Abstract
The aim of this study was to identify any association between serum uric acid and smoking status using data from the Seventh Korea National Health and Nutrition Examination Survey (KNHANES VII-1) 2016 of the Korean population.
This study used a cross-sectional design and analyzed 5609 subjects aged ≥ 19 years among 8150 participants enrolled in the KNHANES VII-1 2016. Smoking status was classified into current smokers, never smokers, and ex-smokers. Hyperuricemia was defined as > 7.0 mg/dL for men and > 6.0 mg/dL of serum uric acid for women. Association between smoking and serum uric acid/hyperuricemia was assessed by Pearson's or Spearman's correlation analyses and multivariate logistic regression analysis showing odds ratio (OR) and 95% confidence interval (CI).
A significant difference in serum uric acid according to smoking status was identified in female (P < .001) but not in male subjects (P = .069). In female subjects, current smokers and ex-smokers showed higher serum uric acid than never smokers (P < 0.001 of both). Serum uric acid was associated with smoking status in female but not male subjects (r = 0.057, P = .001 and r = 0.025, P = .220, respectively). There was significant difference of smoking status between female subjects with and without hyperuricemia (P < .001). Current smokers had 2.7 times higher likely to have hyperuricemia in female, compared to never smokers (OR 2.674, 95% CI 1.578 – 4.531, P < .001).
This study revealed that smoking was closely associated with serum uric acid in female but not in male subjects in Korean population.
Keywords: female, hyperuricemia, smoking, uric acid
1. Introduction
Smoking is a modifiable risk factor associated with a variety of chronic diseases including cardiovascular diseases, diabetes mellitus, kidney diseases, and autoimmune or inflammatory disorders.[1–3] These health problems caused by smoking exposure have been attributable to some pathogenic mechanisms including reactive oxygen species (ROS) generation, oxidative stress on target molecules such as lipids and proteins, and pro-inflammatory responses.[3,4] Uric acid has been also considered as a potent risk factor for the development, progression, and mortality of cardiovascular diseases, chronic kidney diseases, and autoimmune or inflammatory disorders.[5–7] This suggests that smoking and uric acid may share a role in pathophysiology in of these chronic diseases, and that the two risk factors may be related.
Numerous investigations addressed the effect of smoking on serum uric acid in diverse study populations. However, a debate over an association between smoking and uric acid has remained. Substantial evidence demonstrates that smoking reduces serum uric acid.[8–11] An interventional study showed that serum uric acid was markedly decreased in current smokers after smoke exposure for five minutes.[8] Some case-control studies revealed significantly lower serum uric acid levels in smokers compared to non-smokers.[9,10] Unlike the harmful effect of smoking on autoimmune rheumatic diseases such as rheumatoid arthritis and systemic lupus erythematosus,[3] current smoking was found to be associated with lower serum uric acid and a subsequent decreased risk of gout in an Asian cohort study.[11] In contrast, the opposite results have also been demonstrated; smoking caused increased serum uric acid in some studies.[12–14] An experimental study using rats exposed to cigarette smoke for 60 days revealed significantly increased serum uric acid compared to unexposed control rats.[12] In addition, clinical evidence that smoking caused elevation of serum uric acid was presented in larger study populations. Serum uric acid in current smokers was markedly higher than in non-smokers in a study to evaluate association between smoking status and subclinical coronary atherosclerosis in the Korean population.[13] An analysis of the relationship between amount of smoking and uric acid indicated that smoking index (pack-years) was closely associated with serum uric acid in asthmatic patients.[14]
Even though earlier studies have demonstrated some evidence of a relationship between smoking and uric acid, an obvious data conflict for the relationship exists. In this study, we assessed whether serum uric acid was associated with smoking status in the Korean population.
2. Subjects and methods
2.1. Subjects
This study was performed using data from the Korea National Health and Nutrition Examination Survey (KNHANES VII-1) 2016, a cross-sectional survey of the Korean population conducted by Korea Centers for Disease Control and Prevention (https://knhanes.cdc.go.kr/knhanes/eng/index.do). Among a total of 8,150 enrolled in the national survey of KNHANES VII-1 2016, 5609 subjects were 19 years and older and answered the questions for smoking status. Results from serum uric acid testing were included in the present study (Fig. 1). However, 773 subjects did not have answers for smoking status or serum uric acid and were excluded. The present study was approved by the Institutional Review Board at Daegu Catholic University Medical Center (CR-18-124). Because individual information of subjects participating in the national survey was anonymized and de-identified, the informed consent requirement for each subject was waived.
