Supplemental Digital Content is available in the text
Keywords: anticoagulation, atrial fibrillation, cognitive decline
Abstract
Background:
It is well known that atrial fibrillation (AF) carried a high risk of cognitive decline, which is independent of stroke or transient ischemic attack (TIA). Whether anticoagulation is associated with reduced risk of cognitive decline in participants with AF still remains controversial. We conducted a systematic review and meta-analysis to explore the effect of anticoagulation on the risk of cognitive decline in patients with AF.
Methods:
We systematically searched the PubMed, Embase and the Cochrane Database for eligible studies published up to January 2018. Risk ratios (RR) with 95% confidence interval (CI) for cognitive decline were extracted, and pooled estimations were calculated using the fixed effects model. Subgroup analyses were further performed.
Results:
Eight relevant articles involved 454,273 patients were ultimately included in this meta-analysis. We found that anticoagulation was associated with reduced risk of cognitive impairment as compared with nonanticoagulation (RR 0.72, 95% CI 0.69–0.75, I2 11.5%). This reduction was still significant after adjustment for stroke and TIA (RR 0.72, 95% CI 0.69–0.74, I2 0.0%). In the subgroup analyses, the incidence of cognitive decline was significantly decreased in those treated with anticoagulation compared to no treatment (RR 0.72, 95% CI 0.69–0.75, I2 0.0%), but the cognitive benefit showed no significant difference between anticoagulant and antiplatelet treatment (RR 1.01, 95% CI 0.68–1.50, I2 46.8%).
Conclusion:
Anticoagulation is associated with cognitive benefit in participants with AF independent of stroke and TIA, but it was not superior to antiplatelet drugs in reducing the risk of cognitive decline.
1. Introduction
Atrial fibrillation (AF) is the most common arrhythmia and the incidence is growing as the population ages.[1] In 2010, following the accelerated speed of population aging, it is estimated that the numbers of patients with AF around the world were 33.5 million, with a higher rate of incidence, prevalence, and AF-associated mortality in developed countries.[2] AF is an established risk factor for developing dementia.[3] Much evidence suggests that AF carries a substantial risk of stroke and transient ischemic attack (TIA), and anticoagulant therapy can significantly reduce this risk,[4] but it is unclear whether anticoagulation can prevent AF-related dementia. Normally if oral anticoagulants (OACs) can reduce the risk of emboli which leads to ischemic stroke, OACs treatment are also supposed to protect against micro emboli which cause silent cerebral infarction (SCI) that eventually lead to cognitive decline. However, effects of stroke on cognition have been shown to be variable.[5] In addition, AF may also be an independent risk factor for cognitive decline and the subsequent development of dementia, even in the absence of stroke and TIA.[6]
Anticoagulation has become the standard treatment to prevent stroke in patients with atrial fibrillation. However, it is not clear whether anticoagulation is associated with a decreased risk of cognitive decline. One study reported that long-term anticoagulant probably protective against the developing dementia in patients with AF.[7] Another study reported inverse results showed anticoagulants were responsible for higher dementia incidence yet unidentified mechanisms.[8] Then, a meta-analysis did not demonstrate a benefit of anticoagulation in decreasing cognitive decline.[9] Now, a large-scale, population-based retrospective cohort study including 444,106 individuals reports that OACs can decrease the risk of dementia in patients with AF.[10] Though much research on AF-related dementia has been conducted, it still remains controversial whether antithrombotic therapy can reduce the risk of dementia in participants with AF. Therefore, this article attempts to estimate the effect of anticoagulation on the risk of dementia in patients with AF by conducting a meta-analysis.
2. Methods
This study is a meta-analysis, all pooled analyses are based on data in the literatures, and thus no information consent and ethical approval are required.
2.1. Literature search
This study was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).[11] PubMed, Embase, and the Cochrane Library were searched for eligible studies published up to January 2018 by 2 independent reviewers. Studies were identified using several combinations of Medical Subject Headings and keywords. Only articles published in English were selected. The search terms were divided into 3 categories:
-
1.
Atrial fibrillation: “atrial fibrillation”, “fibrillation, atrial”, or “auricular fibrillation” or “fibrillation, auricular”.
-
2.
Anticoagulation: “anticoagulation”, “anticoagulant therapy”, “antithrombotic therapy”, “thromboprophylaxis”, “thrombolysis”, “warfarin”, “rivaroxaban∗”, “dabigatran∗”, “apixaban∗”, “edoxaban∗”, “factor Xa inhibi∗”, “antithrombin∗”, “antithrombin∗”, or “anticoagulation agents”.
