Supplemental Digital Content is available in the text
Keywords: butyrate, irritable bowel syndrome, meta-analysis, propionate, volatile fatty acid
Abstract
Background:
Recent studies indicate that gut microbiota disorders potentially contribute to the pathogenesis of irritable bowel syndrome (IBS), which can be partly reflected by fecal short-chain fatty acids (SCFAs) generated from gut microbiota. Previous studies on SCFA alterations in patients with IBS have yielded conflicting results. No prior systematic review has been conducted on the alterations in fecal SCFAs in IBS patients.
Aims:
We performed a meta-analysis to explore and clarify alterations in fecal SCFAs in IBS patients.
Methods:
Case-control studies, randomized controlled trials (RCTs), and self-controlled studies were identified through electronic database searches. The standardized mean difference (SMD) with 95% confidence interval (CI) in fecal SCFA levels between different groups was calculated.
Results:
The proportion of fecal propionate in patients with IBS was significantly higher than in healthy controls (HCs) (SMD = 0.44, 95% CI = 0.12, 0.76). A subgroup analysis showed that the concentration of fecal propionate (SMD = −0.91, 95% CI = −1.41, −0.41) and butyrate (SMD = −0.53, 95% CI = −1.01, −0.04) in patients with constipation-predominant IBS (IBS-C) was significantly lower than that in HCs, and the concentration of fecal butyrate in patients with diarrhea-predominant IBS (IBS-D) was higher than that in HCs (SMD = 0.34, 95% CI = 0.00, 0.67). Finally, we found that restricted diets correlated with fecal butyrate reduction in IBS (SMD = −0.26, 95% CI = −0.51, −0.01).
Conclusions:
In terms of fecal SCFAs, there were differences between patients with IBS and HCs. In IBS-C patients, propionate and butyrate were reduced, whereas butyrate was increased in IBS-D patients in comparison to HCs. Propionate and butyrate could be used as biomarkers for IBS diagnosis.
1. Introduction
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder, with signs and symptoms including cramping, abdominal pain, bloating, gas, and diarrhea or constipation, or both.[1,2] IBS is a chronic condition that requires long-term management.[3–5] Researchers also discovered that disorders of gut microbiota potentially contribute to the pathogenesis of IBS.[6–8] Short-chain fatty acids (SCFAs), the primary metabolite for colonocytes, are produced in the intestinal lumen by commensal anaerobic bacteria via carbohydrate fermentation, which are principle nutrient substrates of the colonic epithelium.[9–11] The fact that SCFAs are the main end-products of colonic bacterial fermentation, they play a role in preserving gut barrier functions, and have immunomodulatory and anti-inflammatory properties,[12] provides a rationale and representative target to measure intestinal health.[9,13]
The cause of IBS remains unknown, and no single treatment is found to be universally applicable to all patients with IBS.[14–16] Fecal SCFAs abnormalities have been reported in patients with IBS, implying that alterations in SCFAs might be related to IBS.[17–19] Hence, many studies have examined the association between IBS and SCFAs.[17,18,20] However, these studies reported varying or even contradictory results; neither the concentrations of SCFAs nor the relative proportions of different SCFAs have shown consistent associations with IBS.[17–22] Therefore, a systematic review and meta-analysis on the alteration in fecal SCFAs in patients with IBS is desirable for clarifying this issue. We aimed to identify the characteristics of fecal SCFAs in patients with IBS and compare them to those in healthy controls (HCs), to determine whether SCFAs can be utilized as biomarkers of disordered gut microbiota in patients with IBS.
2. Materials and methods
Since this study is a meta-analysis of previously published studies, the ethical approval and patient consent are not required.
2.1. Database search and study selection
Studies to be included in the meta-analysis were searched for using PubMed (January 1946 to May 2018), EMBASE, Web of Science, China National Knowledge Infrastructure, and Wanfang database in May 2018. Controlled vocabulary and keyword searching were both used in each database to identify relevant studies. The search terms used to identify potentially related publications included “irritable bowel syndrome,” “IBS,” “short chain fatty acid,” “SCFA,” “volatile fatty acid,” etc., and these were applied to all fields to identify the maximum number of studies and increase the hit rate. Boolean operators (AND, OR, NOT) were used to widen and narrow the search results (see Supplemental Content, which illustrates the full electronic search strategy including all limits used). The titles and abstracts of the retrieved articles were screened for duplicates and for relevance to our topic, and the reference lists of the selected articles were further searched and examined as a source. Abstracts of conference proceedings between 2008 and 2018 were hand-searched to identify potentially eligible studies.
After titles and abstracts screening was completed, full texts of the articles were accessed to further evaluate appropriateness concerning the study question. The articles were assessed independently by two reviewers according to the prospectively defined eligibility criteria. The overall procedure of study selection is depicted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart in Figure 1.
Figure 1.
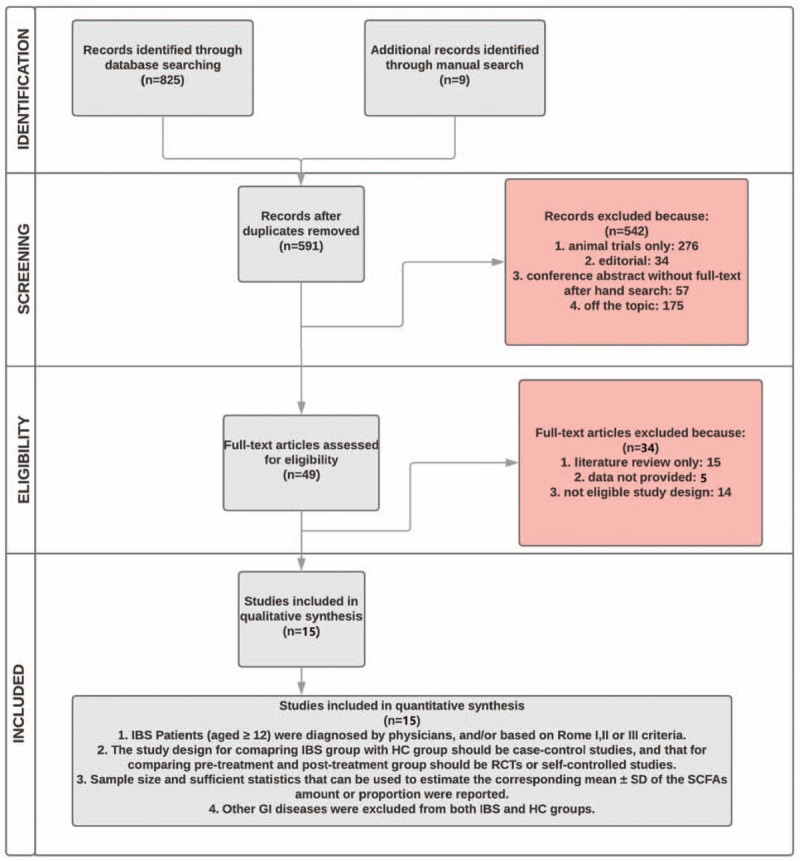
Flowchart of study selection. HC = healthy control, IBS = irritable bowel syndrome, RCT = randomized controlled trial, SCFA = short-chain fatty acids, SD = standard deviation.
