Abstract
The neutrophil–lymphocyte ratio (NLR) is an immune response-related indicator and it is associated with poor prognosis of various cancers. The carbohydrate antigen19-9 (CA19-9) is a tumor-associated antigen and it has prognostic relevance in gallbladder carcinoma (GBC). We aimed to analyze whether preoperative NLR and serum CA19-9 were associated with outcomes of GBC patients after surgery with curative intent.
Between January 2010 and May 2015, 90 resectable GBC patients who underwent curative surgery in our institution were included. All final diagnoses were confirmed by pathologic examination. The demographics, clinical, and histopathology data were analyzed. The Cox regression proportional hazard model and Kaplan–Meier method were used to assess prognostic factors.
The cutoff values of 4.33 and 250.90 U/mL were defined as high NLR and high CA19-9, respectively. The univariate analyses showed that TNM stage, lymph node metastasis, the degree of tumor differentiation, margin status, combined hepatectomy, CA19-9, NLR, and PNI were all associated with overall survival (P < .05). According to the multivariable analysis, NLR (hazard ratio (HR) 3.840, 95% confidence interval (95% CI): 2.122–6.947, P < .001), CA19-9 (HR 2.230, 95% CI: 1.297–3.835, P = .004), TNM stage (HR 3.864, 95% CI: 1.819–8.207, P < .001), lymph node metastasis (HR 1.679, 95% CI: 1.005–2.805, P = .048), and margin status (HR 1.873, 95% CI: 1.063–3.300, P = .030) were independent prognostic factors. The median survival time in low NLR and CA19-9 group was better than high NLR and CA19-9 group (P < .05).
The preoperative NLR and serum CA19-9 are associated with prognosis of patients with GBC. High NLR and high CA19-9 were predictors of poor long-term outcome among patients with GBC undergoing curative surgery.
Keywords: CA19-9, gallbladder carcinoma, NLR, prognostic
1. Introduction
Gallbladder carcinoma (GBC) is the most common malignancy of the biliary tract. It is more common in some developing countries[1] and accounts for about 1% of all cancers in China.[2] Curative resection is the only potentially curative therapeutic option for GBC.[3,4] However, early diagnosis of GBC is difficult because its early symptoms are usually nonspecific and highly invasive character.[5] Therefore, the curative resection rate (22%–38%) is low.[3,4] Under standard treatments, the overall 5-year survival rate of GBC (2.7%–20.1%) is still unsatisfied.[6,7] The prediction of tumor progression after curative resection is limited to the use of histopathological features such as resection margin, differentiation, tumor size, and lymph node metastasis.
Recently, some factors have been reported to be related to a poor prognosis of GBC.[8,9] There is increasing evidence correlating the presence of inflammation with tumor progression and metastasis. The Onodera's prognostic nutrition index (PNI), neutrophil-lymphocyte ratio (NLR), and platelet-lymphocyte ratio (PLR) have been identified as significant predictor for patients’ survival in various different tumors, such as gastric cancer, malignant mesothelioma, and colorectal cancer.[9–11] The carbohydrate antigen 19-9 (CA19-9) is a tumor-associated antigen. It is a widely used marker for biliary and pancreatic tumor. For the diagnosis of GBC, its sensitivity and specificity are 77.5% and 68.7%, respectively.[12] Moreover, CA 19-9 has been reported to be a prognostic marker in various tumors.[13,14] Thus, the purpose of this study was to explore the roles of preoperative NLR and CA19-9 in the prognosis assessment of the patients with curative resection for GBC.
2. Patients and methods
2.1. Patients and data collection
Data from 90 respectable GBC patients after surgery with curative intent at West China Hospital of Sichuan University between January 2010 and May 2015 were reviewed retrospectively. All final diagnoses were confirmed by pathologic examination. The resection with curative intent was defined as R0 or R1 resections.[15] Clinical and pathological data were collected, including age, sex, body mass index (BMI), and serum parameters such as hemoglobin, white blood cell count (WBC count), platelet count, total bilirubin, CA19-9, carcinoembryonic antigen (CEA), nutritional, and inflammatory markers such as PNI, NLR, and PLR, type of resection and tumor differentiation were analyzed. The measurement of serum parameters was performed within 1 week before the operation. In our study, hemoglobin, WBC, platelet count (PLT), neutrophil, and lymphocyte count were determined with XE-2100 and XE-5000 systems (Sysmex Corporation, Kobe, Japan). Serum Albumin (ALB) was determined with a cobas 8000 analyser (Roche, Mannheim, Germany). Serum CA 19-9 and CEA were determined with a cobas E601 system (Roche, Mannheim, Germany). CA19-9 > 37 U/mL and CEA > 3.4 ng/mL were the upper limits of normal. The PNI consists of serum ALB and lymphocyte count, reflecting the inflammation and nutritional status of the host. The NLR was defined as the ratio of neutrophil the count to the lymphocyte count, and the PLR was defined as the ratio of the PLT to the lymphocyte count. Staging was according to AJCC 7th edition criteria for GBC.[16] This study complied with the standards of the Helsinki Declaration and current ethical guideline and was approved by the Institutional Ethical Board of West China Hospital of Sichuan University.
