment score did not identify any moderators which significantly affected the heterogeneity, and the heterogeneity within most subgroups was also high, indicating that future studies should explore more factors which may affect the prevalence of depression among patients with MI, such as social support and a history of previous psychiatric disorders. However, subgroup analyses found that the heterogeneity among unmarried subjects was low, suggesting that it is better for future studies exploring depression among patients with MI to stratify the subjects according to marital status to keep homogeneity. Second, meta-regression analysis according to the timing of depression assessment was not performed in this study since the timing of depression assessment varied across studies and hence difficult to classify. Nevertheless, evidence showed that the prevalence of depression did not decrease significantly with the elapse of time since MI.[9,40,50] Furthermore, it is worth noting here that all eligible studies except 1 were hospital-based, which may preclude generalizing the results of this study to population-based studies. Also, caution should be applied since subgroup analyses were performed univariately without adjustment for potential confounders.
5. Conclusions
The pooled prevalence of depression among patients with MI was 28.70% (95% CI: 22.39–35.46%) and differed significantly by region, tool used to identify depression, study quality, sex, race, anterior MI, and diabetes status. High heterogeneity was observed across all included studies. In addition, except for the married subjects, high heterogeneity was observed across studies within all subgroups. Future prospective studies with high quality are still needed to explore more factors affecting the prevalence of depression as well as clarify the associations of MI characteristics and cardiovascular risk factors with depression among patients with MI.
Acknowledgments
The authors are grateful to all authors of the full-text articles included in this meta-analysis. This study was supported by the National Natural Science Foundation of China (Grant No. 81774016).
Author contributions
Conceptualization: Mingchi Luo.
Data curation: Limin Feng, Lifeng Li, Wennan Liu.
Formal analysis: Limin Feng, Lifeng Li, Wennan Liu, Jianzhou Yang, Qing Wang.
Funding acquisition: Limin Feng.
Investigation: Limin Feng, Wennan Liu, Qing Wang, Le Shi.
Methodology: Limin Feng, Wennan Liu, Jianzhou Yang, Le Shi.
Resources: Qing Wang, Le Shi.
Software: Limin Feng, Le Shi.
Supervision: Mingchi Luo.
Validation: Lifeng Li, Jianzhou Yang, Qing Wang, Mingchi Luo.
Writing – original draft: Limin Feng.
Writing – review & editing: Lifeng Li, Jianzhou Yang, Mingchi Luo.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: BCDRS = Brief Carroll Depression Rating Scale, BDI = Beck Depression Inventory, CES-D = Center for Epidemiologic Studies Depression Scale, CHD = coronary heart disease, CI = confidence interval, CVD = cardiovascular disease, DIS = modified version of the National Institute of Mental Health Diagnostic Interview Schedule, HADS = Hospital Anxiety and Depression Scale, MI = myocardial infarction, PHQ-9 = 9-question Primary Care Evaluation of Mental Disorders Brief Patient Health Questionnaire, RCT = randomized controlled trial, SCID = Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition, SD = standard deviation, SDS = Zung Self-Rating Depression Scale, UK = United Kingdom, USA = United States of America, WHO = World Health Organization.
The authors have no conflicts of interest to disclose.
References
- [1].http://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) Accessed August 18, 2018. [Google Scholar]
- [2].Mendis S, Thygesen K, Kuulasmaa K, et al. World Health Organization definition of myocardial infarction: 2008–09 revision. Int J Epidemiol 2011;40:139–46. [DOI] [PubMed] [Google Scholar]
- [3].Gidron Y, Gilutz H, Berger R, et al. Molecular and cellular interface between behavior and acute coronary syndromes. Cardiovasc Res 2002;56:15–21. [DOI] [PubMed] [Google Scholar]
- [4].Kala P, Hudakova N, Jurajda M, et al. Depression and anxiety after acute myocardial infarction treated by primary PCI. PLoS One 2016;11:e0152367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].DiSante JL, Bires AM, Cline TW, et al. An analysis of the prevalence of depression post-myocardial infarction. Crit Care Nurs Q 2017;40:124–36. [DOI] [PubMed] [Google Scholar]
- [6].Hosseini SH, Ghaemian A, Mehdizadeh E, et al. Contribution of depression and anxiety to impaired quality of life in survivors of myocardial infarction. Int J Psychiatry Clin Pract 2014;18:175–81. [DOI] [PubMed] [Google Scholar]
- [7].Meijer A, Conradi HJ, Bos EH, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis of 25 years of research. Gen Hosp Psychiatry 2011;33:203–16. [DOI] [PubMed] [Google Scholar]