Final inclusion criteria for all studies were as follows:
-
(1)
the diagnosis of IBS was established by physicians using an accepted reference standard, and/or was based on the Rome I, II, or III criteria;
-
(2)
the study design for comparing patients with IBS versus HCs was that of case-control, and that for comparing pretreatment with posttreatment was randomized controlled trial (RCT) or self-controlled study;
-
(3)
data were sufficient to calculate the standardized mean difference (SMD) with 95% confidence interval (CI);
-
(4)
other gastrointestinal diseases were excluded from both the IBS and HCs groups. Studies conducted on pediatric patients (age < 12 years) and animals were not included in our meta-analysis.
2.2. Data extraction
To reduce the reporting error and bias in data manipulation, relevant data were extracted by two reviewers independently; these data included publication year, country in which the research was conducted, study design (e.g., case-control studies, RCTs, and self-controlled studies), the size of the IBS and HC groups, basic characteristics of the study population (age, male/female percentage, and IBS subtypes), length of treatment, diagnostic criteria for IBS, major technique used for fecal SCFAs detection, and the measurement of fecal SCFAs as the main outcome parameters. Extracted data were then crossed over and validated separately, and disagreements between reviewers were resolved through discussion.
2.3. Quality assessment
Quality assessment of the studies was performed based on the Newcastle-Ottawa Scale (NOS) for case control studies and Cochrane Collaboration tool for RCTs to accommodate the methodology of fecal SCFAs concentration or proportion as a criterion. As of the NOS, three separate scores were assigned for the selection, comparability, and exposure (i.e., laboratory methods) domains, where the sum of these three scores was equal to the total NOS. The lowest and highest scores based on the NOS were 0 and 9, respectively. A total NOS score ≥6 indicated good research quality.
2.4. Statistical analysis
All data analyses were conducted with the R Studio (version 3.4.3, R Studio Inc., Boston, USA), and the “meta” package (version 4.9-1) was used to combine the studies. A P-value of 0.05 was used as the cutoff for statistical significance in all analyses. In addition, SMD with 95% CIs were used as a measure of effect size and calculated from studies that contained mean, median, maximum, minimum, range, standard deviation (SD), and interquartile range. As for studies where the results were expressed as mean and 95% CI,[21,23] we performed data conversion using the method described in Chapter 7 of the Cochrane Handbook[24]; as for those studies where median, upper quartile, and lower quartile or range were given as the outcome measures,[25–27] Wan's method[28] was applied to obtain the estimation of mean and SD. The final meta-analytical results are displayed graphically using forest plots.
Statistically, heterogeneity was tested by the Q test, Tau2, and I2 indicator, which can be used to assess whether the heterogeneity observed among effect sizes could be attributed to random chance or if other non-investigated variables, such as discrepancy of test equipment and changes in eating habits, other than IBS may play a role. Among them, I2 statistics represent the percentage of effect size heterogeneity that cannot be explained by random chance, but by the other factors noted above. The random effects model was used according to the DerSimonian and Laird (Random-effects) method to account for different sources of variation among studies if significant heterogeneity existed, otherwise the fixed effect model was used.
The potential for “small study effects,” including publication bias, was examined by visual inspection of funnel plots, in which the standard error was plotted against the net change for each study, and funnel plot symmetry was assessed with Egger's test.
3. Results
3.1. Study selection and quality assessment
As shown in the flow chart (Fig. 1), a total of 834 citations were obtained initially, and 591 of them were included in the next round for review of titles and abstracts after the removal of duplicates. When screened for on-topic articles, 276 of the studies were conducted on animals rather than humans; 91 were editorials or conference abstracts, and 175 were irrelevant to the topic. Thus, the remaining 49 studies were retrieved for full-text scrutiny. Of the 49 full-text articles, 37 were excluded for various reasons: 15 were literature reviews; eight failed to provide appropriate data; 14 did not meet our requirement for the trial design. The final candidates then went through reviewers’ scrutiny for quality assessment based on predefined methodology, and all of them showed decent qualities to conduct a meta-analysis. Finally, a total of 15 studies were included in the meta-analysis.[18–21,23,25–27,29–34] References of these 15 studies were hand searched, and no other eligible studies were identified. Two reviewers in our team had perfect agreement in selecting the 15 studies.
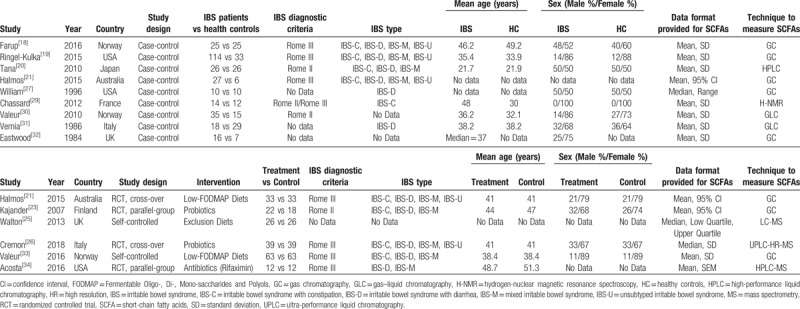
3.2. Study characteristics
Table 1 summarizes the characteristics of the included studies. The majority of the included studies involved age-matched and sex-matched analyses, and all these studies detected SCFAs in fecal samples collected from patients with IBS and HCs. Halmos et al[21] conducted both a case-control study and an RCT; thus, their data were included twice, as both a case-control study and an RCT, as shown in Table 1. Concerning the geographical distribution of the studies, three were conducted in the United States, nine in Europe, one in Australia, and one in Asia. The sex distribution was not balanced in eight studies. Concerning the techniques for detecting fecal SCFAs, eight studies used gas chromatography, four used liquid chromatography, two used gas-liquid chromatography, and one used hydrogen-nuclear magnetic resonance spectroscopy.