2.2. Study grouping and surgery
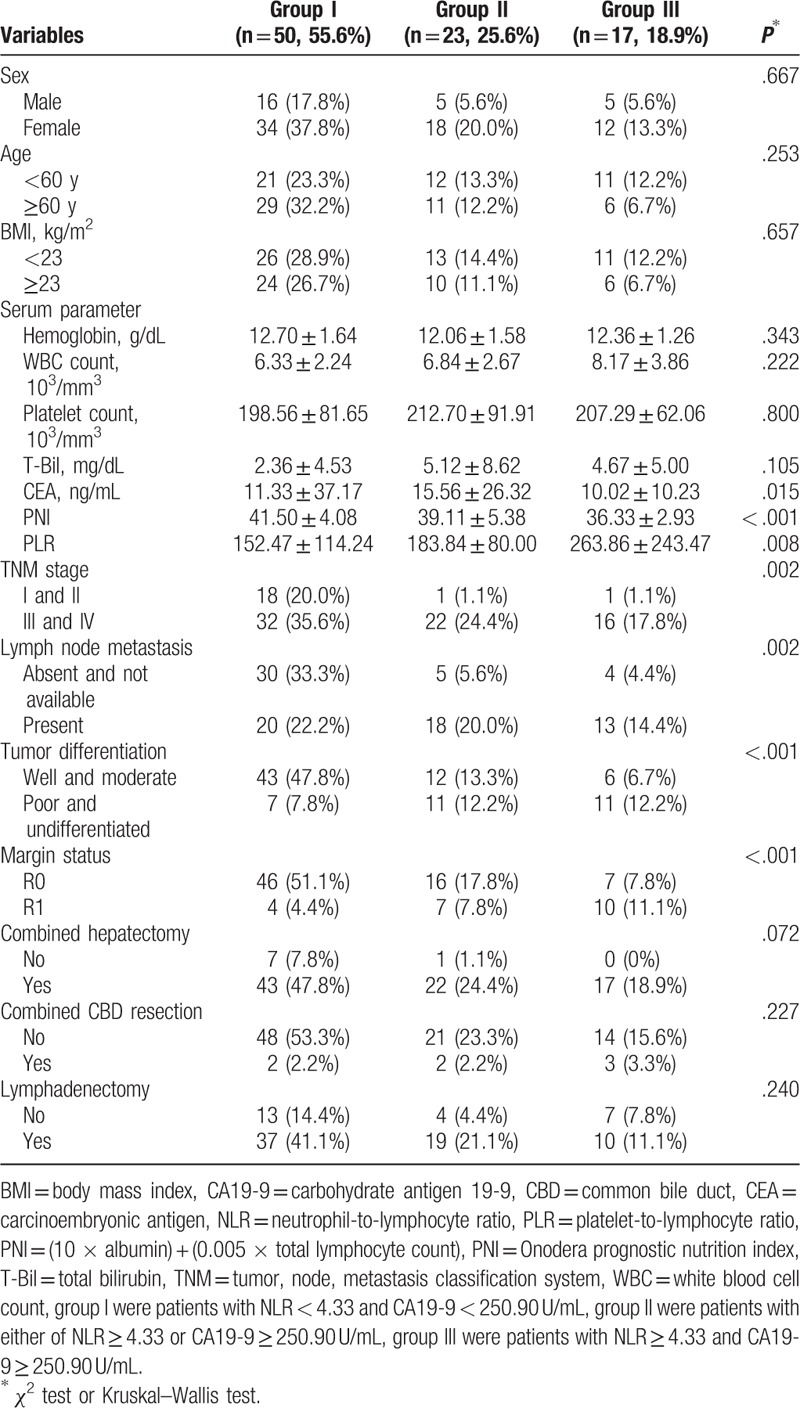
We used mean value as the cutoff point of NLR and CA19-9. The cutoff values of NLR and CA19-9 were 4.33 and 250.90 U/mL, respectively. Patients were classified using the cutoff values of NLR and CA19-9 and were divided into 4 different groups as follows: the high NLR group (NLR ≥ 4.33), the low NLR group (NLR < 4.33), the high CA19-9 group (CA19-9 ≥ 250.90 U/mL), and the low CA19-9 group (CA19-9 < 250.90 U/mL). As a combined utilization of NLR and CA 19-9, patients were divided into 3 groups: patients with NLR < 4.33 and CA19-9 < 250.90 U/mL were group I, patients with either of NLR ≥ 4.33 or CA19-9 ≥ 250.90 U/mL were group II, and patients with NLR ≥ 4.33 and CA19-9 ≥ 250.90 U/mL were group III. For Tis and T1a GBC, simple cholecystectomy is an appropriate treatment to obtain curative resection. For T1b-T4 GBC, radical surgery was performed with curative intent. When tumor had invaded the surrounding organs including common bile duct (CBD), colon, and omentum, the goal of surgical intervention was to achieve a negative margin. In this study, we did not include patients with vascular resection and reconstruction.
2.3. Definition of margin status and follow-up
The definition for R0 margin status was a radical resection without the microscopic involvement. Microscopic and macroscopic residual tumor was defined as R1 and R2, respectively. Follow-up of patients were performed through outpatient visit or by telephone. The overall survival (OS) period was defined as the interval from the date of the initial diagnosis of GBC to death or last followed-up. Patients who were alive at the last visit (May 2017) were considered the censored data.
2.4. Statistical analysis
Continuous variables were presented as the mean ± standard deviation (SD) and were analyzed using Student t test or Kruskal–Wallis test. Categorical variables were expressed as numbers and percentages and were compared by Pearson χ2 test and Fisher exact test. Univariate analysis was performed to evaluate the prognostic relevance of preoperative parameters. The multivariate Cox regression proportional hazard model was used to assess variables significant on univariate analysis. Survival was estimated using the Kaplan–Meier method compared by the log-rank test. P < .05 was considered statistically significant in all analyses. The Statistical analysis was carried out with the SPSS version 16.0 (SPSS Inc, Chicago, IL).