-
3.
Cognitive function: “cognitive function”, “function, cognitive”, “cognitive impairment”, “dementia”, or “cognitive decline”.
The references of included studies were manually checked for eligible studies.
2.2. Selection criteria
Studies were eligible for inclusion if they reported sufficient data on outcomes associated with estimation of effect sizes and 95% confidence interval (CI) and fulfilled all of the following criteria:
-
1.
Patients with AF received anticoagulant therapy for at least 6 months.
-
2.
The length of follow-up above 6 months (including regular patient visits, telephone follow-up, and medical/hospital records review according to local policies.
-
3.
AF prior to dementia diagnosis > 6 months.
-
4.
Outcome of interest was clearly reported or calculated from the published results.
Studies were excluded if they were:
-
1.
Patients with other complications which confound the results.
-
2.
Did not reported data about cognitive decline or only have brain imaging results.
-
3.
Patients with cognitive impairment before anticoagulation.
-
4.
Concomitant use of antiplatelet and anticoagulation.
-
5.
Less than 100 patients.
-
6.
Did not present original data, or were editorials, case reports, or systematic reviews.
Where there were any divergences or uncertainties, decision was made after discussion.
2.3. Data extraction
Two reviewers independently screened articles according to inclusive criteria. Baseline characteristics (country, time period, sex, age, number of participants), inclusion/exclusion criteria, intervention, the length of follow-up, cognitive measures, RR or hazard ratio, and 95% CI were extracted from studies.[12]
2.4. Study quality assessment
The Newcastle–Ottawa Scale (NOS) was used to assess the risk of bias of observational studies.[13] Evaluation scales are categorized into 3 dimensions including selection, comparability, and outcome (for cohort studies). NOS is highly recommended by the Cochrane collaboration and widely used in the quality assessment of nonrandomized studies. A star rating system is adopted to determine the quality of included studies based on 8 items. Star ratings range between 0 up to 9 stars. The quality of each study included in the present meta-analysis can be classified into low (0–3 stars), medium (4–6 stars), or high (7–9 stars). The Cochrane Collaboration's tool was used to assess quality of randomized control trials. Discrepancies were settled by consensus.
2.5. Statistical analysis
We used Stata 12.0 software (Stata Corp, College Station, TX) to perform fixed effects meta-analyses using the Der-Simonian and Laird model for summary relative risks (RRs). When the incidence of dementia is low (<10%), we assumed the odds ratio was very similar to the RR; otherwise we preferred converting the odds ratio to a RR.[14] We referred to hazard ratio from Cox proportional hazards regression models as RR. We chose to use hazard ratios adjusted for multiple factors when some studies reported hazard ratios. If not, we would take full advantage of raw data to yield unadjusted RR. The process of converting raw data into risk ratio was shown in S1 Table. Heterogeneity was estimated using chi-square based on Q test and I2 statistic, where I2 values < 50% and P value > .1 suggest low heterogeneity, and I2 > 50% and P < .1 indicate high heterogeneity. RRs were estimated using a random effects model with high heterogeneity. Otherwise, a fixed effects model was adopted. Subgroup analyses was performed based on antithrombotic therapy, research design and adjusted for confounding factors. Sensitivity analyses were performed by excluding single study and recalculating the RRs for the remaining studies. Publication bias in meta-analysis is examined by visually checking for asymmetry in funnel plots or by Egger method. All P (2-tailed) values < .05 were considered statistically significant and 95% CIs were calculated for RRs.
3. Results
3.1. Study and patient characteristics
Our search initially identified 859 references, of which 226 were retrieved for more detailed evaluation, and 8 articles (6 full texts, 2 conference abstracts) with a total of 454,273 patients were ultimately included in the meta-analysis.[10,15–21] One conference abstract was excluded for duplicate report.[7] Another conference abstract was eliminated for no sufficient data to further analysis.[22] One paper with subsequent subgroup analyses was excluded for less than 100 patients.[23] One study from China was excluded for the limited data,[24] and the risk of dementia among patients with AF was significantly lower than else over a mean follow-up of 3.6 years (overall were 0.61%/year, no therapy, aspirin, and warfarin were 1.04%/y, 0.69%/y, and 0.14%/y respectively). Patients with AF treated by concomitant use of antiplatelet and anticoagulation were not included in this study. The flowchart of literature search and the process of article selection were shown in Fig. 1. The main characteristics of studies included in the meta-analysis are described in Table 1.
Figure 1.