Table 1.
Characteristics of the included case-control studies.
3.3. Analysis of overall effect
The results of our meta-analysis (Table 2) are displayed in detailed forest plots as Figures 2–4.
Table 2.
Summary of meta-analytical results.
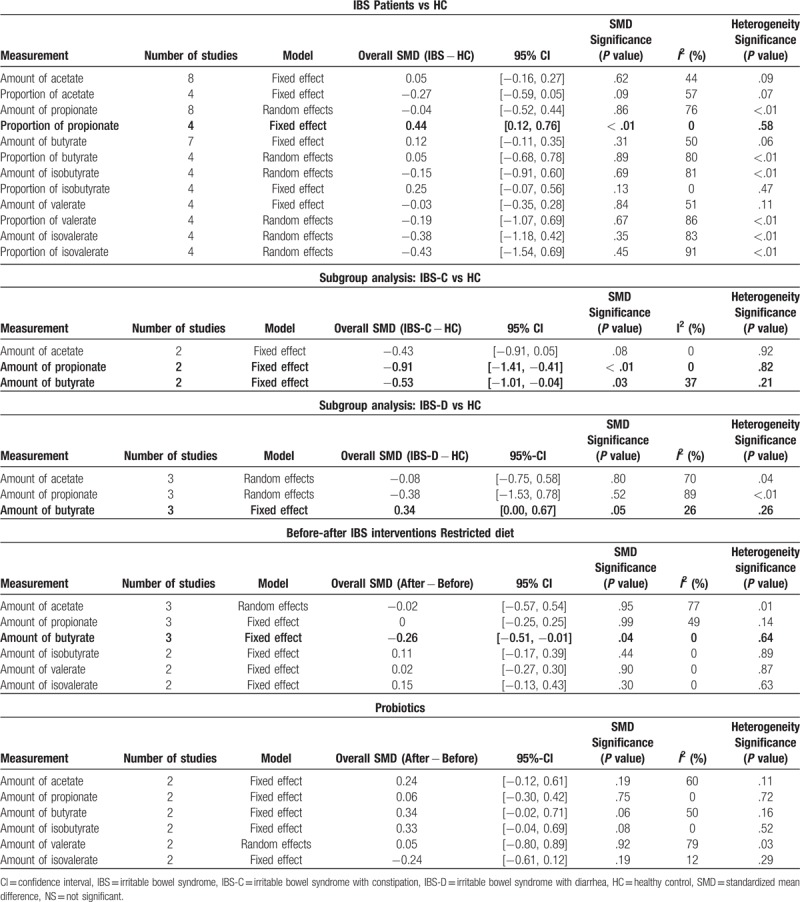
Figure 2.

Forest plots of alterations of fecal short-chain fatty acids in patients with irritable bowel syndrome versus healthy controls: (A-1) concentration of acetate, (A-2) proportion of acetate, (B-1) concentration of propionate, (B-2) proportion of propionate, (C-1) concentration of butyrate, (C-2) proportion of butyrate, (D-1) concentration of iso-butyrate, (D-2) proportion of iso-butyrate, (E-1) concentration of valerate, (E-2) proportion of valerate, (F-1) concentration of iso-valerate, and (F-2) proportion of iso-valerate. CI = confidence interval, SMD = standardized mean difference.
Figure 4.
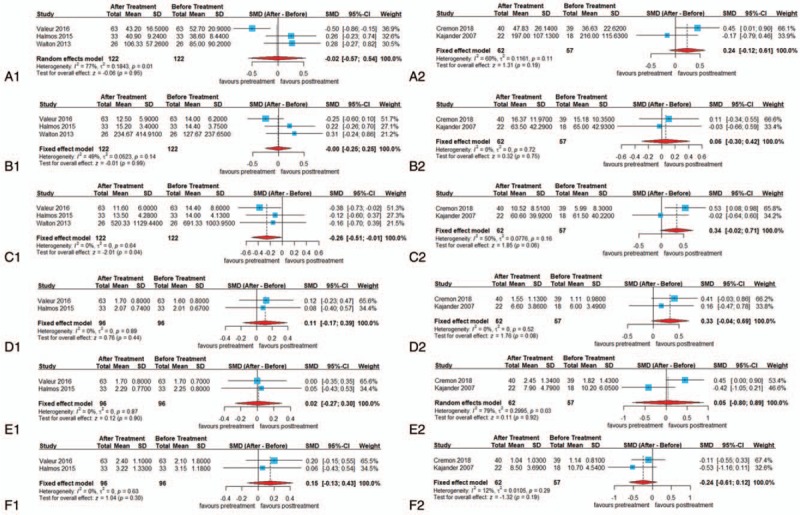
Forest plots of alterations of fecal short-chain fatty acids concentration in patients with irritable bowel syndrome after different treatments: (A-1) acetate after restricted diet, (A-2) acetate after probiotics treatment, (B-1) propionate after restricted diet, (B-2) propionate after probiotics treatment, (C-1) butyrate after restricted diet, (C-2) butyrate after probiotics treatment, (D-1) iso-butyrate after restricted diet, (D-2) iso-butyrate after probiotics treatment, (E-1) valerate after restricted diet, (E-2) valerate after probiotics treatment, (F-1) iso-valerate after restricted diet, and (F-2) iso-valerate after probiotics treatment. CI = confidence interval, SMD = standardized mean difference.