- [8].Bush DE, Ziegelstein RC, Tayback M, et al. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am J Cardiol 2001;88:337–41. [DOI] [PubMed] [Google Scholar]
- [9].Hanssen TA, Nordrehaug JE, Eide GE, et al. Anxiety and depression after acute myocardial infarction: an 18-month follow-up study with repeated measures and comparison with a reference population. Eur J Cardiovasc Prev Rehabil 2009;16:651–9. [DOI] [PubMed] [Google Scholar]
- [10].Kurdyak PA, Chong A, Gnam WH, et al. Depression and self-reported functional status: impact on mortality following acute myocardial infarction. J Eval Clin Pract 2011;17:444–51. [DOI] [PubMed] [Google Scholar]
- [11].Agarwal M, Trivedi JK, Sinh PK, et al. Depression in patients of myocardial infarction--a cross-sectional study in northern India. J Assoc Physicians India 2011;59:636–8. 643. [PubMed] [Google Scholar]
- [12].Drago S, Bergerone S, Anselmino M, et al. Depression in patients with acute myocardial infarction: influence on autonomic nervous system and prognostic role. Results of a five-year follow-up study. Int J Cardiol 2007;115:46–51. [DOI] [PubMed] [Google Scholar]
- [13].Pitzalis MV, Iacoviello M, Todarello O, et al. Depression but not anxiety influences the autonomic control of heart rate after myocardial infarction. Am Heart J 2001;141:765–71. [DOI] [PubMed] [Google Scholar]
- [14].Myers V, Gerber Y, Benyamini Y, et al. Post-myocardial infarction depression: increased hospital admissions and reduced adoption of secondary prevention measures–a longitudinal study. J Psychosom Res 2012;72:5–10. [DOI] [PubMed] [Google Scholar]
- [15].Lane D, Carroll D, Ring C, et al. Effects of depression and anxiety on mortality and quality-of-life 4 months after myocardial infarction. J Psychosom Res 2000;49:229–38. [DOI] [PubMed] [Google Scholar]
- [16].Parakh K, Thombs BD, Fauerbach JA, et al. Effect of depression on late (8 years) mortality after myocardial infarction. Am J Cardiol 2008;101:602–6. [DOI] [PubMed] [Google Scholar]
- [17].Watkins LL, Blumenthal JA, Carney RM. Association of anxiety with reduced baroreflex cardiac control in patients after acute myocardial infarction. Am Heart J 2002;143:460–6. [DOI] [PubMed] [Google Scholar]
- [18].Frasure-Smith N, Lesperance F, Gravel G, et al. Social support, depression, and mortality during the first year after myocardial infarction. Circulation 2000;101:1919–24. [DOI] [PubMed] [Google Scholar]
- [19].Thombs BD, Bass EB, Ford DE, et al. Prevalence of depression in survivors of acute myocardial infarction. J Gen Intern Med 2006;21:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Doyle F, McGee H, Conroy R, et al. Systematic review and individual patient data meta-analysis of sex differences in depression and prognosis in persons with myocardial infarction: a MINDMAPS study. Psychosom Med 2015;77:419–28. [DOI] [PubMed] [Google Scholar]
- [21].Gan Y, Gong Y, Tong X, et al. Depression and the risk of coronary heart disease: a meta-analysis of prospective cohort studies. BMC Psychiatry 2014;14:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wu Q, Kling JM. Depression and the risk of myocardial infarction and coronary death: a meta-analysis of prospective cohort studies. Medicine 2016;95:e2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].https://www.ncbi.nlm.nih.gov/books/NBK35156/ Accessed July 15, 2018. [Google Scholar]
- [24].Yang LS, Zhang ZH, Sun L, et al. Prevalence of suicide attempts among college students in China: a meta-analysis. PLoS One 2015;10:e0116303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hu J, Dong Y, Chen X, et al. Prevalence of suicide attempts among Chinese adolescents: a meta-analysis of cross-sectional studies. Compr Psychiatry 2015;61:78–89. [DOI] [PubMed] [Google Scholar]
- [26].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Huo Q, Zhang N, Yang Q. Epstein-Barr virus infection and sporadic breast cancer risk: a meta-analysis. PLoS One 2012;7:e31656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lauzon C, Beck CA, Huynh T, et al. Depression and prognosis following hospital admission because of acute myocardial infarction. CMAJ 2003;168:547–52. [PMC free article] [PubMed] [Google Scholar]
- [29].Dickens C, McGowan L, Percival C, et al. Depression is a risk factor for mortality after myocardial infarction: fact or artifact? J Am Coll Cardiol 2007;49:1834–40. [DOI] [PubMed] [Google Scholar]
- [30].Parashar S, Rumsfeld JS, Reid KJ, et al. Impact of depression on sex differences in outcome after myocardial infarction. Circ Cardiovasc Qual Outcomes 2009;2:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hosseini SH, Yousefnejad K, Tabiban S, et al. Effects of depression and anxiety symptoms on cardiac mortality following myocardial infarction: a 2-year follow-up. Int J Psychiatry Clin Pract 2011;15:91–6. [DOI] [PubMed] [Google Scholar]