Figure 1.
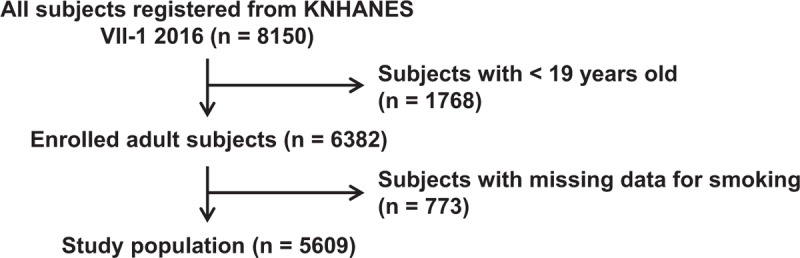
Flow chart showing study population.
2.2. Data collection
The data selected in the present study were used in a previous study assessing the association between uric acid and osteoarthritis.[15] Data from KNHANES VII-1 2016 used in this study comprised age (years), gender, lifetime alcohol intake (per year or none), systolic blood pressure (SBP, mm Hg), diastolic blood pressure (DBP, mmHg), weight (kg), height (cm), and body mass index (BMI, kg/m2). Laboratory findings for fasting glucose (mg/dL), blood urea nitrogen (BUN, mg/dL), creatinine (mg/dL), and high sensitivity C-reactive protein (hs-CRP, mg/L) were also used, as shown in Table 1. Serum uric acid (mg/dL) was measured by the calorimetry (uricase) method using a Hitachi Automatic Analyzer 7600–210 (Hitachi Medical Corporation, Tokyo, Japan).
Table 1.
General characteristics in enrolled subjects (n = 5609).
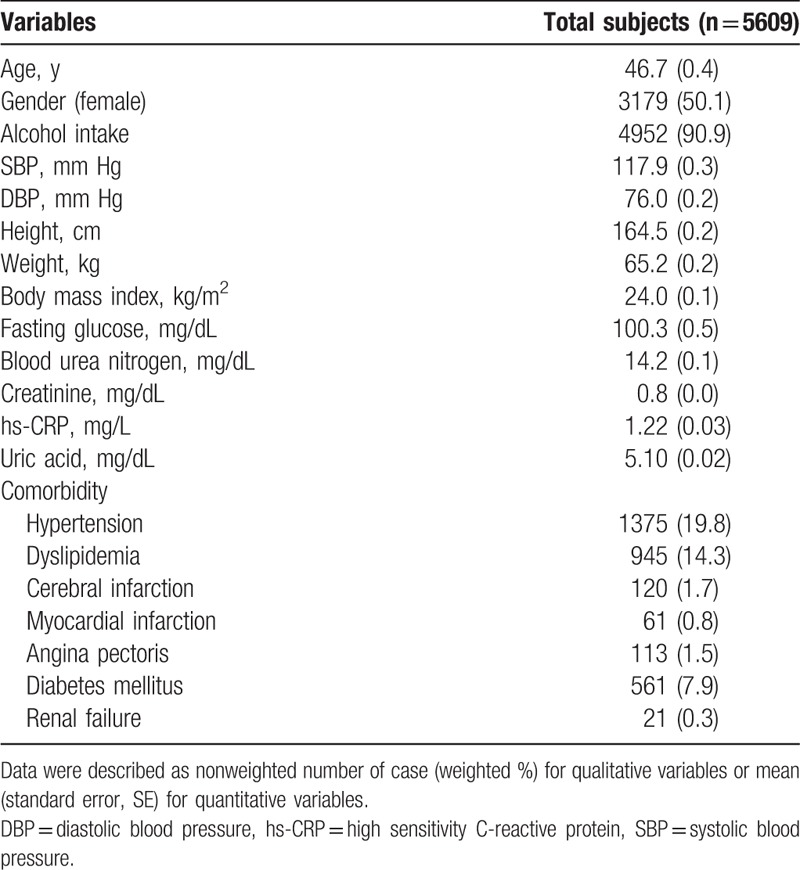
KNHANES VII-1 2016 data assessed comorbid conditions diagnosed by physicians such as hypertension, dyslipidemia, cerebral infarction, myocardial infarction, angina pectoris, diabetes mellitus, and renal failure. Definitions of comorbidities were described in the guidelines for KNHANES users.
