Abstract
Background:
A high-fidelity task simulator for cricothyroidotomy was created using data from a 3-dimensional (3D) computed tomography scan using a 3D printer. We hypothesized that this high-fidelity cricothyroidotomy simulator results in increased proficiency for needle cricothyroidotomy compared with conventional simulators.
Methods:
Cricothyroidotomy-naive residents were recruited and randomly assigned to 2 groups, including simulation training with a conventional simulator (Group C) and with a high-fidelity simulator (Group 3D). After simulation training, participants performed cricothyroidotomy using an ex vivo porcine larynx fitted with an endoscope to record the procedure. The primary outcomes were success rate and procedure time. The secondary outcome was a subjective measure of the similarity of the simulator to the porcine larynx.
Results:
Fifty-two residents participated in the study (Group C: n = 27, Group 3D: n = 25). There was no significant difference in the success rate or procedure time between the 2 groups (success rate: P = .24, procedure time: P = .34). There was no significant difference in the similarity of the simulators to the porcine larynx (P = .81).
Conclusion:
We developed a high-fidelity simulator for cricothyroidotomy from 3D computed tomography data using a 3D printer. This anatomically high-fidelity simulator did not have any advantages compared with conventional dry simulators.
Keywords: cricothyroidotomy, simulation training, three-dimensional printing technology
1. Introduction
Difficult airway management guidelines recommend cricothyroidotomy as the last resort for the “cannot intubate, cannot oxygenate” (CICO) situation.[1,2] Cricothyroidotomy is an unfamiliar technique to many anesthesiologists, because of little opportunity to perform this in the clinical setting.[3] Therefore, most anesthesiologists must practice cricothyroidotomy with simulation training.[4]
Simulators for cricothyroidotomy are classified into 2 types, wet and dry. The dry simulators (torso type) have a simple cylinder shape, and wet simulators are typically ex vivo porcine larynxes. Both dry and wet simulators are in common use, and the porcine larynx is believed to be most similar to a human larynx.[5] However, the porcine larynx has the disadvantage of short-term usability due to tissue decay. Most anesthesiologists use the torso type simulator.
Cricothyroidotomy is categorized into 2 techniques, the open cricothyroidotomy and the needle cricothyroidotomy. The open surgical cricothyroidotomy is considered a standard technique because of its reliability in securing the airway compared to the needle cricothyroidotomy, especially during the CICO situation.[6,7] Determining the location of the cricothyroid membrane during open cricothyroidotomy is more accurate than needle cricothyroidotomy.[7] Complications of needle cricothyroidotomy include securing a false airway, bleeding, and posterior tracheal wall penetration, which can lead to esophageal injury, mediastinal bleeding, and pneumothorax.[8]
The needle cricothyroidotomy is fast, convenient, and familiar to anesthesiologists.[9] The open cricothyroidotomy takes more time compared to the needle cricothyroidotomy, but is generally considered more reliable in securing the airway.[10] If the needle cricothyroidotomy can be improved to achieve high success rate and low complication rate, this technique may be more useful in clinical practice.[11]
Recent advances in 3-dimensional (3D) printing technology allow one to create a simulator which is anatomically the same as a human trachea.[12,13] In this study, we used data from a 3D computed tomography (CT) scan to make a cricothyroidotomy simulator with a 3D printer. We hypothesized that a high fidelity dry cricothyroidotomy simulator may allow trainees to achieve greater proficiency in the performance of a needle cricothyroidotomy compared with conventional simulators.
2. Methods
This study was approved by the Ethics Committee of Kyorin University (no. 9299) and registered in UMIN-CTR (R000028449). Participants were recruited as volunteers from among residents at Kyorin University Hospital. Written informed consent was obtained all participants. The high-fidelity cricothyroidotomy simulator was made by a 3D printer using CT scan data (Fig. 1). Conventional cricothyroidotomy simulators used in the study were the Cricoid Stick Trainer (Laerdal Medical Japan, Tokyo, Japan) and Smiths Medical Tracheostomy Head (Smiths Medical Japan Ltd, Tokyo, Japan) which are commonly used human larynx simulators. The needle cricothyroidotomy kit used in the study was the QuickTrach (Smiths Medical Japan Ltd, Tokyo, Japan).
