Supplemental Digital Content is available in the text
Keywords: bismuth quadruple therapy, Helicobacter pylori, high dose dual therapy, meta-analysis
Abstract
Aim:
The aim of this study was to perform a systematic review and meta-analysis on high-dose dual therapy (HDDT) versus bismuth quadruple therapy (BQT) for Helicobacter pylori infection.
Methods:
Comparing HDDT to BQT were identified from PubMed, EMBASE, Cochrane library, CNKI, and Wanfang databases in Chinese up to March 2018. Statistical analyses were conducted using Review Manager 5.3 to compare the efficacy and side effects of these 2 therapies for H pylori infection. Dichotomous data were pooled to score the relative risk (RR) with 95% confidence intervals (CIs).
Results:
Four randomized clinical trials (RCTs) including 829 patients with a diagnosis of H pylori infection were assessed. Overall the meta-analysis showed that both HDDT and BQT achieved similar efficacy of intention-to-treat (ITT) eradication rate, 85.5% versus 87.2%, RR 1.01 (95% CI: 0.96–1.06), P = .63, and of per-protocol (PP) eradication rate, 88.4% versus 91.5%, RR 1.00 (95% CI: 0.96–1.04), P = .99, and adherence 97.8% versus 95.0%, RR 1.01 (95% CI: 0.99–1.04), P = .32, but side effects were more likely in BQT (14.4% vs 40.4%, RR 0.42 (95% CI: 0.32–0.54), P <.00001).
Conclusion:
Both HDDT and BQT can achieve similar eradication rate for H pylori infection and adherence, and generally HDDT causes fewer side effects.
1. Introduction
Helicobacter pylori treatment still remains a challenge.[1–4] Vaccination is the best option to H pylori but now we do not have it! Thus antibiotic therapy is preferable than other options.[5–10] Despite initial successes, there has been an unacceptable level in H pylori triple eradication therapies currently due to increased antibiotic resistance, especially that to clarithromycin, metronidazole, and levofloxacin.[1–10] Therefore, it is crucial to use H pylori eradication regimens with high efficacy and less adverse events.
Bismuth quadruple therapy (BQT), consisting of a proton pump inhibitor (PPI), bismuth, and 2 antibiotics (amoxicillin and clarithromycin or metronidazole et al), has been recommended in most current H pylori treatment guidelines as a first-line regimen.[2,7–10] For example, both BQT and non-bismuth quadruple therapies were recommended as first-line strategies for H pylori infection by Maastricht V/Florence Consensus Report guidelines and the Toronto Consensus for the Treatment of H pylori Infection in Adults.[2,8] BQT was recommended as 1 common solution in patients with a penicillin allergy by H pylori Management in ASEAN: the Bangkok Consensus Report.[7] The Fifth Chinese National Consensus Report on the management of H pylori infection has also recommended BQT as the main empirical therapy for H pylori eradication.[10] Furthermore, Maastricht-V Consensus Report recommended BQT with no need for drug-sensitive test.[8] However, in some regions, BQT is not available.[2] Moreover, BQT has relatively high side effects.
Up to now, the global prevalence of primary and the acquired H pylori resistance to amoxicillin are still generally rare.[11–14] The actual efficacy of amoxicillin/PPI dual therapy that has been used in several areas remains controversial partly because of differences in doses and dosing frequency.[13,15–35] Actually, high-dose dual therapy (HDDT), defined as amoxicillin ≥2.0 g/day, amoxicillin or PPI gave 3 or 4 times daily, or administration of both amoxicillin and PPI 4 times daily for 14 days, has resulted in greater efficacy (i.e., over 90%).[32,34–38] Several clinical trials have reported the H pylori eradication rates of the HDDT compared with BQT.[27,35,39–41]
In this study, we performed this meta-analysis to compare the efficacy and safety of 2 H pylori eradication regimens, HDDT and BQT.
2. Materials and methods
2.1. Eligibility criteria
Studies included in the meta-analysis met the following criteria:
-
(1)
studies designed as randomized controlled trials or controlled clinical trials;
-
(2)
studies enrolling diagnosed H pylori infection patients, regardless of gender, age, or race;
-
(3)
studies comparing HDDT and BQT, not necessarily in a blind manner; and
-
(4)
studies with similar end-points of interests, including efficacy (intention-to-treat (ITT) eradication rate, per-protocol (PP) eradication rate and adherence) and drug-related toxicity (incidence of side effects), and reported relative risk (RR) with corresponding 95% confidence intervals (CIs).
2.2. Exclusion criteria
The following exclusion criteria were set:
-
(1)
studies not comparing HDDT and BQT;
-
(2)
Randomized clinical trials (RCTs) in which patients received either HDDT or BQT in combination with other drugs;
-
(3)
studies with inappropriate statistical methods or duplicated or overlapped data in multiple reports;
-
(4)
studies from which meaningful statistical data could not be extracted; and
-
(5)
studies that were animal studies, non-clinical studies, case reports, reviews, or letters.