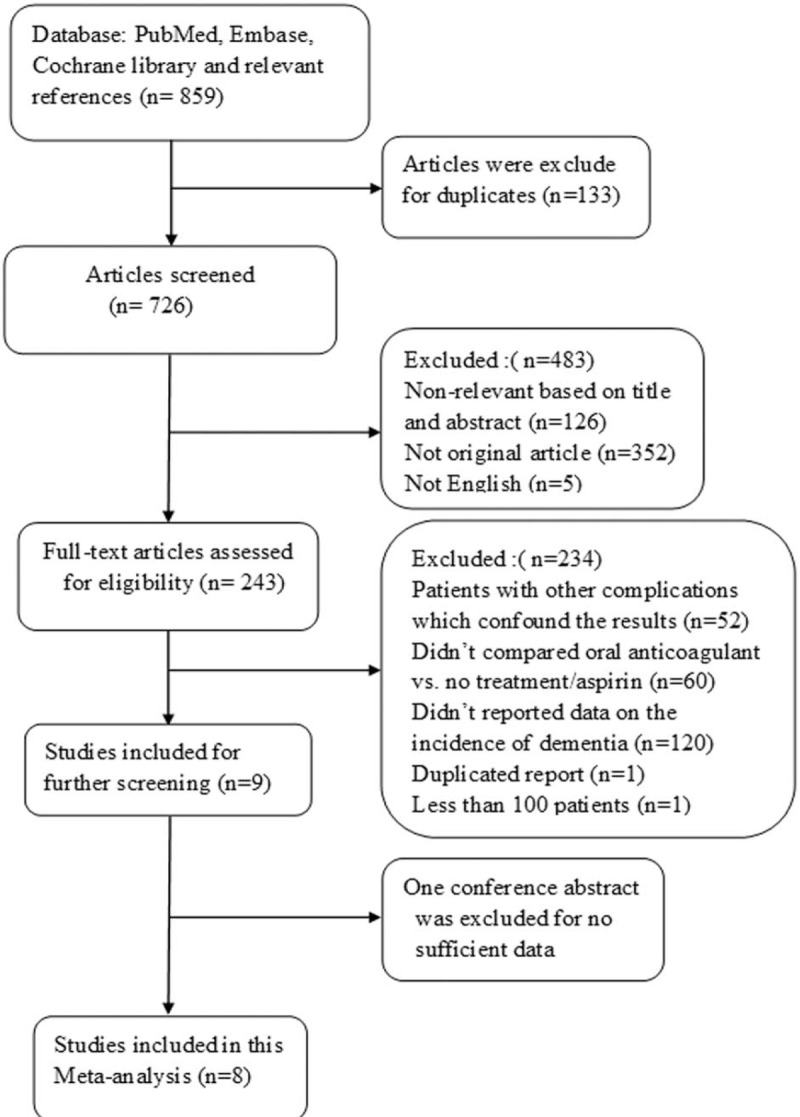
Flowchart of literature search and the process of article selection.
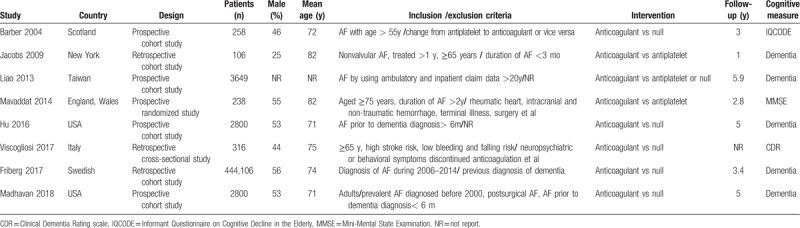
Table 1.
Characteristics of studies included in the systematic review.
3.2. Study quality
Among 7 observational studies, 6 were considered to be of good quality (NOS score 7–9), one of fair quality, and none of poor quality (Table S2). The risk of bias across the randomized control trial was considered low (Table S3). Studies included in this meta-analysis were from a series of international centers and the date of publication ranged from 2004 to 2018. The mean age of participants ranged from 71 to 82 years. The mean follow-time ranged from 1 to 5.9 years. Of 8 studies, 5 were prospective studies, and remaining 3 were retrospective studies. One study[17] reported the effect of antithrombotic therapy (including anticoagulant and antiplatelet) on the incidence of dementia in elderly patients with atrial fibrillation. Two studies[16,18] made a comparison between anticoagulant and antiplatelet on the effects of cognitive function. The remaining 5 reports[10,15,19–21] only focused on the relationship between anticoagulation and cognitive function.
