Supplemental Digital Content is available in the text
Keywords: mortality, nursing homes, sarcopenia, survival
Abstract
Little is known about the prognostic value of the strength, assistance walking, rise from a chair, climb stairs, and falls questionnaire (SARC-F) and SARC-F combined with calf circumference (SARC-CalF) among elderly nursing home residents.
To compare the prognostic value of SARC-F and SARC-CalF for mortality in this population.
We conducted a prospective study in four nursing homes in western China. Sarcopenia was estimated using SARC-F and SARC-CalF, respectively. Nutrition status, activities of daily living, and other covariates were evaluated. The survival status was collected via medical records and telephone interviews at the 12th month after the baseline investigation. We used multivariate Cox proportional-hazard models to calculate the hazard ratio (HR) and 95% confidence interval (CI) for 1-year all-cause mortality by SARC-F-defined sarcopenia and SARC-CalF-defined sarcopenia, separately.
We included 329 participants (median age: 85 years). The prevalences of SARC-F-defined sarcopenia and SARC-CalF-defined sarcopenia were 39.8% and 46.8%, respectively. During the 1-year follow-up period, 73 participants (22.7%) died. The mortality was 29.0% and 18.3% in the participants with or without SARC-F-defined sarcopenia, respectively (P = .025). The mortality was 26.6% and 19.0% in the participants with or without SARC-CalF-defined sarcopenia, respectively (P = .105). After adjusted for the relevant confounders including malnutrition, SARC-F-defined sarcopenia was independently associated with an increased risk of 1-year mortality (adjusted HR: 2.08; 95% CI: 1.27–3.42). However, SARC-CalF-defined sarcopenia was not an independent predictor of 1-year mortality (adjusted HR: 1.54; 95% CI: 0.95–2.47).
Sarcopenia is highly prevalent in Chinese elderly nursing home residents according to SARC-F or SARC-CalF. SARC-F-defined sarcopenia appears to be better for predicting the 1-year mortality of Chinese nursing home residents than SARC-CalF-defined sarcopenia.
1. Introduction
Sarcopenia, a new geriatric syndrome, is featured by the loss of skeletal muscle mass, strength, and physical performance.[1] It is prevalent in elderly adults, especially in nursing home residents.[2] Previous studies demonstrated that the prevalence of sarcopenia varied from 29% to 85.4% in nursing home residents.[3–6] However, the prevalence of sarcopenia in Chinese nursing home residents remains unclear.
Sarcopenia has been associated with many negative outcomes, such as functional disability, falls, health care costs, and even death.[7] The association between sarcopenia and mortality in community-dwelling elderly adults has been well documented.[8,9] However, the evidence addressing sarcopenia and mortality in nursing home residents is controversial. For example, a retrospective study revealed that there was no significant difference in 1-year mortality between patients with or without sarcopenia.[10] On the contrary, another prospective study indicated that sarcopenia independently increased all-cause mortality in Turkish nursing home residents.[11] Furthermore, there is currently no study addressing sarcopenia and mortality in Chinese nursing home residents.
According to the current guidelines, the diagnosis of sarcopenia is device-dependent (e.g., computed tomography [CT] or dual-energy x-ray analysis [DXA] for estimating muscle mass) and may not be feasible in some cases (e.g., walking tests for bedridden elders).[12,13] Therefore, sarcopenia is under-diagnosed in the real world, especially in nursing home residents, and sarcopenia screening tools are supposed to be useful for these elders.[14]
As a classic screening tool for sarcopenia, the strength, assistance walking, rise from a chair, climb stairs, and falls questionnaire (SARC-F) has been validated in different populations since being developed in 2013.[15–18] A recent study demonstrated that SARC-F could also be implemented in long-term care facilities.[19] In that study, the SARC-F had a sensitivity of 18.2% and a specificity of 78.7% when compared with the European Working Group on Sarcopenia in Older People (EWGSOP) criteria.[19] Additionally, Barbosa-Silva and colleagues reported that adding calf circumference (CC) to the SARC-F (named SARC-CalF) improved SARC-F's diagnostic accuracy, especially the sensitivity.[20] Our team also found that SARC-CalF significantly improved the sensitivity and overall diagnostic accuracy of SARC-F for screening sarcopenia in Chinese community-dwelling older adults.[21] Most recently, SARC-CalF has been applied in nursing home residents and showed a sensitivity of 77.4% and a specificity of 89.8% when compared with the EWGSOP criteria.[22]
In nursing home residents, sarcopenia has been proven to be related to adverse clinical outcomes including mortality.[23,24] A good screening tool of sarcopenia is therefore supposed to be valuable for predicting these clinical outcomes too. However, it is unclear whether SARC-F (or SARC-CalF) can predict mortality or not in nursing home residents. Therefore, we conducted a prospective study to compare the prognostic value of SARC-F and SARC-CalF for predicting mortality in this population.
2. Methods
2.1. Study design and population
We conducted a prospective observational study. The baseline investigation was from September to November 2016. We continuously recruited elderly adults (aged ≥ 70 years) living in 4 nursing homes in Chengdu City, China. Individuals with the following conditions were excluded:
-
1.
unable to communicate with interviewers;
-
2.
living in nursing homes less than 2 weeks;
-
3.
refuse to join this study.
