Abstract
This retrospective study investigated the risk factors of exudative retinal detachment (ERD) occurring after vitrectomy performed to treat proliferative diabetic retinopathy (PDR).
All patients were treated with vitrectomy for PDR. Patients with history(s) of the following were excluded: ocular surgery (except phacoemulsification combined with intraocular lens implantation or retinal laser photocoagulation); ocular trauma; systemic diseases; ocular diseases; uveitis; scleritis; tumor; congenital ocular disorders; or others.
Included were 205 eyes of 169 patients, of whom 18 (8.78%) developed ERD with varying degrees of exudative choroidal detachment after 1 to 3 days. Binary logistic regression showed the following association with the development of ERD: lower serum albumin concentration (P = .001); without intravitreal anti-vascular endothelial growth factor (anti-VEGF) drug injection before vitrectomy (P = .044); and history of phacoemulsification combined with intraocular lens implantation (P = .046). No association was found with gender; age; systolic pressure; diastolic pressure; panretinal photocoagulation; intraocular pressure on the 1st postoperative day; intraocular pressure on the 2nd postoperative day; serum albumin concentration; or blood urea nitrogen.
Risk factors for ERD after vitrectomy for PDR include low serum albumin concentration, without history of intravitreal anti-VEGF drug injection before surgery, and a history of phacoemulsification combined with intraocular lens implantation.
Keywords: exudative retinal detachment, proliferative diabetic retinopathy, vitrectomy
1. Introduction
Exudative retinal detachment (ERD) is a common manifestation of the late stage of certain ocular diseases, for example, Coats disease, familial exudative vitreoretinopathy, Vogt–Koyanagi–Harada disease, uveitis, scleritis, tumor, and congenital ocular disorders. The pathogenesis of ERD is believed to involve widespread damage of retinal and/or choroidal capillary endothelial cells and retinal pigment epithelial cells. Such cell damage or death leads to a large amount of fluid exudation, and then fluid accumulation in the subretinal space. In addition, glaucoma surgery may induce suprachoroidal hemorrhage.[1–3]
A sudden decompression of the globe during or after surgery can also develop into severe ERD and even suprachoroidal hemorrhage, and is thought to be the possible mechanism of ERD induction after ocular surgeries.[3] To avoid a sudden drop in intraocular pressure (IOP), it is necessary to ensure that it is stabilized in the normal range before and after the operation, especially during surgery. Yet, ERD still can occur after vitrectomy.
Unlike the above, we studied ERD, which was established based on the history of PDR vitrectomy. In addition, retinal detachment, one of the characteristic fundus findings, occurs more often in the lower section than in the upper section. Subretinal fluid can flow depending on the posture of the patient, and was more often when sitting upright. The detached retina is usually spherical, and the surface is smooth without stretch wrinkles and with no retinal holes (Fig. 1). ERD was often accompanied by varying degrees of exudative choroidal detachment, and its fundus features are very easy to distinguish. ERD is a relatively rare postoperative complication after vitrectomy, its specific mechanism has not been clearly reported and the number of reports or studies is very limited. The present retrospective study investigated the risk factors of ERD as a complication of vitrectomy performed for PDR (vitreous hemorrhage, proliferative vitreoretinopathy, or traction detachment).
Figure 1.
