Abstract
Background:
Obstructive sleep apnea (OSA) is common among patients with chronic kidney disease (CKD). CKD may increase the risk of OSA, and OSA may increase the risk of renal injury. Nasal continuous positive airway pressure (nCPAP) is the standard treatment for OSA. However, the effect of nCPAP on the progression of CKD is unclear.
Methods:
A total of 395 patients with stage 3/4 CKD were initially examined, and 269 patients (148 non-OSA cases; 79 mild OSA cases; 42 moderate/severe OSA cases) were analyzed after implementation of the exclusion criteria. The severity of OSA was determined by polysomnography (PSG). Fifty-two OSA patients (32 mild OSA cases; 20 moderate/severe OSA cases) received nCPAP treatment for 12 months. Variables associated with OSA severity and estimated glomerular filtration rate (eGFR) were evaluated before and after the 12-month nCPAP treatment.
Results:
Among all 269 CKD patients, body mass index (BMI), and eGFR had significant associations with OSA severity. Age, BMI, apnea–hypopnea index (AHI), mean SaO2%, and SaO2 <90% monitoring time had independent associations with lower eGFR. The 12-month nCPAP treatment significantly reduced the rate of eGFR decline. Univariate and multivariate analysis indicated that age, BMI, AHI, mean SaO2%, and SaO2 <90% monitoring time were independently associated with reduced eGFR. Furthermore, nCPAP treatment significantly improved eGFR, AHI, mean SaO2, and SaO2 <90% monitoring time in patients with mild OSA, and improved systolic/diastolic blood pressure, urinary protein level, eGFR, AHI, mean SaO2, and SaO2 <90% monitoring time for patients with moderate/severe OSA.
Conclusion:
This study of patients with CKD and OSA indicated that nCPAP therapy significantly ameliorated CKD progression, especially in those with moderate/severe OSA.
Keywords: apnea/hypopnea index, chronic kidney disease, continuous positive airway pressure, estimated glomerular filtration rate, obstructive sleep apnea
1. Introduction
Obstructive sleep apnea (OSA) is a common condition in patients with chronic kidney disease (CKD),[1–5] and previous research indicated its prevalence is much higher among CKD patients (up to 65%) than in the general population (20%).[6–8] Many clinical studies have demonstrated a relationship between OSA and renal dysfunction. In particular, patients with OSA often have increased levels of urinary albumin excretion, glomerular hyperfiltration, and proteinuria.[9–11] There is also a high rate of OSA in patients with end-stage renal disease (ESRD).[12–15] In addition, OSA may contribute to the progression of CKD, because hypoxia can lead to renal tubule interstitial injury during the progression to ESRD.[16] Other reports demonstrated a correlation between the increase of nocturnal hypoxia and the decrease of kidney function over time, and reported that OSA is a significant predictor of accelerated loss of kidney function.[17]
Nasal continuous positive airway pressure (nCPAP) is the primary treatment used to improve nocturnal desaturation in patients with OSA, and this therapy provides relief from symptoms such as headaches, snoring, daytime sleepiness, and elevated blood pressure (BP).[18,19] There is also evidence that untreated OSA increases the risk of CDK, but that nCPAP therapy can significantly decrease the mortality of patients with OSA.[20,21] However, the effect of nCPAP on the progression of CKD in patients with OSA is unclear. In this study, we investigated the effects of a 12-month nCPAP therapy on the progression of CKD in patients with OSA.
2. Materials and methods
2.1. CKD patients
This non-randomized cohort study was approved by the local ethics committees. The 395 participants were all patients with stages 3/4 CKD (estimated glomerular filtration rate [eGFR] between 15 and 60 mL/min per 1.73 m2) and were initially enrolled from January 2011 to June 2015 in the Department of Otolaryngology at the Second Hospital of Shangdong University and the Department of Nephrology in Tongji Hospital of Tongji Medical College. A total of 269 patients were ultimately analyzed, after exclusion of patients: who were hospitalized for acute complications, such as acute kidney injury (AKI), acute heart failure, stroke, or infection (n = 38); who declined to participate due to economic reasons (n = 42); who had diagnoses of chronic obstructive pulmonary disease (n = 9); or who were lost to follow-up, had missing data, discontinued intervention, or had a malignancy, a psychiatric disorder, or hyperparathyroidism (n = 37). Informed consent was obtained from each patient prior enrollment, and an education session was given to all participants (Fig. 1).