All participants signed the written informed consent. The Research Ethics Committee of Sichuan University approved our study protocol.
2.2. Sarcopenia screening
We applied both SARC-F and SARC-CalF questionnaires to screen sarcopenia. The 2 questionnaires were performed by trained nurses via face-to-face interviews. SARC-F includes 5 items, namely strength, assistance in walking, rising from a chair, climbing stairs, falls, whereas SARC-CalF has an additional item, namely CC. The CC was measured by trained nurses using a millimeter graded tape when the participants were in supine position, with their left knee raised and calf at right angles to the thigh.
The contents of SARC-F and SARC-CalF are presented in Supplementary Table 1. A total score of SARC-F ≥4 and a total score of SARC-CalF ≥11 indicate sarcopenia.[20,25]
2.3. Nutrition status assessment
Trained nurses evaluated the participants’ nutrition status using the Mini Nutritional Assessment (MNA) questionnaire.[26] A total score of MNA ≥23.5, 17–23.5, and <17 indicates normal nutrition status, malnutrition risk (MR), and malnutrition (MN), respectively.
2.4. Activities of daily living
Trained nurses evaluated the activities of daily living (ADL) of each participant using the Physical Self-Maintenance Scale (PSMS) developed by Lawton and Brody.[27] The PSMS includes 6 items: eating, dressing, grooming, walking, bathing, and toileting. The options for each item are “can do by myself”, “some difficulty but can do by myself”, “need some help from other people”, and “cannot do by myself”, In this study, impairment was defined as “cannot do by myself” or “need some help from other people”. The ADL disability was defined as having an impairment in any of the 6 items.
2.5. Other covariates
We collected the following information from the medical records: age, gender, smoking, alcohol drinking, body mass index (BMI), comorbidities (hypertension, ischemic heart disease, chronic heart failure, chronic obstructive pulmonary disease, diabetes, stroke, cancer of any type, osteoarthritis, Parkinson disease, cognitive impairment, and depression).
2.6. Follow-up
At the 12th month after the baseline investigation, we collected the survival status of the participants via the medical records in the four nursing homes. For those who left the nursing homes where they lived at the baseline, the survival status was collected via telephone interviews. Time to death was calculated as the period between the baseline investigation and the date of death.
2.7. Statistical analysis
The statistical analysis in this study was performed using SPSS version 20.0 (IBM Inc., Chicago, IL). A P value of <.05 indicates statistical significance. The categorical data were presented as counts (percentages). The continuous data in this study were all skewed distributed, therefore, these data were presented as the median (interquartile range). The differences between groups were compared using the Pearson chi-squared test or Fisher exact test for the categorical data where appropriate and the Mann-Whitney U test for the continuous data.
We used multivariate Cox proportional-hazard models to calculate the hazard ratio (HR) and 95% confidence interval (CI) for 1-year all-cause mortality by SARC-F-defined sarcopenia and SARC-CalF-defined sarcopenia, separately. Model 1 adjusted for age and gender. Model 2 further adjusted for the chronic diseases that were significantly different between the sarcopenia group and the non-sarcopenia group. Because a previous study indicated that the association between sarcopenia and mortality in nursing home residents could be influenced by malnutrition,[11] we further adjusted nutrition status, BMI, and CC in Model 3. In these models, the age, BMI, and CC were treated as continuous data, whereas the other variables were treated as categorical data. In addition, survival curves were estimated using the Kaplan-Meier method and the difference between the curves was compared using the log-rank test.
3. Results
3.1. Baseline characteristics of the study population
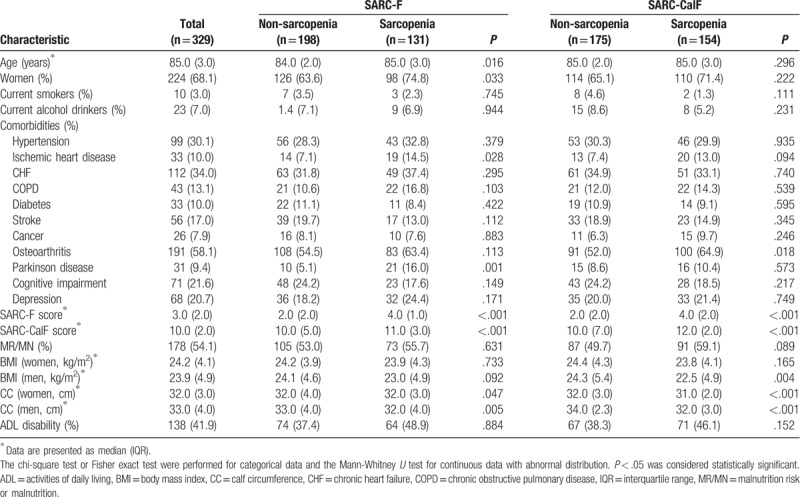
The flow diagram of this study is presented in Figure 1. A total of 329 participants (224 women and 105 men) were included in the baseline investigation. The median age of the participants was 85.0 years (range: 73–95 years). One hundred thirty-eight participants (41.9%) had ADL disability and 178 participants (54.1%) had malnutrition risk or malnutrition (MR/MN). The baseline characteristics of the study population are presented in Table 1.
Figure 1.