2.3. Definition of smoking status
In the survey of KNHANES VII-1 2016, smoking status was recorded in the self-reported manner. Classification of smoking status was done on the base of a well-designed questionnaire used at the time of study participation. The smoking status for all participants was classified into three groups: current smokers, ex-smokers, and never smokers. Subjects who had never smoked were classified into never smokers. Those who smoked at the time of the survey were classified into current smokers, regardless of the amount of cigarettes smoked per day or the total amount of cigarettes smoked. Ex-smoker was defined as subjects who had ever smoked and did not smoke at the time of registration regardless of duration of stopping smoking or the total amount of smoking.
2.4. Statistical analysis
The samples and variables used in statistical analysis were weighted, multistage clustered, and stratified. In the descriptive analysis, the data were described as the mean and standard error (SE) for quantitative variables. Unweighted frequency and weighting rates (%) were used for qualitative variables. Categorical variables in clinical information were compared among smoking status groups such as never smokers, ex-smokers, and current smokers and also compared between subjects with and without hyperuricemia by the chi-square test or Fisher's exact test. An analysis of variance (ANOVA) using a generalized linear model of composite sample was used to determine the difference for clinical variables according to smoking status. Post Hoc analysis using Bonferroni method was performed in comparison for serum uric acid between two groups among three smoking status. A multivariate logistic regression analysis was used to determine clinical variables associated with hyperuricemia by determining odds ratios (ORs) and 95% confidence intervals (CIs). Hyperuricemia was defined as > 7.0 mg/dL for men and > 6.0 mg/dL of serum uric acid for women.[15] Correlation between serum uric acid and clinical variables was performed using Pearson's correlation coefficient (r) for continuous variables and Spearman's rho coefficient for categorical variables. The significance level was less than 0.05 for P values. All statistical analysis was performed using the IBM SPSS Statistics 19.0 software (IBM Corp, Armonk, NY).
3. Results
3.1. General characteristics of study population
The general characteristics of a total of 5609 subjects [weighted number = 38198176.2 (SE 958562.3)] are illustrated in Table 1, composed with male subjects [unweighted number = 2430, weighted number = 19063525.6 (SE 528383.1)] and female subjects [unweighted number = 3179, weighted number = 19134650.6 (SE 541285.9)]. Among 5609 subjects, never smokers, ex-smokers, and current smokers were 3386 (56.3%), 1176 (21.1%), and 1047 (22.6%), respectively.
3.2. Comparison of serum uric acid and clinical variables according to smoking status
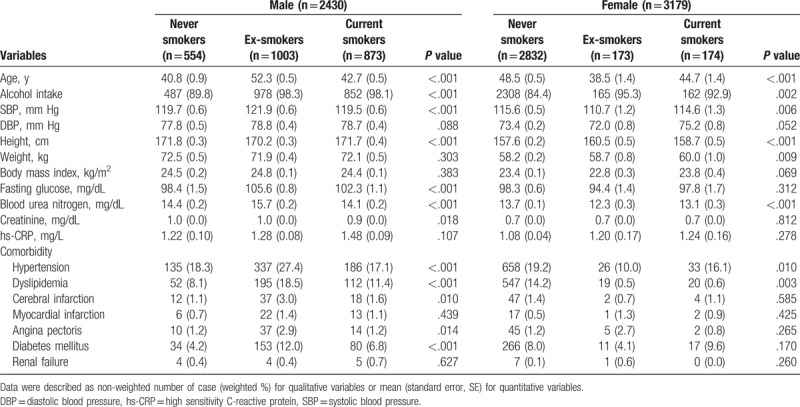
Mean serum uric acid of total subjects was 5.10 mg/dL (SE 0.02), of male subjects was 5.83 mg/dL (SE 0.03), and of female subjects was 4.36 mg/dL (SE 0.02). Comparing serum uric acid according to smoking status, there is no significant difference of serum uric acid among 3 smoking status in male subjects [5.94 (SE 0.07) for never smokers, 5.73 (SE 0.05) for ex-smokers, and 5.86 (SE 0.05) for current smokers, P = .069 by ANOVA] (Fig. 2). In addition, no differences of serum uric acid between two smoking status were also noted in the post hoc analysis. In contrast, serum uric acid among smoking status in female subjects was markedly different among smoking status [4.33 (SE 0.02) for never smokers, 4.47 (SE 0.09) for ex-smokers, and 4.69 (SE 0.09) for current smokers, P < .001 by ANOVA] showing the highest levels in current smokers and the lowest levels in never smokers. In addition, Post Hoc analysis showed only difference of serum uric acid between ex-smokers or never smokers and current smokers (P < .001 of both), without difference between ex-smokers and never smokers. Comparison results of other clinical variables including age, alcohol intake, blood pressure, BMI, fasting glucose, blood urea nitrogen, creatinine, and comorbidities are also described in Table 2.