Figure 1.

Simulator made with 3-dimensional (3D) printer. The frame of the simulator is based on data from 3-dimensional computed tomography scan data (local ethical committee approved). Face validity of the simulator was verified independently by 2 anesthesiologists. The simulator is equipped with an endoscope to observe inside the lumen of the simulated trachea.
Cricothyroidotomy-experienced residents were excluded from the study. Participants were randomly assigned to 2 groups, including simulation training with one of the 2 conventional simulators (Group C) and training with the high-fidelity simulator made with a 3D printer (Group 3D). Participants were randomly assigned to the 2 groups using a computer-generated random number.
Participants were given a lecture to review the relevant anatomy and details of the procedure for cricothyroidotomy. Simulation training was performed in separate areas to blind what type of simulator was being used compared with other participants. Each group had a senior anesthesiologist as an instructor. The 2 instructors switched groups randomly during each session. The technique for cricothyroidotomy was demonstrated by the instructors using each simulator. Practice in the technique for cricothyroidotomy in each group was performed under an instructor's supervision. The participants practiced cricothyroidotomy 10 times.[14] After the practice session, participants moved to the test area. During the test, each participant was evaluated for proficiency in performing a cricothyroidotomy on an ex vivo porcine larynx. The porcine larynx was placed in a box and covered with artificial skin (BioSKIN, Regina Fashion Supply Co Ltd, Saitama, Japan). An endoscope was placed into the porcine trachea, and the intratracheal views were recorded on a computer (Fig. 2). The video was recorded with sequential numbers. Two investigators, who did not participate in the test, observed and evaluated the recorded videos to determine whether the procedure was performed successfully or failed, and looked for evidence of incorrect technique.
Figure 2.
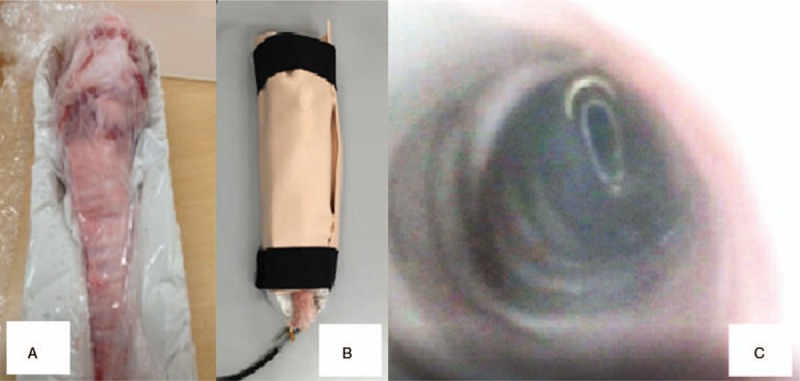
Porcine larynx and endoscopic observation. A, The porcine larynx fixed in a box. B, The larynx and box are covered with artificial skin. C, Endoscopic observation of the porcine larynx. The internal view is recorded by a computer.
Successful cricothyroidotomy was defined as a needle insertion that penetrates the cricothyroid membrane without touching or penetrating the posterior tracheal wall. Once the needle tip is located inside the trachea, the cannula remains inside the trachea. Failed cricothyroidotomy was defined as the needle penetrating at the wrong site (not the cricothyroid membrane), incomplete penetration of the cricothyroid membrane, the needle tip not inside the trachea (penetration of posterior tracheal wall) or the cannula did not remain inside the trachea. If the participant could not successfully perform cricothyroidotomy within 3 minutes, it was deemed a failed cricothyroidotomy. Procedure time was defined from the time of issuing the starting signal until connecting the catheter after placing the cannula inside the trachea.
After finishing the simulation session, participants completed a questionnaire which included questions about self-confidence in their skill for cricothyroidotomy and a subjective measure of realism comparing the dry simulators to the porcine larynx. The primary outcome was success rate and procedure time. The secondary outcome was similarity of the dry simulator to the porcine larynx.