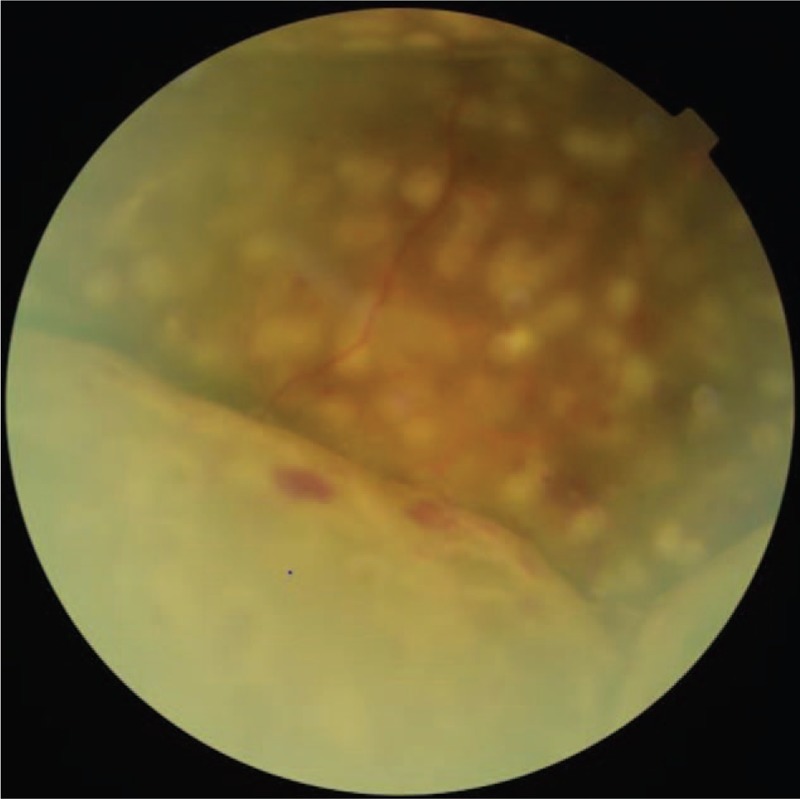
A representative fundus photograph of exudative retinal detachment.
2. Methods
The Ethics Committee of Second Hospital of Jilin University approved this retrospective study, which was performed in accordance with the tenets of the Declaration of Helsinki.
2.1. Patients
This retrospective study analyzed the outcomes of 169 consecutive patients with PDR (205 eyes) who underwent vitrectomy at the Fundus Department of Second Hospital of Jilin University between December 2015 and April 2017. Excluded were all patients who underwent ocular surgery (except phacoemulsification combined with intraocular lens implantation and retinal laser photocoagulation). Other criteria for exclusion were a history of any of the following: ocular trauma; systemic disease such as nephropathy, gestational hypertension syndrome, hematological, or cardiocerebrovascular disease; or ocular diseases including other vascular diseases of the eye, uveitis, scleritis, tumor, or congenital ocular disorders. All surgeries were performed by a vitreoretinal surgeon.
2.2. Data collection
A complete ophthalmologic evaluation of both eyes, including anterior segment examination and dilated fundoscopy, was performed for all patients before surgery. In addition, color Doppler flow imaging was performed, especially for those whose fundus was invisible because of vitreous hemorrhage or cataract, to exclude other retinopathy and/or choroidopathy that might compromise the effect of the vitrectomy. A fundus fluorescein angiogram was performed as required. Best-corrected visual acuity and IOP were obtained with care. IOP, routine blood and urine, coagulation convention, and liver renal function were within the normal range for all patients who received surgery. Blood pressure, history of cataract surgery and retinal laser photocoagulation, as well as the use of anti-vascular endothelial growth factor (anti-VEGF) drugs before vitrectomy were recorded.
The following clinical information was also collected from the medical records: age; gender; blood pressure; history of cataract surgery or retinal laser photocoagulation; serum albumin concentration (serum albumin normal value range from 40 to 55 g/L); panretinal photocoagulation (PRP); use of anti-VEGF drugs before vitrectomy; serum creatinine; and blood urea nitrogen; IOP on the 1st postoperative day; and IOP on the 2nd postoperative day. These parameters were analyzed as risk factors for ERD. Because ERD occurs in the early postoperative period, color Doppler flow imaging was not performed regularly to prevent intraocular infection. Long-term vision was not analyzed due to limited long-term and regular follow-ups.
2.3. Pre- and postoperative medication
Routine tests for irrigation of the lacrimal passages were used to exclude dacryocystitis. The pupil of each eye was kept dilated before vitrectomy with a mydriatic agent. Levofloxacin eye drops (0.5%) were applied 4 times daily for 2 to 3 days before surgery. All eyes were washed with an iodophor (1%) prior to the surgery. At the end of the surgery, tobramycin and dexamethasone ophthalmic ointment was coated onto the conjunctival sac. Postoperative medicine included a prednisolone acetate ophthalmic suspension (1%) and pranoprofen for anti-inflammation and levofloxacin eye drops (0.5%) for anti-infection prescribed 4 to 6 times daily for 2 weeks. Tobramycin and dexamethasone ophthalmic ointment was used for anti-inflammation and anti-infection before sleep for 2 weeks. Drug application was adjusted as appropriate.