Figure 2.
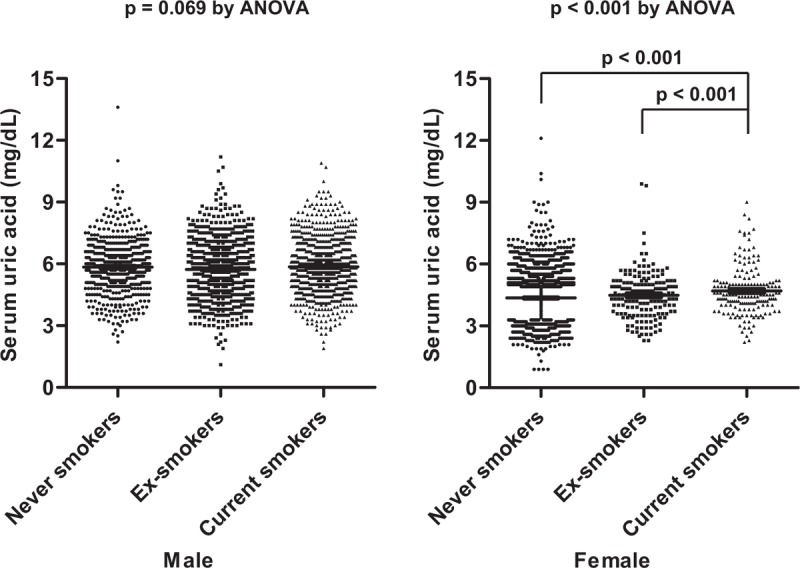
Comparison of serum uric acid among smoking status based on gender. The data were illustrated as mean and standard error. ANOVA = analysis of variance.
Table 2.
Univariate analysis for variables among smoking status.
3.3. Determination of clinical variables associated with serum uric acid
In male subjects, serum uric acid was associated with younger age, alcohol intake, higher DBP, higher BMI, lower fasting glucose, higher BUN, and higher creatinine (Table 3). Smoking status was found not to be associated with serum uric acid in male (r = 0.025, P = .220). Serum uric acid was positively associated with age, blood pressure, BMI, fasting glucose, BUN, creatinine, and hs-CRP in female subjects. In female population, there was significant correlation between serum uric acid and smoking status (r = 0.057, P = .001).
Table 3.
Multivariate correlation analysis for serum uric acid.
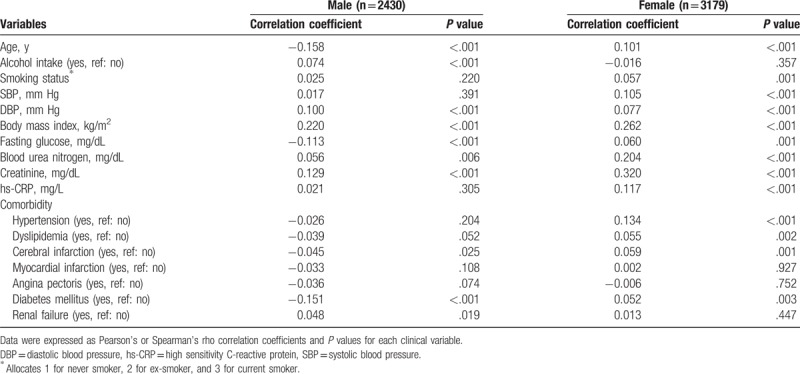
Concerning relationship between serum uric acid and comorbidity, the presence of hypertension, dyslipidemia, cerebral infarction, and diabetes mellitus were associated with increased serum uric acid in female. In contrast, serum uric acid was negatively associated with diabetes mellitus, but positively associated with renal failure in male subjects.