3. Results
In our study, 90 patients who underwent surgery with curative intent for GBC were included. There were 64 females (71.1%) and 26 males (28.9%). The mean values of all serum parameters and the results of univariate analysis of OS are shown in Table 1. We used mean value as the cutoff point of each parameter. The cutoff values of NLR and CA19-9 were 4.33 and 250.90 U/mL, respectively. The univariate analysis demonstrated that CA19-9 (P < .001), NLR (P < .001), PNI (P = .001), TNM (tumor, node, metastasis classification system) stage (P < .001), lymph node metastasis (P < .001), the degree of tumor differentiation (P < .001), margin status (P < .001), and combined hepatectomy (P = .001) were significant prognostic factors (Table 1). Therefore, the above factors were related to the survival of patients with GBC. The multivariate analysis indicated that NLR (hazard ratio (HR) = 3.840, P < .001), CA19-9 (HR = 2.230, P = .004), TNM stage (HR = 3.864, P < .001), lymph node metastasis (HR = 1.679, P = .048), and margin status (HR = 1.873, P = .030) were independent prognostic factors in the patients with GBC (Table 2).
Table 1.
Univariate analysis of overall survival.

Table 2.
Multivariate analysis of overall survival.
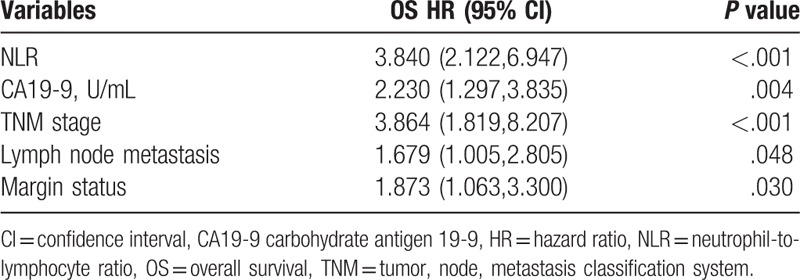
3.1. Clinical pathological factors of patients with NLR < 4.33 compared with NLR ≥ 4.33
The relationship between clinical pathological factors and NLR is presented in Table 3. There was no significant difference in sex, age, BMI, hemoglobin, platelet count, total bilirubin, CEA, combined hepatectomy, combined CBD resection, and lymphadenectomy of the patients with high NLR group compared with low NLR group (P > .05). However, with respect to WBC count, CA19-9, PNI, PLR, TNM stage, lymph node metastasis, the degree of tumor differentiation and margin status, there were significant difference between the high and low NLR groups (P < .05).
Table 3.
Clinical pathological factors of patients with NLR < 4.33 compared with NLR ≥ 4.33.

3.2. Clinical pathological factors of patients with CA19-9 < 250.90 u/mL compared with CA19-9 ≥ 250.90 u/mL