2.4. Surgical technique
Standard pars plana vitrectomy (PPV) was completed under local anesthesia for all patients. A high-speed vitreous cutter (5000 cycles/min) was employed. The vitreous gel and vitreoretinal adhesions were revealed by intravitreal injection of triamcinolone acetonide using a 25-gauge 3-port system in the vitrectomy. Dissection, segmentation, delamination of the fibrovascular membrane, and posterior vitreous surface removal were performed mainly with vitreoretinal forceps in 25-gauge vitrectomy and a high-speed vitrectomy cutter in 25-gauge vitrectomy. Intraoperative bleeding was controlled either by endodiathermy or by increasing the irrigation bottle height.
A perfusion system was utilized to maintain IOP. Shaving of the vitreous base and blood clots in the peripheral vitreous skirt was performed upon scleral depression. Iatrogenic tears were avoided. An endolaser was applied to complete PRP up to the equator, even to the ora serrata. According to the reattachment of the retina, the intraocular tamponade consisted of balanced salt solution, sterilized air, different concentrations of C3F8 or silicone oil.
Most of the sclerotomy sites were closed well after gently smoothing the incisions. If the IOP could not be kept stable because of leakage of the sclerotomy sites, then suture of the sclera was performed. Patients who had undergone vitrectomy were instructed to remain face down for 3 to 14 days according to different intraocular tamponade to press the retina. It was not required if the intraocular tamponade is balanced salt solution.
2.5. Statistical analysis
All findings were evaluated using SPSS (Statistical Package for Social Sciences) 20.0 software. The categorical factors were compared by the Chi-squared test, while normally distributed data were compared using independent sample t test between patients with or without ERD. Binary logistic regression was used to investigate an association between the incidence of ERD and different influencing factors. P < .05 was considered statistically significant.
3. Results
The results of the Chi-squared test showed that gender was not associated with ERD as a complication of vitrectomy performed to treat PDR (P = .387) (Table 1). However, the relevance of a history of phacoemulsification combined with intraocular lens implantation in this complication was statistically significant (P = .026). Patients who had a history of cataract surgery were more likely to have ERD, but there was no association between a history of retinal laser photocoagulation and the incidence of ERD (P = .394).
Table 1.
Categorical factors tested Chi-squared test in the study population.
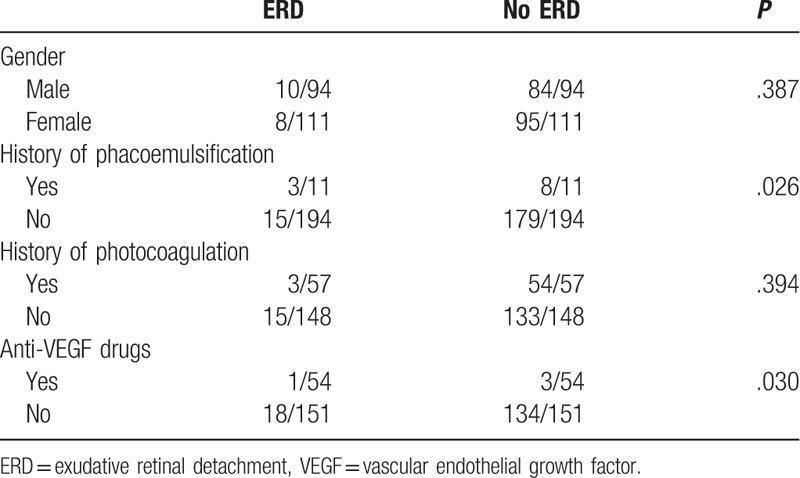
Anti-VEGF drugs such ranibizumab (Lucentis) (13) or conbercept (41) were administered to the patients whose fundus neovascularization may compromise the vitrectomy, 3 to 7 days prior to the surgery. An association between the use of anti-VEGF drugs before vitrectomy and ERD was statistically significant (P = .030), as patients who had a history of intravitreal anti-VEGF drug injection were less likely to have ERD.
