Abstract
Complex regional pain syndrome (CRPS) and fibromyalgia (FM) share many features. Both can cause severe pain and are considered to have a mechanism of action, including dysfunction of the sympathetic nervous system. However, they have clinical differences in pain range and degree. The present study aimed to find neurophysiologic differences between CRPS and FM using quantitative electroencephalography (QEEG). Thirty-eight patients with CRPS and 33 patients with FM were included in the analysis. Resting-state QEEG data were grouped into frontal, central, and posterior regions to analyze for regional differences. General linear models were utilized to test for group differences in absolute and relative powers. As a result, the CRPS group relative to FM group showed lower total absolute powers in the beta band (F = 5.159, P < .05), high beta (F = 14.120, P < .05), and gamma band (F = 15.034, P < .05). There were no significant differences between 2 groups in the delta, theta, and alpha bands. The present findings show that the CRPS and FM groups differ mainly in the high frequency, which may reflect their distinct pathophysiology and symptomatology. Our study suggests that the QEEG differences can be clinically useful in assessing brain function in patients with CRPS and FM.
Keywords: beta band, complex regional pain syndrome, fibromyalgia, gamma band, high beta band, quantitative electroencephalography
1. Introduction
Chronic pain afflicts people of all ages. A recent study conducted in South Korea found that 87.7% of women and 63.8% of men aged over 60 years experienced chronic pain.[1] Chronic pain is distinguished from acute pain by its duration; pain that lasts for more than 3 months is classified as chronic pain.[2] Chronic pain is also defined when the pain is severe or persistent after a tissue injury is restored.[3]
Complex regional pain syndrome (CRPS) is a chronic pain disorder in which severe pain occurs at a specific site after trauma. Most patients with CRPS show abnormal sudomotor activity, edema, and trophic skin changes.[4] In addition, patients with CRPS experience a spectrum of painful sensations, including hyperalgesia or mechanical, cold, or heat allodynia.[5] As described in a series of publications by Schwartzman et al,[6] CRPS is a chronic condition, characterized by severe neuropathic pain typically preceded by an injury (77% of cases) or surgical procedure (11%), but sometimes occurring without antecedent tissue damage. The mechanism of action of CRPS is thought to involve dysfunction of the sympathetic nervous system (SNS), although its etiology is poorly defined.[7] It is believed that there is a complex interplay between inflammatory mediators and the SNS, including sympathetic neurons releasing norepinephrine, which acts on adrenergic receptors, causing an increase in the amount of proinflammatory cytokines produced, ultimately leading to peripheral pain sensitization.[8]
Fibromyalgia (FM) is a disorder in which skeletal muscle or adjacent fibrous tissue is painful, or becomes so in response to use or physical pressure.[9] FM is characterized by chronic widespread pain and diffuse tenderness.[10] Most patients with FM complain of subjective symptoms such as anxiety, fatigue, forgetfulness, nonrestorative sleep, difficulty concentrating, and psychologic distress.[9] Similar to CRPS, the exact mechanism underlying FM remains unknown, but a central, sympathetically mediated mechanism is suspected.[11] Various psychologic interventions for pain have been employed to counter central sensitization and hyperactivity of the SNS.[12] The characteristics of FM suggest that it should be classified as dysfunctional pain, reflecting a malfunction in sensory processing within the central nervous system (CNS).
The CRPS and FM share many features: both are chronic pain disorders that can cause severe pain, and both are considered to have a mechanism of action involving dysfunction of the SNS. However, CRPS is more intense, being marked by burning, aching pain, and exhaustion, and a highly localized area of pain. Relative to CRPS, FM is associated with less intense and generally widespread pain, and tenderness in the musculoskeletal system. In addition, unlike FM, CRPS is usually characterized by changes in skin color and temperature at the site of the original tissue injury, suggesting local sympathetic hyperactivity.[9,13] However, there is no recognized biologic basis for distinguishing between CRPS and FM. Thus, in this study, we used quantitative electroencephalography (QEEG) to investigate neurophysiologic differences between patients with CRPS and FM.