2.3. Search strategy
PubMed, Embase, Cochrane library, CNKI, and Wanfang databases in Chinese were searched up to March 2018 to identify studies comparing HDDT with BQT for H pylori-infected patients. The search strategy included terms: Helicobacter pylori or H pylori, amoxicillin, dual therapy, bismuth, and quadruple therapy. No limitation was used during the literature search. The references of eligible studies were reviewed for additional studies. The reporting of this study follows the PRISMA guidelines.[42]
2.4. Study selection and data extraction
Two authors independently extracted the relevant data from each included study. Disagreement was resolved by consensus, and then the accuracy was checked by the third author. We extracted the following information from included studies:
-
(1)
name of the first author, year of publication, and trials types;
-
(2)
methods used to confirm H pylori infection and eradication;
-
(3)
number of subjects, therapeutic regimens; and
-
(4)
main outcomes including ITT eradication rate, PP eradication rate, adherence, and side effects.
2.5. Risk of bias
Two investigators separately rated the quality of retrieved studies. The quality of RCTs was assessed by Jadad quality scale.[43] Funnel plots were constructed to evaluate the risk of publication bias.
2.6. Statistical analysis
The endpoints of interest in the pooled analysis were eradication rate, compliance, and side effects. A sensitivity analysis was also performed to examine the impact on the overall results, depending on the heterogeneity across the included studies. Between-study heterogeneity was evaluated using I2 statistic.[44] I2 value larger than 50% suggested high degree of heterogeneity, less than 50% means low or moderate degree of heterogeneity.[45] Both ITT and PP analyses were used for clinical outcomes. Summary RRs were calculated by using random-effect models when there was high heterogeneity among studies. Otherwise, fixed-effect models were used. P values less than .05 were considered to be statistically significant. Statistical analyses were conducted using Review Manager Version 5.3 software (Revman; The Cochrane collaboration Oxford, United Kingdom). Findings of our meta-analysis were shown in forest plots. Publication bias was assessed using funnel plot.
3. Results
3.1. Study selection
In all, 159 studies were obtained from the original search algorithm, of which 128 were excluded because they were not RCTs, duplicated, or irrelevant to the current analysis. Thirty-one studies were evaluated, of which 26 were further excluded because amoxicillin or PPI was given less than 3 times per day or the given dose of amoxicillin was <2.0 g/day in the dual therapy, and 4 studies did not use BQT as control. Furthermore, 1 study in which amoxicillin or PPI did not be given 4 times daily for 14 days in HDDT was excluded.
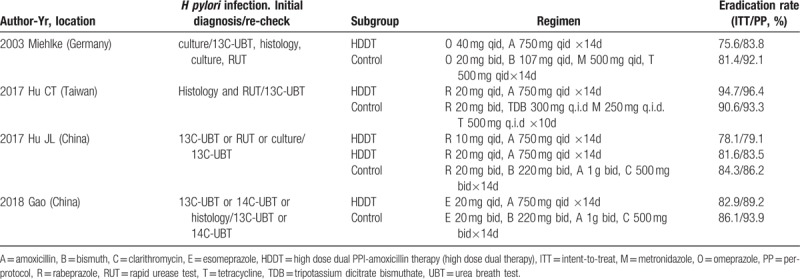
Finally, 4 prospective RCTs (without publication bias, Figure S1) including 829 participants (455 were treated with HDDT and 374 with BQT), met the inclusion criteria and were included in the meta-analysis. The flowchart of study selection is shown in Figure 1. The characteristics of the included studies are summarized in Table 1. Gao et al.'s study[39] and Hu JL et al's study[40] had a high risk for bias and the other 2 studies[26,35] had an unclear risk for bias (Figure S2, S3).
Figure 1.
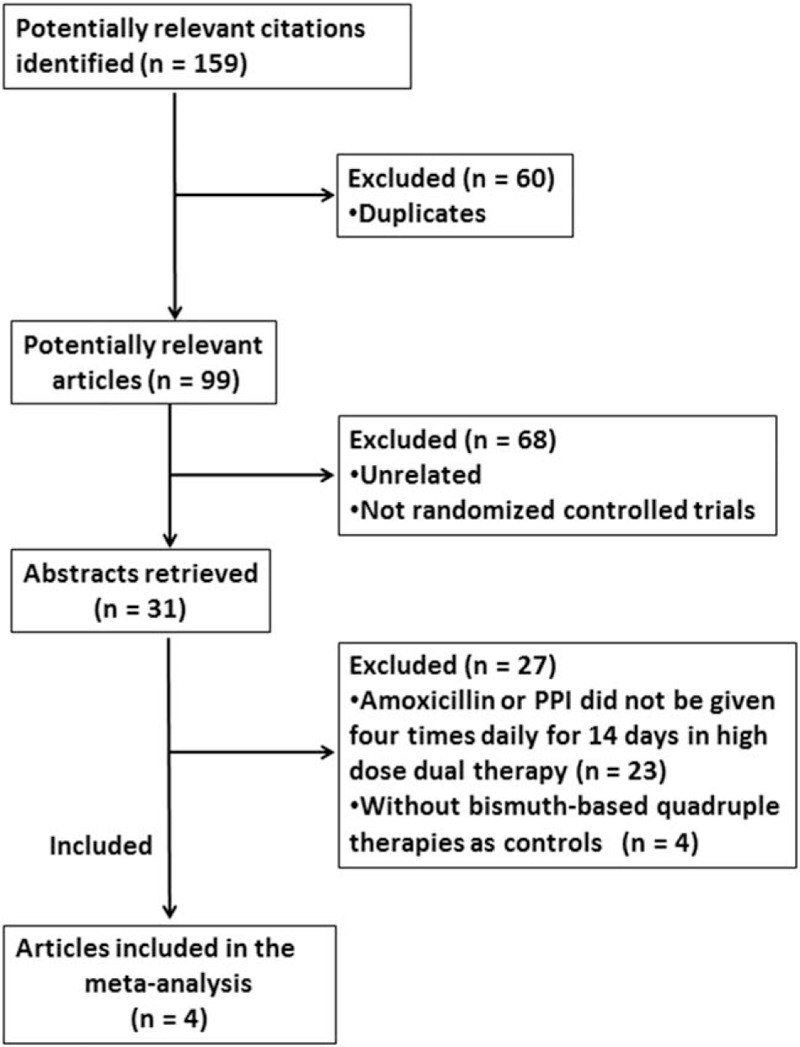
Study selection.