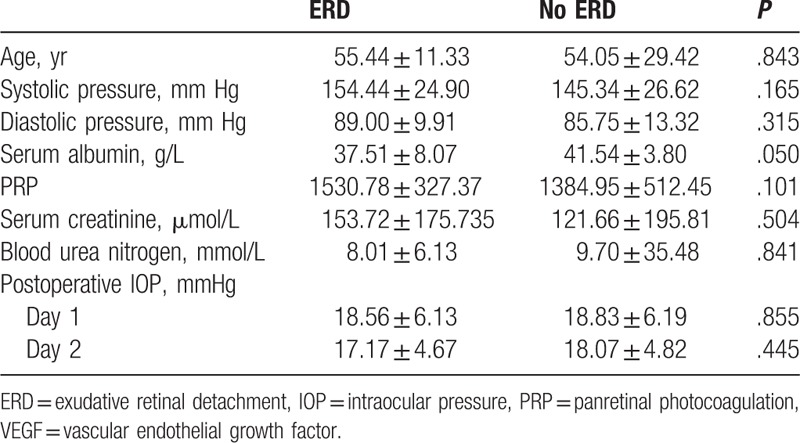
Blood pressure, serum albumin, PRP, serum creatinine, and blood urea nitrogen were controlled within the range required for vitrectomy. The independent sample t test revealed that lower serum albumin concentration (P = .050) was associated with the incidence of ERD (Table 2). No association was found between ERD and age (P = .843), systolic pressure (P = .165), diastolic pressure (P = .315), PRP (P = .101), serum creatinine (P = .179), or blood urea nitrogen (P = .700), IOP on the 1st postoperative day (P = .855), or IOP on the 2nd postoperative day (P = .445).
Table 2.
Data factors tested independent sample t test in the study population.
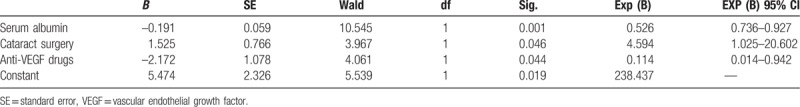
Binary logistic regression was applied to analyze an association between the incidence of ERD and relevant factors, and to determine the relatively independent hazards (Table 3). The binary logistic regression showed that lower serum albumin concentration (P = .001), the use of anti-VEGF drugs before vitrectomy (P = .044), and a history of phacoemulsification combined with intraocular lens implantation (P = .046) were associated with the incidence of ERD.
Table 3.
Logistic regression coefficients of variables.
4. Discussion
Plasma proteins are mainly composed of albumin, globulin, and fibrinogen. Globulin is associated with immune function. Fibrinogen is mainly related to the coagulation process. Serum albumin is the most abundant, low-molecular-weight, high-solubility plasma protein, accounting for 40% to 60% of total plasma proteins. One of the main functions of plasma proteins is the formation of plasma colloid osmotic pressure, in which 75% to 80% comes from albumin. A decrease in serum albumin concentration leads to tissue edema by decreasing plasma colloid osmotic pressure.
A compromise of the inner blood-retinal barrier (BRB) is believed to trigger diabetic retinopathy (DR). BRB is tight and restrictive in nature. It acts as a physiologic barrier and regulates the ion, protein, and water balance of the retina. The BRB is composed of an inner and outer layer. Tight junctions are present between retinal capillary endothelial cells in the inner BRB and retinal pigment epithelial cells in the outer BRB. The BRB maintains the physiology of the retina to support normal visual functions. Compromise of the BRB is crucial in the pathogenesis of retinal diseases.[4] The central mechanism of altered BRB function is a change in the permeability characteristics of the retinal endothelial cells.[5,6] A study showed that low serum albumin concentration enhances the permeability of the vessels by decreasing plasma colloid osmotic pressure,[5] which is in accord with our results.