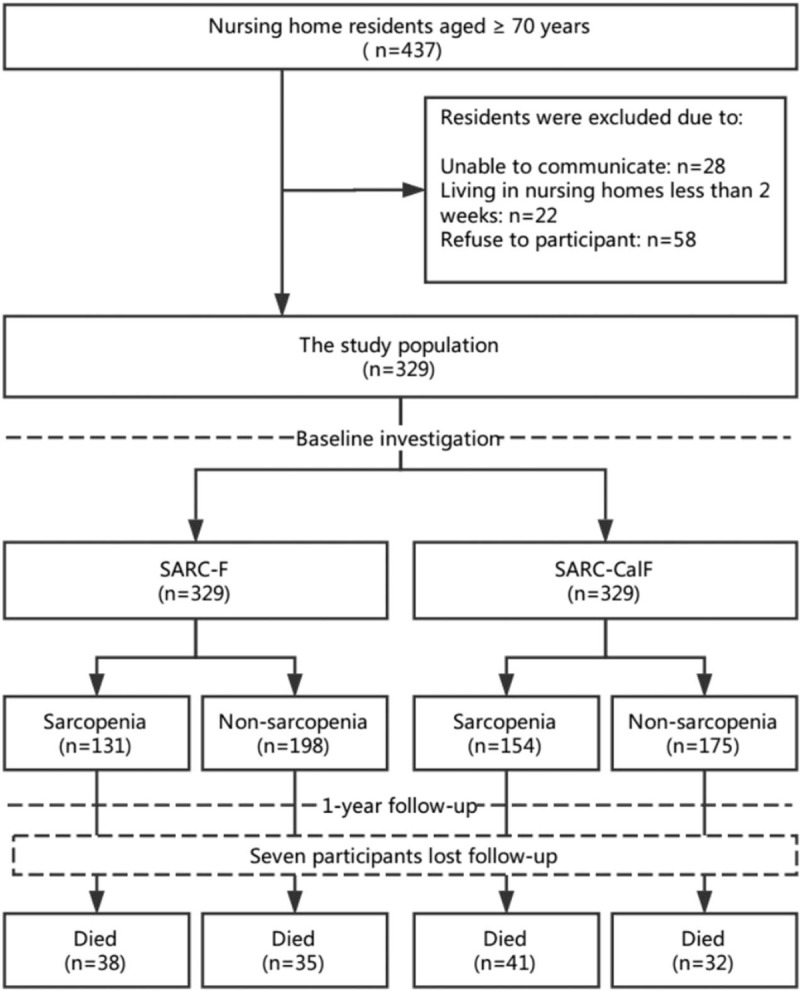
The study flow diagram.
Table 1.
Baseline characteristics of the study population.
3.2. The prevalence of sarcopenia
According to the SARC-F, 131 participants (39.8%) were sarcopenic. The prevalence of SARC-F-defined sarcopenia was 31.4% in men and 43.8% in women (P = .033). Participants with SARC-F-defined sarcopenia were older and more prone to ischemic heart disease and Parkinson disease compared with those without sarcopenia (Table 1).
According to the SARC-CalF, 154 participants (46.8%) were sarcopenic. The prevalence of SARC-CalF-defined sarcopenia was 41.9% in men and 49.1% in women (P = .222). Individuals with SARC-CalF-defined sarcopenia were more prone to osteoarthritis compared with those without sarcopenia (Table 1).
The overlap of SARC-F-defined sarcopenia and SARC-CalF-defined sarcopenia is presented in Figure 2. A total of 110 participants (33.4%) were identified as sarcopenia by both SARC-F and SARC-CalF.
Figure 2.
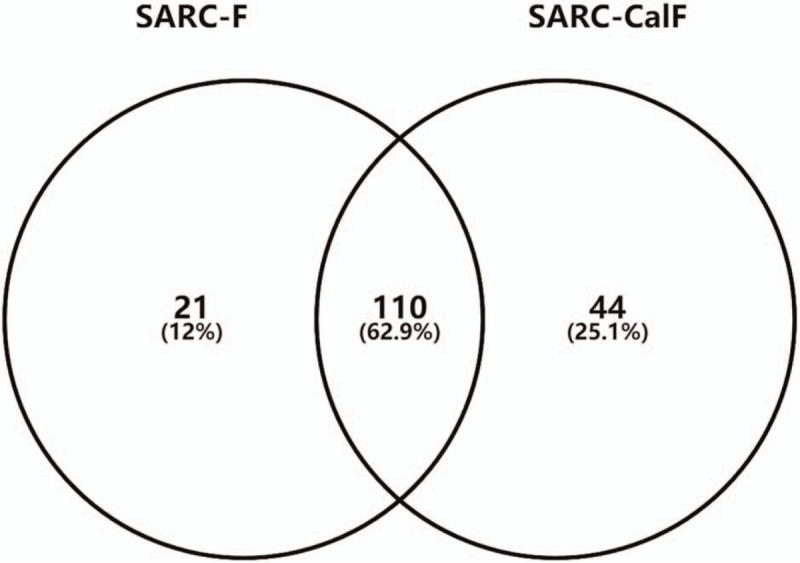
The overlap of SARC-F-defined sarcopenia and SARC-CalF-defined sarcopenia. SARC-CalF = SARC-F combined with calf circumference, SARC-F = Strength, Assistance walking, Rise from a chair, Climb stairs, and Falls questionnaire.