Table 1.
Study characteristics.
3.2. Meta-analysis results
3.2.1. Overall eradication rate
A fixed- effects model was used to pool the ITT eradication rate data since the heterogeneity across the 4 studies was low (χ2 = 3.38, P = .34, I2 = 11%). The pooled eradication rate was 85.5% (95% CI 82.3%–88.7%) in HDDT groups compared to 87.2% (95% CI 83.8%–90.6%) in BQT groups. The pooling data did not achieve advantage in the HDDT or BQT groups (RR = 1.01, 95%CI = 0.96–1.06, P = .63) (Fig. 2).
Figure 2.
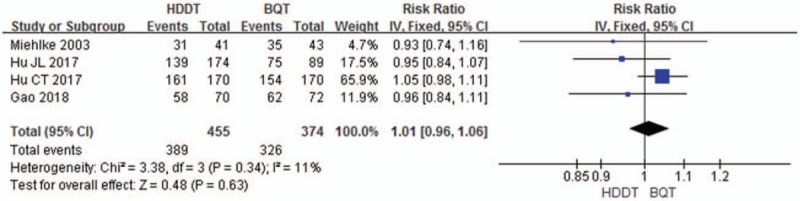
Forest plot for comparison of HDDT with BQT. Outcome: Helicobacter pylori eradication rate (intention-to-treat). BQT = bismuth quadruple therapy, HDDT = high-dose dual therapy.
A fixed- effects model was also used to pool the PP eradication rate data, since the heterogeneity across the 4 studies was low (χ2 = 4.85, P = .18, I2 = 38%). The pooled eradication rate was 88.4% (95% CI 85.4%–91.4%) in HDDT groups compared to 91.5% (95% CI 88.7%–94.4%) in BQT groups. Results showed that there were no significant differences between HDDT and BQT were observed (RR = 1.00, 95% CI: 0.96–1.04, P = .99; Fig. 3).
Figure 3.
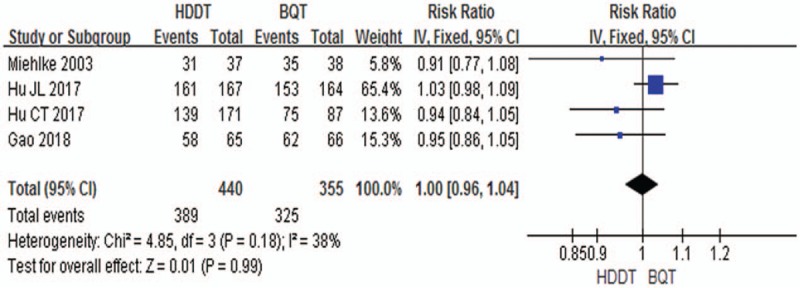
Forest plot for comparison of HDDT with BQT. Outcome: Helicobacter pylori eradication rate (per-protocol). BQT = bismuth quadruple therapy, HDDT = high-dose dual therapy.
3.2.2. Compliance
Both therapies showed a high compliance rate, with 96.7% (95% CI 95.1%–98.3%) for HDDT and 94.9% (95% CI 92.7%–97.2%) for BQT. No significant difference was observed (RR = 1.01, 95% CI 0.99–1.04, P = .32; Fig. 4).
Figure 4.
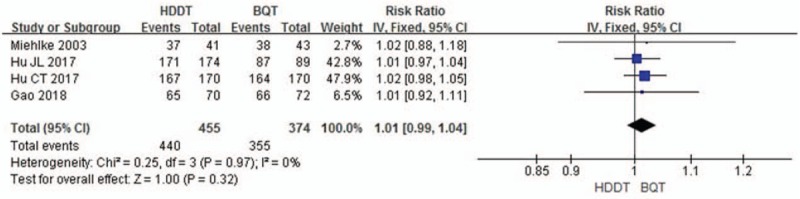
Forest plot for comparison of HDDT with BQT. Outcome: compliance. BQT = bismuth quadruple therapy, HDDT = high-dose dual therapy.
