Abstract
Radiation therapy can have adverse effects on normal tissue and cause chronic ulcers. The purpose of this study was to compare breast cancer patients who underwent single-stage reconstruction with patients who underwent 2-stage reconstruction for chronic radiation-induced necrotic ulcers of the chest wall.
This retrospective study comprised of 50 patients with chronic radiation-induced chest wall ulcers who underwent chest wall reconstruction in our hospital between January 2002 and January 2016. All patients developed ulcers after undergoing breast cancer surgery, followed by radiation therapy. These patients were divided into 2 groups: group A, patients who underwent debridement and reconstruction with tissue flaps simultaneously in a single-stage procedure; group B, patients who underwent debridement and omentum majus tamping in the 1st stage, followed by surgical reconstruction with skin grafting or flap transfer 2 weeks later. The postoperative complications and outcomes were evaluated and compared.
These patients were followed up for 48 to 55 months (mean: 50 months), and overall survival was 98%. One patient in group A died of septicemia 5 days after the operation. Six patients in group A developed flap infection, among which 4 patients progressed to flap necrosis (group A: 6/25 vs group B: 0/25; P = .000).
Compared to single-stage reconstruction, surgical reconstruction in 2 stages was safer and more effective in treating chronic radiation-induced ulcers of the chest wall, and is associated with fewer postoperative complications. The omentum majus flap was the most ideal tissue for the repair of these defects.
Keywords: breast cancer, chest wall defect, chest wall reconstruction, chronic radiation-induced ulcer, staged reconstruction
1. Introduction
Radiotherapy can occasionally be complicated by adverse effects such as dermatitis, radiation-induced ulcers and osteoradionecrosis. The incidence of chronic radiation-induced chest wall ulcers is about 25 to 30%. There were many risk factors such as the doses, time and self-condition. The regimens of radiation being more than 70Gy/7 week/30 times could cause such ulcers. These radiation-related complications are difficult to treat, because radiation reduces blood supply, induces fibrosis and directly impairs cellular repair potential in irradiated tissues. Decreased angiogenesis and persistently high concentrations of matrix metalloproteinase, which reflect a hostile molecular environment for cell replication after injury, have been found in irradiated tissues.[1–4] Usually, ulcers tend to improve and heal with adequate local wound care. However, radiation-induced ulcers tend to progress and worsen due to the underlying ischemia, accompanying infection and lower viability of granulation tissues.[5] Patients who have undergone radiotherapy after breast cancer surgery often develop chronic radiation-induced ulcers of the chest wall, which subsequently becomes infected. Chronic radiation dermatitis can last for months or years with continuous suppuration. Furthermore, conservative therapies are often ineffective in these patients. Moreover, simple skin grafting often fails due to the poor state of the wound bed. With advancements in microsurgical reconstruction and the wider availability of tissue flaps, free-tissue transfer has become a common and reliable method for treating radiation-induced ulcers.[6–8] The application of microvascular free-tissue transfer allows the surgeon to select tissues that are most suitable for the size and shape of the defect. However, in some patients, an acceptable recipient vessel could not be found near the irradiated area, such as when ulceration occurs in atrophic areas.[9,10] Thus, the optimal reconstruction method varies among individuals, and depends on the functional and esthetic conditions of each case. The key points for the successful reconstruction of radiation-induced ulcers are complete resection of the affected tissue, followed by coverage with a well-vascularized tissue. It should be noted that even with the grafting of a well-vascularized tissue, infection and necrosis often occur postoperatively, and can result in treatment failure.
The surgical reconstruction of radiation-induced ulcers can be performed as a 1-stage or 2-stage procedure. The former is more rapid, but is also more prone to complications, while the latter is safe, but requires repeat anesthesia and operation. The purpose of the present study is to retrospectively review our 10-year experience with radiation-induced ulcers of the chest wall. We sought to compare patients who underwent single-stage reconstruction with patients who underwent 2-stage reconstruction for radiation-induced necrotic ulcers of the chest wall.
2. Patients and methods
2.1. Patients
This retrospective study comprised of 50 women with chronic radiation-induced ulcers, who underwent treatment at the Shanghai Ninth People's Hospital Affiliated to Shanghai Jiaotong University between January 2004 and January 2016 (Table 1). All patients received radiotherapy for breast cancer. Patients who developed ulcers within 3 months after radiotherapy were excluded from the present study, because of the edema and swelling generally still existing. These patients were divided into 2 groups based on the reconstruction method: group A, 1-stage reconstruction; group B, 2-stage reconstruction. The present study was approved by the Research Ethics Committee of Jiaotong University (Shanghai, People's Republic of China; Approval number: RL2016098347), and written informed consent was obtained from all patients. All postoperative complications and follow-up outcomes were analyzed and compared between these 2 study groups.