3.3. Mortality of the study population
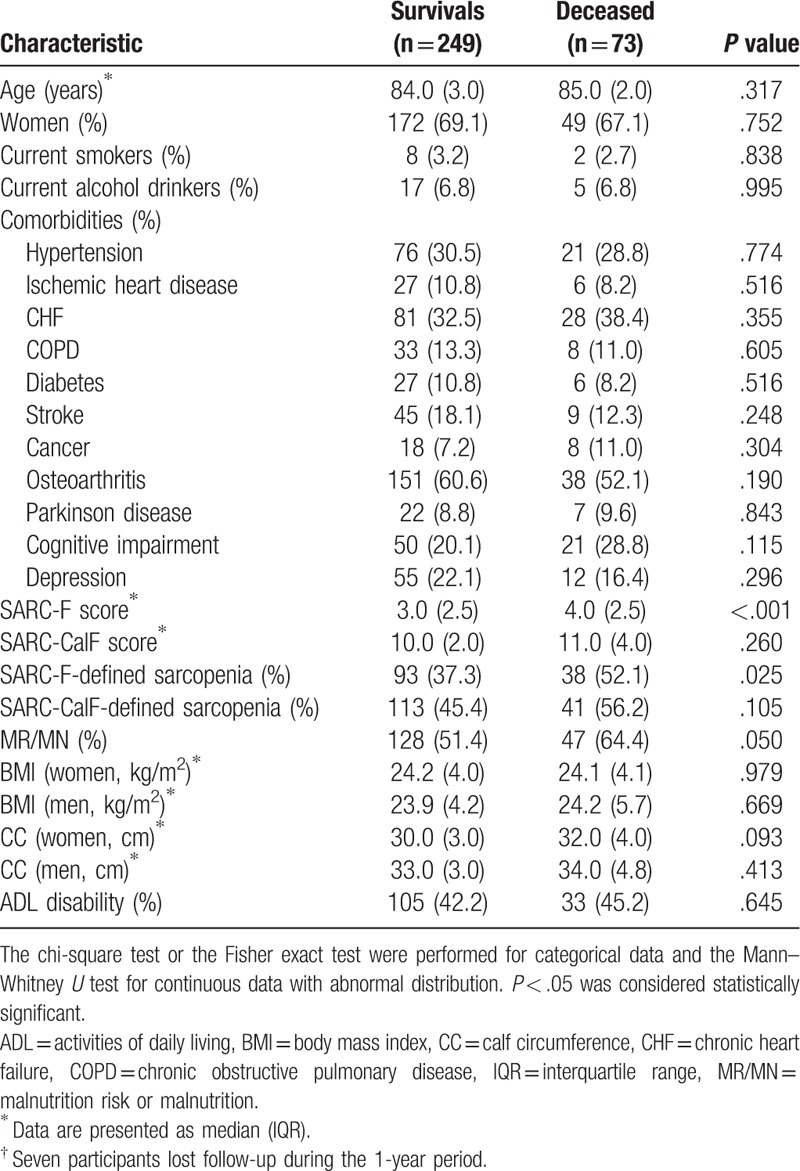
Seven participants were lost during the 1-year follow-up, which led to a final study population of 322 participants. No significant difference was identified between the included participants and those who lost follow-up with respect to median age, median SARC-F score, and median SARC-CalF score at the baseline. Seventy-three participants (22.7%) died during the 1-year follow-up, including 24 men and 49 women (23.8% vs 22.2%, P = .752). Table 2 shows the characteristics of the final study population according to the survival status.
Table 2.
Characteristics of the study population according to survivors and deceased at the end of a 1-year follow-up†.
3.4. Association between SARC-F-defined sarcopenia and mortality
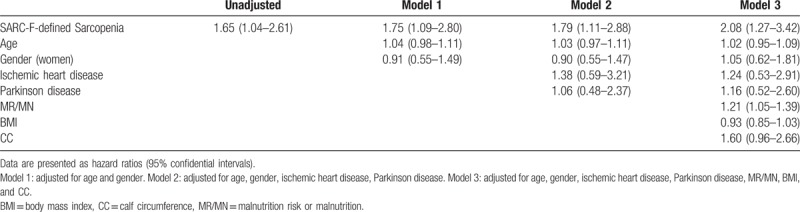
The mortality was 29.0% and 18.3% in the participants with or without SARC-F-defined sarcopenia, respectively (P = .025). As indicated in Table 3, SARC-F-defined sarcopenia was an independent predictor of 1-year mortality in the unadjusted model (HR: 1.65; 95% CI: 1.04–2.61). After adjusted for the potential confounders, the association between SARC-F-defined sarcopenia and 1-year mortality remained significant (Table 3). In the fully adjusted model (Model 3), SARC-F-defined sarcopenia was also independently associated with an increased risk of 1-year mortality (fully adjusted HR: 2.08; 95% CI: 1.27–3.42).
Table 3.
Association between SACR-F-defined sarcopenia and mortality according to Cox Regression Models adjusted for potential confounders.
The survival curves of the participants with or without SARC-F-defined sarcopenia are shown in Figure 3. The survival curves were significantly different according to the log-rank test (P = .001).
Figure 3.