Figure 1.
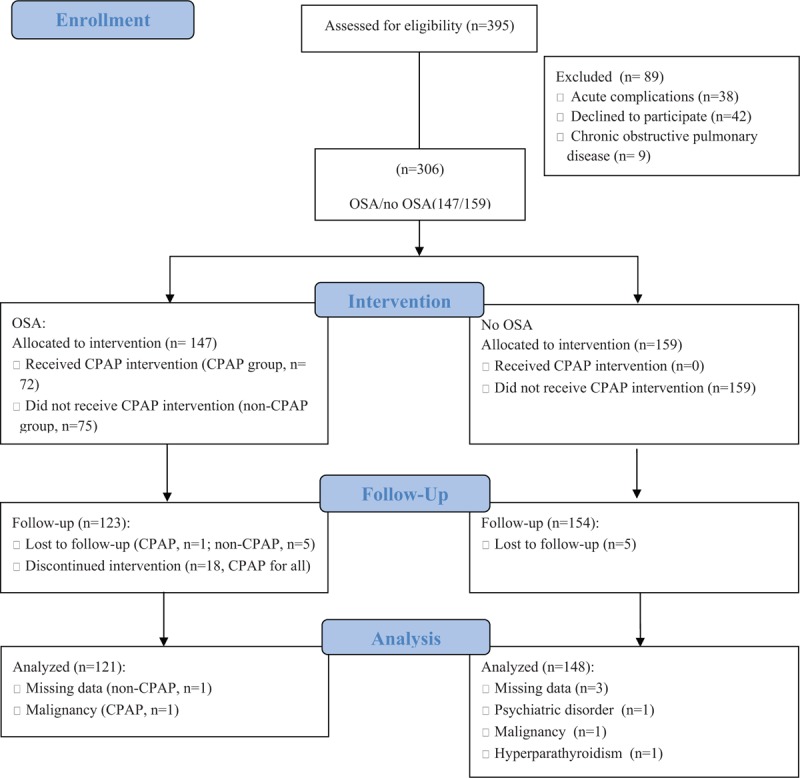
CONSORT 2010 flow diagram. In this study, 395 patients with stages 3 to 4 CKD were enrolled from January 2011 to June 2015. Finally, 121 patients with OSA and 148 patients without OSA were analyzed. CKD = chronic kidney disease, OSA = obstructive sleep apnea.
2.2. OSA examination
After elimination of participants due to the first 3 exclusion criteria, full-night polysomnography (PSG, Respironics, Murrysville, PA) was performed in 306 CKD patients to evaluate the apnea–hypopnea index (AHI), according to the recommendations of the American Academy of Sleep Medicine. Apnea was defined as cessation of breathing for a minimum of 10 seconds. Hypopnea was defined as a reduction of airflow from baseline of >50%, with a 4% or greater reduction in arterial oxygen saturation (SaO2) for a minimum of 10 seconds. AHI was defined as the number of apnea and hypopnea episodes per hour of sleep. After examination of the PSG results and application of the fourth exclusion criterion, 269 patients were analyzed and divided into 3 groups: no OSA (AHI < 5/h), mild OSA (5/h ≤ AHI < 15/h), and moderate/severe SA (AHI ≥ 15/h).
2.3. CPAP intervention
Based on patient choice, 72 patients preferred to receive a 12-month nCPAP treatment with an auto-titrating device (pressure: 4–12 cm H2O, AirSense 10 AutoSet, ResMed, Ltd., Bella Vista, Australia). Patients were encouraged to use a nasal mask (MirageFX, ResMed, Ltd.) and a heated humidification system (HumidAir, ResMed, Ltd.). The auto-pressure modus was used during the entire study period. After initiation of nCPAP therapy by a trained study nurse, further support was provided by a web-based telemedicine system (AirView, ResMed, Ltd.). The telemedicine data were screened by a specially trained study nurse, who examined adherence, mask leakage, and residual apneas each week, and established telephone contact if necessary. The first follow-up study visit was scheduled for 3 months after initiation of nCPAP treatment. Continued intervention was defined as use of nCPAP for 4 hours per night during 70% of the recorded nights.[15] Ultimately, 52 patients (mild OSA: n = 32; moderate/severe OSA: n = 20) completed the 12-month nCPAP treatment, and 20 OSA patients were excluded because of loss to follow-up, discontinued intervention, missing data, or malignancy.