Our study revealed that ERD may be related to a history of phacoemulsification combined with intraocular lens implantation. The mechanism is not clear, but we conjecture that it may be related to the following. The blood ocular barrier is composed of the blood aqueous barrier and the BRB, which are anatomically close and influence each other. The postoperative inflammatory response has been attributed to the disruption of the blood aqueous barrier by phacoemulsification. In turn, the compromised blood aqueous barrier or its breakdown caused by anterior segment inflammation can lead to extensive protein leakage into the anterior chamber.[7] While no anterior segment complications were found in the cataract surgery, we suspect disruption of the BRB due to the release of inflammatory mediators to the vitreous cavity. Clinical studies show no significant progress in mild-to-moderate nonproliferative DR (NPDR) after cataract surgery, but risk of progression of severe NPDR or PDR after cataract surgery increased.[8] On the contrary, patients who underwent phacoemulsification combined with intraocular lens implantation had a small pupil size, and were prone to posterior synechia according to our clinical experience. Such an association may be related to the dissecting of the posterior synechia during surgery and use of the iris hook. Taken together, BAB and BRB damage after cataract surgery in DR patients, coupled with surgical stimulation, can lead to ERD in severe cases.
The consensus is that VEGF causes breakdown of the BRB, stimulates endothelial cell growth and neovascularization, and increases vascular permeability in the ischemic retina.[4] Thus, it takes part in the pathogenesis of DR. It is well established that VEGF enhances permeability and endothelial cell migration/proliferation in angiogenesis and vascular inflammation. VEGF amplifies the inflammatory response through a cascade of cyclical reactions,[9] namely leukocyte activation and cytokine release, through the promotion of endothelial cell expression of intercellular adhesion molecule-1, leading to a further increase in VEGF. Intraocular delivery of anti-VEGF therapies are a common treatment strategy for advanced DR.[10] Preoperative use of intravitreal anti-VEGF drugs in vitrectomy for PDR may further facilitate hemostasis and shorten the operative time. We speculate that adjunctive use of intravitreal anti-VEGF drugs is beneficial to reduce the incidence rate of ERD after vitrectomy for PDR. Our current results confirmed an association between a history of anti-VEGF drug use before vitrectomy and ERD.
The PRP is an established treatment for patients with PDR,[11,12] as it inhibits the expression of some cytokines to reduce retinal vascular leakage and neovascularization.[13] PRP is conventionally performed during vitrectomies. In our study, spots on the retina were 200 to 500 μm, and the duration of the application was 0.15 to 0.2 seconds; there were 1000 to 2500 spots. Acute thermal destruction in the retina was reported in PRP and was likely associated with subsequent inflammation. Studies have suggested that proliferation of pro-inflammatory cytokines, such as IL-6, was found after PRP, causing endothelial barrier dysfunction[14] and increasing endothelial permeability,[15] and resulting in ERD. All burns were located at the defects at the junction of the inner and outer segments of photoreceptors and apical retinal pigment epithelium.[16] Surgical complications were reported in vitreous surgery, in which PPV without pretreatment of PRP, including vitreous hemorrhages or retinal detachment under PPV.[17] Unfortunately, some patients underwent preoperative retinal laser photocoagulation (3/18 in ERD, 54/187 in no ERD), and a conclusion has not been made regarding an association between PRP and ERD.
5. Conclusion
In conclusion, this study suggests that the risk factors for ERD after vitrectomy for PDR include low serum albumin, preoperative use of intravitreal anti-VEGF drugs, and a history of phacoemulsification combined with intraocular lens implantation. In other words, patients with PDR with low serum albumin do not use anti-VEGF drugs before PPV, and have a history of phacoemulsification combined with intraocular lens implantation are easily to have ERD after PPV. Cook et al's[18] experimental retinal detachment results revealed that photoreceptor degeneration after retinal detachment occurs through apoptosis, usually associated with intrinsic, programmed cell death mechanisms, especially in the first 3 days.
Xu and Chen[19] verified the apoptosis of retinal photoreceptor cells; after retinal detachment, the nutrients and growth factors needed for survival of photoreceptor cells were reduced or deprived. Therefore, retinal detachment, especially macular detachment, is always accompanied by a loss of optimally corrected visual acuity,[20] and ERD may have adverse effects on postoperative visual acuity. ERD is self-cured, and local use of corticosteroids and oculentum atropinae can accelerate recovery.