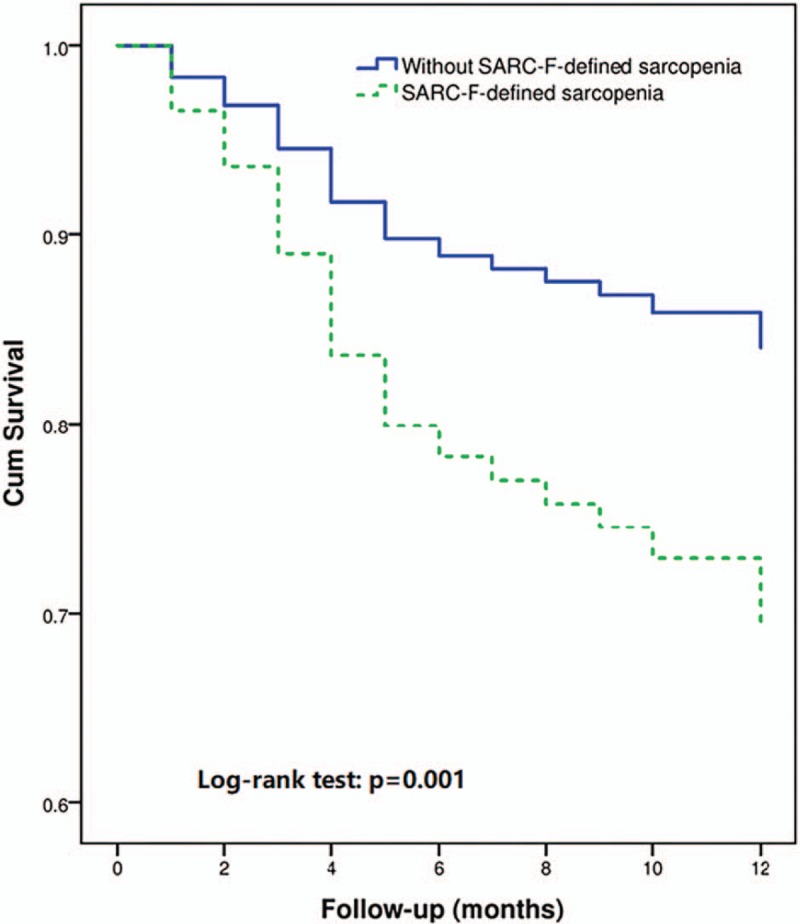
Survival curves of the study population according to sarcopenia defined by SARC-F at baseline. SARC-F = Strength, Assistance walking, Rise from a chair, Climb stairs, and Falls questionnaire.
3.5. Association between SARC-CalF-defined sarcopenia and mortality
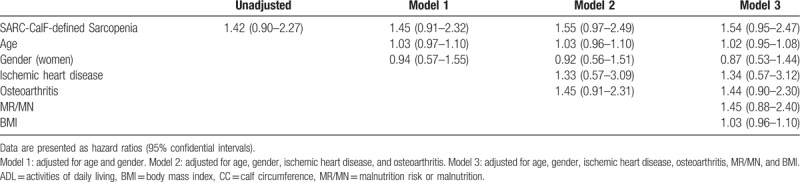
The mortality was 26.6% and 19.0% in the participants with or without SARC-CalF-defined sarcopenia, respectively (P = .105). As indicated in Table 4, SARC-CalF-defined was not an independent predictor of 1-year mortality in either the unadjusted or the adjusted models (fully adjusted HR: 1.54; 95% CI: 0.95–2.47).
Table 4.
Association between SACR-CalF-defined sarcopenia and mortality according to Cox Regression Models adjusted for potential confounders.
The survival curves of the participants with or without SARC-CalF-defined sarcopenia are shown in Figure 4. The log-rank test indicated that the survival curves were not significantly different (P = .201).
Figure 4.
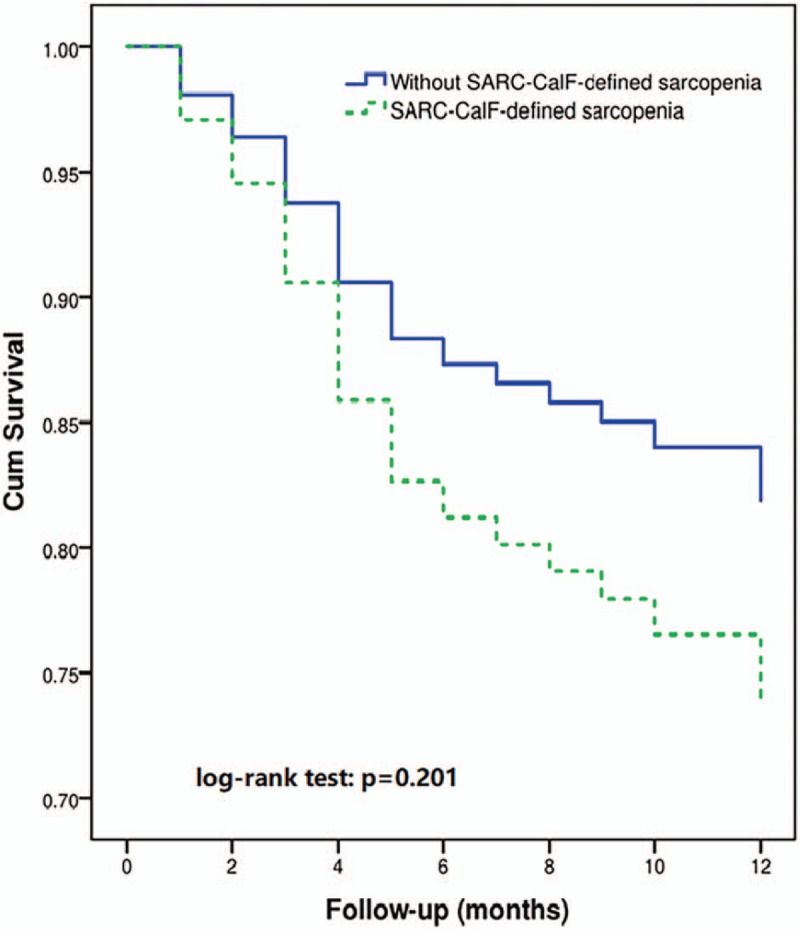
Survival curves of the study population according to sarcopenia defined by SARC-CalF at baseline. SARC-CalF = SARC-F combined with calf circumference.