2.4. Anthropometric and laboratory data
The anthropometric and laboratory data of the subjects, including age, sex, body mass index (BMI), CKD etiology, medications (angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, aldosterone receptor antagonists, and statins), serum creatinine, and serum albumin, were obtained from their medical records. The association of eGFR with these clinical characteristics was evaluated in all 52 patients who completed treatment.
2.5. Statistical analysis
All continuous values are expressed as means ± standard deviations (SDs), and differences were assessed using the independent-samples t test or a one-way analysis of variance (ANOVA). Categorical variables are expressed as numbers and percentages, and differences were assessed using the chi-square test. Univariate and multivariate linear regression analyses were used to identify factors independently associated with eGFR. All eGFR values are in units of “mL/min/1.73 m2.” According to the univariate analysis, age, BMI, hypertension, diabetes mellitus, AHI, mean SaO2, SaO2 <90% monitoring time were explanatory variables. Variables with P values <.05 in the univariate model were entered into the multivariate model. A P value <.05 was considered statistically significant. All data were analyzed using the SPSS version 11.0 (Chicago, IL).
3. Results
3.1. Baseline characteristics of patients with stratification by OSA severity
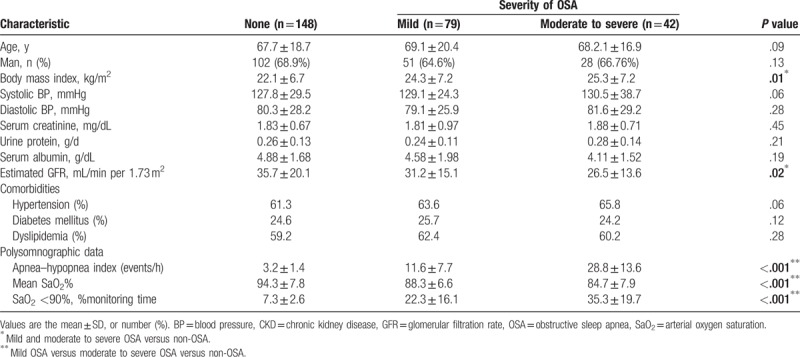
Table 1 summarizes the baseline characteristics of the 269 patients. There were 148 patients (55.02%) without OSA, 70 patients (29.37%) with mild OSA, and 42 patients (15.61%) with moderate/severe OSA. The BMI increased with OSA severity (no OSA: 22.1 ± 6.7, mild OSA: 24.3 ± 7.2, moderate/severe OSA: 25.3 ± 7.2; P = .001) and the eGFR declined with increasing OSA severity (no OSA: 35.7 ± 20.1, mild OSA: 31.2 ± 15.1, moderate/severe OSA: 26.5 ± 13.6, P = .02). Moreover, all 3 PSG parameters (AHI, mean SaO2, and SaO2 <90% monitoring time) deteriorated significantly with the increasing of severity of the OSA (P < .001 for all). However, patients with and without OSA had no significant differences in age, sex, systolic BP, diastolic BP, serum creatinine, and urinary protein.
Table 1.
Baseline characteristics of 269 patients with CKD stratified by the severity of OSA.
3.2. Univariate and multivariate analysis of factors associated with eGFR
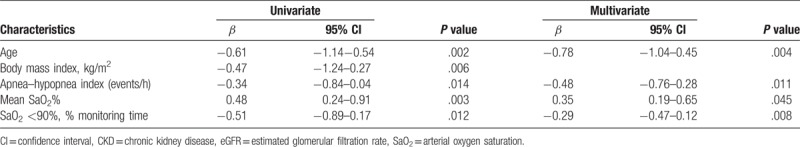
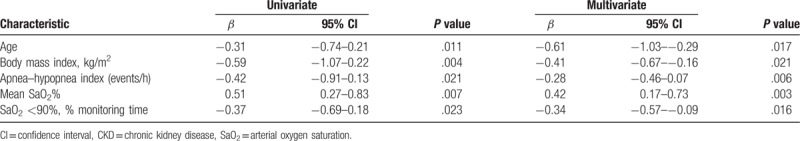
We used a linear regression model, with univariate and multivariate analyses, to identify risk factors associated with eGFR in 269 patients (Table 2). The univariate analysis indicated that advanced age, high BMI, high AHI, low mean SaO2%, and long SaO2 <90% monitoring time were significantly associated with lower eGFR. The multivariate analysis indicated that advanced age, high AHI, low mean SaO2%, and long SaO2 <90% monitoring time were also independently associated with lower eGFR.