In QEEG, EEG data are quantitatively analyzed to generate brain maps, which are then used to estimate brain function; analog EEG data are converted to a digital format using a computer. The conventional method of QEEG is spectrum analysis, in which fast Fourier transformation converts a complex waveform into a signal showing the amplitude per cycle.[14]
In a previous study using QEEG, patients with FM showed reduced absolute power in low- to mid-frequency waves (delta, theta, and alpha) in the frontal area, and elevated high-frequency (beta) relative spectral power in frontal and central areas compared to a normal control group.[15] Delta, theta, and alpha activities are associated with cognitive performance.[16,17] It suggests that the absolute power is lower in the low- to mid-frequency range in patients with FM compared to normal controls, indicating cognitive deterioration. According to research showing that patients with CRPS have cognitive deficits,[18] we predicted that patients with CRPS would show reduced absolute power in the low- to mid-frequency range. On the contrary, beta activity is known to increase as beta band power decreases during the preparation and execution of voluntary movements, and when movement must be sustained or spontaneously suppressed.[19–21] Whereas FM is associated with a central mechanism and elevated beta wave activity, patients with CRPS may exhibit lower beta wave activity because the condition is hypothesized to relate mainly to peripheral nerve injury.
To date, there have been QEEG studies on FM but not on CRPS. Thus, we herein explored commonalities and differences between CRPS and FM based on QEEG data. We aimed to determine the characteristics distinguishing the two disorders through an analysis of physiologic parameters. We hypothesized that patients with FM were expected to show higher beta wave activity than those with CRPS.
2. Materials and methods
2.1. Participants
We analyzed the QEEG data of 38 patients diagnosed with CRPS and 33 diagnosed with FM; all data were from patients who visited Seoul National University Hospital, Seoul, Korea, between 2014 and 2016. Patients with CRPS were diagnosed at the Department of Anesthesiology based on the “Budapest criteria.” An anesthesiologist at the Department of Anesthesiology diagnosed patients with FM based on the FM criteria of the American College of Rheumatology. The study protocol was approved by the Seoul National University Hospital Institutional Review Board (Seoul, Republic of Korea). The acquisition of the informed consent from the patients was waived due to the retrospective nature of this study.
2.2. EEG recordings
The participants were seated in a resting state in an isolated and sound-shielded room connected to a recording room via a 1-way glass window. The EEG recordings lasted for 10 minutes: 4 minutes with the eyes closed, 2 minutes with the eyes open, and 4 additional minutes with eyes closed.
The EEG data were acquired using a SynAmps2 Amplifier (Compumedics, Abbotsford, Australia) and NeuroScan 4.3 (Compumedics). Twenty-one electrodes were attached to the scalp (Cz, FPz, Fz, Oz, Pz, T4, O2, T6, C4, P4, Fp2, F8, F4, T3, O1, T5, C3, P3, Fp1, F7, and F3) according to the international 10 to 20 system, with 2 electrodes used for tracking eye movements. Reference electrodes (single-channel bipolar electrodes) were attached to the mastoids. The ground channel was located between FPz and Fz. The signals were sampled at a frequency of 500 Hz. The electrode impedance was below 5 kΩ, and the EEG signal was band-pass filtered at 0.1 to 60 Hz using the NeuroScan 4.3 device; recordings were transferred to NeuroGuide software (ver 2.5.5; Applied Neuroscience, Inc, St Petersburg, FL) for spectral analysis in a 32-bit file format, and 19 sites were selected from the set within NeuroGuide: FP1, F3, F7, Fz, FP2, F4, F8, T3, C3, Cz, T4, C4, T5, P3, O1, Pz, T6, P4, and O2. Artifact removal was performed offline using the artifact rejection toolbox of NeuroGuide. The EEG recordings were also visually inspected to eliminate eye muscle movements and other artifacts, and artifact-free epochs of approximately 90 seconds, under eyes-closed conditions, were selected for spectral analysis. The absolute (uV2) and relative (%) power components were smoothed using fast Fourier transformation and averaged using the spectral analysis system of NeuroGuide in 7 frequency bands: delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz), high beta (25–30 Hz), gamma (30–40 Hz), and high gamma (40–50 Hz).
2.3. Statistical analysis
Statistical analyses were performed using SPSS software (ver 23.0; IBM Corp, Armonk, NY), and P-value <.05 was considered statistically significant. The activity at each of the 19 selected sites was classified into 3 categories, by region: frontal (FP1, F3, F7, Fz, FP2, F4, and F8), central (T3, C3, Cz, T4, and C4), and posterior (T5, P3, O1, Pz, T6, P4, and O2). Because there were more than 2 dependent variables, a multivariate analysis (MANOVA) was performed to statistically analyze the values by group and area.