3.4. Determination of variables associated with hyperuricemia
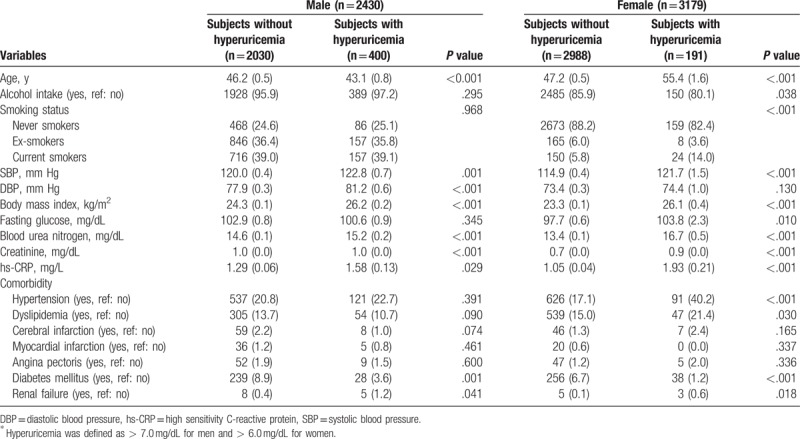
Comparison of variables between subjects with and without hyperuricemia was shown in Table 4. Some variables such as age, SBP, BMI, BUN, creatinine, hs-CRP, diabetes mellitus, and renal failure were significantly different between two groups in both male and female subjects in common. DBP in male and fasting glucose and hypertension in female subjects was different between those with and without hyperuricemia. Smoking status in only female but not male subjects was significantly different between them (P < .001), showing that hyperuricemia in current smokers was more frequent than non-hyperuricemia.
Table 4.
Univariate analysis for hyperuricemia∗.
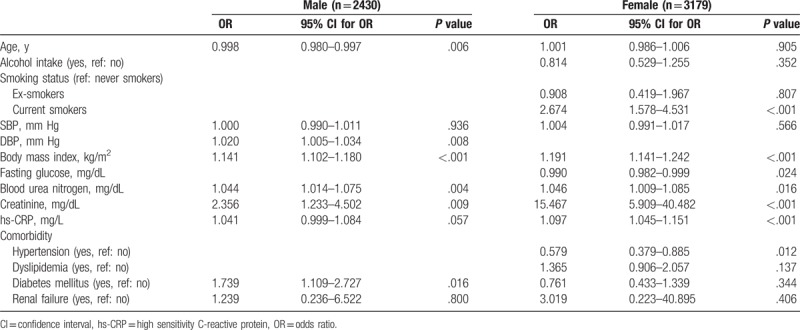
In the multivariate logistic regression analysis, hyperuricemia was found to be associated with BMI, BUN, and creatinine at both male and female subjects (Table 5). Male subjects with younger age and higher DBP and diabetes mellitus showed significant association with hyperuricemia. In contrast, lower fasting glucose, higher hsCRP, and hypertension were associated with hyperuricemia in female population. In female subjects, smoking status, especially current smoking, was significantly associated with hyperuricemia (OR 2.674, 95% CI 1.578 – 4.531, P < .001).
Table 5.
Multivariate logistic regression analysis for hyperuricemia.