2.1. Statistical analysis
There have been no studies comparing proficiency differences between simulators for cricothyroidotomy training, so objective data for a power calculation are difficult to obtain. The fourth National Audit Project on major airway complications reported about half of needle cricothyroidotomy attempts resulted in failure.[8] We set the expected success rate at 90%. The sample size required for 80% power at α = 0.05 was estimated to be 38 participants. In this study, we recruited 50 participants to account for drop-outs and exclusions.
Success rate of cricothyroidotomy was analyzed using Fisher exact test calculated with R software (version 3.4.4., open-source software for statistics).[15] Procedure time was expressed as the median [first quartile–third quartile], evaluated with the Mann–Whitney U test using GraphPad Prism 7 (MDF Co., Tokyo, Japan). Similarity of the simulators to the porcine larynx was evaluated in the postseminar questionnaire using a 5-point Likert scale (1: exactly same, 2: almost the same, 3: cannot say similar or not, 4: slightly different, 5: completely different). The similarity was expressed as the median [first quartile–third quartile], and evaluated with Student t test using R software (version 3.4.4., open-source software for statistics). A P value < .05 was considered statistically significant.
3. Results
Fifty-two residents participated in the study (Fig. 3). All participants met all inclusion criteria. In group C, 13 participants used the Cricoid Stick Trainer (Laerdal Medical Japan, Tokyo, Japan) and 14 used the Smiths Medical Tracheostomy Head (Smiths Medical Japan Ltd, Tokyo, Japan). There was no significant difference in the success rate for cricothyroidotomy comparing Group C and Group 3D (Table 1, P = .24). There was no statistically significant difference in success rate with subgroup analysis comparing the 2 conventional simulators used (P = .45).
Figure 3.
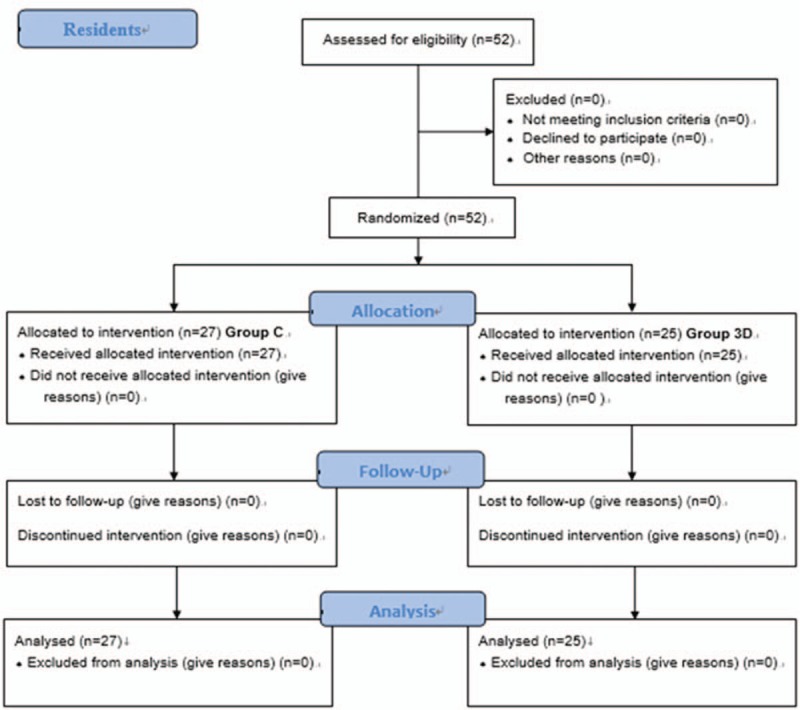
CONSORT flow diagram.
Table 1.
Success rate and complications of cricothyroidotomy in a porcine larynx.
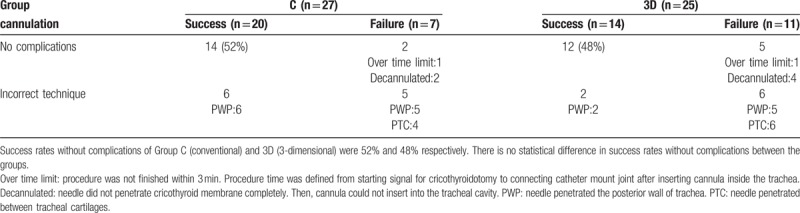
One participant in each group failed cannulation because of exceeding the time limit. Cannulation failure due to inadequate penetration of the cricothyroid membrane was observed in the both groups. Posterior wall puncture (Fig. 4) occurred in both groups, and there was no significant difference in the rate between the groups (P = .25). Penetrating the tracheal cartilage, almost the same as percutaneous needle tracheostomy, was observed in both groups.