3. Results
3.1. Demographic characteristics of the study participants
The average age of the 38 patients with CRPS (23 males and 15 females) was 40.49 ± 12.10 years, and that of the 33 patients with FM (11 males and 22 females) was 43.18 ± 14.03 years. There was no significant difference in age between the groups, but there was a difference in sex (t = −0.859, P < .05). Therefore, sex was included as a covariate in the MANOVA.
3.2. EEG activity
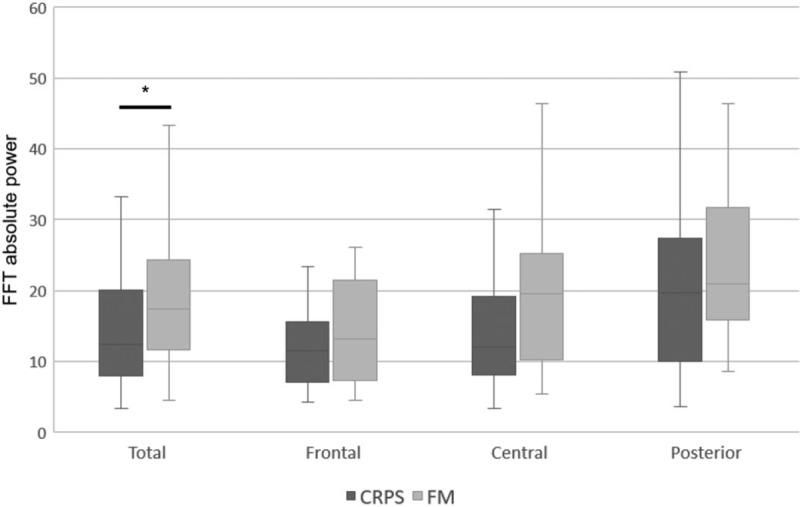
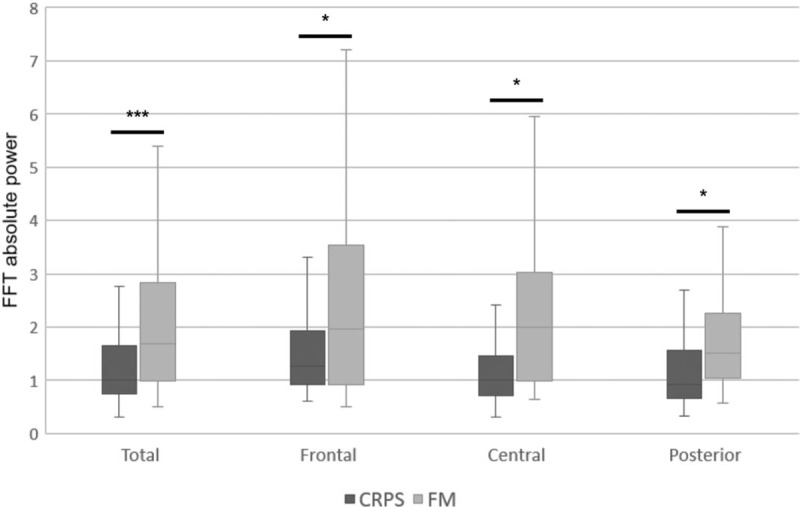
Table 1 shows that there was a main effect of group in terms of the absolute power of the high-frequency EEG signals (beta, high beta, and gamma bands). In all areas, the CRPS group showed lower absolute power in the beta band (CRPS group, 15.941; FM group, 20.588; P < .05) and high beta band (CRPS, 1.302; FM, 2.466; P < .05) (Figs. 1 and 2, respectively), and in the gamma band in frontal and central areas (CRPS, 0.193; FM, 0.291; P < .05) (Fig. 3). In addition, we found a main effect of group on the theta/beta ratio. The CRPS group showed a higher delta/alpha ratio in central (CRPS, 0.777; FM, 0.496; P < .05) and posterior (CRPS, 0.424; FM, 0.297; P < .05) areas, and a higher theta/beta ratio overall (CRPS, 1.194; FM, 0.882; P < .05). We found no significant difference in low- to mid-frequency EEG signals (delta, theta, or alpha bands), and no group-by-region interaction for absolute power or the theta/beta ratio.