4. Discussion
To our knowledge, this study is the first to investigate the prevalence of sarcopenia in Chinse nursing home residents using SARC-F and SARC-CalF. And this is also the first prospective study to compare the predictive capacity of SARC-F and SARC-CalF for mortality in nursing home residents. Our study indicates that sarcopenia is highly prevalent in Chinese elderly nursing home residents according to either SARC-F or SARC-CalF. SARC-F appears to be a useful tool in nursing homes to predict 1-year mortality even after an adjustment for the potential confounders including malnutrition. However, sarcopenia, defined by SARC-CalF, cannot predict 1-year mortality in our study population.
Previous systematic reviews have confirmed the association between sarcopenia and mortality in community-dwelling older adults[9,28] and in patients with different types of cancer.[29,30] However, we found only four previous studies addressing sarcopenia and mortality in nursing home residents.[10,11,24,31] One retrospective study found that sarcopenia, defined by low muscle mass that was estimated using a 24-hour creatinine excretion method, was not associated with 1-year mortality in elderly nursing home residents.[10] However, sarcopenia, defined by the EWGSOP criteria, was independently associated with an increased risk of 6-month mortality in nursing home residents according to a prospective study.[24] Similarly, another prospective study also found that sarcopenia, defined by the EWGSOP criteria, was associated with 1-year mortality in Turkish nursing home residents.[31] Furthermore, a recent prospective study demonstrated that sarcopenia, defined by the EWGSOP criteria, was significantly associated with 2-year mortality among elderly nursing home residents in Turkey; however, this association was not significant anymore after an adjustment of MNA scores.[11] Our study showed that SARC-F-defined sarcopenia was independently associated with an increased risk of mortality even after adjusting for the relevant confounders including malnutrition, but SARC-CalF-defined sarcopenia was not. It is difficult to compare these results due to the significant clinical heterogeneity across studies. However, considering the high prevalence of sarcopenia in nursing homes, the predictive capacity of sarcopenia for mortality and other important outcomes (e.g., functional disability and falls) in this special population deserves further studies in the future.
SARC-F has been translated into Chinese,[32] Japanese,[15] Korean,[33] and Spanish.[34] It has been well validated in various study populations.[15–17,33] However, most previous studies regarding SARC-F were conducted in community-dwelling older adults. Only Kotlarczyk and colleagues reported that SARC-F-defined sarcopenia was 21.3% among female long-term care residents in the United States.[19] The evidence regarding the predictive capacity of SARC-F for mortality is also limited. Only 1 prospective study indicated that SARC-F-defined sarcopenia was associated with 4-year mortality among community-dwelling senior in Taiwan.[18] Our study provided new evidence on the predictive value of SARC-F for mortality in nursing home residents.
SARC-F only includes items regarding muscle strength and function, SARC-CalF also includes an additional item regarding muscle mass, therefore, SARC-CalF is theoretically supposed to be more accurate than SARC-F for estimating sarcopenia. However, unlike SARC-F, SARC-CalF (as a novel screening tool) has not been well validated. Our previous study indicated that SARC-CalF significantly improved the overall diagnostic accuracy of SARC-F for sarcopenia in Chinese community-dwelling older adults.[21] In addition, SARC-CalF has been proven to be valuable for screening sarcopenia in nursing homes.[22] However, the current study did not support the predictive capacity of SARC-CalF for mortality in nursing home residents. Therefore, further studies are needed to evaluate the validity and reliability of SARC-CalF in nursing home residents.
In this study, compared with SARC-F, SARC-CalF appeared to overestimate sarcopenia (46.8% versus 39.8%). One possible reason was the cutoff point of CC in SARC-CalF, which were 34 and 33 cm for men and women, respectively.[20] The optimal cutoff points for CC to estimate low skeletal muscle mass remains unclear; however, it may vary in different ethnic populations. For example, 33 and 32 cm were the proposed cutoff points of CC to estimate low muscle mass in elderly men and women in Taiwan.[35] In Turkish elderly adults, 33 cm was supposed to be the optimal cutoff point of CC for both men and women.[36] In our study population, the median of CC was 33 and 32 cm in men and women, therefore, the cutoff points of CC in SARC-CalF may not suitable for our study population.