Table 2.
Univariate and multivariate analysis for eGFR levels in 269 patients with CKD.
3.3. Change of eGFR after 12 months
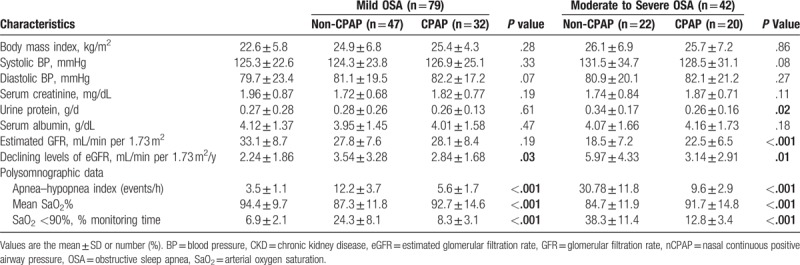
Fifty-two of the 121 CKD patients with OSA (mild OSA: 32 cases; moderate/severe OSA: 20 cases) underwent 12-month nCPAP treatment based on individual patient choice (Table 3). Relative to the nCPAP group, the non-CPAP group had a greater decline of eGFR for those with mild OSA (3.54 ± 3.28/y vs 3.14 ± 2.91/y, P = .01) and for those with moderate/severe OSA (5.97 ± 4.33/y vs 2.84 ± 1.68/y, P = .03). In addition, among moderate/severe OSA patients at 12 months, the nCPAP group had a significantly higher eGFR than the non-CPAP group (22.5 ± 6.5 vs 18.5 ± 7.2, P < .001), and a lower level of urinary protein (0.26 ± 0.16 vs 0.34 ± 0.17, P = .02). Also at 12 months, the PSG results of the nCPAP group were significantly better than those of the non-CPAP group.
Table 3.
The characteristics of 269 patients with CKD after 12-month non-CPAP/CPAP treatment.
3.4. Univariate and multivariate analysis of factors associated with eGFR decline
We performed univariate and multivariate analyses of 269 CKD patients with OSA to identify factors independently associated with eGFR decline over 12 months (Table 4). The univariate analyses indicated that age, AHI, BMI, mean SaO2%, and SaO2 <90% monitoring time were significantly associated with a greater eGFR decline. Moreover, the multivariate analysis indicated that age, AHI, BMI, mean SaO2%, and SaO2 <90% monitoring time were also independently associated with eGFR decline.
Table 4.
Univariate and multivariate analysis for the declining levels of eGFR in 269 patients with CKD.
3.5. Characteristics of patients after 12-month CPAP treatment
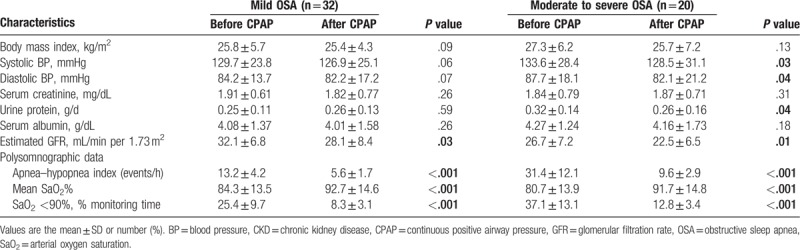
We also compared the clinical parameters of the 52 patients before and after the 12-month nCPAP treatment (Table 5). After treatment, patients with moderate/severe OSA (n = 20) had a lower mean systolic BP (128.5 ± 31.1 mmHg vs 133.6 ± 28.4 mmHg, P = .03), a lower mean diastolic BP (82.1 ± 21.2 mmHg vs 87.7 ± 18.1 mmHg, P = .04), and a lower urinary protein level (0.26 ± 0.16 vs 0.32 ± 0.14, P = .04). The 12-month treatment was also associated with decreased eGFR in those with mild OSA (n = 32; 32.1 ± 6.8 vs 28.1 ± 8.4, P = .03) and moderate/severe OSA (26.7 ± 7.2 vs 22.5 ± 6.5, P = .01). Moreover, there were also significant improvements in PSG results after nCPAP treatment for those with mild OSA and moderate/severe OSA. Characteristics such as BMI, BP, serum creatinine, urinary protein, and serum albumin did not change significantly in patients with OSA after the 12 months.