3.3. Meta-analyses
Anticoagulation was associated with a significant reduction of cognitive impairment as compared with nonanticoagulation (RR 0.72, 95% CI 0.69–0.75, I2 11.5%). This reduction was still significant after adjustment for stroke and TIA (RR 0.72, 95% CI 0.69–0.74, I2 0.0%). In the subgroup analyses, the incidence of cognitive decline was significantly decreased in those treated with anticoagulation compared to no treatment (RR 0.72, 95% CI 0.69–0.75, I2 0.0%), but the cognitive benefit of anticoagulant showed no significant difference compared with antiplatelet treatment in patients with AF (RR 1.01, 95% CI 0.68–1.50, I2 46.8%). When stratified by research design, anticoagulation decreased the risk of cognitive decline in prospective studies (RR 0.81, 95% CI 0.71–0.93, I2 8.8%), and the same was true in retrospective studies. The above results were all showed in Fig. 2.
Figure 2.
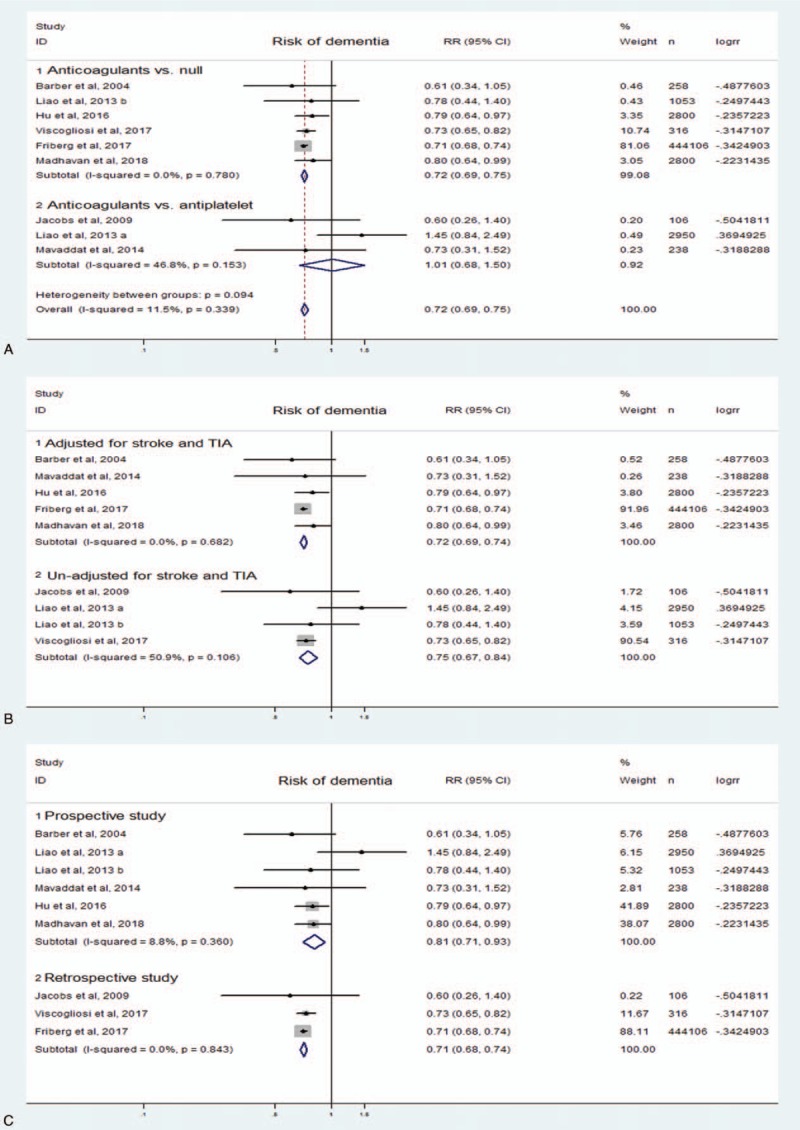
Fixed-effects meta-analysis of risk of dementia in patients with atrial fibrillation treated by anticoagulation or not. Subgroup analyses of (A) antithrombotic therapy, (B) adjusted for confounding factors, and (C) research design.
A sensitivity analysis was performed by systematically excluding studies one by one in turn to identify whether each individual contribution to a significant impact on pooled values. Estimates of 95% CI ranged from 0.68 to 0.84. The result was shown in Fig. 3. Publication bias of included studies was detected by Egger regression test and funnel plot. The shape of funnel plot showed no significant asymmetry (Fig. 4), and the result of Egger test suggested that no significant evidence of publication bias existed (t = 1.43, P = .20).
Figure 3.