There was no significant difference in sex, age, BMI, hemoglobin, WBC count, platelet count, total bilirubin, CEA, lymph node metastasis, combined hepatectomy, combined CBD resection, and lymphadenectomy between the high CA19-9 group and low CA19-9 group (P > .05) (Table 4). In contrast, we found significant differences in NLR, PNI, PLR, TNM stage, the degree of tumor differentiation, and margin status of the patients with high CA19-9 group compared with low CA 19-9 group (P < .05) (Table 4).
Table 4.
Clinical pathological factors of patients with CA19-9 < 250.90 U/mL compared with CA19-9 ≥ 250.90 U/mL.
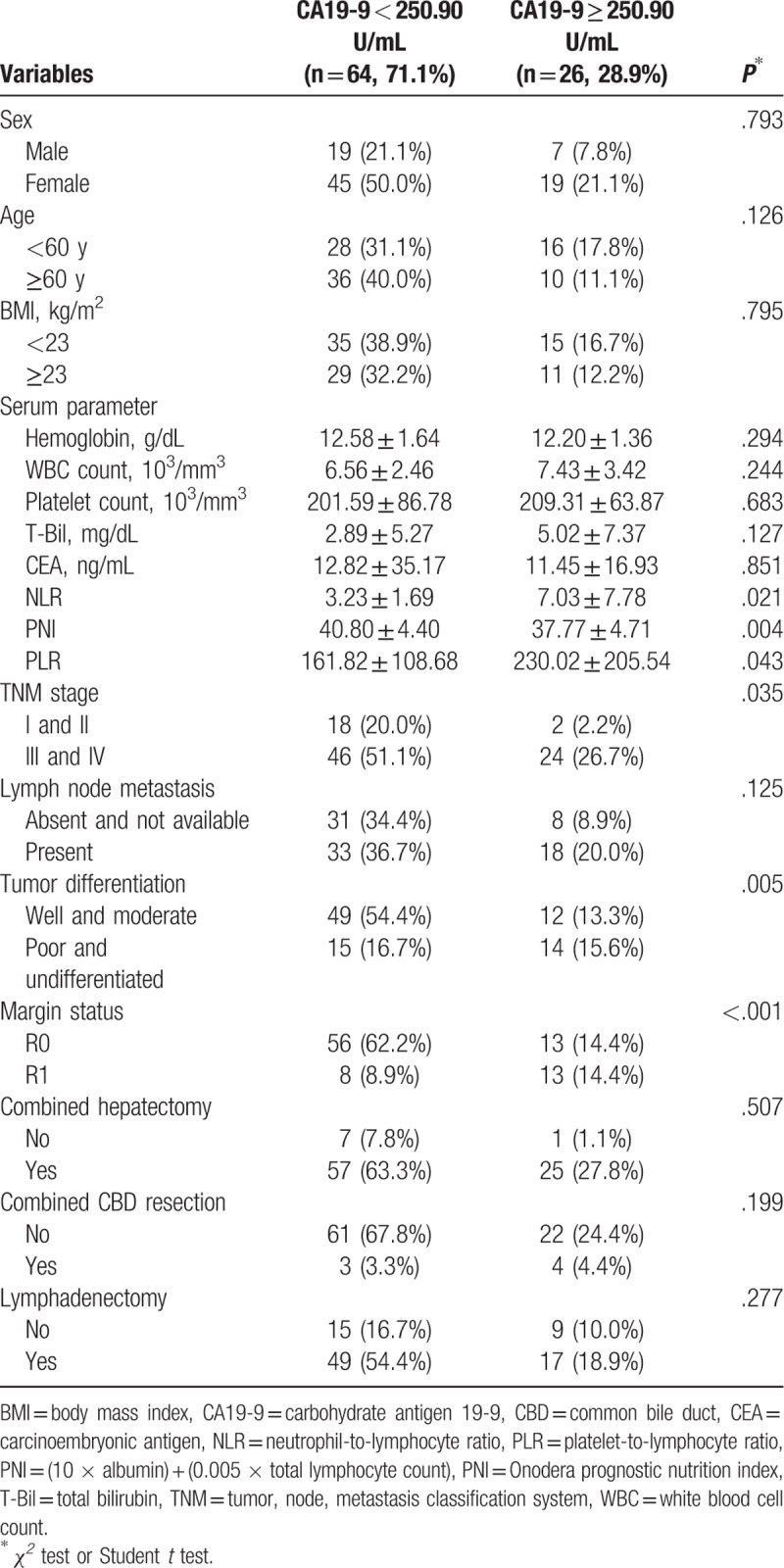
3.3. Comparison of clinical pathological factors of GBC patients grouped according to NLR and CA19-9
The results indicated that there was no significant difference in sex, age, BMI, hemoglobin, WBC count, platelet count, total bilirubin, combined hepatectomy, combined CBD resection, and lymphadenectomy for each group (P > .05). However, we found significant differences in CEA, PNI, PLR, TNM stage, lymph node metastasis, the degree of tumor differentiation, and margin status for each group (P < .05) (Table 5).
Table 5.
Comparison of clinical pathological factors of gallbladder carcinoma patients grouped according to NLR and CA19-9.
3.4. Survival analysis
During the follow-up, 79 patients (87.8%) died and 11 (12.2%) were censored at the last follow-up. Among the entire cohort, the 1-, 3-, and 5-year OS were 68.9%, 23.1%, and 10.7%, respectively (Fig. 1). Patients in the low NLR group (26 months) had a better median survival time compared with patients in the high NLR group (10 months; P < .001, Fig. 2). Similar to NLR groups, we found patients in the low CA19-9 group (25 months) had a better median survival time compared with patients in the high CA19-9 group (10 months; P < .001, Fig. 3). Moreover, the median survival times in the high NLR and high CA19-9 group (group I) and the low NLR and low PLR group (group III) were 28 months and 9 months, respectively. Comparison of postoperative survival between 2 groups showed statistical significance (P < .001, Fig. 4).
Figure 1.
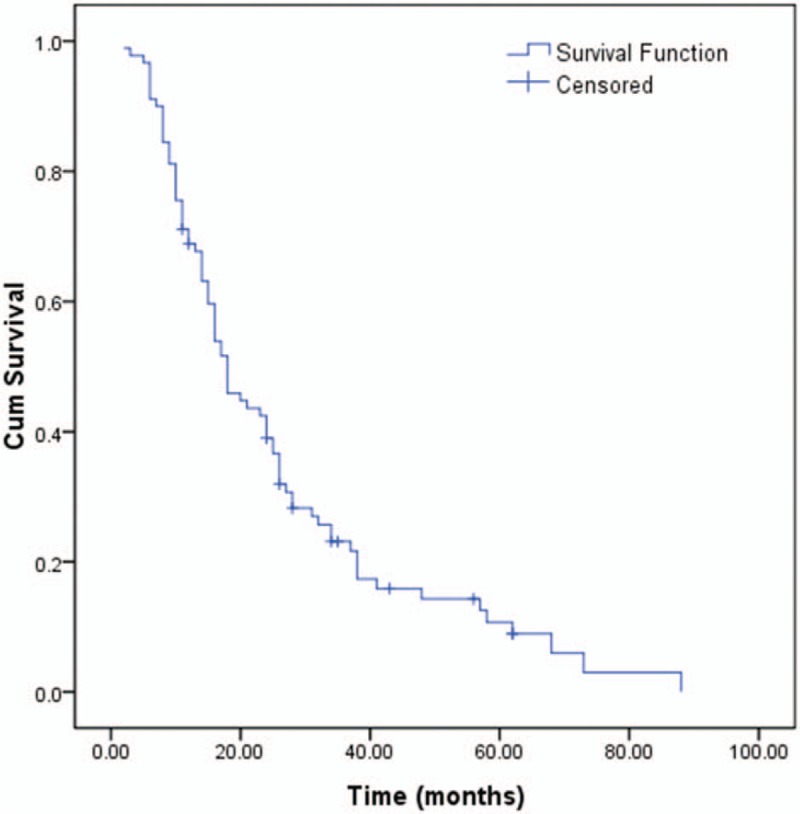
Overall survival of gallbladder carcinoma.
Figure 2.