Table 1.
Quantitative electroencephalography results of the study participants.
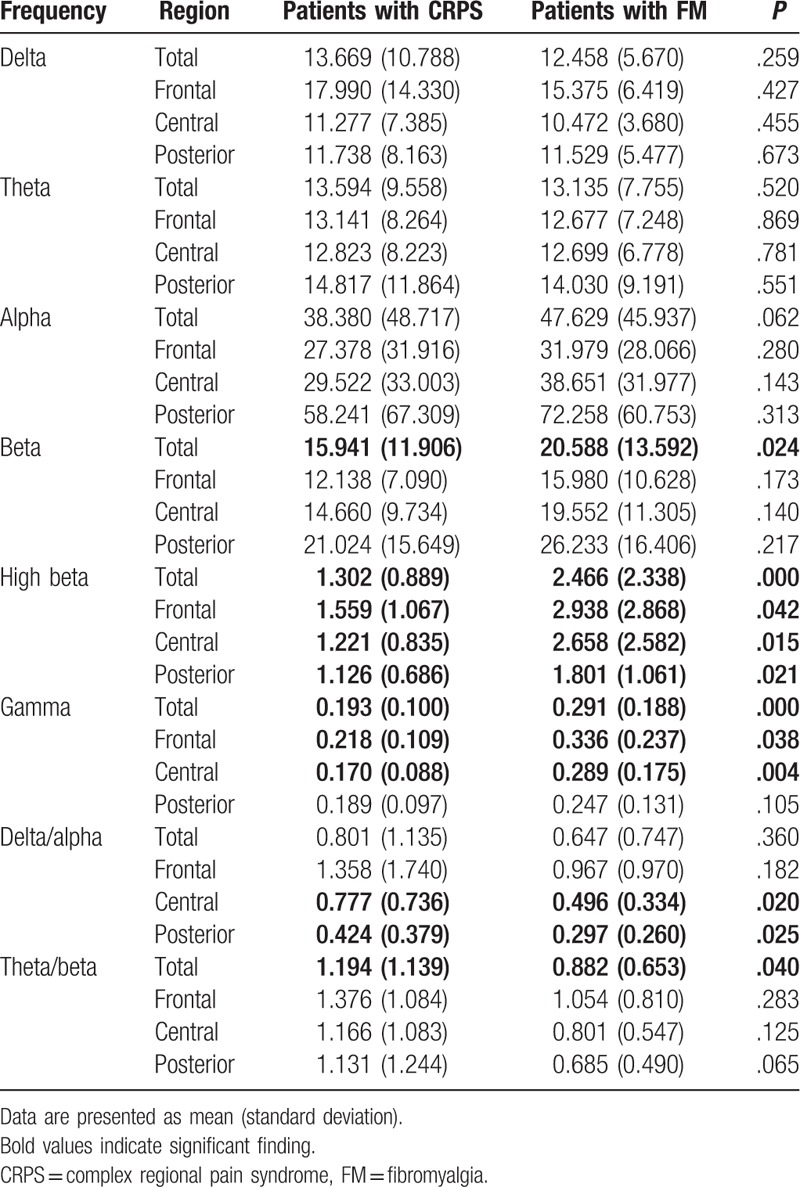
Figure 1.
Differences of the absolute power in the beta band between complex regional pain syndrome (CRPS) and fibromyalgia (FM) group. The CRPS group relative to FM group showed lower absolute power in the beta band. ∗P < .05. FFT = fast fourier transform.
Figure 2.
Differences of the absolute power in the high beta band between complex regional pain syndrome (CRPS) and fibromyalgia (FM) group. The CRPS group relative to FM group showed lower absolute power in the high beta band. ∗∗∗P < .001, ∗P < .05. FFT = fast fourier transform.
Figure 3.
Differences of the absolute power in the gamma band between complex regional pain syndrome (CRPS) and fibromyalgia (FM) group. The CRPS group relative to FM group showed lower absolute power in the gamma band. ∗∗∗P < .001, ∗∗P < .01, ∗P < .05. FFT = fast fourier transform.