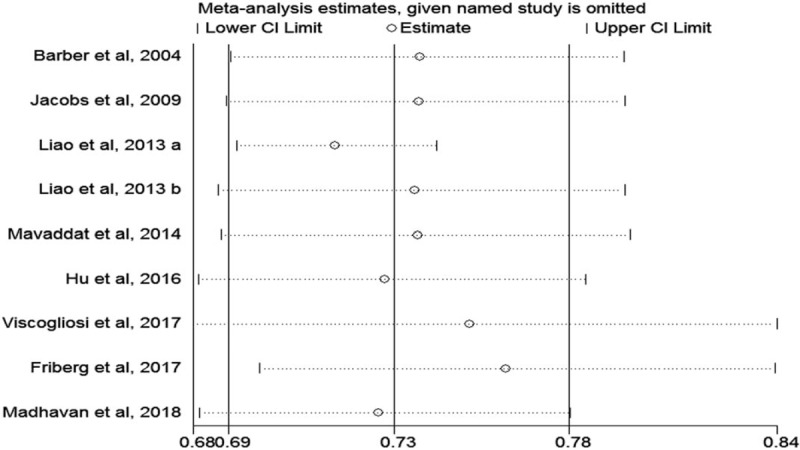
A sensitivity analyses of the included studies.
Figure 4.
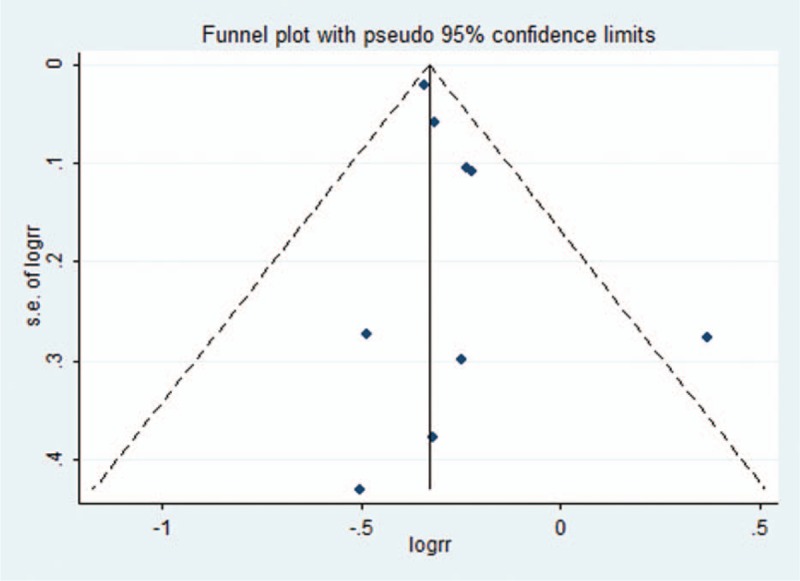
Funnel plots of publication bias.
4. Discussion
This meta-analysis based on the overall RRs suggested that anticoagulation carried a lower risk of subsequent dementia in patients with AF compared to other therapy. The association would not be weakened and anticoagulation still brings additional benefits of cognitive protection after adjustment for stroke and TIA. Nevertheless, no definite evidence has been brought forward to show whether antiplatelet treatment is noninferior to anticoagulant with regard to prevention of dementia in patients with AF. Both retrospective and prospective analysis demonstrated a protective cognitive effect from anticoagulation in AF.
AF carried a high risk of cognitive decline and dementia,[25] and increases with age.[1] A great deal of research explores the possible etiology responsible for cognitive impaired and dementia in participants with AF, but the exact mechanisms behind this association are less known. Previous research revealed that approximately a third of stroke survivors took a high risk of cognitive impairment in their first month after the event.[26,27] The descending rate of cognitive function after stroke was significantly faster in comparison with the prestroke rate. Recently, a prospective study showed that AF increases the risk of dementia and is associated with accelerated cognitive decline even though the period of low-incidence AF.[28] More and more evidence confirm that the risk of cerebrovascular embolic events is linked to an increased risk of cognitive decline and dementia in patients with AF.