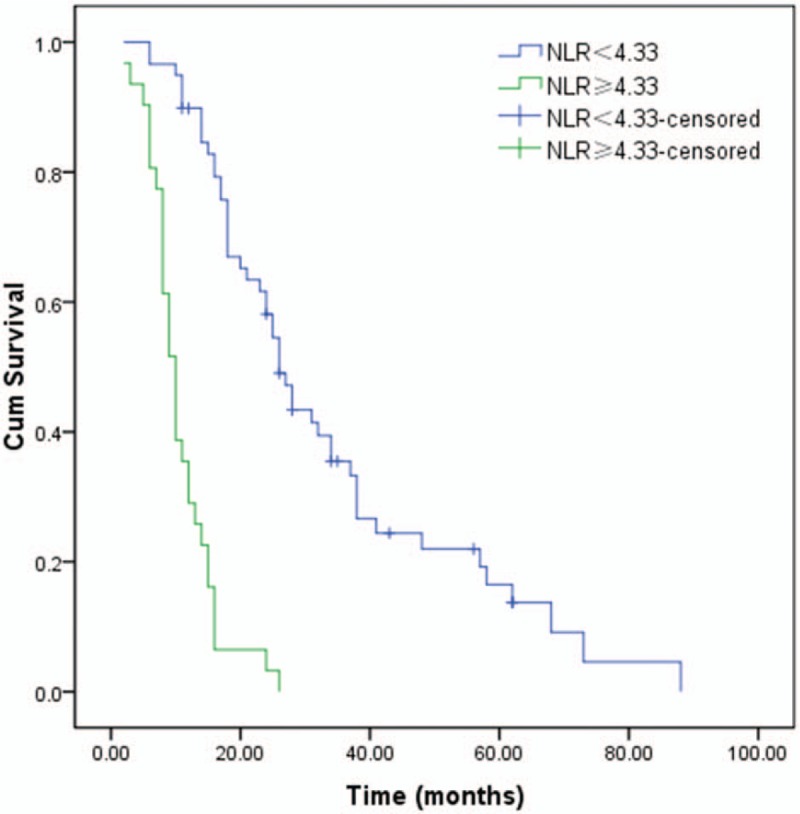
The univariate survival analysis for the survival time of patients in the low (NLR < 4.33) and high (NLR ≥ 4.33) NLR group by Kaplan–Meier (P < .001). NLR = neutrophil-lymphocyte ratio.
Figure 3.
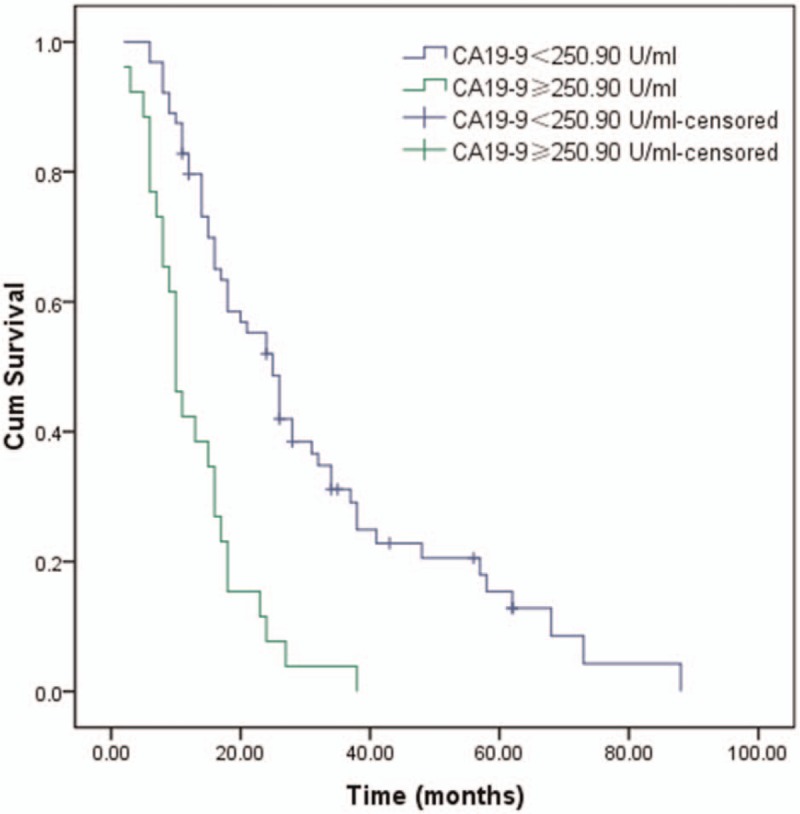
The univariate survival analysis for the survival time of patients in the low (CA19-9 < 250.90 U/mL) and high (CA19-9 ≥ 250.90 U/mL) CA19-9 group by Kaplan–Meier (P < .001).
Figure 4.