Figure 4.
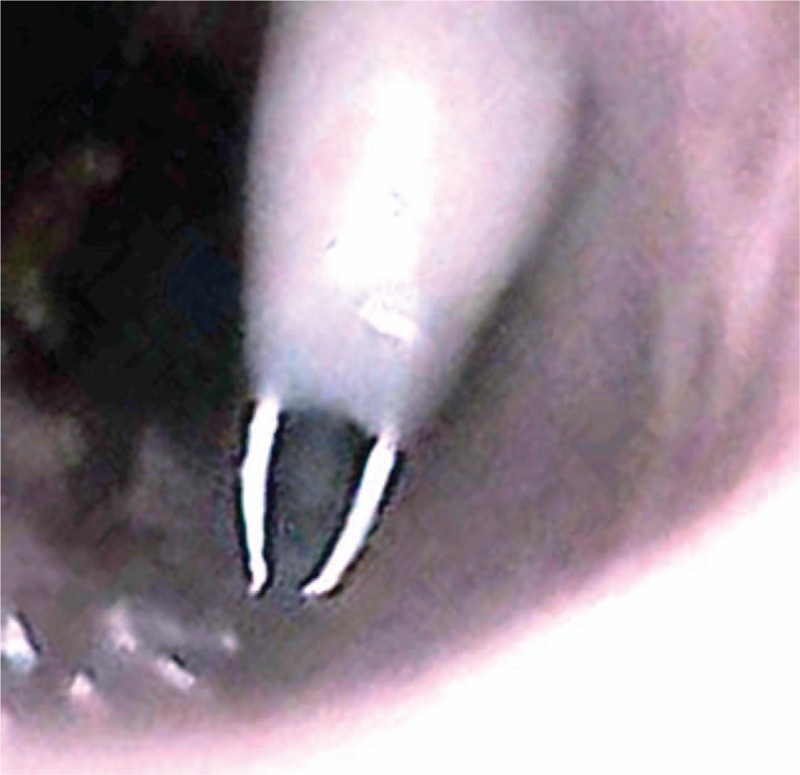
Needle in the posterior wall of the simulated trachea.
There was no significant difference in procedure time between the 2 groups (Group C; median 45 [40–51] s, Group 3D; 49 [40–62] s, P = .34). Participants reported no significant difference in the similarity of the simulators to the porcine larynx (Group C; 4 [3–4], Group 3D; 3 [3–4], P = .81).
4. Discussion
Clinical investigators conducting simulation studies have tried to improve the proficiency in performing cricothyroidotomy.[16,17] Dry simulators are considered to be low-fidelity simulators. Wet simulators, using ex vivo animal or cadaver larynxes, are generally considered to be high-fidelity simulators.[18] Some studies showed that training with a low-fidelity simulator is effective to improve skills for performing cricothyroidotomy.[19] Clinical skills were evaluated using a cadaver larynx as the most high-fidelity simulator.[19]
In the present study, we developed a new dry simulator, which closely resembles a human larynx made using data from a 3D CT scan and produced with 3D printing technology. The shape of the simulated larynx is almost the same as a human larynx. The results of this study show no significant difference in success rate for cricothyroidotomy after participants trained with either a conventional simulator or the high-fidelity simulator made with a 3D printer. The porcine larynx is anatomically similar to the human larynx, but not exactly the same. The porcine thyroid cartilage protrudes at the lower edge.[20] In contrast, the human thyroid cartilage has no protrusion at the lower edge. This anatomical difference between a human and porcine thyroid cartilage makes a difference in the cricothyroid membrane space. This may explain why the high-fidelity simulator does not have an advantage over training with conventional simulators.