What plausible therapy should be utilized to decrease the risk of cognitive decline and dementia? Anticoagulation protects against emboli and significantly reduces the incidence of stroke and silent cerebral ischemia, which is recognized as a potential approach to prevent this type of dementia.[16] However, a systematic review and meta-analysis failed to suggest a protective effect of anticoagulation on dementia among AF patients.[9] This may be explained by the complex interactions between AF and dementia. Besides, it was also subject to confounding factor, and a limited number of included articles. Previous research has shown that although anticoagulation can prevent stroke, it may also lead to intracerebral hemorrhage, thereby aggravating cognitive decline.[8] A large retrospective cohort study even more recently published demonstrated improvement in cognitive decline in those treated with atrial fibrillation.[10] Our meta-analysis includes this large study and demonstrated suggested a significant benefit of subsequent cognitive function from anticoagulation. Although antiplatelet therapy currently plays a very limited role in the prevention of stroke in AF and most of the guidelines restrict it in this setting,[29–31] but the effect on dementia is still not clearly understood. Previous research demonstrated antiplatelet drugs could reduce the incidence of dementia in patients with atrial fibrillation.[17] Our results indicated that antiplatelet therapy was noninferior to anticoagulant on prevention of dementia in individuals with AF (RR 1.01, 95% CI 0.68–1.50, I2 46.8%). One explanation could be that no detailed description of antiplatelet therapy in patients with nonanticoagulation among many studies, which may confound the results. AF has also been identified as a risk factor for dementia, which is independent of stroke or TIA. This is because decreased cardiac output may lead to chronic cerebral hypoperfusion.[25,32] But neither antiplatelet nor anticoagulated therapy contributes to the prevention of hypoperfusion. As a result, we confer that antiplatelet therapy and anticoagulation therapy may not differ in the prevention of AF-related cognitive decline in the absence of stroke.
We explore the protective effects on cognitive function from anticoagulation after adjustment for stoke and TIA, and the result showed that anticoagulated therapy significantly reduces the occurrence of cognitive impairment. The most recent review on vascular risk factors and dementia suggested multiple possible mechanisms might account for the association between AF and dementia.[33] Much research shows silent brain infarcts which contributed greatly to cognitive impairment in stroke-free patients.[6,34,35] As for symptomatic strokes, micro-embolism was expected to be related to dementia. Gaita et al[36] suggested that most microcerebral infarcts detected by magnetic resonance imaging do not manifest as clinical stroke. This may be a potential explanation for stroke-free patients with dementia. Anticoagulation therapy can prevent subclinical stroke, and thus reduce the risk of cognitive decline.
It is common to see cerebral microbleeds among patients with AF, whether they are treated with anticoagulation or not.[37] Oral anticoagulation perhaps leads to an increased prevalence of cerebral microbleeds.[38] This is partly because warfarin users carried a higher risk of associated cerebral hemorrhage in comparison with no antithrombotic treatment. One possible mechanism causing cognitive decline in AF patients is chronic cerebral injury which has been driven by long-term exposure to microbleeds or microembolism. In patients taking oral anticoagulant therapy, how can we make the balance between safety and efficacy? It is critical for the clinician to calculate the time in the therapeutic range (TTR) for warfarin use in patients with AF. TTR is closely related to improved efficacy and safety of long-term warfarin use.[39] The non-vitamin-K novel oral anticoagulants (NOACs) were recommended as effective alternatives to warfarin for stroke prevention and their efficacy and safety had been confirmed in several studies.[40,41] Unfortunately, related information among most studies included in this meta-analysis was not available. More observational prospective studies with long-term follow-up are warranted to confirm cognitive benefit of OACs in patients with AF apart from stroke prevention, and compare the risk of cognitive decline across patients with AF initiating different OACs.
There are some limitations in this meta-analysis. First, mean follow-time ranged from 1 to 5.9 years and varies greatly. Second, although large sample size was included in this meta-analysis, it mainly derived from a retrospective study. As most of subjects included in this meta-analysis come from retrospective studies, we would anticipate that dementia rates would have been overestimated. Therefore, protective effects of anticoagulation against dementia may be magnified. More observational prospective studies with long term follow-up are warranted to confirm the cognitive effect that anticoagulant therapy may help to prevent or postpone dementia in patients with AF. Third, we perform to choose RR adjusted for multiple factors including at least stroke and TIA, a residual, potential, or unmeasured confounding factor which is inherent to all observational studies, is confounding by indication. It may exist and possibly plays a main role in comparison with the no anticoagulation group. Fourth, the meta-analyses were mostly based on observational studies with only 1 randomized controlled study. The result should be interpreted cautiously. Maybe this question is perfectly answered through conducting a randomized placebo-controlled trial with this purpose, but such a study would not be approved due to ethics. At last, there is slightly high heterogeneity that is why we perform sensitivity analysis and subgroup analysis.
5. Conclusion
Anticoagulation is associated with cognitive benefit in participants with AF independent of stroke and TIA. No definite evidence at present indicated that antiplatelet therapy is inferior to anticoagulant with regard to prevention of dementia in people with AF. More observational prospective studies with long-term follow-up are warranted to confirm cognitive benefit of OACs in patients with AF apart from stroke prevention, and compare the risk of cognitive decline across patients with AF initiating different OACs.