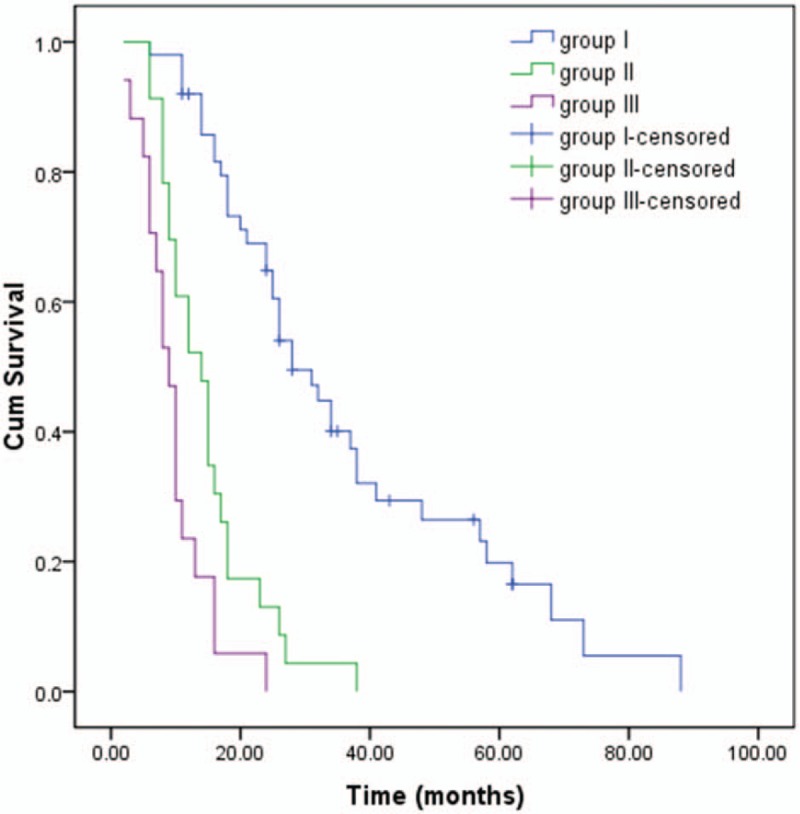
Patients were divided into 3 groups according to NLR and CA19-9. Kaplan–Meier survival curves of gallbladder carcinoma patients with different groups (P < .001).
4. Discussion
Prognosis for GBC is typically poor. The most accurate and effective predictor of long-term survival for postoperative GBC patients is currently pathological TNM stage. However, it can only be properly assessed after surgery. In recent years, some other clinical pathological factors have been found to be related to GBC prognosis and metastasis.[17] However, it remains controversial whether they have a good clinical significance. Furthermore, it needs to be pointed out these also can only be evaluated postoperatively. In our study, we evaluated the preoperative parameters to predict subsequent prognosis after surgery for GBC. Our study demonstrated that elevated NLR and CA19-9 values can be the simple and effective means of predicting the prognosis of GBC before surgery. We found that the baseline NLR and CA19-9 (mean value) in our institute were independent prognostic determinants for OS (NLR, HR 3.840, 95% CI: 2.122–6.947, P < .001; CA19-9, HR 2.230, 95% CI: 1.297–3.835, P = .004). Compared with TNM stage, NLR and CA19-9 as prognostic markers in clinical practice have many advantages, such as easy measurement, good replicability and low cost, application in preoperative assessment, and so on.
In our study, we found the preoperative NLR was an important independent prognostic factor of GBC patient. Neutrophils play pivotal roles in tumorigenesis and have a significant impact on the microenvironment of tumor. It can produce various cytokines and chemokines, which influence the activation and recruitment of inflammatory cells and play an important role in tumor cell proliferation and metastasis.[18] In contrast, lymphocytes can monitor the immune system of tumors and also prevent tumor cells from maturing.[19] It has been reported that the reduction of total lymphocytes in blood is an indicator of adverse outcomes in patients with pancreatic cancer.[20] In other words, NLR is an amplified risk factor for predicting of the systematic inflammation conditions. Consistently, NLR has been shown to be a potential prognostic factor in various tumors including lung, breast cancer, liver, stomach, and colorectum.[21,22] The relationship between cancer progression and systemic inflammation has been supported and NLR is getting more attractive, because NLR is readily measurable in peripheral blood and directly reflects the systemic host inflammatory response.
NLR is related to GBC prognosis and is a potential prognostic indicator for GBC. Zhang et al[9] investigated the importance of NLR in 316 GBC patients. The authors established 2.61 as the cutoff value of NLR, and their results demonstrated that NLR was a prognostic indicator for patients with GBC. Beal et al[23] analyzed the data of 187 patients and concluded that NLR was associated with the prognosis of GBC patients. Zhang et al[24] reported NLR and PLR were closely associated with the prognosis of GBC patients. The cutoff values of NLR in the Zhang, Beal, and Zhang studies were 2.61, 5.0, and 1.94, respectively. There is still considerable variation about which cutoff value of NLR can be used to stratify the treatment option. This may be attributed to differences in study population, primary site of cancer, and whether values relate to patients following curative resection. In our study, we determined a threshold value of mean value (4.33) as the cutoff value. The multivariate analysis demonstrated that there was a significant correlation between NLR ≥ 4.33 and poor clinical outcome. Moreover, it highlighted the independent value of this parameter. Additional studies are required for final establishment of the optimal cutoff value of NLR for clinical application.
CA19-9 is a tumor-associated antigen, initially reported by Koprowski et al.[25] It has been shown that CA19-9 is a prognostic indicator in GBC.[26] However, the optimal cutoff value has remained controversial. In our analysis, we used 250.90 U/mL as the cutoff point of CA19-9. In multivariate analysis, it was proven to be an independent prognostic factor. Moreover, Selvakumar et al[27] demonstrated that surgery after neoadjuvant chemotherapy was workable and might improve survival in selected patients. Another study reported that neoadjuvant therapy in unresectable GBC resulted in a 15% resectability rate.[28] The use of CA19-9 might provide opportunity for guiding personalized neoadjuvant therapy in patients with GBC.
Based on the combined utilization of NLR and CA19-9, individualized prediction of postoperative prognosis was available, such as good in group I, moderate in group II, and dismal in group III. In patients with elevation of NLR and CA19-9 (group III, dismal), implementation of aggressive procedures should be carefully evaluated, considering the decreased prognostic benefits and increased postoperative morbidity. On the contrary, in patients without elevation of either NLR or CA19-9 (group I, good), surgeons should not easily make concessions to palliative resection, even when the invasion is extended on radiological imaging.