4. Discussion
Over several decades, several studies investigated whether smoking influences serum uric acid, generating some debates about positive, negative, or no association between smoking status and serum uric acid. Some clinical and experimental studies provided substantial evidence that smoking may reduce serum uric acid.[8–10] In contrast, opposite findings, that smoking can increase serum uric acid concentration, have been reported.[12–14] In this study, we identified that serum uric acid level in current smokers and ex-smokers was significantly higher than that in never smokers in female subjects but not male subjects in the analysis of data from the KNHANES VII-1 2016 of the Korean population, as shown in Figure 2. In addition, we also found that current smokers were associated with hyperuricemia in only female population. An increasing effect of smoking on serum uric acid was also consistently observed in other Asian study populations including Japan and Nepal.[16,17]
With respect to the effect of smoking on serum uric acid, several mechanisms have been suggested for lowering uric acid by smoking. Uric acid has dual physiological properties as both an antioxidant and oxidant.[18,19] Uric acid is a potent physiologic scavenger and antioxidant for ROS and free radicals in vitro. Cigarette smoke contains diverse ROS, toxic materials, and free radicals including nicotine, carbon monoxide, nitric oxide, nitrogen dioxide, and peroxynitrite, subsequently generating oxidative stress and immune system disturbance.[3,4,20] The lower serum uric acid during smoking may be due to the antioxidant action for ROS and free radicals produced by smoking.[8] A similar consistency was observed with decreased concentrations of other antioxidants such as ascorbic acid, nitrate, and β-cryptoxanthin in smoking compared to non-smoking subjects.[8,21,22] In contrast, serologic markers for oxidative stress such as superoxide dismutase, glutathione peroxidase, catalase, xanthine oxidase, and malondialdehyde (MDA) were significantly higher in smokers.[22,23] Based on these mechanisms, a dose-dependent effect of smoking on uric acid was demonstrated. The number of cigarettes smoked/day and the duration of smoking were inversely associated with uric acid in smokers.[9,10] However, some evidence that gradual decrease of serum uric acid was not found according to smoking habits such as light, moderate, to heavy smokers has been reported in different larger study population.[24,25] Serum uric acid in moderate to heavy smokers was shown to be higher than in light smokers in larger study populations. While debatable, these data suggest that, in general, lower uric acid levels in smokers are associated with exhaustion of antioxidants.
In contrast, possible mechanisms explaining how smoking increases serum uric acid have also been suggested. Deleterious effects of smoking on renal function and structure have been reported in diverse study populations.[12,26,27] EL-Safty et al revealed that urinary total protein and cytoplasmic enzyme glutathione S-transferase (GST) among smokers were higher than among nonsmokers.[26] Electron microscopic morphometric analysis demonstrated that smoking status was associated with alternations of renal function in patients with type 2 diabetes mellitus. This was assessed by albumin excretion rate and glomerular and glomerular basement membrane structures.[27] Pekmez et al[12] demonstrated via light microscopy that renal tissue from rats exposed to cigarette smoke for 60 days showed mesangial cell proliferation and degeneration in the proximal tubules, which lead to reduced renal function and structural impairment. In the same experiment, renal tissue expression of MDA, a marker of oxidative stress, was higher in smoke-exposed rats than in unexposed control rats. Consistently, renal expression of antioxidant enzymes including superoxide dismutase and glutathione peroxidase was markedly increased in rats exposed to cigarette smoke. This contradicts another experimental study that showed a lowering effect of uric acid level after transient exposure to cigarette smoke in humans.[8] These findings indicate that the nephrotoxic effect of long-term smoking exposure may be mainly due to the induced changes in serum uric acid level rather than uric acid's antioxidant effect. Our result of this study also supports increasing effect of smoking on serum uric acid.
Serum uric acid level is determined by genetic factors such as renal urate transporters SLC2A9, URAT1, and ABCG2, which are responsible for hyperuricemia. Nongenetic risk factors including age, gender, ethnicity, dietary pattern, obesity, and comorbidity also play a role.[28,29] Some studies demonstrating a positive or negative relationship between smoking habits and serum uric acid did not fully consider confounders such as alcohol consumption, body mass index, ethnicity, and other comorbidities. In the present study, we assessed whether serum uric acid could be affected by smoking habits. We found a positive association between serum uric acid and smoking status in only female subjects [corrrelation coefficient (r) = 0.057, P = .001] that was independent of confounders such as age, body mass index, fasting glucose, creatinine, and hs-CRP. We did not identify an association between smoking habits and serum uric acid in male subjects. In terms of association between ex-smoking and serum uric acid, some studies demonstrated the highest serum uric acid in ex-smokers as opposed to current or never smokers.[30,31] These findings were prominent in male subjects. In addition, smoking cessation among persistent smokers induced slight increase in serum uric acid in white women,[32] suggesting that serum uric acid may be influenced by smoking cessation.