We suggest preoperative improvement of serum albumin by increasing protein intake. To gain better surgical effects and reduce complications, patients who have more neovascularization in the fundus may be advised to treat with intravitreal anti-VEGF drugs before vitrectomy, if they can accept it. Lastly, people with DR, especially severe NPDR or PDR, should not undergo cataract surgery before the vitrectomy unless the PRP operation is performed 1st.
Although this study showed that the spots of PRP were not significantly different between the groups, with or without ERD, our clinical experience indicated that as the number of spots of PRP increased, the risk of ERD increased. It is important to adjust PRP spots according to different conditions of the patients: if they have the risk factors of ERD, it is appropriate to reduce the spots of PRP in vitrectomy, and complete PRP timely after surgery. If dioptric media is not clear, or there is a risk of bleeding again after vitrectomy, regardless of whether there are risk factors of ERD, the PRP should be completed during the vitrectomy, to avoid missing treatment opportunities.
The main limitation of this study is the small sample size. The risk factors of ERD after PPV, especially the influence of low serum albumin and PRP spots, were found. Although the latter has not been confirmed in this study, patients’, who underwent PPV after April 2017, PRP points during the operation have been controlled at about 1200. Albumin concentration has also been controlled to within the normal range, and no recurrence of ERD has been seen in the past year. Follow-ups were on-going; future study with larger sample size was warranted, especially to further confirm the impact of PRP.
Author contributions
Conceptualization: Di-Fang Sun, Yan-Li Wang, Bin Wang, Chun-Ling Xu, Gong Zhang, Jia Li, Xiao-Meng Zhang.
Data curation: Di-Fang Sun, Yan-Li Wang, Bin Wang, Chun-Ling Xu, Gong Zhang, Jia Li, Xiao-Meng Zhang.
Formal analysis: Di-Fang Sun, Yan-Li Wang, Bin Wang, Chun-Ling Xu, Gong Zhang, Jia Li, Xiao-Meng Zhang.
Funding acquisition: Di-Fang Sun, Yan-Li Wang, Bin Wang, Chun-Ling Xu, Gong Zhang, Jia Li, Xiao-Meng Zhang.
Investigation: Di-Fang Sun, Yan-Li Wang, Bin Wang, Chun-Ling Xu, Gong Zhang, Jia Li, Xiao-Meng Zhang.
Methodology: Di-Fang Sun, Yan-Li Wang, Bin Wang, Chun-Ling Xu, Gong Zhang, Jia Li, Xiao-Meng Zhang.
Project administration: Di-Fang Sun, Yan-Li Wang, Bin Wang, Chun-Ling Xu, Gong Zhang, Jia Li, Xiao-Meng Zhang.
Resources: Di-Fang Sun, Yan-Li Wang, Bin Wang, Chun-Ling Xu, Gong Zhang, Jia Li, Xiao-Meng Zhang.
Software: Di-Fang Sun, Yan-Li Wang, Bin Wang, Chun-Ling Xu, Gong Zhang, Jia Li, Xiao-Meng Zhang.
Supervision: Di-Fang Sun, Yan-Li Wang, Bin Wang, Chun-Ling Xu, Gong Zhang, Jia Li, Xiao-Meng Zhang.
Validation: Di-Fang Sun, Yan-Li Wang, Bin Wang, Chun-Ling Xu, Gong Zhang, Jia Li, Xiao-Meng Zhang.
Visualization: Di-Fang Sun, Yan-Li Wang, Bin Wang, Chun-Ling Xu, Gong Zhang, Jia Li, Xiao-Meng Zhang.
Writing – original draft: Di-Fang Sun, Yan-Li Wang, Bin Wang, Chun-Ling Xu, Gong Zhang, Jia Li, Xiao-Meng Zhang.
Writing – review & editing: Di-Fang Sun, Yan-Li Wang, Bin Wang, Chun-Ling Xu, Gong Zhang, Jia Li, Xiao-Meng Zhang.