3.3.1. Primary outcomes: alteration in fecal SCFAs concentration or proportion in patients with IBS compared with HCs
The proportion of fecal propionate in patients with IBS was significantly higher compared with in HCs (SMD = 0.44, 95% CI = 0.12, 0.76, P < .05; heterogeneity: I2 = 0%, P = .58) (Fig. 2). In the subgroup analysis based on different subtypes of IBS, the forest plots showed that the concentration of fecal propionate and butyrate in patients with IBS-C was significantly lower than that in HCs (propionate: SMD = −0.91, 95% CI = −1.41, −0.41, P < .05; heterogeneity: I2 = 0%, P = .82; butyrate: SMD = −0.53, 95% CI = −1.01, −0.04, P < .05; heterogeneity: I2 = 37%, P = .21). However, it is noteworthy that in patients with IBS-D, the concentration of fecal butyrate was higher than that in HCs (SMD = 0.34, 95% CI = 0.00, 0.67, P = .0506; heterogeneity: I2 = 26%, P = .26) (Fig. 3). In this comparison, the P-value was .0506, representing borderline significance, which might be attributed to an insufficient number of studies. For butyrate, the opposite results in patients with IBS-C and IBS-D when compared with HCs could account for the insignificant SMD result and the medium heterogeneity of the fecal butyric concentration comparison between all patients with IBS and HCs, indicating that it is necessary to investigate IBS separately based on subtypes. No significant differences in other fecal SCFAs were found in the comparison between patients with IBS (or the subtypes of IBS) and HCs.
Figure 3.
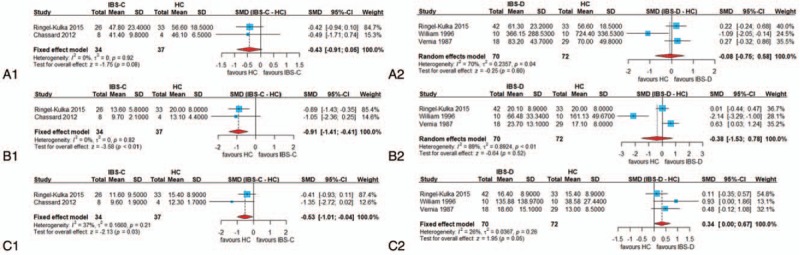
Forest plots of alterations of fecal short-chain fatty acids concentration in patients with different subtypes of irritable bowel syndrome (IBS) versus healthy controls (HC): (A-1) acetate in constipation-predominant irritable bowel syndrome (IBS-C) vs HC, (A-2) acetate in diarrhea-predominant irritable bowel syndrome (IBS-D) vs HC, (B-1) propionate in IBS-C vs HC, (B-2) propionate in IBS-D vs HC, (C-1) butyrate in IBS-C vs HC, and (C-2) butyrate in IBS-D vs HC. CI = confidence interval, SMD = standardized mean difference
3.3.2. Secondary outcomes: effect of different IBS interventions on fecal SCFAs
Currently available treatments can be categorized into three groups: (1) dietary intervention, (2) drug therapy, and (3) psychological and other treatments.[35] Among the above treatments, it is recognized that probiotics, antibiotics, and dietary intervention can affect the gut microbiota. Thus, our comprehensive literature review included these three treatments, but only a portion of the studies provided usable data for analysis. Additionally, all included studies were not conducted based on subtyped of IBS, thus subgroup analysis could not be performed.
Three RCTs and two self-controlled studies where probiotics or restricted diets including diets low in Fermentable Oligo-, Di-, Mono-saccharides And Polyols (FODMAPs) were used as major intervention methods were entered into our meta-analysis. As for the RCTs, the concentration of fecal SCFAs from the treatment group and placebo group at the end of the treatment period was chosen as the major outcome measurement; in terms of the self-controlled studies, the concentration of fecal SCFAs in patients with IBS before and after receiving the corresponding treatment was considered as the major outcome measure, since there was no placebo group.
The result showed that the restricted diets led to a significant reduction in the concentration of fecal butyrate in the IBS group compared with pre-intervention (SMD = −0.26, 95% CI = −0.51, −0.01, P < .05; heterogeneity: I2 = 0%, P = .64) (Fig. 4). Interestingly, among primary outcome measures, fecal butyrate was higher in patients with IBS-D and lower in patients with IBS-C. After treatment with restricted diets in patients with IBS (un-typed), fecal butyrate decreased. One possible explanation is that though patients with IBS were un-typed, patients with IBS-D represented nearly half of the included patients (68/139) from the extractable data. Thus, the results may be more characteristic of IBS-D.
Because there was only one study where SCFAs were measured in patients with IBS treated with antibiotics (rifaximin), a corresponding meta-analysis could not be performed. In this study, Acosta et al[34] designed an RCT comparing rifaximin with placebo in 24 non-constipated patients with IBS. Results showed no significant changes in stool content of the individual SCFAs (acetate or propionate). However, there was a marginally significant reduction (P = .061) in the fecal butyrate concentration in the rifaximin group.
3.4. Publication bias
To assess publication bias, Egger's test was conducted, and a funnel plot was drawn (Fig. 5). The funnel plot and Egger's test result included at least seven studies. Based on the plot and test result, there was no publication bias for the concentration of acetate (P = .1657), propionate (P = .2397), and butyrate (P = .6404). The assessment of the rest of the fecal SCFAs measurements might be underpowered, as there were no adequate clinical trials.
Figure 5.