This study had some limitations and a notable strength. Despite the large dataset representing the Korean population, only the relationship between smoking and serum uric acid was identified. Because KNHANES VII-1 2016 data were cross-sectional, we could not assess whether smoking would have influenced serum uric acid in longitudinal studies. Another limitation is that we did not provide sufficient data to accurately quantify the amount of smoking. Also, trusting self-reported smoking quantities is questionable. The amount of smoking according to smoking device (cigarette smoking, electronic cigarette smoking, or pipe smoking) and smoking pattern (active and passive) may need to be calculated differently. In cross-sectional studies, more accuracy may be gained by using alternative tests such as urine nicotine rather calculating pack-years. Despite these limitations, our study may be beneficial. The large dataset representing the entire Korean population provides a robust result regarding the association between serum uric acid and smoking status.
In conclusion, this study found that smoking status was associated with serum uric acid only in female subjects when using data from KNHANES VII-1 2016. Our result implicates smoking as another environmental factor in determining serum uric acid. The effect, however, is not as dramatic as that of genetic risk factors such as renal urate transporters and other non-genetic factors including age, gender, and comorbidity. Further longitudinal and prospective studies are needed to confirm the association between smoking and serum uric acid.
Acknowledgment
The authors thank for use of the data for the Seventh Korea National Health and Nutrition Examination Survey (KNHANES VII-1) 2016 provided by the Korea Centers for Disease Control and Prevention.
Author contributions
Conceptualization: Seong-Kyu Kim.
Data curation: Jung-Yoon Choe.
Formal analysis: Seong-Kyu Kim.
Funding acquisition: Seong-Kyu Kim.
Resources: Jung-Yoon Choe.
Writing – original draft: Seong-Kyu Kim.
Writing – review & editing: Seong-Kyu Kim, Jung-Yoon Choe.
Seong-Kyu Kim orcid: 0000-0002-7780-0167.
Footnotes
Abbreviations: ANOVA = analysis of variance, BMI = body mass index, BUN = blood urea nitrogen, CIs = confidence intervals, DBP = diastolic blood pressure, hs-CRP = high sensitivity C-reactive protein, KNHANES = Korea National Health and Nutrition Examination Survey, MDA = malondialdehyde, ORs = odds ratios, ROS = reactive oxygen species, SBP = systolic blood pressure, SE = standard error.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1D1A1B03933687).
The authors have no conflicts of interest to disclose.
References
- [1].Ockene IS, Miller NH. Cigarette smoking, cardiovascular disease, and stroke: a statement for healthcare professionals from the American Heart Association. American Heart Association Task Force on Risk Reduction. Circulation 1997;96:3243–7. [DOI] [PubMed] [Google Scholar]
- [2].Chang SA. Smoking and type 2 diabetes mellitus. Diabetes Metab J 2012;36:399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun 2010;34:J258–65. [DOI] [PubMed] [Google Scholar]
- [4].van der Vaart H, Postma DS, Timens W, et al. Acute effects of cigarette smoke on inflammation and oxidative stress: a review. Thorax 2004;59:713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kang DH, Chen W. Uric acid and chronic kidney disease: new understanding of an old problem. Semin Nephrol 2011;31:447–52. [DOI] [PubMed] [Google Scholar]
- [6].Feig DI. Serum uric acid and the risk of hypertension and chronic kidney disease. Curr Opin Rheumatol 2014;26:176–85. [DOI] [PubMed] [Google Scholar]
- [7].Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med 2008;359:1811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tsuchiya M, Asada A, Kasahara E, et al. Smoking a single cigarette rapidly reduces combined concentrations of nitrate and nitrite and concentrations of antioxidants in plasma. Circulation 2002;105:1155–7. [DOI] [PubMed] [Google Scholar]
- [9].Haj Mouhamed D, Ezzaher A, Neffati F, et al. Effect of cigarette smoking on plasma uric acid concentrations. Environ Health Prev Med 2011;16:307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hanna BE, Hamed JM, Touhala LM. Serum uric Acid in smokers. Oman Med J 2008;23:269–74. [PMC free article] [PubMed] [Google Scholar]