Table 5.
The characteristics of 52 patients with combined CKD and OSA before/after 12-month CPAP treatment.
4. Discussion
Hypoxia is related to the deterioration of renal function, and chronic hypoxia is a defining characteristic of the final common pathway leading to ESRD.[22,23] We also found a close association between hypoxia and CKD progression. In particular, our study of patients with CKD and OSA indicated that as OSA became more severe (based on AHI, mean SaO2%, and SaO2 <90% monitoring time), eGFR also decreased significantly (Table 1). Moreover, old age, high BMI, high AHI, low mean SaO2%, and long SaO2 <90% monitoring time were significantly associated with lower eGFR (Table 2). This supports previous research findings that multiple factors are associated with CKD progression.[22–28]
Although nCPAP treatment is considered the most effective strategy for treatment of hypoxia in OSA patients, no studies have yet definitively established the effect of nCPAP treatment on the progression of CKD in patients with OSA. To our knowledge, the present study is the first to examine the relationship of nCPAP treatment with progression of CKD. We also compared changes in eGFR levels of OSA patients receiving and not receiving nCPAP treatment. Among patients with mild OSA and moderate/sever OSA, the decline of eGFR was significantly greater in the non-CPAP group (Table 3). Moreover, age, BMI, and indicators of hypoxia (AHI, mean SaO2%, and SaO2 <90% monitoring time) were also independently associated with CKD progression (Table 4). These results are consistent with previous studies which found that CKD and OSA have reciprocal effects on each other,[22,23] and also indicate that amelioration of hypoxia in patients with CKD and OSA by nCPAP treatment may help to slow the progression of renal disease.
In addition to causing hypoxia, OSA may promote CKD progression by several other mechanisms. We found that BMI was significantly associated with severity of OSA and low eGFR (Tables 1 and 2). These results are consistent with previous studies which showed that obesity was associated with OSA, and was also a risk factor for progression to renal failure.[24–27] However, the 12-month nCPAP treatment used in the present study did not reduce patient BMI. Therefore, we considered that this small difference would be one of the risk factors of renal failure but not the major progressive factor. Furthermore, elevated BP is considered a major risk factor for CKD progression. Recent studies demonstrated that OSA caused hypertension by activation of the renin-angiotensin system and the sympathetic nervous system.[18,19] However, we found no evidence of an association between hypertension and CKD progression in our patients. Patients with hypertension are typically treated with angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers, and this might explain our finding of no relationship between BP and declining of eGFR. Notably, our patients with moderate/severe OSA who received the nCPAP treatment experienced significant declines of systolic and diastolic BP at 12 months (Table 5). In agreement, other studies reported that even a short daily usage of CPAP provides some clinical benefits for the cardiovascular systems of patients with OSA.[28] Therefore, nCPAP treatment protected kidney function, and also reduced the cardiovascular complications of patients with CKD and OSA because it reduced urinary protein excretion and BP.
It was noteworthy that eGFR was lower in patients with mild OSA and moderate/severe OSA after the 12-month nCPAP treatment (Table 5). This indicates that CKD apparently continues to progress despite the use of nCPAP. In other words, nCPAP treatment appears to slow the decline of eGFR in CKD patients with OSA, but does not prevent or reverse CKD progression (Table 3). Other factors, such as age, BMI, and BP, also affect CKD progression.[22–28] On the other hand, we found that nCPAP treatment dramatically improved the status of CKD patients with severe hypoxia and moderate/severe OSA, but provided little benefit for CKD patients with mild hypoxia and mild OSA. Therefore, among those with moderate/severe OSA, eGFR was significantly higher and urinary protein excretion was significantly lower in the nCPAP group; but among those with mild OSA, the nCPAP and non-CPAP groups had no significant differences of eGFR and urinary protein (Table 3).