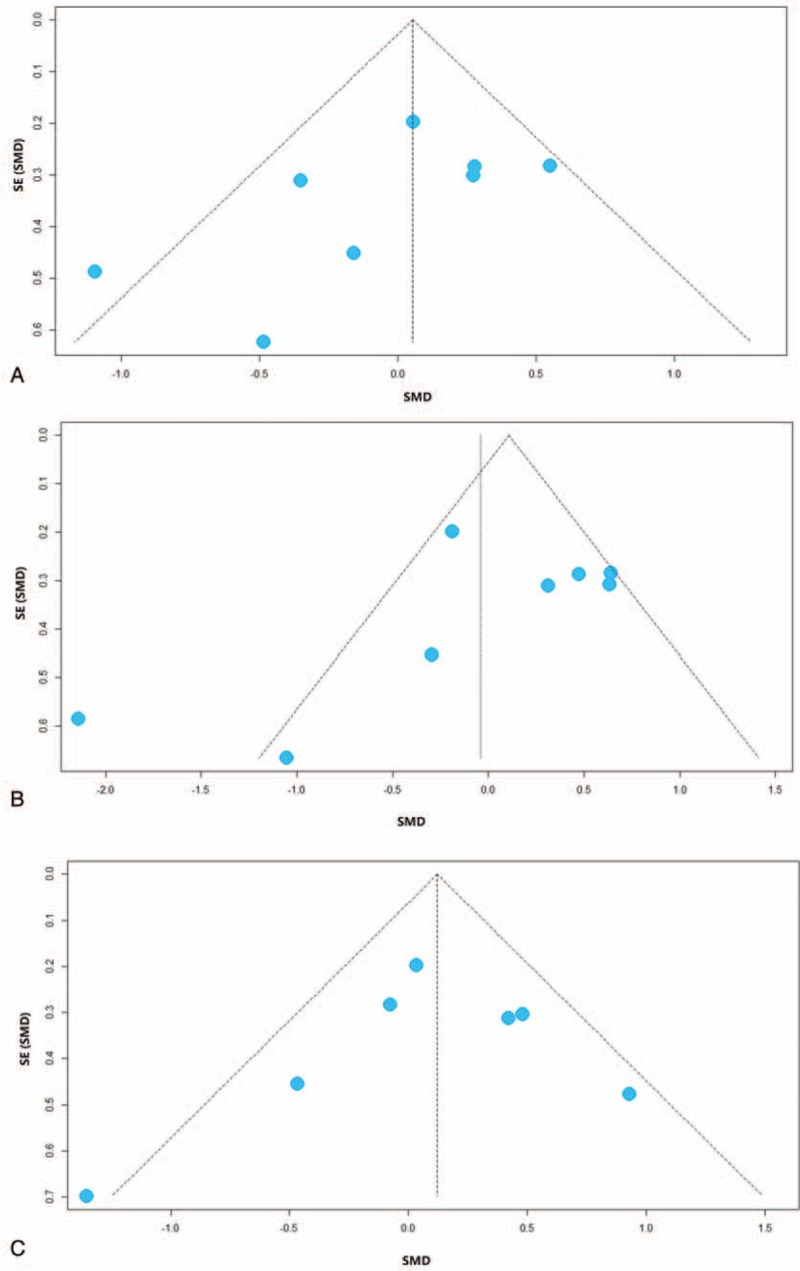
Funnel plots of included studies analyzing the concentration of fecal short-chain fatty acids from patients with irritable bowel syndrome versus healthy controls: (A) acetate, (B) propionate, (C) butyrate. SE = standard error, SMD = standardized mean difference.
3.5. Findings from studies that failed to offer data for our meta-analysis
During our comprehensive literature search, we also identified studies where the comparisons of fecal SCFAs were conducted but data could not be extracted. For such cases, we summarize the studies below to provide a full-scope view of the published literature on the topic.
In addition to the concentration and proportion of fecal SCFAs, Farup et al[18] calculated the propionate/butyrate ratio and the differences between propionic acid and butyric acid (mmol/l and molar %), aiming to identify potential biomarkers for the diagnosis of IBS. The newly-created indicators showed statistically significant differences, and “Propionic Acid Minus Butyric Acid (mmol/l)” was the best parameter to discriminate between the IBS group and the control group.
In Hustoft's study,[36] a simultaneous reduction of butyric acid and Fecalibacterium prausnitzii, a major commensal butyrate producer, was observed after 3 weeks of the low-FODMAP diet, and could be considered a biomarker of intestinal conditions in adults.
A clinical study conducted by Gargari et al characterized fecal samples collected at 4-weeks intervals from 40 IBS patients by SCFAs quantification.[17] They found that the fecal levels of SCFAs clearly distinguished the IBS-C samples from the IBS-D and IBS unsubtyped (IBS-U) samples. Acetate and propionate were significantly higher in patients with IBS-D compared to healthy controls, whereas acetate and valerate were significantly lower in patients with IBS-C than controls. In detail, the levels of acetate, butyrate, propionate, and valerate were significantly higher in patients with IBS-D than in those with IBS-C. In addition, fecal concentrations of acetate, butyrate, and propionate were higher in patients with IBS-U than in those with IBS-C. Globally, the total concentration of SCFAs was significantly higher in IBS-D and lower in IBS-C compared to healthy controls. That study was a comprehensive study covering all types of IBS. Unfortunately, the study did not provide suitable data for analysis.
Staudacher et al conducted a 4-week RCT in which patients with IBS were randomized to ingest foods high in fermentable carbohydrate or to continue their habitual diet.[37] Results suggested that there was no difference in individual fecal SCFAs between groups at baseline or after 4 weeks.
4. Discussion
The prevalence of IBS is high, and IBS seriously affects patients’ quality of life. The treatment of IBS primarily aims to improve symptoms, and there are no specific and targeted drugs available to date. The pathogenesis of IBS is very complex, including intestinal dysmotility, visceral hypersensitivity, intestinal inflammation, mental and psychological abnormalities, and other factors that are associated with intestinal dysfunction caused by intestinal flora and their metabolites. Therefore, it is important to determine the status of intestinal flora in patients with IBS. Compared with HCs, patients with IBS have significant changes in the structure of the gut microbiota, showing a decline in the diversity of the flora and abundance of certain bacteria species. Because the disorder of intestinal flora is related to the occurrence of symptoms, the detection of intestinal flora in patients with IBS may play a key role in treatment. SCFAs, the products of gut bacteria, can help us to identify patient's intestinal condition.
To our knowledge, no prior systematic review and meta-analysis has investigated the concentration or proportionate alteration in fecal SCFAs in patients with IBS to date. Our analysis limited its assessment to two measurements (concentration and proportion) of fecal SCFAs and included nine case-control studies, three RCTs, and three self-controlled studies.
4.1. Summary of evidence
In the current meta-analysis, we identified a significant increase in the proportion of fecal propionate among total fecal SCFAs of patients with IBS. In addition, the symptoms and gut motility in patients with IBS-C and IBS-D were different which also influence the SCFA concentration. Thus, a subgroup analysis was performed based on different subtypes of IBS to resolve the heterogeneous effects. Results of subgroup analysis indicate that the concentrations of propionate and butyrate are lower in the fecal samples collected from patients with IBS-C compared to HCs; the concentration of butyrate in patients with IBS-D is higher, though the p-value was of only borderline significance. More trials and studies are needed to validate these findings.
Most butyrate producers in the human colon belong to the Phylum Firmicutes, in particular of clostridial clusters IV and XIVa.[38–40] In HCs, these clusters’ change with aging.[41] One possible explanation for the different results between IBS-C and IBS-D is that they are characterized by a different distribution of intestinal microbiota. For instance, Clostridiales OTUs were enriched in IBS-C samples and negatively correlated with fecal SCFAs propionate and butyrate.[17,42,43] The Roseburia–E. rectale group, a predominant butyrate-producing bacterial group of in the human gut were detected at significantly lower levels in IBS-C patients compared with HCs, which may explain the decrease in concentration of butyrate in IBS-C patients.[29,44] In one study comparing patients with IBS-C and IBS-D,[19] patients with IBS-D were characterized by increased colonic fermentation, leading to higher fecal levels of SCFAs, thereby stimulating intestinal motility and reducing transit time, which, in turn, can lead to altered bacteria in the colon.[17,45] Analysis of SCFAs yielded significantly different results according to IBS subtypes, and SFCA levels can thus plausibly be considered valid microbial signatures.