- [11].Gee Teng G, Pan A, Yuan JM, et al. Cigarette smoking and the risk of incident gout in a prospective cohort study. Arthritis Care Res (Hoboken) 2016;68:1135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pekmez H, Ogeturk M, Ozyurt H, et al. Ameliorative effect of caffeic acid phenethyl ester on histopathological and biochemical changes induced by cigarette smoke in rat kidney. Toxicol Ind Health 2010;26:175–82. [DOI] [PubMed] [Google Scholar]
- [13].Kim JA, Chun EJ, Lee MS, et al. Relationship between amount of cigarette smoking and coronary atherosclerosis on coronary CTA in asymptomatic individuals. Int J Cardiovasc Imaging 2013;29suppl 1:21–8. [DOI] [PubMed] [Google Scholar]
- [14].Abdulnaby NK, Sayed AO, Shalaby NM. Predictive value of serum uric acid in hospitalized adolescents and adults with acute asthma. Ther Clin Risk Manag 2016;12:1701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim SK, Kwak SG, Choe JY. Serum uric acid level is not associated with osteoarthritis in Korean population: data from the Seventh Korea National Health and Nutrition Examination Survey 2016. Rheumatol Int 2018;38:2077–85. [DOI] [PubMed] [Google Scholar]
- [16].Fukuhara A, Saito J, Sato S, et al. The association between risk of airflow limitation and serum uric acid measured at medical health check-ups. Int J Chron Obstruct Pulmon Dis 2017;12:1213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jha JC, Maharjan BR, Adhikari D, et al. Cigarette smoke induced oxidative insult in local population of Pokhara. Kathmandu Univ Med J (KUMJ) 2007;5:511–7. [PubMed] [Google Scholar]
- [18].Ames BN, Cathcart R, Schwiers E, et al. Uric acid provides an antioxidant defense in humans against oxidant- and radical caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA 1981;78:6858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sautin YY, Nakagawa T, Zharikov S, et al. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol 2007;293:C584–96. [DOI] [PubMed] [Google Scholar]
- [20].Yanbaeva DG, Dentener MA, Creutzberg EC, et al. Systemic effects of smoking. Chest 2007;131:1557–66. [DOI] [PubMed] [Google Scholar]
- [21].Dietrich M, Block G, Norkus EP, et al. Smoking and exposure to environmental tobacco smoke decrease some plasma antioxidants and increase gamma-tocopherol in vivo after adjustment for dietary antioxidant intakes. Am J Clin Nutr 2003;77:160–6. [DOI] [PubMed] [Google Scholar]
- [22].Shah AA, Khand F, Khand TU. Effect of smoking on serum xanthine oxidase, malondialdehyde, ascorbic acid and (-tocopherol levels in healthy male subjects. Pak J Med Sci 2015;31:146–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kumar A, Biswas UK. Smoking is associated with reduced serum paraoxonase, antioxidants and increased oxidative stress in normolipidaemic acute myocardial infarct patients. Heart Asia 2011;3:115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Okubo Y, Miyamoto T, Suwazono Y, et al. An association between smoking habits and blood pressure in normotensive Japanese men. J Hum Hypertens 2002;16:91–6. [DOI] [PubMed] [Google Scholar]
- [25].Gordon T, Kannel WB, Dawber TR, et al. Changes associated with quitting cigarette smoking: the Framingham Study. Am Heart J 1975;90:322–8. [DOI] [PubMed] [Google Scholar]
- [26].EL-Safty IA, Gadallah M, Shouman AE, et al. Subclinical nephrotoxicity caused by smoking and occupational silica exposure among Egyptian industrial workers. Arch Med Res 2003;34:415–21. [DOI] [PubMed] [Google Scholar]
- [27].Baggio B, Budakovic A, Dalla Vestra M, et al. Effects of cigarette smoking on glomerular structure and function in type 2 diabetic patients. J Am Soc Nephrol 2002;13:2730–6. [DOI] [PubMed] [Google Scholar]
- [28].Merriman TR, Dalbeth N. The genetic basis of hyperuricaemia and gout. Joint Bone Spine 2011;78:35–40. [DOI] [PubMed] [Google Scholar]
- [29].Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet 2016;388:2039–52. [DOI] [PubMed] [Google Scholar]
- [30].Onat A, Can G, Örnek E, et al. Elevated serum uric acid in nondiabetic people mark pro-inflammatory state and HDL dysfunction and independently predicts coronary disease. Clin Rheumatol 2013;32:1767–75. [DOI] [PubMed] [Google Scholar]
- [31].Tomita M, Mizuno S, Yokota K. Increased levels of serum uric acid among ex-smokers. J Epidemiol 2008;18:132–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Friedman GD, Siegelaub AB. Changes after quitting cigarette smoking. Circulation 1980;61:716–23. [DOI] [PubMed] [Google Scholar]