In post-training questionnaires, participants did not subjectively notice any difference between the 2 conventional simulators used and the high-fidelity simulator made with 3D printing technology compared with the porcine larynx. These results suggest that a porcine larynx may not be like a human larynx. The porcine larynx may not be as high-fidelity a simulator as a human cadaver larynx.[21] A study comparing a cadaver larynx with conventional simulators showed that the cadaver larynx was significantly higher fidelity than other simulators.[22] In Japan, cadaveric training is restricted by law, making cadaver training essentially impossible.
Inexpensive cricothyroidotomy simulators were developed to reduce the direct costs of simulation training. These low-cost simulators have been made using a cardboard toilet paper roll,[4] and a plastic tracheal model using 3D printing technology.[13] The latter is a simple cylinder-shaped trachea, different from the tracheal simulator we created. However, the efficacy of low-cost low-fidelity simulators for training has not been well studied. While some may intuitively believe that higher-fidelity simulators provide superior training, it is also possible that the fidelity of the simulator may not be an important factor in developing proficiency in simulation training. This requires further study comparing simulators. If this is found to be true, it would have significant implications for training programs seeking cost-effective training equipment.
We designed a simulator equipped with an endoscope to allow direct observation inside the simulated trachea, to determine the proficiency of cricothyroidotomy by evaluating the trainee's skill. However, there was no difference in success rate or the rate of inserting the needle into the posterior wall between the 2 groups. It is possible that the success rate and rate of posterior wall puncture may not be improved, even though the operator performs the appropriate procedure for needle cricothyroidotomy. These metrics may not adequately reflect proficiency in the technique.
This study has acknowledged limitations. An important limitation of the study is that by its design, the generalizability of these findings may be limited. The model used in the simulation was made from imaging data of a human larynx, while the wet lab portion of the research used an ex vivo porcine larynx. The ex vivo porcine larynx has been considered as a high-fidelity simulator of a human larynx. However, the porcine larynx may not be a high-fidelity simulator of a human larynx which would limit the applicability of this model.
We developed a new type of simulator for cricothyroidotomy based on data from 3D computed tomography scan data using a 3D printer. Simulation training with this anatomically correct simulator did not translate to improved performance of cricothyroidotomy on a porcine trachea compared with training with conventional dry simulators. Further studies are needed to determine the optimal simulator to improve clinical performance of this procedure.
Acknowledgments
The authors express their gratitude to the residents who participated in the study.
Author contributions
Conceptualization: Joho Tokumine, Alan Kawarai Lefor, Takayuki Asao.
Data curation: Kunitaro Watanabe.
Formal analysis: Kunitaro Watanabe, Tomoko Yorozu.
Investigation: Atsuko Katayama, Harumasa Nakazawa.
Methodology: Atsuko Katayama, Harumasa Nakazawa, Kunitaro Watanabe.
Project administration: Harumasa Nakazawa.
Software: Kunitaro Watanabe.
Supervision: Takayuki Asao, Tomoko Yorozu.
Validation: Kunitaro Watanabe, Takayuki Asao, Tomoko Yorozu.
Writing – original draft: Atsuko Katayama.
Writing – review & editing: Joho Tokumine, Alan Kawarai Lefor.
Joho Tokumine orcid: 0000-0003-3481-2085.
Footnotes
Abbreviations: 3D = three-dimensional, CICO = cannot intubate, cannot oxygenate, CT = computed tomography, UMIN-CTR = University hospital Medical Information Network – Clinical Trials Registry.
The authors have no conflicts of interest to disclose.