3.2.3. Side effects
The overall side effect rate was 14.4% (95% CI 11.0%–17.8%) for HDDT and 40.4% (95% CI 35.0%–45.8%) for BQT. The pooling side-effects data did achieve advantage in the HDDT therapy (RR = 0.42, 95% CI = 0.32–0.54, P <.00001) without significant statistical heterogeneity (χ2 = 1.60, P = .45, I2 = 0%). In other words, HDDT therapy compared to BQT therapy did reduce the rate of side-effects (Fig. 5).
Figure 5.
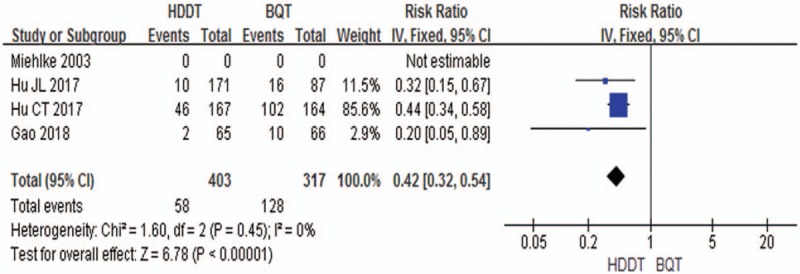
Forest plot for comparison of HDDT with BQT. Outcome: side effects. BQT = bismuth quadruple therapy, HDDT = high-dose dual therapy.
3.3. Sensitivity analysis
In the sensitivity analysis, by removing 1 study at a time, the statistical significance of the pooled RR was not changed.
4. Discussion
H pylori infection is one of the most common chronic bacterial infections in humans and causes chronic progressive gastric inflammation and a variety of diseases, including peptic ulcer disease and gastric cancer.[2,5–10] Antibiotic resistance is thought to be the key element to consider in H pylori treatment.[1–7,12,14] Resistance occurs by mutations which are errors during the replication of H pylori DNA. This can occur during the treatment of H pylori infection, but also when taking the antibiotic for another infection.[46] So far, resistance rates of H pylori to antibiotics, such as clarithromycin, metronidazole, and levofloxacin, have reached alarming levels worldwide.[1,3,4,11,12,14]
Due to the global prevalence of primary and the acquired H pylori resistance to amoxicillin are still generally rare,[11–14] a combination of PPI and amoxicillin dual therapy have been evaluated to treat H pylori infection for many areas, while the efficacy of this dual therapy is under controversial.[13,15–36] Many studies in which H pylori-infected patients were treated with standard-dose amoxicillin (2 g/d or less) and PPI once or twice daily did not achieve satisfying results of H pylori eradication rate compared with the standard triple therapy.[13,17–21,28,31,33,47–50]
There are several explanations for the decrease in the efficacy of H pylori eradication. On 1 hand, amoxicillin is time-dependent semi-synthetic penicillin and could be much better absorbed to blood after oral administration. Plasma levels in excess of the minimum inhibitory concentrations were maintained for 6 to 8 hours, so plasma concentrations of amoxicillin cannot be achieved by only a single oral dose of 1 g amoxicillin twice daily.[51] On the other hand, responding to amoxicillin is strongly affected by gastric pH value. H pylori are much more likely sensitive to amoxicillin when gastric pH value is high (pH >6). Gastric pH value is associated with the dose of PPI, dosing frequency and drug type.[38,52]
Accordingly, researches from different areas of the world have investigated the optimal designs of the dual (PPI and amoxicillin) therapy.
-
(1)
Increasing the dose and dosing frequency of amoxicillin alone. For example, Schwartz et al compared a triple therapy with PPI and amoxicillin dual therapy. They found that when lansoprazole was given 30 mg twice daily in combination with amoxicillin 1 g 3 times daily, the cure rate of H pylori was only 53% with ITT analysis.[15]
-
(2)
Modifying the dose and dosing frequency of PPI, and increasing gastric pH alone. For example, Attumi et al treated H pylori infected patients with high dose extended-release lansoprazole 120 mg twice daily in combination with amoxicillin 1 g twice daily for 14 days. They found that the success rates of both PP and ITT treatment were only 53.8%.[18]
-
(3)
Increasing PPI and amoxicillin simultaneously, and achieving satisfying effect.[32,34,35,37,39] A previous multi-center RCT in 1995 has demonstrated that the ITT eradication rate was 91% treated with 40 mg omeprazole 3 times a day and 750 mg amoxicillin 3 times a day.[37] Yang et al showed that the eradication rates of H pylori were 96.6% (PP) and 95.3% (ITT) using HDDT (rabeprazole 10 mg and amoxicillin 750 mg, 4 times/day for 14 days).[34] While, Hu et al demonstrated that the eradication rate of H pylori was 96.4% (PP) and 94.7% (ITT), respectively, treated with HDDT (rabeprazole 20 mg and amoxicillin 750 mg, 4 times/day for 14 days).[35]
HDDT means increasing PPI and amoxicillin simultaneously to achieve satisfied eradication rate of H pylori infection. Up to now, there is no standard HDDT, so different researchers have adopted different specific schemes. In Yang's study, both amoxicillin and PPI were given 4 times daily for 14 days.[34] A recent meta-analysis defined HDDT as taking amoxicillin ≥2.0 g/day, amoxicillin or PPI 3 or 4 times daily.[36]
In our meta-analysis, we treated both amoxicillin and PPI be given 4 times daily for 14 days (Yang's criteria) as the standard, running RCT comparing HDDT with BQT. We found 4 RCTs met Yang's criteria,[34] the efficacy of H pylori eradication show ITT and PP eradication rates were 75.6% to 94.7% and 81.2% to 96.4%, and the combined eradication rates are 85.5% (ITT) and 88.4% (PP) respectively, no significant differences compared HDDT with BQT were observed (Figs. 2 and 3). We found that both HDDT and BQT can achieve similar eradication rates for H pylori infection.