Author contributions
Conceptualization: Decai Zeng, Chunxiao Su, Ying Tan, Ji Wu.
Formal analysis: Decai Zeng, Chunlan Jiang, Ji Wu.
Funding acquisition: Ji Wu.
Methodology: Decai Zeng, Chunlan Jiang, Chunxiao Su.
Software: Ying Tan.
Writing – original draft: Decai Zeng.
Writing – review & editing: Decai Zeng, Chunlan Jiang, Chunxiao Su, Ying Tan, Ji Wu.
Supplementary Material
Footnotes
Abbreviations: AF = atrial fibrillation, CI = confidence interval, NOACs = the non-vitamin-K novel oral anticoagulants, NOS = Newcastle–Ottawa Scale, OACs = oral anticoagulants, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RR = risk ratios, RRs = summary relative risks, SCI = silent cerebral infarction, TIA = transient ischemic attack, TTR = time in therapeutic range.
This study was supported in part by National Natural Science Foundation of China (Grant No. 81760314).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Staerk L, Sherer JA, Ko D, et al. Atrial fibrillation: epidemiology, pathophysiology, clinical outcomes. Circ Res 2017;120:1501–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kwok CS, Loke YK, Hale R, et al. Atrial fibrillation and incidence of dementia: a systematic review and meta-analysis. Neurology 2011;76:914–22. [DOI] [PubMed] [Google Scholar]
- [4].Lopez-Lopez JA, Sterne JAC, Thom HHZ, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ 2017;359:j5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tang EY, Amiesimaka O, Harrison SL, et al. Longitudinal effect of stroke on cognition: a systematic review. J Am Heart Assoc 2018;7: e006443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen LY, Lopez FL, Gottesman RF, et al. Atrial fibrillation and cognitive decline-the role of subclinical cerebral infarcts: the atherosclerosis risk in communities study. Stroke 2014;45:2568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hu T, Madhavan M, Gersh BJ, et al. Warfarin therapy is associated with lower risk of dementia in patients with incident atrial fibrillation in a community based cohort. Eur Heart J 2015;36:863.25205528 [Google Scholar]
- [8].Meranus D, Kukull W. Antithrombotic medication use and dementia incidence among people with mild cognitive impairment and atrial fibrillation. Alzheimer's Dement 2013;9:612. [Google Scholar]
- [9].Moffitt P, Lane DA, Park H, et al. Thromboprophylaxis in atrial fibrillation and association with cognitive decline: systematic review. Age Ageing 2016;45:767–75. [DOI] [PubMed] [Google Scholar]
- [10].Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2018;39:453–60. [DOI] [PubMed] [Google Scholar]
- [11].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Internal Med 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
- [12].Altman DG, Bland JM. How to obtain the confidence interval from a P value. BMJ 2011;343:d2090. [DOI] [PubMed] [Google Scholar]
- [13].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [14].Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280:1690–1. [DOI] [PubMed] [Google Scholar]
- [15].Barber M, Tait RC, Scott J, et al. Dementia in subjects with atrial fibrillation: hemostatic function and the role of anticoagulation. J Thromb Haemost 2004;2:1873–8. [DOI] [PubMed] [Google Scholar]
- [16].Jacobs LG, Billett HH, Freeman K, et al. Anticoagulation for stroke prevention in elderly patients with atrial fibrillation, including those with falls and/or early-stage dementia: a single-center, retrospective, observational study. Am J Geriatr Pharmacother 2009;7:159–66. [DOI] [PubMed] [Google Scholar]
- [17].Liao MT, Lin LY, Lin JL. Did warfarin and antiplatelet reduce the incidence of dementia in patients with atrial fibrillation: a nationwide cohort study. Europace 2013;15:ii120. [PMC free article] [PubMed] [Google Scholar]
- [18].Mavaddat N, Roalfe A, Fletcher K, et al. Warfarin versus aspirin for prevention of cognitive decline in atrial fibrillation: randomized controlled trial (Birmingham Atrial Fibrillation Treatment of the Aged Study). Stroke 2014;45:1381–6. [DOI] [PubMed] [Google Scholar]
- [19].Hu TY, Madhavan M, Gersh BJ, et al. Lower time in therapeutic range on warfarin correlates with incident dementia in patients with atrial fibrillation. Heart Rhythm 2016;13:S598–9. [Google Scholar]