4. Discussion
To our knowledge, this study is the 1st to compare EEG data between patients with CRPS and FM. The CRPS group relative to FM group showed lower absolute power in the beta and high beta bands, overall and in frontal, central, and posterior areas. The absolute power was also low in the gamma band in frontal and central areas. Two groups showed neurophysiologic differences mainly in high frequency band rather than low- to mid-frequency band.
It is well established that beta band power decreases during the preparation and execution of voluntary movements.[19–21] Recent studies showed that bursts of beta activity were associated with strengthened sensory feedback during static motor control, and reduced beta activity during movement.[22] Beta activity also increases when movement must be inhibited or voluntarily suppressed.[23] Elsewhere, an increase in beta activity induced by transcranial alternating current stimulation over the motor cortex slowed subjects’ movements.[24] In addition, abnormally high beta activity has been hypothesized to be indicative of a hyper-aroused CNS, stress, inability to relax, and anxiety. In this study, higher beta wave activity was observed in patients with FM than in patients with CRPS. Presumably, this reflects the central sensitization seen in idiopathic FM, which differs from the peripheral sensitization that characterizes CRPS following pain-induced brain changes.
The gamma band is associated with the simultaneous processing of information from different brain areas, and with cognitive processes such as perception, attention, and memory.[25] In disease states, these fast-frequency brain waves often appear distorted compared to those of control subjects.[26] Furthermore, different aspects of gamma oscillatory activity are thought to be related to abnormal brain activity, and perhaps also to the pathophysiology of various disorders. Reduced gamma power in CRPS relative to FM might be associated with comorbid psychiatric disorders in patients with the former disease.
Additionally, studies using QEEG have shown increased beta bands in patients with psychiatric disorders.[27] One example is that the symptoms of agitated depression were linked to reversals of beta waves.[28] In addition, a previous study reported an increment of the level of attention in patients with depression when treated by QEEG using the neurofeedback system.[29] Considering that patients with CRPS or FM more likely have psychiatric disorders, including depressive and anxiety disorders,[30,31] the increment of the spectral high bands can be associated with diverse psychiatric pathologies. It is necessary to pay attention to existing co-occurrence and their distinction.
In this study, the CRPS group showed a higher delta/alpha ratio in central and posterior areas, and a higher overall theta/beta ratio, compared to the patients with FM. The delta/beta ratio is known to be important in recovery from disease and the outcome of therapy.[32,33] The higher delta/alpha ratio of our CRPS group suggests that treatment was not successful. The theta/beta ratio is known to be negatively associated with cognition and attention.[34,35] In this study, the CRPS group showed a higher theta/beta ratio than the FM group, indicating that cognitive and attention are more impaired in the former condition.
There are several limitations to this study. First, given its retrospective cross-sectional design, it was difficult to interpret the correlation between the mechanism underlying pain in CRPS and FM and the QEEG data. Furthermore, patients were selected based only on the main diagnosis documented in their medical records, such that the effects of other, coexisting diseases cannot be excluded. Third, the generalizability of the results is limited because the progression of symptoms and neurologic changes over the disease course may affect patient outcomes. Moreover, only the patient groups were included in the study and there was no control group. Although difficult to recruit, it is advantageous to include large numbers of patients with CRPS and FM and healthy controls prospectively in studies thereon. It should also be noted that most of our patients were taking antipsychotic medications, the effects of which likely acted as a confounding factor. The relationship between the patients’ pain levels and the QEEG data could not be confirmed because no pain scale, such as the Numeric Rating Scale, was used to assess pain at the time of presentation. Finally, for the statistical analysis, a MANOVA was performed on EEG data measured at 1 electrode and classified into 3 categories by area; however, the mutual influence of the electrodes cannot be completely ruled out.
In this study, we observed no differences between the CRPS and FM patient groups in delta, theta, or alpha waves, but there were differences in the beta, high beta, and gamma bands. This suggests that the similarities and differences between the 2 diseases are reflected in EEG data. Thus, we conclude that QEEG analysis is instructive in showing differences between CRPS and FM, and that it has the potential to be clinically useful for assessing brain function in these patient groups. In subsequent studies, it will be important to compare patients with healthy controls to validate the present results.
Author contributions
Conceptualization: Soo-Hee Choi, Joon Hwan Jang, Jee Youn Moon, Do-Hyung Kang.