H pylori resistance to antibiotics plays a key role in the failure of the treatment. Bismuth can improve eradication rates without resistance, and is safe for short-term used.[53] There is synergistic effect of bismuth combined with antibiotics, so the addition of bismuth might enhance the effectiveness of triple therapies.[54] Bismuth can also partly overcome H pylori resistance to clarithromycin,[55–57] metronidazole,[58,59] and levofloxacin.[60,61] On the other hand, unlike clarithromycin-containing triple therapy or levofloxacin-containing triple therapy, the efficacy of HDDT will not gradually decrease with the use of amoxicillin in terms of that H pylori resistance to amoxicillin, both primary and acquired, is rare.[11–14] Therefore we included studies regardless of first-line or rescue therapy for H pylori infection in this meta-analysis.
BQTs are believed to perform better than other H pylori eradication therapies.[54–62] Currently, strong consensus was reached that classic BQT (PPI-bismuth-tetracycline-metronidazole) has been recommended for H pylori infection.[2,7,9,10] Despite Zhang et al[63] and Wu et al[64] have conducted meta-analysis of studies comparing BQT with quinolone-based (moxifloxacin, levofloxacin) triple therapy for H pylori eradication, and they found that quinolone-based triple regimen is more effective and well tolerated than BQT in the treatment. Recently, resistance rates of H pylori to quinolone were over 30%, and the rescue therapy with PPI-amoxicillin-levofloxacin still failed in >20% of patients.[65,66]
In addition, side effects were more likely in BQT than in HDDT (Fig. 5). A high rate of adverse events with BQT may decrease its compliance. Bismuth agents and tetracycline are also not available in some geographic areas. Moreover, H pylori showed higher risk of secondary resistance after treatment failure of the BQT than HDDT, which makes it harder with rescue treatment for H pylori eradication. Therefore, HDDT is an effective therapy in patients who are not allergic to penicillin; while for patients who are allergic to penicillin, BQT is a good treatment option for H pylori infection.
There are some limitations of our study. First, the patients were enrolled in RCTs are mainly from Asia. The response to HDDT might be affected by CYP2C19 polymorphisms, so further research needs to be taken among different people. Second, all included trials were not carried out in a blind manner, which may lead to heterogeneity among included studies. Finally, all RCTs in this meta-analysis used the different kinds of PPIs, and 1 study had 2 PPI dosage in HDDT group, and different dosage and antibiotics in BQT group, resulting in publication bias.
In conclusion, our findings showed that HDDT was comparable to BQT for H pylori infection. HDDT is effective and safe. In geographical areas with high antibiotic resistance, empirical treatment with HDDT would potentially achieve higher eradication rates (for non-penicillin-allergic patients) because the overall rate of amoxicillin resistance is low worldwide. Future research should be directed in comparing the 2 therapies, also in terms of different antibiotics composition and therapies based on antibiotic susceptibility testing.
Author contributions
Yang X et al high dose dual therapy versus bismuth quadruple therapy for H pylori: a meta-analysis;
Yang X, Wang JX, and Gao CP acquired, analyzed and interpreted the data, and drafted the article;
Gao CP contributed to conception and design of the study;
Han SX critically revised the manuscript; and all authors approved the final version.
Conceptualization: Xue Yang, Cai-Ping Gao.
Data curation: Jin-xia Wang, Sheng-Xi Han.
Formal analysis: Jin-xia Wang, Sheng-Xi Han.
Funding acquisition: Cai-Ping Gao.
Investigation: Xue Yang, Cai-Ping Gao.
Methodology: Sheng-Xi Han.
Software: Jin-xia Wang, Sheng-Xi Han.
Writing – original draft: Xue Yang.
Writing – review & editing: Xue Yang, Cai-Ping Gao.
Supplementary Material
Footnotes
Abbreviations: BQT = bismuth quadruple therapy, CIs = confidence intervals, H pylori = Helicobacter pylori, HDDT = high-dose dual therapy, ITT = intention-to-treat, PP = per-protocol, RCTs = randomized clinical trials.
National Natural Science Foundation of China (81001083);
Sichuan Academy of Science & Sichuan Provincial People's Hospital (2016LY06).
Helicobacter pylori (H pylori) treatment still remains a challenge. Currently, bismuth quadruple therapy (BQT) has been widely used to eradicate H pylori. High dose dual therapy (HDDT) is an alternative treatment with high efficacy. Our meta-analysis revealed that both BQT and HDDT can achieve similar eradication rate and adherence, and generally HDDT causes fewer side effects.
The authors deny any conflict of interest.
Supplemental Digital Content is available for this article.