A secondary result of our meta-analysis showed that a restricted diet (including low-FODMAP diets) leads to a significant reduction of the concentration of butyrate in the fecal samples collected from patients with IBS. These acids are mainly produced within the proximal colon as a result of carbohydrate fermentation, and reduced concentrations are probably a consequence of reduced amounts of carbohydrates. Concerning other interventions, after treatment with probiotics, the concentration of fecal butyrate was higher, but this difference was not significant. It is interesting that different interventions can cause different changes in SCFAs. Probiotics can improve the gut microbiota, favoring the co-existence of probiotic strains with other bifidobacteria and with butyrate-producing gut bacteria in the human colon. Studies showed that bifidogenic effects could cause a butyrogenic effect in the human colon, that is , an enhancement of colon butyrate production.[29,46] More research is needed to verify this conclusion.
4.2. Potential mechanism of SCFAs in IBS
An abundance of evidence has demonstrated that SCFAs play an important role in gut health. SCFAs can affect the gut directly through enterocytes or by being absorbed by the gut epithelium into the blood, playing a key role in the maintenance of gut and immune homeostasis by (1) altering chemotaxis and phagocytosis, (2) inducing reactive oxygen species, (3) changing cell proliferation and function, (4) inducing anti-inflammatory, anti-tumorigenic, and antimicrobial effects, and (5) altering gut integrity.[9,10,13,47] A layer of mucus and functional tight junction proteins, such as ZO-1 and occludin, between epithelial cells contributes to tissue integrity by limiting physical access to bacteria and gut permeability.[48,49] Impaired gut integrity plays a critical role in the mechanism of IBS. Studies have shown that supplementation with butyrate or propionate modulates gut permeability. The possible underlying mechanisms could be HDAC inhibition or the stimulation of GPR41, GPR43, or GPR109.
The highest level of SCFAs is found in the proximal colon, and thus, fecal SCFAs can reflect the colonic conditions to a certain degree. Stool specimens are easy to obtain, and the techniques for SCFAs detection are well-established and can be used to monitor the gut noninvasively and expediently.
Acetate, propionate, and butyrate are the major SCFAs released through fermentation of fiber and resistant starches; their molar ratio of production in the colon is 60:25:15.[50] Changes in colonic SCFAs are a highly complex and dynamic process. The complicated and delicate interaction between the colon and microbiota may also control the proportion and levels of SCFAs in the gut lumen. Interventions, altering the balance of colonic bacteria may accordingly modulate the production and degradation of SCFAs. It would be clinically meaningful to investigate the alteration in SCFAs as an indicator of the intestinal microbial ecosystem.
4.3. Strengths and weaknesses
The strengths of this review include the comprehensive literature search and assessment for all types of bias. In the first part of our meta-analysis investigating the concentration or proportion of alteration in fecal SCFAs in IBS, only nine studies were finally included, with four studies including less than 20 patients with IBS and two studies reporting median, range, or 95% CI rather than mean and standard deviation for SMD calculation. Moreover, there were three RCTs and three self-controlled studies in the second part of our analysis evaluating the effect of different IBS interventions on fecal SCFAs, and only one provided the exact combination of mean and standard deviation for SMD calculation. Therefore, Wan's methodology, as well as the estimation method described in Cochrane Handbook Chapter 7.7 were utilized to obtain a reasonable estimation of mean and SD, which increased the number of usable studies.
Furthermore, three of fifteen studies investigated the concentration or proportion of fecal SCFAs based on different IBS subtypes, which allowed for subgroup analysis with a thorough and deep view of IBS. As products of the intestinal flora, SCFAs reflected the status of the flora. Patients with different subtypes of IBS may have different dominant bacteria species.[51,52] Thus, propionate and butyrate decreased in IBS-C patients and butyrate increased in IBS-D patients. The current method to evaluate microbiota disorder involves 16S rRNA gene sequencing analysis of bacteria which is costly, complicated, and time-consuming. Our results suggested that the fecal SCFAs could be a new biomarker for microbiota disorder in IBS patients and facilitate diagnosis.
Concerning weaknesses of the current analysis, the measurement of fecal SCFAs was not consistent in the included studies, with only three studies measuring both the concentration and proportion of fecal SCFAs; this diluted the sample size to some extent. On the grounds that the concentration and proportional results could not be combined, they were meta-analyzed separately. Additionally, the diagnostic criteria of IBS have been modified intermittently. IBS with alternating constipation and diarrhea and IBS-U were not analyzed due to a lack of data. Most patients included in the analysis were from Europe, Australia, and North America, and only one included Asian patients with IBS. Thus, the findings might be limited to specific geographic regions and might not be fully applicable to Asian patients.
In addition, several other factors influence the outcome of the study. We used fecal SCFAs to measure the intestinal products of microbiota. The collection, storage, and treatment of stool may result in experimental loss because some SCFAs were volatile. Thus, fecal SCFAs might not accurately reflect the acids levels in the colon. However, fecal SCFAs were the most widely-reported outcome measures in studies assessing colonic SCFA levels. Moreover, the dietary habits might have an influence on the amounts and types of SCFAs in the stool.[53] The SCFAs were produced by intestinal bacterial fermentation of fiber in the colon. The quantity and species of colonic bacteria, along with the substrate (poorly absorbed carbohydrates) determine the SCFAs produced. For instance, high-fiber diets might affect the concentration of SCFAs.[54] We need more studies to explore the correlation between particular bacterial strains and relative concentrations of individual SCFAs. Also, normal values for healthy individuals are needed to be established in large-scale studies.
According to the meta-analysis results, IBS subtypes and diets greatly influence the concentration and relative proportion of fecal SCFAs in patients with IBS. Future studies investigating diet and IBS subtypes are essential in order to investigate whether a correlation exists between IBS and fecal SCFAs. More studies from Asia are required to provide a more comprehensive view on IBS.