Footnotes
Abbreviations: BRB = blood-retinal barrier, DR = diabetic retinopathy, ERD = exudative retinal detachment, IL = interleukin, IOP = intraocular pressure, NPDR = nonproliferative diabetic retinopathy, PDR = proliferative diabetic retinopathy, PPV = pars plana vitrectomy, PRP = panretinal photocoagulation.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Zheng XX, Jiang YR. The effect of intravitreal bevacizumab injection as the initial treatment for Coats’ disease. Graefes Arch Clin Exp Ophthalmol 2014;252:35–42. [DOI] [PubMed] [Google Scholar]
- [2].Stanga PE, Jaberansari H, Bindra MS, et al. Transcleral drainage of subretinal fluid, anti-vascular endothelial growth factor, and wide-field imaging-guided laser in coats exudative retinal detachment. Retina 2016;36:156–62. [DOI] [PubMed] [Google Scholar]
- [3].Kim YC, Lee SY, Kim KS. Exudative retinal detachment following strabismus surgery in Sturge-Weber syndrome. Indian J Ophthalmol 2015;63:554–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pusparajah P, Lee LH, Abdul Kadir K. Molecular markers of diabetic retinopathy: potential screening tool of the future? Front Physiol 2016;7:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fujioka S, Karashima K, Nishikawa N, et al. Optic disk manifestation in diabetic eyes with low serum albumin: late fluorescein staining and high blood flow velocities in the optic disk. Jpn J Ophthalmol 2004;48:59–64. [DOI] [PubMed] [Google Scholar]
- [6].Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res 2013;34:19–48. [DOI] [PubMed] [Google Scholar]
- [7].Chee SP, Ti SE, Sivakumar M, et al. Postoperative inflammation: extracapsular cataract extraction versus phacoemulsification. J Cataract Refract Surg 1999;25:1280–5. [DOI] [PubMed] [Google Scholar]
- [8].Cetin EN, Yildirim C. Adjuvant treatment modalities to control macular edema in diabetic patients undergoing cataract surgery. Int Ophthalmol 2013;33:605–10. [DOI] [PubMed] [Google Scholar]
- [9].Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res 2011;30:343–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wirostko B, Wong TY, Simo R. Vascular endothelial growth factor and diabetic complications. Prog Retin Eye Res 2008;27:608–21. [DOI] [PubMed] [Google Scholar]
- [11].Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991;98:766–85. [PubMed] [Google Scholar]
- [12].Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology 1991;98:741–56. [DOI] [PubMed] [Google Scholar]
- [13].Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994;331:1480–7. [DOI] [PubMed] [Google Scholar]
- [14].Desai TR, Leeper NJ, Hynes KL, et al. Interleukin-6 causes endothelial barrier dysfunction via the protein kinase C pathway. J Surg Res 2002;104:118–23. [DOI] [PubMed] [Google Scholar]
- [15].Maruo N, Morita I, Shirao M, et al. IL-6 increases endothelial permeability in vitro. Endocrinology 1992;131:710–4. [DOI] [PubMed] [Google Scholar]
- [16].Muqit MM, Gray JC, Marcellino GR, et al. In vivo laser-tissue interactions and healing responses from 20- vs 100-millisecond pulse Pascal photocoagulation burns. Arch Ophthalmol 2010;128:448–55. [DOI] [PubMed] [Google Scholar]
- [17].Shimura M, Yasuda K, Nakazawa T, et al. Panretinal photocoagulation induces pro-inflammatory cytokines and macular thickening in high-risk proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2009;247:1617–24. [DOI] [PubMed] [Google Scholar]
- [18].Cook B, Lewis GP, Fisher SK, et al. Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci 1995;36:990–6. [PubMed] [Google Scholar]
- [19].Xu GZ, Chen RJ. The mechanism of photoreceptor cell death after exudative retinal detachment. Shanghai Med J 2001;24:714–6. [Google Scholar]
- [20].Ranty ML, Carpentier S, Cournot M, et al. Ceramide production associated with retinal apoptosis after retinal detachment. Graefes Arch Clin Exp Ophthalmol 2009;247:215–24. [DOI] [PubMed] [Google Scholar]