References
- [1].Thung I, Aramin H, Vavinskaya V, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther 2016;43:514–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fallone CA, Chiba N, van Zanten SV, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016;151:51–69. [DOI] [PubMed] [Google Scholar]
- [3].Ang TL, Fock KM, Ang D, et al. The changing profile of Helicobacter pylori antibiotic resistance in Singapore: a 15-year study. Helicobacter 2016;21:261–5. [DOI] [PubMed] [Google Scholar]
- [4].Zhang YX, Zhou LY, Song ZQ, et al. Primary antibiotic resistance of Helicobacter pylori strains isolated from patients with dyspeptic symptoms in Beijing: a prospective serial study. World J Gastroenterol 2015;21:2786–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Smith S, Boyle B, Brennan D, et al. The Irish Helicobacter pylori working group consensus for the diagnosis and treatment of H pylori infection in adult patients in Ireland. Eur J Gastroenterol Hepatol 2017;29:552–9. [DOI] [PubMed] [Google Scholar]
- [6].Talebi Bezmin Abadi A. Helicobacter pylori treatment: new perspectives using current experience. J Glob Antimicrob Resist 2017;8:123–30. [DOI] [PubMed] [Google Scholar]
- [7].Mahachai V, Vilaichone RK, Pittayanon R, et al. Helicobacter pylori management in ASEAN: the Bangkok consensus report. J Gastroenterol Hepatol 2018;33:37–56. [DOI] [PubMed] [Google Scholar]
- [8].Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017;66:6–30. [DOI] [PubMed] [Google Scholar]
- [9].Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori Infection. Am J Gastroenterol 2017;112:212–39. [DOI] [PubMed] [Google Scholar]
- [10].Liu WZ, Xie Y, Lu H, et al. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter 2018;23:1–7. [DOI] [PubMed] [Google Scholar]
- [11].Gao W, Cheng H, Hu F, et al. The evolution of Helicobacter pylori antibiotics resistance over 10 years in Beijing, China. Helicobacter 2010;15:460–6. [DOI] [PubMed] [Google Scholar]
- [12].Savoldi A, Carrara E, Graham DY, et al. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology 2018;155:1372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schwartz H, Krause R, Sahba B, et al. Triple versus dual therapy for eradicating Helicobacter pylori and preventing ulcer recurrence: a randomized, double-blind, multicenter study of lansoprazole, clarithromycin, and/or amoxicillin in different dosing regimens. Am J Gastroenterol 1998;93:584–90. [DOI] [PubMed] [Google Scholar]
- [14].Shiota S, Reddy R, Alsarraj A, et al. Antibiotic resistance of Helicobacter pylori among male United States veterans. Clin Gastroenterol Hepatol 2015;13:1616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Attumi TA, Graham DY. Increasing the duration of dual amoxicillin plus omeprazole Helicobacter pylori eradication to 6 weeks: a pilot study. J Gastroenterol Hepatol 2012;27:59–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Attumi TA, Graham DY. High-dose extended-release lansoprazole (dexlansoprazole) and amoxicillin dual therapy for Helicobacter pylori infections. Helicobacter 2014;19:319–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bell GD, Bate CM, Axon AT, et al. Addition of metronidazole to omeprazole/amoxycillin dual therapy increases the rate of Helicobacter pylori eradication: a double-blind, randomized trial. Aliment Pharmacol Ther 1995;9:513–20. [DOI] [PubMed] [Google Scholar]
- [18].Bell GD, Bate CM, Axon AT, et al. Symptomatic and endoscopic duodenal ulcer relapse rates 12 months following Helicobacter pylori eradication treatment with omeprazole and amoxycillin with or without metronidazole. Aliment Pharmacol Ther 1996;10:637–44. [DOI] [PubMed] [Google Scholar]
- [19].Cottrill MR, McKinnon C, Mason I, et al. Two omeprazole-based Helicobacter pylori eradication regimens for the treatment of duodenal ulcer disease in general practice. Aliment Pharmacol Ther 1997;11:919–27. [DOI] [PubMed] [Google Scholar]
- [20].Delchier JC, Elamine I, Goldfain D, et al. Omeprazole-amoxycillin versus omeprazole-amoxycillin-clarithromycin in the eradication of Helicobacter pylori. Aliment Pharmacol Ther 1996;10:263–8. [DOI] [PubMed] [Google Scholar]
- [21].Goh KL, Peh SC, Parasakthi N, et al. Omeprazole 40 mg o.m. combined with amoxycillin alone or with amoxycillin and metronidazole in the eradication of Helicobacter pylori. Am J Gastroenterol 1994;89:1789–92. [PubMed] [Google Scholar]
- [22].Harford W, Lanza F, Arora A, et al. Double-blind, multicenter evaluation of lansoprazole and amoxicillin dual therapy for the cure of Helicobacter pylori infection. Helicobacter 1996;1:243–50. [DOI] [PubMed] [Google Scholar]
- [23].Kagaya H, Kato M, Komatsu Y, et al. High-dose ecabet sodium improves the eradication rate of Helicobacter pylori in dual therapy with lansoprazole and amoxicillin. Aliment Pharmacol Ther 2000;14:1523–7. [DOI] [PubMed] [Google Scholar]