4.4. Clinical utility
Intestinal microbiome disorder was one of the most important clinical manifestations in IBS patients.[55] Regulating intestinal flora and restoring homeostasis were critical therapeutic methods. Physicians could use the fecal SCFA analysis to determine the status of gut microbiota in patients. According to the results of fecal SCFAs, the individualized combination of prebiotics and probiotics could be administered to patients.[56]
5. Conclusions
Alterations in fecal SCFAs in patients with IBS were observed compared with HCs, and the proportion of propionate was significantly higher in patients with IBS. Further subgroup analysis found that IBS subtypes have dissimilar fecal levels of SCFAs. The concentration of propionate and butyrate were both significantly lower in IBS-C, and the concentration of butyrate was higher in IBS-D. Treatment with a restricted diet, including the low-FODMAP diet, could significantly decrease the concentration of fecal butyrate in patients with IBS.
More studies and patients are needed to provide a more comprehensive subgroup analysis to explain the heterogeneity of the included studies. Additionally, future studies and patients are warranted to explore the mechanisms behind the changes in different patient groups. Our results shed some light on the potential use of fecal SCFAs as a diagnostic biomarker for IBS and for individualized treatment.
Acknowledgments
We are indebted to the authors of the primary studies.
Author contributions
Data curation: Qinghua Sun, Qiong Jia, Lijin Song.
Formal analysis: Qinghua Sun.
Investigation: Qiong Jia, Lijin Song.
Software: Lijin Song.
Supervision: Liping Duan.
Writing – original draft: Qinghua Sun.
Writing – review & editing: Liping Duan.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, FODMAP = Fermentable Oligo-, Di-, Mono-saccharides And Polyol, HC = healthy control, IBS = irritable bowel syndrome, IBS-C = constipation-predominant irritable bowel syndrome, IBS-D = diarrhea-predominant irritable bowel syndrome, IBS-U = IBS unsubtyped., NOS = Newcastle-Ottawa Scale, RCT = randomized controlled trial, SCFA = short-chain fatty acid, SMD = standardized mean difference.
The study was supported by grant from “National Natural Science Foundation of China” (81670491) and “The Capital Health Research and Development of Special” (2016-2-4093).
No potential conflicts of interest relevant to this article were reported.
Supplemental Digital Content is available for this article.
References
- [1].Guthrie E, Creed F, Fernandes L, et al. Cluster analysis of symptoms and health seeking behaviour differentiates subgroups of patients with severe irritable bowel syndrome. Gut 2003;52:1616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 2006;130:1377–90. [DOI] [PubMed] [Google Scholar]
- [3].Evangelista S. Benefits from long-term treatment in irritable bowel syndrome. Gastroenterol Res Pract 2012;2012:936960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Müller-Lissner S, Kamm MA, Musoglu A, et al. Safety, tolerability, and efficacy of tegaserod over 13 months in patients with chronic constipation. Am J Gastroenterol 2006;101:2558–69. quiz 2671. [DOI] [PubMed] [Google Scholar]
- [5].Müller-Lissner S, Holtmann G, Rueegg P, et al. Tegaserod is effective in the initial and retreatment of irritable bowel syndrome with constipation. Aliment Pharmacol Ther 2005;21:11–20. [DOI] [PubMed] [Google Scholar]
- [6].Ghoshal UC, Srivastava D. Irritable bowel syndrome and small intestinal bacterial overgrowth: meaningful association or unnecessary hype. World J Gastroenterol 2014;20:2482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kennedy PJ, Cryan JF, Dinan TG, et al. Irritable bowel syndrome: a microbiome-gut-brain axis disorder? World J Gastroenterol 2014;20:14105–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jeffery IB, O’Toole PW, Ohman L, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2012;61:997–1006. [DOI] [PubMed] [Google Scholar]
- [9].Tan J, McKenzie C, Potamitis M, et al. The role of short-chain fatty acids in health and disease. Adv Immunol 2014;121:91–119. [DOI] [PubMed] [Google Scholar]
- [10].Natarajan N, Pluznick JL. From microbe to man: the role of microbial short chain fatty acid metabolites in host cell biology. Am J Physiol Cell Physiol 2014;307:C979–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ahmed I, Greenwood R, Costello Bde L, et al. An investigation of fecal volatile organic metabolites in irritable bowel syndrome. PLoS One 2013;8:e58204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Velasquez-Manoff M. Gut microbiome: the peacekeepers. Nature 2015;518:S3–11. [DOI] [PubMed] [Google Scholar]
- [13].Vinolo MA, Rodrigues HG, Nachbar RT, et al. Regulation of inflammation by short chain fatty acids. Nutrients 2011;3:858–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Weaver KR, Melkus GD, Henderson WA. Irritable bowel syndrome. Am J Nurs 2017;117:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA 2015;313:949–58. [DOI] [PubMed] [Google Scholar]
- [16].Anastasi JK, Capili B, Chang M. Managing irritable bowel syndrome. Am J Nurs 2013;113:42–52. quiz 54, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gargari G, Taverniti V, Gardana C, et al. Fecal Clostridiales distribution and short-chain fatty acids reflect bowel habits in irritable bowel syndrome. Environ Microbiol 2018;20:3201–13. [DOI] [PubMed] [Google Scholar]
- [18].Farup PG, Rudi K, Hestad K. Faecal short-chain fatty acids – a diagnostic biomarker for irritable bowel syndrome? BMC Gastroenterol 2016;16:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ringel-Kulka T, Choi CH, Temas D, et al. Altered colonic bacterial fermentation as a potential pathophysiological factor in irritable bowel syndrome. Am J Gastroenterol 2015;110:1339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tana C, Umesaki Y, Imaoka A, et al. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil 2010;22:512–9. e114–5. [DOI] [PubMed] [Google Scholar]
- [21].Halmos EP, Christophersen CT, Bird AR, et al. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015;64:93–100. [DOI] [PubMed] [Google Scholar]
- [22].Philpott H, Nandurkar S, Lubel J, et al. Alternative investigations for irritable bowel syndrome. J Gastroenterol Hepatol 2013;28:73–7. [DOI] [PubMed] [Google Scholar]