Our study also had limitations. First, all study subjects were from 2 hospitals, suggesting our results may have limited generalizability. However, our results were consistent with those of several other studies. Second, during the study period, certain potential risk factors (uncontrolled hypertension) could have influenced renal function. Third, receipt of nCPAP treatment was not randomized, but was a personal decision of each patient (due to economic reasons). This could lead to referral bias.
In summary, the current non-randomized cohort study provides evidence that OSA contributes to CKD progression, and that age, BMI, apnea–hypopnea index (AHI), mean SaO2%, and SaO2 <90% monitoring time were independently associated with lower eGFR. Moreover, comparing OSA patients who received and did not receive nCPAP treatment indicated greater declines of eGFR among non-CPAP patients with mild OSA and among non-CPAP patients with moderate/severe OSA. These results indicate that a 12-month nCPAP therapy significantly slowed the progression of CKD in patients with CKD and OSA.
Acknowledgments
The authors acknowledge the helpful comments from our reviewers.
Author contributions
Conceptualization: Chengcheng Liu, Miaoqing Zhao, Ming Xia.
Data curation: Xiaoming Li, Hao Zhang, Dianshui Sun.
Formal analysis: Min Han.
Funding acquisition: Dianshui Sun, Ming Xia.
Investigation: Xiaoming Li, Chengcheng Liu, Hao Zhang, Jie Zhang, Miaoqing Zhao, Ming Xia, Min Han.
Project administration: Dianshui Sun.
Resources: Xiaoming Li, Jie Zhang.
Software: Chengcheng Liu, Hao Zhang, Min Han.
Writing – original draft: Miaoqing Zhao.
Footnotes
Abbreviations: AHI = apnea–hypopnea index, AKI = acute kidney injury, ANOVA = analysis of variance, BMI = body mass index, BP = blood pressure, CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate, ESRD = end-stage renal disease, GFR = glomerular filtration rate, nCPAP = nasal continuous positive airway pressure, OSA = obstructive sleep apnea, PSG = polysomnography, SaO2 = arterial oxygen saturation, SD = standard deviation.
XL and CL have contributed equally to this paper.
This study was supported by the Clinical Medicine Science and Technology Innovation Project of Jinan City (No. 201506005), a grant of the Key research and development plan of Shandong Province (2016GSF201155, 2016GSF201077, and 2016GSF201140), and the plan of Qingdao medical scientific research guidance for 2016 (No.2016-WJZD057). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors report no conflicts of interest.
the author(s) of this work have nothing to disclose.
References
- [1].Ddm N, Ahmed SB, Ahs L, et al. Declining kidney function increases the prevalence of sleep apnea and nocturnal hypoxia. Chest 2012;141:1422–30. [DOI] [PubMed] [Google Scholar]
- [2].Sakaguchi Y, Shoji T, Kawabata H, et al. High prevalence of obstructive sleep apnea and its association with renal function among nondialysis chronic kidney disease patients in Japan: a cross-sectional study. Clin J Am Soc Nephrol 2011;6:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Markou N, Kanakaki M, Myrianthefs P, et al. Sleep-disordered breathing in nondialyzed patients with chronic renal failure. Lung 2006;184:43–9. [DOI] [PubMed] [Google Scholar]
- [4].Roumelioti ME, Buysse DJ, Sanders MH, et al. Sleep-disordered breathing and excessive daytime sleepiness in chronic kidney disease and hemodialysis. Clin J Am Soc Nephrol 2011;6:986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kraus MA, Hamburger RJ. Sleep apnea in renal failure. Adv Perit Dial 1997;13:88–92. [PubMed] [Google Scholar]
- [6].Fletcher EC. Obstructive sleep apnea and the kidney. J Am Soc Nephrol 1993;4:1111–21. [DOI] [PubMed] [Google Scholar]
- [7].Hanly P. Sleep apnea and daytime sleepiness in end-stage renal disease. Semin Dial 2004;17:109–14. [DOI] [PubMed] [Google Scholar]
- [8].Fleischmann G, Fillafer G, Matterer H, et al. Prevalence of chronic kidney disease in patients with suspected sleep apnoea. Nephrol Dial Transplant 2010;25:181–6. [DOI] [PubMed] [Google Scholar]