- [24].Kim SY, Jung SW, Kim JH, et al. Effectiveness of three times daily lansoprazole/amoxicillin dual therapy for Helicobacter pylori infection in Korea. Br J Clin Pharmacol 2012;73:140–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Laine L, Stein C, Neil G. Limited efficacy of omeprazole-based dual and triple therapy for Helicobacter pylori: a randomized trial employing “optimal” dosing. Am J Gastroenterol 1995;90:1407–10. [PubMed] [Google Scholar]
- [26].Miehlke S, Hansky K, Schneider-Brachert W, et al. Randomized trial of rifabutin-based triple therapy and high-dose dual therapy for rescue treatment of Helicobacter pylori resistant to both metronidazole and clarithromycin. Aliment Pharmacol Ther 2006;24:395–403. [DOI] [PubMed] [Google Scholar]
- [27].Miehlke S, Kirsch C, Schneider-Brachert W, et al. A prospective, randomized study of quadruple therapy and high-dose dual therapy for treatment of Helicobacter pylori resistant to both metronidazole and clarithromycin. Helicobacter 2003;8:310–9. [DOI] [PubMed] [Google Scholar]
- [28].Pieramico O, Zanetti MV, Innerhofer M, et al. Omeprazole-based dual and triple therapy for the treatment of Helicobacter pylori infection in peptic ulcer disease: a randomized trial. Helicobacter 1997;2:92–7. [DOI] [PubMed] [Google Scholar]
- [29].Ren L, Lu H, Li HY, et al. New dual therapy for primary treatment of Helicobacter pylori infection: a prospective randomized study in Shanghai, China. J Dig Dis 2014;15:622–7. [DOI] [PubMed] [Google Scholar]
- [30].Saita H, Murakami M, Takahashi Y, et al. Factors influencing Helicobacter pylori eradication with 2 week combination therapy of lansoprazole and amoxycillin: intragastric distribution of colonization and gastric mucosal atrophy. J Gastroenterol Hepatol 1998;13:725–31. [DOI] [PubMed] [Google Scholar]
- [31].Schmid CH, Whiting G, Cory D, et al. Omeprazole plus antibiotics in the eradication of Helicobacter pylori infection: a meta-regression analysis of randomized, controlled trials. Am J Ther 1999;6:25–36. [DOI] [PubMed] [Google Scholar]
- [32].Shirai N, Sugimoto M, Kodaira C, et al. Dual therapy with high doses of rabeprazole and amoxicillin versus triple therapy with rabeprazole, amoxicillin, and metronidazole as a rescue regimen for Helicobacter pylori infection after the standard triple therapy. Eur J Clin Pharmacol 2007;63:743–9. [DOI] [PubMed] [Google Scholar]
- [33].Wong BC, Xiao SD, Hu FL, et al. Comparison of lansoprazole-based triple and dual therapy for treatment of Helicobacter pylori-related duodenal ulcer: an Asian multicentre double-blind randomized placebo controlled study. Aliment Pharmacol Ther 2000;14:217–24. [DOI] [PubMed] [Google Scholar]
- [34].Yang JC, Lin CJ, Wang HL, et al. High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin Gastroenterol Hepatol 2015;13:895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hu C-T, Tung C-C, Lin C-J, et al. Efficacy of high-dose dual therapy versus bismuthcontaining quadruple therapy for first-line treatment of Helicobacter pylori infection and an interim report of multi-center, randomized control study. Gastroenterology 2017;152:S182–3. [Google Scholar]
- [36].Gao CP, Zhou Z, Wang JZ, et al. Efficacy and safety of high-dose dual therapy for Helicobacter pylori rescue therapy: a systematic review and meta-analysis. J Dig Dis 2016;17:811–9. [DOI] [PubMed] [Google Scholar]
- [37].Bayerdorffer E, Miehlke S, Mannes GA, et al. Double-blind trial of omeprazole and amoxicillin to cure Helicobacter pylori infection in patients with duodenal ulcers. Gastroenterology 1995;108:1412–7. [DOI] [PubMed] [Google Scholar]
- [38].Furuta T, Shirai N, Kodaira M, et al. Pharmacogenomics-based tailored versus standard therapeutic regimen for eradication of H. pylori. Clin Pharmacol Ther 2007;81:521–8. [DOI] [PubMed] [Google Scholar]
- [39].Gao CP, Xiao X, Liu PX, et al. High-dose amoxicillin/esomeprazole dual therapy as a first-line therapy for Helicobacter pylori eradication. World Chin J Digestol 2018;26:353–9. [Google Scholar]
- [40].Hu JL, Yang J, Zhou YB, et al. Optimized high-dose amoxicillin-proton-pump inhibitor dual therapies fail to achieve high cure rates in China. Saudi J Gastroenterol 2017;23:275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sapmaz F, Kalkan IH, Atasoy P, et al. A non-inferiority study: modified dual therapy consisting higher doses of rabeprazole is as successful as standard quadruple therapy in eradication of Helicobacter pylori. Am J Ther 2017;24:e393–8. [DOI] [PubMed] [Google Scholar]