- [32].Wheeler A, Beltrame J, Tucker G, et al. Depression and 5-year mortality in patients with acute myocardial infarction: analysis of the IDACC database. Aust N Z J Psychiatry 2012;46:669–75. [DOI] [PubMed] [Google Scholar]
- [33].Larsen KK, Christensen B, Nielsen TJ, et al. Post-myocardial infarction anxiety or depressive symptoms and risk of new cardiovascular events or death: a population-based longitudinal study. Psychosom Medicine 2014;76:739–46. [DOI] [PubMed] [Google Scholar]
- [34].Schleifer SJ, Macari-Hinson MM, Coyle DA, et al. The nature and course of depression following myocardial infarction. Arch Intern Med 1989;149:1785–9. [PubMed] [Google Scholar]
- [35].Johansson I, Karlson BW, Grankvist G, et al. Disturbed sleep, fatigue, anxiety and depression in myocardial infarction patients. Eur J Cardiovasc Nurs 2010;9:175–80. [DOI] [PubMed] [Google Scholar]
- [36].Crowe JM, Runions J, Ebbesen LS, et al. Anxiety and depression after acute myocardial infarction. Heart Lung 1996;25:98–107. [DOI] [PubMed] [Google Scholar]
- [37].Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA 1993;270:1819–25. [PubMed] [Google Scholar]
- [38].Kaufmann MW, Fitzgibbons JP, Sussman EJ, et al. Relation between myocardial infarction, depression, hostility, and death. Am Heart J 1999;138(3 Pt 1):549–54. [DOI] [PubMed] [Google Scholar]
- [39].Shiotani I, Sato H, Kinjo K, et al. Depressive symptoms predict 12-month prognosis in elderly patients with acute myocardial infarction. J Cardiovasc Risk 2002;9:153–60. [DOI] [PubMed] [Google Scholar]
- [40].Welin C, Lappas G, Wilhelmsen L. Independent importance of psychosocial factors for prognosis after myocardial infarction. J Intern Med 2000;247:629–39. [DOI] [PubMed] [Google Scholar]
- [41].Boeschoten RE, Braamse AMJ, Beekman ATF, et al. Prevalence of depression and anxiety in Multiple Sclerosis: a systematic review and meta-analysis. J Neurol Sci 2017;372:331–41. [DOI] [PubMed] [Google Scholar]
- [42].Li Z, Li Y, Chen L, et al. Prevalence of depression in patients with hypertension: a systematic review and meta-analysis. Medicine 2015;94:e1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Matte DL, Pizzichini MM, Hoepers AT, et al. Prevalence of depression in COPD: a systematic review and meta-analysis of controlled studies. Respir Med 2016;117:154–61. [DOI] [PubMed] [Google Scholar]
- [44].Krebber AM, Buffart LM, Kleijn G, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology 2014;23:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Watts S, Prescott P, Mason J, et al. Depression and anxiety in ovarian cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open 2015;5:e007618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Watts S, Leydon G, Birch B, et al. Depression and anxiety in prostate cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open 2014;4:e003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Stubbs B, Aluko Y, Myint PK, et al. Prevalence of depressive symptoms and anxiety in osteoarthritis: a systematic review and meta-analysis. Age Ageing 2016;45:228–35. [DOI] [PubMed] [Google Scholar]
- [48].Williams R, Murray A. Prevalence of depression after spinal cord injury: a meta-analysis. Arch Phys Med Rehabil 2015;96:133–40. [DOI] [PubMed] [Google Scholar]
- [49].Cameron EE, Sedov ID, Tomfohr-Madsen LM. Prevalence of paternal depression in pregnancy and the postpartum: an updated meta-analysis. J Affect Disord 2016;206:189–203. [DOI] [PubMed] [Google Scholar]
- [50].Lacerda-Pinheiro SF, Pinheiro Junior RF, Pereira de Lima MA, et al. Are there depression and anxiety genetic markers and mutations? J Affect Disord 2014;168:387–98. [DOI] [PubMed] [Google Scholar]
- [51].Zhang L, Fu T, Yin R, et al. Prevalence of depression and anxiety in systemic lupus erythematosus: a systematic review and meta-analysis. BMC Psychiatry 2017;17:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Matcham F, Rayner L, Steer S, et al. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology 2013;52:2136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kornstein SG. Gender differences in depression: implications for treatment. J Clin Psychiatry 1997;58Suppl 15:12–8. [PubMed] [Google Scholar]
- [54].Goodwin RD, Gotlib IH. Gender differences in depression: the role of personality factors. Psychiatry Res 2004;126:135–42. [DOI] [PubMed] [Google Scholar]
- [55].Poynter B, Shuman M, Diaz-Granados N, et al. Sex differences in the prevalence of post-stroke depression: a systematic review. Psychosomatics 2009;50:563–9. [DOI] [PubMed] [Google Scholar]
- [56].Shanmugasegaram S, Russell KL, Kovacs AH, et al. Gender and sex differences in prevalence of major depression in coronary artery disease patients: a meta-analysis. Maturitas 2012;73:305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609–13. [DOI] [PubMed] [Google Scholar]
- [58].Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001;135:982–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