In previous studies, it has been reported that higher TNM stage, lymph node metastasis, and a positive resection margin predict poor denouement in GBC patients.[29] Patients were divided into stage T1/T2 and stage T3/T4 groups because of the small study group. Our study supports these findings because we found significantly lower survival in stage T3/T4, tumors with lymph node metastasis, and patients with R1 resection. This was confirmed by Cox regression analyses, indicating that TNM stage, lymph node metastasis, and margin status were independent prognostic determinants for OS (TNM stage, HR 3.864, 95% CI: 1.819–8.207, P < .001; lymph node metastasis, HR 1.679, 95% CI: 1.005–2.805, P = .048; margin status, HR 1.873, 95% CI: 1.063–3.300, P = .030). Some authors reported that lymph node status has always been one of the strongest predictors of a poor prognosis in postoperative GBC.[30,31] Lymph node metastases are common for GBC. The incidence of lymph node metastases increases along with the T stage, reaching 60% to 80% in stage T3–4.[32,33] Lymph node status has been conventionally described in 3 ways: location of the metastatic lymph node, number of metastatic lymph nodes, and ratio of metastatic lymph nodes to the number of retrieved lymph nodes.[34] A study by Amini et al[35] demonstrated that among the patients who had 4 or more lymph nodes examined, the log odds of metastatic lymph node had the best discrimination ability. Curative resection is the only effective treatment for GBC. In our study, margin status was an independent prognostic factor for OS. As expected, patients with R1 resection had a significantly worse survival than R0 resection. Therefore, we believe that R0 resection should be attempted whenever possible on the premise of ensuring patient safety.
However, there were some limitations to be taken into account in the present study. First, our study was performed in a retrospective design. Second, it was a single-center sample size. Furthermore, our study did not integrate NLR, CA19-9, and other statistically significant variables into a new equation to increase the sensitivity and specificity, and it lacked a validation group for consolidation. Further multicenter, larger prospective studies are required to validate our findings. Meanwhile, the cutoff values of preoperative NLR and CA19-9 should be determined in a prospective manner. Further studies should also focus on proposing equations to improve the sensitivity and specificity of NLR, CA19-9 and other statistically significant variables, and establishing validation group for consolidation.
In conclusion, the preoperative NLR and CA19-9 in our institute were independent prognostic determinants for OS. That is to say, the preoperative NLR and CA19-9 are associated with prognosis of GBC patients and may be useful for the evaluation of prognosis of patients with GBC. High NLR and high CA19-9 were predictors of poor long-term outcome among patients with GBC undergoing curative surgery. Further independent prospective trials should be requested to confirm these results.
Author contributions
FL and H-JH contributed to the data acquisition and drafted the manuscript. W-JM, QY, and J-KW contributed to data acquisition. F-YL contributed to the study design and revised the manuscript. Manuscript is approved by all authors for publication.
Conceptualization: Fu-yu Li.
Data curation: Wen-Jie Ma, Qin Yang, Jun-Ke Wang.
Writing – original draft: Fei Liu, Hai-Jie Hu.
Footnotes
Abbreviations: ALB = Serum Albumin, BMI = body mass index, CA19-9 = carbohydrate antigen19-9, CBD = common bile duct, CEA = carcinoembryonic antigen, GBC = gallbladder carcinoma, HR = hazard ratio, NLR = neutrophil-lymphocyte ratio, OS = overall survival, PLR = platelet-lymphocyte ratio, PLT = platelet, PNI = Onodera's prognosticnutrition index, SD = standard deviation, TNM = tumor, node, metastasis classification system, WBC = white blood cell.
FL and H-JH contributed equally to this work and were the co-first authors.
This study was funded by the National Nature Science of China (30801111), Science and Technology Support Project of Sichuan Province [No. 2014SZ0002-10], and Applied Basic Research Project of Sichuan Province (2018JY0019).
The authors have no conflicts of interest to disclose.