- [20].Viscogliosi G, Ettorre E, Chiriac IM. Dementia correlates with anticoagulation underuse in older patients with atrial fibrillation. Arch Gerontol Geriatr 2017;72:108–12. [DOI] [PubMed] [Google Scholar]
- [21].Madhavan M, Hu TY, Gersh BJ, et al. Efficacy of warfarin anticoagulation and incident dementia in a community-based cohort of atrial fibrillation. Mayo Clin Proc 2018;93:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Petroni R, Di Mauro M, Pezzi L, et al. Inadequacy of oral anticoagulation is a crucial factor for development of dementia in patients with permanent atrial fibrillation. Eur Heart J 2015;36:1080. [Google Scholar]
- [23].Formiga F, Ferrer A, Mimbrera D, et al. High rate of anticoagulation therapy in oldest old subjects with atrial fibrillation: the octabaix study. J Am Med Directors Assoc 2012;13:8–10. [DOI] [PubMed] [Google Scholar]
- [24].Wong CK, Huang D, Li WH, et al. Antithrombotic strategies and new-onset dementia in patients with atrial fibrillation. Europace 2017;19:iv44. [Google Scholar]
- [25].Kalantarian S, Stern TA, Mansour M, et al. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med 2013;158(5 Pt 1):338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Levine DA, Galecki AT, Langa KM, et al. Trajectory of cognitive decline after incident stroke. JAMA 2015;314:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mijajlovic MD, Pavlovic A, Brainin M, et al. Post-stroke dementia: a comprehensive review. BMC Med 2017;15:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Singh-Manoux A, Fayosse A, Sabia S, et al. Atrial fibrillation as a risk factor for cognitive decline and dementia. Eur Heart J 2017;38:2612–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:2071–104. [DOI] [PubMed] [Google Scholar]
- [30].Cairns JA, Connolly S, McMurtry S, et al. Committee CCSAFG. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: prevention of stroke and systemic thromboembolism in atrial fibrillation and flutter. Canad J Cardiol 2011;27:74–90. [DOI] [PubMed] [Google Scholar]
- [31].Group JCSJW. Guidelines for pharmacotherapy of atrial fibrillation (JCS 2008): digest version. Circ J 2010;74:2479–500. [DOI] [PubMed] [Google Scholar]
- [32].Kalaria R. Similarities between Alzheimer's disease and vascular dementia. J Neurol Sci 2002;203–204:29–34. [DOI] [PubMed] [Google Scholar]
- [33].Diener HC. Does atrial fibrillation lead to cognitive decline and dementia? Eur Heart J 2017;38:2619–20. [DOI] [PubMed] [Google Scholar]
- [34].Knecht S, Oelschlager C, Duning T, et al. Atrial fibrillation in stroke-free patients is associated with memory impairment and hippocampal atrophy. Eur Heart J 2008;29:2125–32. [DOI] [PubMed] [Google Scholar]
- [35].Bellmann B, Fiebach JB, Guttmann S, et al. Incidence of MRI-detected brain lesions and neurocognitive function after electrical cardioversion in anticoagulated patients with persistent atrial fibrillation. Int J Cardiol 2017;243:239–43. [DOI] [PubMed] [Google Scholar]
- [36].Gaita F, Corsinovi L, Anselmino M, et al. Prevalence of silent cerebral ischemia in paroxysmal and persistent atrial fibrillation and correlation with cognitive function. J Am Coll Cardiol 2013;62:1990–7. [DOI] [PubMed] [Google Scholar]
- [37].Saito T, Kawamura Y, Tanabe Y, et al. Cerebral microbleeds and asymptomatic cerebral infarctions in patients with atrial fibrillation. J Stroke Cerebrovasc Dis 2014;23:1616–22. [DOI] [PubMed] [Google Scholar]
- [38].Horstmann S, Mohlenbruch M, Wegele C, et al. Prevalence of atrial fibrillation and association of previous antithrombotic treatment in patients with cerebral microbleeds. Eur J Neurol 2015;22:1355–62. [DOI] [PubMed] [Google Scholar]
- [39].Jacobs V, Woller SC, Stevens S, et al. Time outside of therapeutic range in atrial fibrillation patients is associated with long-term risk of dementia. Heart Rhythm 2014;11:2206–13. [DOI] [PubMed] [Google Scholar]
- [40].Marzec LN, Wang J, Shah ND, et al. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol 2017;69:2475–84. [DOI] [PubMed] [Google Scholar]
- [41].Gadsboll K, Staerk L, Fosbol EL, et al. Increased use of oral anticoagulants in patients with atrial fibrillation: temporal trends from 2005 to 2015 in Denmark. Eur Heart J 2017;38:899–906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