- [9].Yaşar ZA, Ucar ZZ, Demir AU, et al. Does CPAP therapy alter urinary albumin level in adult patients with moderate to severe obstructive sleep apnea syndrome. Sleep Breath 2014;18:525–32. [DOI] [PubMed] [Google Scholar]
- [10].Adeseun GA, Rosas SE. The impact of obstructive sleep apnea on chronic kidney disease. Curr Hypertens Rep 2010;12:378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ting H, Liou CM, Shih TS, et al. Obstructive sleep apnea rather than diabetes or obesity associated with proteinuria in late mid-aged male workers: a decision tree analysis. Sleep Breath 2015;19:1167–74. [DOI] [PubMed] [Google Scholar]
- [12].Zoccali C, Mallamaci F, Tripepi G. Nocturnal hypoxemia predicts incident cardiovascular complications in dialysis patients. J Am Soc Nephrol 2002;13:729–33. [DOI] [PubMed] [Google Scholar]
- [13].Masuda T, Murata M, Honma S, et al. Sleep-disordered breathing predicts cardiovascular events and mortality in hemodialysis patients. Nephrol Dial Transplant 2011;26:2289–95. [DOI] [PubMed] [Google Scholar]
- [14].Tang SC, Lam B, Yao TJ, et al. Sleep apnea is a novel risk predictor of cardiovascular morbidity and death in patients receiving peritoneal dialysis. Kidney Int 2010;77:1031–8. [DOI] [PubMed] [Google Scholar]
- [15].Benz RL, Pressman MR, Hovick ET, et al. Potential novel predictors of mortality in end-stage renal disease patients with sleep disorders. Am J Kidney Dis 2000;35:1052–60. [DOI] [PubMed] [Google Scholar]
- [16].Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol 2006;17:17–25. [DOI] [PubMed] [Google Scholar]
- [17].Ahmed SB, Ronksley PE, Hemmelgarn BR, et al. Nocturnal hypoxia and loss of kidney function. PLoS One 2011;6:e19029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zalucky AA, Nicholl DD, Hanly PJ, et al. Nocturnal hypoxemia severity and renin-angiotensin system activity in obstructive sleep apnea. Am J Respir Crit Care Med 2015;192:873–80. [DOI] [PubMed] [Google Scholar]
- [19].Foster GE, Hanly PJ, Ahmed SB, et al. Intermittent hypoxia increases arterial blood pressure in humans through a Renin-Angiotensin system-dependent mechanism. Hypertension 2010;56:369–77. [DOI] [PubMed] [Google Scholar]
- [20].Abdel-Kader K, Dohar S, Shah N, et al. Resistant hypertension and obstructive sleep apnea in the setting of kidney disease. J Hypertens 2012;30:960–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Abuyassin B, Sharma K, Ayas NT, et al. Obstructive sleep apnea and kidney disease: a potential bidirectional relationship. J Clin Sleep Med 2015;11:915–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Puckrin R, Iqbal S, Zidulka A, et al. Renoprotective effects of continuous positive airway pressure in chronic kidney disease patients with sleep apnea. Int Urol Nephrol 2015;47:1839–45. [DOI] [PubMed] [Google Scholar]
- [23].Koga S, Ikeda S, Yasunaga T, et al. Effects of nasal continuous positive airway pressure on the glomerular filtration rate in patients with obstructive sleep apnea syndrome. Intern Med 2013;52:345–9. [DOI] [PubMed] [Google Scholar]
- [24].Wiggins KJ, Johnson DW. The influence of obesity on the development and survival outcomes of chronic kidney disease. Adv Chronic Kidney Dis 2005;12:49–55. [DOI] [PubMed] [Google Scholar]
- [25].Gámez-Méndez AM, Vargas-Robles H, Arellano-Mendoza M, et al. Early stage of obesity potentiates nitric oxide reduction during the development of renal failure. J Nephrol 2014;27:281–7. [DOI] [PubMed] [Google Scholar]
- [26].Komura H, Nomura I, Kitamura K, et al. Gender difference in relationship between body mass index and development of chronic kidney disease. BMC Res Notes 2013;6:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Romero-Corral A, Caples SM, Lopez-Jimenez F, et al. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest 2010;137:711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Linz D, Mahfoud F, Linz B, et al. Effect of obstructive respiratory events on blood pressure and renal perfusion in a pig model for sleep apnea. Am J Hypertens 2014;27:1293–300. [DOI] [PubMed] [Google Scholar]