References
- [1].Sharma A, Sharma KL, Gupta A, et al. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: recent update. World J Gastroenterol 2017;23:3978–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Liu YB, He XW, Wang JW, et al. Establishment of liver metastasis model of human gallbladder cancer and isolation of the subpopulation with high metastatic potential. Zhonghua Yi Xue Za Zhi 2006;86:2117–21. [PubMed] [Google Scholar]
- [3].Ito H, Matros E, Brooks DC, et al. Treatment outcomes associated with surgery for gallbladder cancer: a 20-year experience. J Gastrointest Surg 2004;8:183–90. [DOI] [PubMed] [Google Scholar]
- [4].Puhalla H, Wild T, Bareck E, et al. Long-term follow-up of surgically treated gallbladder cancer patients. Eur J Surg Oncol 2002;28:857–63. [DOI] [PubMed] [Google Scholar]
- [5].Batra Y, Pal S, Dutta U, et al. Gallbladder cancer in India: a dismal picture. J Gastroenterol Hepatol 2005;20:309–14. [DOI] [PubMed] [Google Scholar]
- [6].Manfredi S, Benhamiche AM, Isambert N, et al. Trends in incidence and management of gallbladder carcinoma: a population-based study in France. Cancer 2000;89:757–62. [DOI] [PubMed] [Google Scholar]
- [7].Zeng H, Zheng R, Guo Y, et al. Cancer survival in China, 2003-2005: a population-based study. Int J Cancer 2015;136:1921–30. [DOI] [PubMed] [Google Scholar]
- [8].Agrawal S, Lawrence A, Saxena R. Does CA 19-9 have prognostic relevance in gallbladder carcinoma (GBC)? J Gastrointest Cancer 2018;49:144–9. [DOI] [PubMed] [Google Scholar]
- [9].Zhang L, Wang R, Chen W, et al. Prognostic significance of neutrophil to lymphocyte ratio in patients with gallbladder carcinoma. HPB (Oxford) 2016;18:600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jeong JH, Lim SM, Yun JY, et al. Comparison of two inflammation-based prognostic scores in patients with unresectable advanced gastric cancer. Oncology 2012;83:292–9. [DOI] [PubMed] [Google Scholar]
- [11].He W, Yin C, Guo G, et al. Initial neutrophil lymphocyte ratio is superior to platelet lymphocyte ratio as an adverse prognostic and predictive factor in metastatic colorectal cancer. Med Oncol 2013;30:439. [DOI] [PubMed] [Google Scholar]
- [12].Shukla VK, Gurubachan, Sharma D, et al. Diagnostic value of serum CA242, CA 19-9, CA 15-3 and CA 125 in patients with carcinoma of the gallbladder. Trop Gastroenterol 2006;27:160–5. [PubMed] [Google Scholar]
- [13].Luo G, Jin K, Cheng H, et al. Carbohydrate antigen 19-9 as a prognostic biomarker in pancreatic neuroendocrine tumors. Oncol Lett 2017;14:6795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Feng F, Tian Y, Xu G, et al. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC Cancer 2017;17:737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Regimbeau JM, Fuks D, Bachellier P, et al. Prognostic value of jaundice in patients with gallbladder cancer by the AFC-GBC- 2009 study group. Eur J Surg Oncol 2011;37:505–12. [DOI] [PubMed] [Google Scholar]
- [16].Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual, 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- [17].Wang JW, Peng SY, Li JT, et al. Identification of metastasis-associated proteins involved in gallbladder carcinoma metastasis by proteomic analysis and functional exploration of chloride intracellular channel 1. Cancer Lett 2009;281:71–81. [DOI] [PubMed] [Google Scholar]
- [18].Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res 2011;1:2411–6. [DOI] [PubMed] [Google Scholar]
- [19].Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004;21:137–48. [DOI] [PubMed] [Google Scholar]
- [20].Fogar P, Sperti C, Basso D, et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas 2006;32:22–8. [DOI] [PubMed] [Google Scholar]
- [21].Halazun KJ, Hardy MA, Rana AA, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg 2009;250:141–51. [DOI] [PubMed] [Google Scholar]
- [22].Kishi Y, Kopetz S, Chun YS, et al. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol 2009;16:614–22. [DOI] [PubMed] [Google Scholar]
- [23].Beal EW, Wei L, Ethun CG, et al. Elevated NLR in gallbladder cancer and cholangiocarcinoma—making bad cancers even worse: results from the US Extrahepatic Biliary Malignancy Consortium. HPB (Oxford) 2016;18:950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang Y, Jiang C, Li J, et al. Prognostic significance of preoperative neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in patients with gallbladder carcinoma. Clin Transl Oncol 2015;17:810–8. [DOI] [PubMed] [Google Scholar]
- [25].Koprowski H, Steplewski Z, Mitchell K, et al. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet 1979;5:957–71. [DOI] [PubMed] [Google Scholar]
- [26].Hong EK, Kim KK, Lee JN, et al. Surgical outcome and prognostic factors in patients with gallbladder carcinoma. Korean J Hepatobiliary Pancreat Surg 2014;18:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Selvakumar VP, Zaidi S, Pande P, et al. Resection after neoadjuvant chemotherapy in advanced carcinoma of the gallbladder: a retrospective study. Indian J Surg Oncol 2015;6:16–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Agrawal S, Mohan L, Mourya C, et al. Radiological downstaging with neoadjuvant therapy in unresectable gall bladder cancer cases. Asian Pac J Cancer Prev 2016;17:2137–40. [DOI] [PubMed] [Google Scholar]
- [29].Bartlett DL, Fong Y, Fortner JG, et al. Long-term results after resection for gallbladder cancer. Implications for staging and management. Ann Surg 1996;224:639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Miura F, Asano T, Amano H, et al. New prognostic factor influencing long-term survival of patients with advanced gall-bladder carcinoma. Surgery 2010;148:271–7. [DOI] [PubMed] [Google Scholar]
- [31].Shimada H, Endo I, Fujii Y, et al. Appraisal of surgical resection of gallbladder cancer with special reference to lymph node dissection. Langenbecks Arch Surg 2000;385:509–14. [DOI] [PubMed] [Google Scholar]
- [32].Miyakawa S, Ishihara S, Horiguchi A, et al. Biliary tract cancer treatment: 5,584 results from the Biliary Tract Cancer Statistics Registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg 2009;16:1–7. [DOI] [PubMed] [Google Scholar]
- [33].Fong Y, Wagman L, Gonen M, et al. Evidence-based gallbladder cancer staging: changing cancer staging by analysis of data from the National Cancer Database. Ann Surg 2006;243:767–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sakata J, Shirai Y, Wakai T, et al. Number of positive lymph nodes independently determines the prognosis after resection in patients with gallbladder carcinoma. Ann Surg Oncol 2010;17:1831–40. [DOI] [PubMed] [Google Scholar]
- [35].Amini N, Kim Y, Wilson A, et al. Prognostic implications of lymph node status for patients with gallbladder cancer: a multi-institutional study. Ann Surg Oncol 2016;23:3016–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