Our study has some limitations. First, although we have carefully adjusted for many important confounders, unmeasured factors (e.g., polypharmacy) may bias the results. Second, we did not compare SARC-F (and SARC-CalF) with common sarcopenia criteria, such as the EWGSOP[12] or the Asia Working Group for Sarcopenia (AWGS)[9] criteria. In fact, the sensitivity and specificity of the SARC-F and SARC-CalF were not very good, therefore, some participants in both the SARC-F and SARC-CalF groups might have no sarcopenia. On the other hand, both the SARC-F and SARC-CalF might miss some participants who actually had sarcopenia. This might bias our results. Third, our study only focused on mortality, the predictive capacity of SARC-F and SARC-CalF for other important outcomes, such as quality of life and the incidence of disability, should be evaluated in prospective studies in the future. Fourth, our results were based on a sample population of Chinese elderly adults aged 70 years or older living in nursing homes. It should be cautious to apply these results to other populations. Last, just like all cohort studies, selective survival before entry into the study population needs to be considered.
5. Conclusion
Sarcopenia, defined by SARC-F or SARC-CalF, is highly prevalent in a study population of Chinese elderly nursing home residents. SARC-F-defined sarcopenia appears to be better for predicting the 1-year mortality of Chinese nursing home residents than SARC-CalF-defined sarcopenia. The applications of SARC-F and SARC-CalF in the nursing home residents need to be further evaluated in the future.
Acknowledgments
We thank all individuals who worked as volunteers in this study for their support.
Author contributions
Conceptualization: Ming Yang, Huairong Tang.
Data curation: Yanli Zeng, Huairong Tang.
Formal analysis: Ming Yang, Jiaojiao Jiang, Yanli Zeng, Huairong Tang.
Investigation: Jiaojiao Jiang.
Methodology: Jiaojiao Jiang, Huairong Tang.
Writing – original draft: Ming Yang.
Writing – review & editing: Huairong Tang.
Supplementary Material
Footnotes
Abbreviations: ADL = activities of daily living, AWGS = Asia working group for sarcopenia, BMI = body mass index, CC = calf circumference, CI = confidence interval, CT = computed tomography, DXA = dual-energy x-ray analysis, EWGSOP = European working group on sarcopenia in older people, MN = malnutrition, MNA = mini nutritional assessment, MR = malnutrition risk, PSMS = physical self-maintenance scale, SARC-CalF = SARC-F combined with calf circumference, SARC-F = strength, assistance walking, rise from a chair, climb stairs, and falls questionnaire.
This study was supported by the Sichuan Medical Association (No. S17054) and the Health and Family Planning Commission of Sichuan Province (No. ZH2018–102). The sponsor had no role in the design, methods, data collection, analysis or preparation of this manuscript.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Cederholm T, Morley JE. Sarcopenia: the new definitions. Curr Opin Clin Nutr Metab Care 2015;18:1–4. [DOI] [PubMed] [Google Scholar]
- [2].Bauer JM, Kaiser MJ, Sieber CC. Sarcopenia in nursing home residents. J Am Med Dir Assoc 2008;9:545–51. [DOI] [PubMed] [Google Scholar]
- [3].Yalcin A, Aras S, Atmis V, et al. Sarcopenia prevalence and factors associated with sarcopenia in older people living in a nursing home in Ankara Turkey. Geriatr Gerontol Int 2016;16:903–10. [DOI] [PubMed] [Google Scholar]
- [4].Senior HE, Henwood TR, Beller EM, et al. Prevalence and risk factors of sarcopenia among adults living in nursing homes. Maturitas 2015;82:418–23. [DOI] [PubMed] [Google Scholar]
- [5].Bahat G, Saka B, Tufan F, et al. Prevalence of sarcopenia and its association with functional and nutritional status among male residents in a nursing home in Turkey. Aging Male 2010;13:211–4. [DOI] [PubMed] [Google Scholar]
- [6].Landi F, Liperoti R, Fusco D, et al. Prevalence and risk factors of sarcopenia among nursing home older residents. J Gerontol Ser A-Biol Sci Med Sci 2012;67:48–55. [DOI] [PubMed] [Google Scholar]
- [7].Marzetti E, Calvani R, Tosato M, et al. Sarcopenia: an overview. Aging Clin Exp Res 2017;29:11–7. [DOI] [PubMed] [Google Scholar]
- [8].Kelley GA, Kelley KS. Is sarcopenia associated with an increased risk of all-cause mortality and functional disability? Exp Gerontol 2017;96:100–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu P, Hao Q, Hai S, et al. Sarcopenia as a predictor of all-cause mortality among community-dwelling older people: a systematic review and meta-analysis. Maturitas 2017;103:16–22. [DOI] [PubMed] [Google Scholar]
- [10].Kimyagarov S, Klid R, Fleissig Y, et al. Skeletal muscle mass abnormalities are associated with survival rates of institutionalized elderly nursing home residents. J Nutr Health Aging 2012;16:432–6. [DOI] [PubMed] [Google Scholar]