References
- [1].Apfelbaum JL, Hagberg CA, Caplan RA, et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2013;118:251–70. [DOI] [PubMed] [Google Scholar]
- [2].Frerk C, Mitchell VS, McNarry AF, et al. Difficult Airway Society intubation guidelines working g: Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth 2015;115:827–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wong DT, Lai K, Chung FF, et al. Cannot intubate-cannot ventilate and difficult intubation strategies: results of a Canadian national survey. Anesth Analg 2005;100:1439–46. [DOI] [PubMed] [Google Scholar]
- [4].Aho JM, Thiels CA, AlJamal YN, et al. Every surgical resident should know how to perform a cricothyrotomy: an inexpensive cricothyrotomy task trainer for teaching and assessing surgical trainees. J Surg Educ 2015;72:658–61. [DOI] [PubMed] [Google Scholar]
- [5].Cho J, Kang GH, Kim EC, et al. Comparison of manikin versus porcine models in cricothyrotomy procedure training. Emerg Med J 2008;25:732–4. [DOI] [PubMed] [Google Scholar]
- [6].Apfelbaum JL, Hagberg CA, Caplan RA, et al. American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2013;2:251–70. [DOI] [PubMed] [Google Scholar]
- [7].Asai T. Surgical cricothyrotomy, rather than percutaneous cricothyrotomy, in “cannot intubate, cannot oxygenate” situation. Anesthesiology 2016;2:269–71. [DOI] [PubMed] [Google Scholar]
- [8].Cook TM, Woodall N, Harper J, et al. Fourth National Audit Project. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: intensive care and emergency departments. Br J Anaesth 2011;5:632–42. [DOI] [PubMed] [Google Scholar]
- [9].Wong DT, Mehta A, Tam AD, et al. A survey of Canadian anesthesiologists’ preferences in difficult intubation and “cannot intubate, cannot ventilate” situations. Can J Anaesth 2014;61:717–26. [DOI] [PubMed] [Google Scholar]
- [10].Kanji H, Thirsk W, Dong S, et al. Emergency cricothyroidotomy: a randomized crossover trial comparing percutaneous techniques: classic needle first versus “incision first”. Acad Emerg Med 2012;19:E1061–7. [DOI] [PubMed] [Google Scholar]
- [11].Murphy C, Rooney SJ, Maharaj CH, et al. Comparison of three cuffed emergency percutaneous cricothyroidotomy devices to conventional surgical cricothyroidotomy in a porcine model. Br J Anaesth 2011;106:57–64. [DOI] [PubMed] [Google Scholar]
- [12].Marro A, Bandukwala T, Mak W. Three-dimensional printing and medical imaging: a review of the methods and applications. Curr Probl Diagn Radiol 2016;45:2–9. [DOI] [PubMed] [Google Scholar]
- [13].Doucet G, Ryan S, Bartellas M, et al. Modelling and manufacturing of a 3D printed trachea for cricothyroidotomy simulation. Cureus 2017;9:e1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wong DT, Prabhu AJ, Coloma M, et al. What is the minimum training required for successful cricothyroidotomy?: a study in mannequins. Anesthesiology 2003;2:349–53. [DOI] [PubMed] [Google Scholar]
- [15].Kanda Y. Investigation of the freely available easy-to-use software ’EZR’ for medical statistics. Bone Marrow Transplant 2013;3:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jayaraman V, Feeney JM, Brautigam RT, et al. The use of simulation procedural training to improve self-efficacy, knowledge, and skill to perform cricothyroidotomy. Am Surg 2014;80:377–81. [PubMed] [Google Scholar]
- [17].Howes TE, Lobo CA, Kelly FE, et al. Rescuing the obese or burned airway: are conventional training manikins adequate? A simulation study. Br J Anaesth 2015;114:136–42. [DOI] [PubMed] [Google Scholar]
- [18].Littlewood KE. High fidelity simulation as a research tool. Best Pract Res Clin Anaesthesiol 2011;25:473–87. [DOI] [PubMed] [Google Scholar]
- [19].Melchiors J, Todsen T, Nilsson P, et al. Preparing for emergency: a valid, reliable assessment tool for emergency cricothyroidotomy skills. Otolaryngol Head Neck Surg 2015;152:260–5. [DOI] [PubMed] [Google Scholar]
- [20].Wysocki J, Kielska E, Janiuk I, et al. Analysis of larynx measurements and proportions in young and adult domestic pigs (Sus scropha domestica). Turk J Vet Anim Sci 2010;34:339–47. [Google Scholar]
- [21].Iverson K, Riojas R, Sharon D, et al. Objective comparison of animal training versus artificial simulation for initial cricothyroidotomy training. Am Surg 2015;81:515–8. [PubMed] [Google Scholar]
- [22].Takayesu JK, Peak D, Stearns D. Cadaver-based training is superior to simulation training for cricothyrotomy and tube thoracostomy. Intern Emerg Med 2017;12:99–102. [DOI] [PubMed] [Google Scholar]