- [23].Kajander K, Krogius-Kurikka L, Rinttilä T, et al. Effects of multispecies probiotic supplementation on intestinal microbiota in irritable bowel syndrome. Aliment Pharmacol Ther 2007;26:463–73. [DOI] [PubMed] [Google Scholar]
- [24].Higgins J, Green S. The Cochrane C, Cochrane Reviewers H. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. 2008. [Google Scholar]
- [25].Walton C, Fowler DP, Turner C, et al. Analysis of volatile organic compounds of bacterial origin in chronic gastrointestinal diseases. Inflamm Bowel Dis 2013;19:2069–78. [DOI] [PubMed] [Google Scholar]
- [26].Cremon C, Guglielmetti S, Gargari G, et al. Effect of Lactobacillus paracasei CNCM I-1572 on symptoms, gut microbiota, short chain fatty acids, and immune activation in patients with irritable bowel syndrome: A pilot randomized clinical trial. United European Gastroenterol J 2018;6:604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Treem WR, Ahsan N, Kastoff G, et al. Fecal short-chain fatty acids in patients with diarrhea-predominant irritable bowel syndrome: in vitro studies of carbohydrate fermentation. J Pediatr Gastroenterol Nutr 1996;23:280–6. [DOI] [PubMed] [Google Scholar]
- [28].Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chassard C, Dapoigny M, Scott KP, et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment Pharmacol Ther 2012;35:828–38. [DOI] [PubMed] [Google Scholar]
- [30].Valeur J, Morken MH, Norin E, et al. Intestinal fermentation in patients with self-reported food hypersensitivity: painful, but protective? Clin Exp Gastroenterol 2010;3:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Vernia P, Latella G, Magliocca FM. Seeking clues for a positive diagnosis of the irritable bowel syndrome. Eur J Clin Invest 1987;17:189–93. [DOI] [PubMed] [Google Scholar]
- [32].Eastwood MA, Walton BA, Brydon WG, et al. Faecal weight, constituents, colonic motility, and lactose tolerance in the irritable bowel syndrome. Digestion 1984;30:7–12. [DOI] [PubMed] [Google Scholar]
- [33].Valeur J, Røseth AG, Knudsen T, et al. Fecal fermentation in irritable bowel syndrome: influence of dietary restriction of fermentable oligosaccharides, disaccharides, monosaccharides and polyols. Digestion 2016;94:50–6. [DOI] [PubMed] [Google Scholar]
- [34].Acosta A, Camilleri M, Shin A, et al. Effects of rifaximin on transit, permeability, fecal microbiome, and organic acid excretion in irritable bowel syndrome. Clin Transl Gastroenterol 2016;7:e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Quigley EM, Fried M, Gwee KA, et al. World Gastroenterology Organisation Global Guidelines Irritable Bowel Syndrome: A Global Perspective Update September 2015. J Clin Gastroenterol 2016;50:704–13. [DOI] [PubMed] [Google Scholar]
- [36].Hustoft TN, Hausken T, Ystad SO, et al. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol Motil 2017;29:e12969. [DOI] [PubMed] [Google Scholar]
- [37].Staudacher HM, Lomer MC, Anderson JL, et al. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr 2012;142:1510–8. [DOI] [PubMed] [Google Scholar]
- [38].Van den Abbeele P, Belzer C, Goossens M, et al. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J 2013;7:949–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 2009;294:1–8. [DOI] [PubMed] [Google Scholar]
- [40].Vital M, Howe AC, Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio 2014;5:e00889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Miquel S, Martín R, Rossi O, et al. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol 2013;16:255–61. [DOI] [PubMed] [Google Scholar]
- [42].Tap J, Derrien M, Törnblom H, et al. Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology 2017;152:111–23. e8. [DOI] [PubMed] [Google Scholar]
- [43].Hayashi H, Sakamoto M, Kitahara M, et al. Diversity of the Clostridium coccoides group in human fecal microbiota as determined by 16S rRNA gene library. FEMS Microbiol Lett 2006;257:202–7. [DOI] [PubMed] [Google Scholar]
- [44].Gobert AP, Sagrestani G, Delmas E, et al. The human intestinal microbiota of constipated-predominant irritable bowel syndrome patients exhibits anti-inflammatory properties. Sci Rep 2016;6:39399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fukumoto S, Tatewaki M, Yamada T, et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol 2003;284:R1269–76. [DOI] [PubMed] [Google Scholar]
- [46].Rivière A, Selak M, Lantin D, et al. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol 2016;7:979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Panzer AR, Lynch SV. Influence and effect of the human microbiome in allergy and asthma. Curr Opin Rheumatol 2015;27:373–80. [DOI] [PubMed] [Google Scholar]
- [48].Qin X, Sheth SU, Sharpe SM, et al. The mucus layer is critical in protecting against ischemia-reperfusion-mediated gut injury and in the restitution of gut barrier function. Shock 2011;35:275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tran CD, Grice DM, Wade B, et al. Gut permeability, its interaction with gut microflora and effects on metabolic health are mediated by the lymphatics system, liver and bile acid. Future Microbiol 2015;10:1339–53. [DOI] [PubMed] [Google Scholar]
- [50].Tazoe H, Otomo Y, Kaji I, et al. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol 2008;59Suppl 2:251–62. [PubMed] [Google Scholar]
- [51].Durbán A, Abellán JJ, Jimenéz-Hernández N, et al. Structural alterations of faecal and mucosa-associated bacterial communities in irritable bowel syndrome. Environ Microbiol Rep 2012;4:242–7. [DOI] [PubMed] [Google Scholar]
- [52].Carroll IM, Ringel-Kulka T, Siddle JP, et al. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil 2012;24:521–30. e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Speranza E, Cioffi I, Santarpia L, et al. Fecal short chain fatty acids and dietary intake in Italian women with restrictive anorexia nervosa: a pilot study. Front Nutr 2018;5:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mayengbam S, Lambert JE, Parnell JA, et al. Impact of dietary fiber supplementation on modulating microbiota-host-metabolic axes in obesity. J Nutr Biochem 2018;64:228–36. [DOI] [PubMed] [Google Scholar]
- [55].Ford AC, Lacy BE, Talley NJ. Irritable bowel syndrome. N Engl J Med 2017;376:2566–78. [DOI] [PubMed] [Google Scholar]
- [56].Boesmans L, Valles-Colomer M, Wang J, et al. Butyrate producers as potential next-generation probiotics: safety assessment of the administration of Butyricicoccus pullicaecorum to healthy volunteers. mSystems 2018;3: e00094-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