- [11].Yalcin A, Aras S, Atmis V, et al. Sarcopenia and mortality in older people living in a nursing home in Turkey. Geriatr Gerontol Int 2017;17:1118–24. [DOI] [PubMed] [Google Scholar]
- [12].Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 2010;39:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Arai H, Akishita M, Chen LK. Growing research on sarcopenia in Asia. Geriatr Gerontol Int 2014;14Suppl 1:1–7. [DOI] [PubMed] [Google Scholar]
- [14].Morley JE, Cao L. Rapid screening for sarcopenia. J Cachexia Sarcopenia Muscle 2015;6:312–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ida S, Murata K, Nakadachi D, et al. Development of a Japanese version of the SARC-F for diabetic patients: an examination of reliability and validity. Aging Clin Exp Res 2017;29:935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tanaka S, Kamiya K, Hamazaki N, et al. Utility of SARC-F for assessing physical function in elderly patients with cardiovascular disease. J Am Med Dir Assoc 2017;18:176–81. [DOI] [PubMed] [Google Scholar]
- [17].Rolland Y, Dupuy C, Abellan Van Kan G, et al. Sarcopenia Screened by the SARC-F questionnaire and physical performances of elderly women: a cross-sectional study. J Am Med Dir Assoc 2017;18:848–52. [DOI] [PubMed] [Google Scholar]
- [18].Wu TY, Liaw CK, Chen FC, et al. Sarcopenia screened with SARC-f questionnaire is associated with quality of life and 4-year mortality. J Am Med Dir Assoc 2016;17:1129–35. [DOI] [PubMed] [Google Scholar]
- [19].Kotlarczyk MP, Perera S, Nace DA, et al. Identifying sarcopenia in female long-term care residents: a comparison of current guidelines. J Am Geriatr Soc 2018;66:316–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Barbosa-Silva TG, Menezes AM, Bielemann RM, et al. Grupo de estudos em composicao corporal e N. Enhancing SARC-F: improving sarcopenia screening in the clinical practice. J Am Med Dir Assoc 2016;17:1136–41. [DOI] [PubMed] [Google Scholar]
- [21].Yang M, Hu X, Xie L, et al. Screening sarcopenia in community-dwelling older Adults: SARC-F vs SARC-F combined with calf circumference (SARC-CalF). J Am Med Dir Assoc 2018;19:277e271-277.e278. [DOI] [PubMed] [Google Scholar]
- [22].Urzi F, Šimunič B, Buzan E. Basis for sarcopenia screening with the SARC-CalF in nursing homes. J Am Med Dir Assoc 2017;18:991.e5–10. [DOI] [PubMed] [Google Scholar]
- [23].Henwood T, Hassan B, Swinton P, et al. Consequences of sarcopenia among nursing home residents at long-term follow-up. Geriatr Nurs 2017;38:406–11. [DOI] [PubMed] [Google Scholar]
- [24].Landi F, Liperoti R, Fusco D, et al. Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc 2012;13:121–6. [DOI] [PubMed] [Google Scholar]
- [25].Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc 2013;14:531–2. [DOI] [PubMed] [Google Scholar]
- [26].Vellas B, Villars H, Abellan G, et al. Overview of the MNA--Its history and challenges. J Nutr Health Aging 2006;10:456–63. [PubMed] [Google Scholar]
- [27].Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–86. [PubMed] [Google Scholar]
- [28].Chang SF, Lin PL. Systematic literature review and meta-analysis of the association of sarcopenia with mortality. Worldviews Evid-Based Nurs 2016;13:153–62. [DOI] [PubMed] [Google Scholar]
- [29].Pamoukdjian F, Bouillet T, Levy V, et al. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: a systematic review. Clinical nutrition (Edinburgh, Scotland) 2017;37:1101–13. [DOI] [PubMed] [Google Scholar]
- [30].Collins J, Noble S, Chester J, et al. The assessment and impact of sarcopenia in lung cancer: a systematic literature review. BMJ Open 2014;4:e003697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Saka B, Ozkaya H, Karisik E, et al. Malnutrition and sarcopenia are associated with increased mortality rate in nursing home residents: a prospective study. Eur Geriatr Med 2016;7:232–8. [Google Scholar]
- [32].Woo J, Leung J, Morley JE. Validating the SARC-F: a suitable community screening tool for sarcopenia? J Am Med Dir Assoc 2014;15:630–4. [DOI] [PubMed] [Google Scholar]
- [33].Kim S, Kim M, Won CW. Validation of the Korean version of the SARC-F questionnaire to assess sarcopenia: Korean frailty and aging cohort study. J Am Med Dir Assoc 2018;19:40–5. e41. [DOI] [PubMed] [Google Scholar]
- [34].Parra-Rodriguez L, Szlejf C, Garcia-Gonzalez AI, et al. Cross-cultural adaptation and validation of the Spanish-language Version of the SARC-F to assess sarcopenia in Mexican community-dwelling older adults. J Am Med Dir Assoc 2016;17:1142–6. [DOI] [PubMed] [Google Scholar]
- [35].Hwang AC, Liu LK, Lee WJ, et al. Calf circumference as a screening instrument for appendicular muscle mass measurement. J Am Med Dir Assoc 2018;19:182–4. [DOI] [PubMed] [Google Scholar]
- [36].Bahat G, Tufan A, Tufan F, et al. Cut-off points to identify sarcopenia according to European working group on sarcopenia in older people (EWGSOP) definition. Clin Nutr 2016;35:1557–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