Data curation: Soo-Hee Choi, Yoo Bin Choi, Hee Kyung Jung, Dasom Lee.
Formal analysis: Jae-Yeon Lee, Ki-Soon Park, Yoo Bin Choi, Hee Kyung Jung, Dasom Lee.
Funding acquisition: Do-Hyung Kang.
Investigation: Yoo Bin Choi, Hee Kyung Jung, Dasom Lee.
Methodology: Soo-Hee Choi, Do-Hyung Kang.
Project administration: Soo-Hee Choi.
Resources: Soo-Hee Choi, Joon Hwan Jang, Jee Youn Moon.
Supervision: Soo-Hee Choi, Do-Hyung Kang.
Validation: Jae-Yeon Lee.
Visualization: Jae-Yeon Lee.
Writing – original draft: Jae-Yeon Lee, Soo-Hee Choi, Ki-Soon Park.
Writing – review & editing: Jae-Yeon Lee, Soo-Hee Choi, Joon Hwan Jang, Jee Youn Moon, Do-Hyung Kang.
Footnotes
Abbreviations: CNS = central nervous system, CRPS = complex regional pain syndrome, FM = fibromyalgia, QEEG = quantitative electroencephalography, SNS = sympathetic nervous system.
J-YL and S-HC contributed equally to this work.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No NRF-2018R1A2B6001806).
The authors have no conflicts of interest to disclose.
References
- [1].Jung CK, Park JY, Kim NS, et al. Status of chronic pain prevalence in the Korean adults. Public Health Weekly Report 2015;8:728–34. [Google Scholar]
- [2].Turk DC, Okifuji A. Psychological factors in chronic pain: evolution and revolution. J Consulting Clin Psychol 2002;70:678. [DOI] [PubMed] [Google Scholar]
- [3].Kang YK. Evaluation and management of chronic pain. J Korean Acad Fam Med 2003;24:103–11. [Google Scholar]
- [4].de Mos M, Huygen FJ, Dieleman JP, et al. Medical history and the onset of complex regional pain syndrome (CRPS). Pain 2008;139:458–66. [DOI] [PubMed] [Google Scholar]
- [5].Jänig W, Baron R. Complex regional pain syndrome: mystery explained? Lancet Neurol 2003;2:687–97. [DOI] [PubMed] [Google Scholar]
- [6].Schwartzman RJ, Erwin KL, Alexander GM. The natural history of complex regional pain syndrome. Clin J Pain 2009;25:273–80. [DOI] [PubMed] [Google Scholar]
- [7].Kuttikat A, Noreika V, Shenker N, et al. Neurocognitive and neuroplastic mechanisms of novel clinical signs in CRPS. Front Human Neurosci 2016;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li W, Shi X, Wang L, et al. Epidermal adrenergic signaling contributes to inflammation and pain sensitization in a rat model of complex regional pain syndrome. Pain 2013;154:1224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wurtman RJ. Fibromyalgia and the complex regional pain syndrome: similarities in pathophysiology and treatment. Metabolism 2010;59:S37–40. [DOI] [PubMed] [Google Scholar]
- [10].Gracely RH, Petzke F, Wolf JM, et al. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum 2002;46:1333–43. [DOI] [PubMed] [Google Scholar]
- [11].Kosek E, Altawil R, Kadetoff D, et al. Evidence of different mediators of central inflammation in dysfunctional and inflammatory pain—interleukin-8 in fibromyalgia and interleukin-1 in rheumatoid arthritis. J Neuroimmunol 2015;280:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Thieme K, Turk DC, Gracely RH, et al. Differential psychophysiological effects of operant and cognitive behavioural treatments in women with fibromyalgia. Eur J Pain 2016;20:1478–89. [DOI] [PubMed] [Google Scholar]
- [13].Littlejohn G. Neurogenic neuroinflammation in fibromyalgia and complex regional pain syndrome. Nat Rev Rheumatol 2015;11:639. [DOI] [PubMed] [Google Scholar]
- [14].Kim S, Shin JE, Kim MJ, et al. Correlation between quantitative electroencephalogram findings and neurocognitive functions in patients with obsessive-compulsive disorder and schizophrenia. Korean J Biol Psychiatry 2016;23:193–8. [Google Scholar]
- [15].Hargrove JB, Bennett RM, Simons DG, et al. Quantitative electroencephalographic abnormalities in fibromyalgia patients. Clin EEG Neurosci 2010;41:132–9. [DOI] [PubMed] [Google Scholar]