- [42].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Moher D, Jadad AR, Tugwell P. Assessing the quality of randomized controlled trials. Current issues and future directions. Int J Technol Assess Health Care 1996;12:195–208. [DOI] [PubMed] [Google Scholar]
- [44].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [45].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013;62:34–42. [DOI] [PubMed] [Google Scholar]
- [47].Koizumi W, Tanabe S, Hibi K, et al. A prospective randomized study of amoxycillin and omeprazole with and without metronidazole in the eradication treatment of Helicobacter pylori. J Gastroenterol Hepatol 1998;13:301–4. [DOI] [PubMed] [Google Scholar]
- [48].Kato S, Takeyama J, Ebina K, et al. Omeprazole-based dual and triple regimens for Helicobacter pylori eradication in children. Pediatrics 1997;100:1–3. [DOI] [PubMed] [Google Scholar]
- [49].Laine L, Frantz JE, Baker A, et al. A United States multicentre trial of dual and proton pump inhibitor-based triple therapies for Helicobacter pylori. Aliment Pharmacol Ther 1997;11:913–7. [DOI] [PubMed] [Google Scholar]
- [50].Tursi A, Cammarota G, Papa A, et al. One-week low-dose triple therapy vs. two-week medium-dose double therapy for H.pylori infection. Hepatogastroenterology 1996;43:859–62. [PubMed] [Google Scholar]
- [51].Barbhaiya R, Thin RN, Turner P, et al. Clinical pharmacological studies of amoxycillin: effect of probenecid. Br J Vener Dis 1979;55:211–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Scott D, Weeks D, Melchers K, et al. The life and death of Helicobacter pylori. Gut 1998;43suppl 1:S56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 2016;65:870–8. [DOI] [PubMed] [Google Scholar]
- [54].Gisbert JP, McNicholl AG. Optimization strategies aimed to increase the efficacy of H. pylori eradication therapies. Helicobacter 2017;22:1–3. [DOI] [PubMed] [Google Scholar]
- [55].Ozturk O, Doganay L, Colak Y, et al. Therapeutic success with bismuth-containing sequential and quadruple regimens in Helicobacter pylori eradication. Arab J Gastroenterol 2017;18:62–7. [DOI] [PubMed] [Google Scholar]
- [56].Kekilli M, Onal IK, Ocal S, et al. Inefficacy of triple therapy and comparison of two different bismuth-containing quadruple regimens as a firstline treatment option for Helicobacter pylori. Saudi J Gastroenterol 2016;22:366–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhang W, Chen Q, Liang X, et al. Bismuth, lansoprazole, amoxicillin and metronidazole or clarithromycin as first-line Helicobacter pylori therapy. Gut 2015;64:1715–20. [DOI] [PubMed] [Google Scholar]
- [58].Chen Q, Zhang W, Fu Q, et al. Rescue therapy for Helicobacter pylori eradication: a randomized non-inferiority trial of amoxicillin or tetracycline in bismuth quadruple therapy. Am J Gastroenterol 2016;111:1736–42. [DOI] [PubMed] [Google Scholar]
- [59].Muller N, Amiot A, Le Thuaut A, et al. Rescue therapy with bismuth-containing quadruple therapy in patients infected with metronidazole-resistant Helicobacter pylori strains. Clin Res Hepatol Gastroenterol 2016;40:517–24. [DOI] [PubMed] [Google Scholar]
- [60].Marin AC, Nyssen OP, McNicholl AG, et al. Efficacy and safety of quinolone-containing rescue therapies after the failure of non-bismuth quadruple treatments for Helicobacter pylori eradication: systematic review and meta-analysis. Drugs 2017;77:765–76. [DOI] [PubMed] [Google Scholar]
- [61].Liao J, Zheng Q, Liang X, et al. Effect of fluoroquinolone resistance on 14-day levofloxacin triple and triple plus bismuth quadruple therapy. Helicobacter 2013;18:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kadayifci A, Uygun A, Polat Z, et al. Comparison of bismuth-containing quadruple and concomitant therapies as a first-line treatment option for Helicobacter pylori. Turk J Gastroenterol 2012;23:8–13. [DOI] [PubMed] [Google Scholar]
- [63].Zhang M, Chen CY, Wang XT, et al. Levofloxacin-based triple therapy versus bismuth-based quadruple therapy in the treatment of Helicobacter pylori as the rescue therapy: a meta analysis. Zhonghua Nei Ke Za Zhi 2017;56:368–74. [DOI] [PubMed] [Google Scholar]
- [64].Wu C, Chen X, Liu J, et al. Moxifloxacin-containing triple therapy versus bismuth-containing quadruple therapy for second-line treatment of Helicobacter pylori infection: a meta-analysis. Helicobacter 2011;16:131–8. [DOI] [PubMed] [Google Scholar]
- [65].Yoon K, Kim N, Nam RH, et al. Ultimate eradication rate of Helicobacter pylori after first, second, or third-line therapy in Korea. J Gastroenterol Hepatol 2015;30:490–5. [DOI] [PubMed] [Google Scholar]
- [66].Gisbert JP, Perez-Aisa A, Rodrigo L, et al. Third-line rescue therapy with bismuth-containing quadruple regimen after failure of two treatments (with clarithromycin and levofloxacin) for H pylori infection. Dig Dis Sci 2014;59:383–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