- [16].Prichep LS, John ER, Ferris SH, et al. Quantitative EEG correlates of cognitive deterioration in the elderly. Neurobiol Aging 1994;15:85–90. [DOI] [PubMed] [Google Scholar]
- [17].Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev 1999;29:169–95. [DOI] [PubMed] [Google Scholar]
- [18].Kang DH, Jung YH, Park SY, et al. The impairment of vocabulary ability in complex regional pain syndrome patients. Int J Pain 2010;1:8–14. [Google Scholar]
- [19].Jasper H, Penfield W. Electrocorticograms in man: effect of voluntary movement upon the electrical activity of the precentral gyrus. Archiv Psychiatr Nervenkr 1949;183:163–74. [Google Scholar]
- [20].Pfurtscheller G, Berghold A. Patterns of cortical activation during planning of voluntary movement. Electroencephalogr Clin Neurophysiol 1989;72:250–8. [DOI] [PubMed] [Google Scholar]
- [21].Pfurtscheller G, Da Silva FL. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 1999;110:1842–57. [DOI] [PubMed] [Google Scholar]
- [22].Lalo E, Gilbertson T, Doyle L, et al. Phasic increases in cortical beta activity are associated with alterations in sensory processing in the human. Exp Brain Res 2007;177:137–45. [DOI] [PubMed] [Google Scholar]
- [23].Androulidakis AG, Kühn AA, Chu Chen C, et al. Dopaminergic therapy promotes lateralized motor activity in the subthalamic area in Parkinson's disease. Brain 2007;130:457–68. [DOI] [PubMed] [Google Scholar]
- [24].Pogosyan A, Gaynor LD, Eusebio A, et al. Boosting cortical activity at beta-band frequencies slows movement in humans. Curr Biol 2009;19:1637–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Uhlhaas P, Pipa G, Lima B, et al. Neural synchrony in cortical networks: history, concept and current status. Front Integr Neurosci 2009;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Uhlhaas PJ, Singer W. High-frequency oscillations and the neurobiology of schizophrenia. Dialogues Clin Neurosci 2013;15:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Coutin-Churchman P, Anez Y, Uzcategui M, et al. Quantitative spectral analysis of EEG in psychiatry revisited: drawing signs out of numbers in a clinical setting. Clin Neurophysiol 2003;114:2294–306. [DOI] [PubMed] [Google Scholar]
- [28].Ribas VR, Ribas RD, Martins HA. The learning curve in neurofeedback of peter van deusen: a review article. Dement Neuropsychol 2016;10:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ribas VR, De Souza MV, Tulio VW, et al. Treatment of depression with quantitative electroencephalography (QEEG) of the TQ-7 neuro-feedback system increases the level of attention of patients. J Neurol Disord 2017;5:2. [Google Scholar]
- [30].Bruehl S. Complex regional pain syndrome. BMJ 2015;351:h2730. [DOI] [PubMed] [Google Scholar]
- [31].Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311:1547–55. [DOI] [PubMed] [Google Scholar]
- [32].Leon-Carrion J, Martin-Rodriguez JF, Damas-Lopez J, et al. Delta–alpha ratio correlates with level of recovery after neurorehabilitation in patients with acquired brain injury. Clin Neurophysiol 2009;120:1039–45. [DOI] [PubMed] [Google Scholar]
- [33].Sheikh N, Wong A, Read S, et al. QEEG may uniquely inform and expedite decisions regarding intra-arterial clot retrieval in acute stroke. Clin Neurophysiol 2013;124:1913–4. [DOI] [PubMed] [Google Scholar]
- [34].Rabiner D. Do QEEG scan results differentiate ADHD from other psychiatric disorders. Attention Res Update 2001;42. [Google Scholar]
- [35].Angelidis A, van der Does W, Schakel L, et al. Frontal EEG theta/beta ratio as an electrophysiological marker for attentional control and its test-retest reliability. Biol Psychol 2016;121:49–52. [DOI] [PubMed] [Google Scholar]