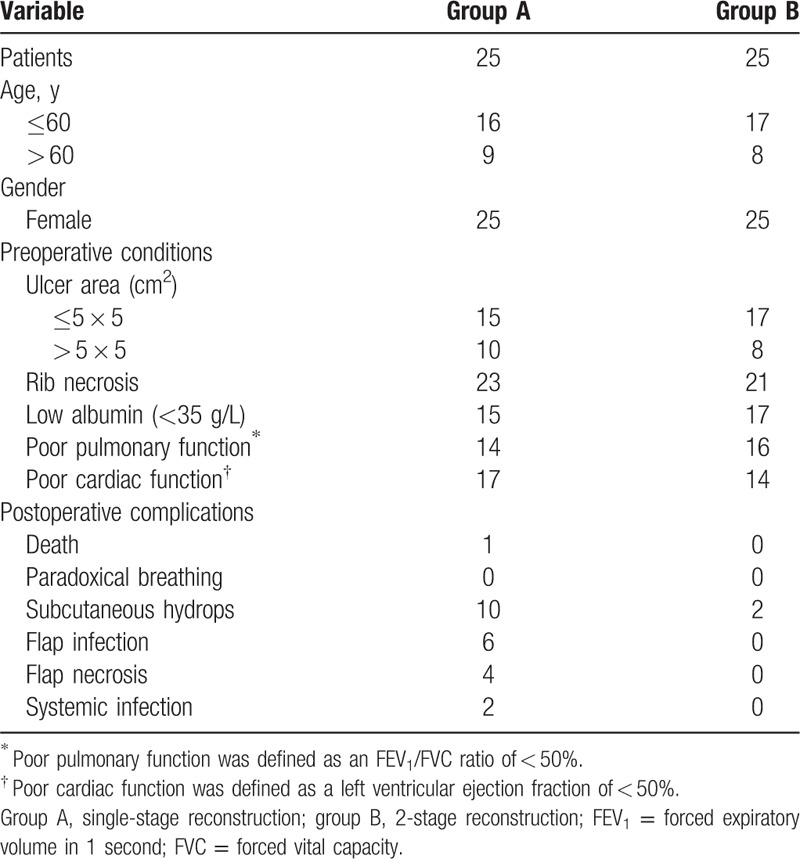
Table 1.
Patient demographics and clinical data.
2.2. Preoperative preparations
2.2.1. General condition
In all patients, the chronic radiation-induced ulcer was accompanied by severe infection, water and electrolyte imbalance, and anemia. Before the reconstruction surgery, patients were treated to induce their white blood cell count and hemoglobin levels (>100 g/L) to return to the normal range. Preoperative albumin level was required to be at least 35 g/L. Patients with chronic anemia were given blood transfusions. These preoperative preparations were undertaken to minimize postoperative complications.
2.3. Preoperative pulmonary function
Preoperative pulmonary function largely determines the duration of postoperative mechanical ventilation and the incidence of pulmonary complications. In the present study, preoperative forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) were required to exceed 60% of the predicted value, and the FEV1/FVC ratio was required to exceed 50%. Arterial blood gas analysis was performed for all patients. Chest computed tomography was performed to evaluate the infection status and ulcer, and plan the operation, if required.
2.4. Multidisciplinary approach
Thoracic surgeons and plastic surgeons discussed each case to anticipate potential intraoperative complications, and jointly developed an operative plan for each patient. The eligibility of each patient for defect repair with free flaps or rotation flaps was carefully evaluated. Preoperative CT angiography is useful to design omental flap. In some patients, multiple flaps were planned. If the 1st flap was found not to be appropriate during the operation, the standby flap was used.
2.5. Surgical procedure
Before surgery, the ulcer was managed with daily wound dressings. Tissue samples from the ulcer tissue were collected preoperatively, and subjected to bacterial culture and antibiotic-sensitivity testing, which is prepared for the choice of post operative antibiotics. Patients in group A underwent debridement and reconstruction with different types of tissue flaps in a single-stage procedure, while patients in group B underwent wide local debridement and omentum majus tamping in the 1st stage. The omentum majus was pulled through a subcutaneous tunnel from the abdomen to the thorax using the open approach. This tunnel was made to be spacious enough to preserve the blood supply of the omentum majus. Occasionally, a metal plate is used for chest cage fixation and a titanium meshed graft is placed over the omental flap, when the ulcer area was more than 6 × 6 cm2. In the 2nd stage, which was approximately 2 weeks after the 1st stage, patients underwent surgical reconstruction with flap transfer or skin grafting, depending on the status of the granulation tissue (Figs. 1–4).
Figure 1.
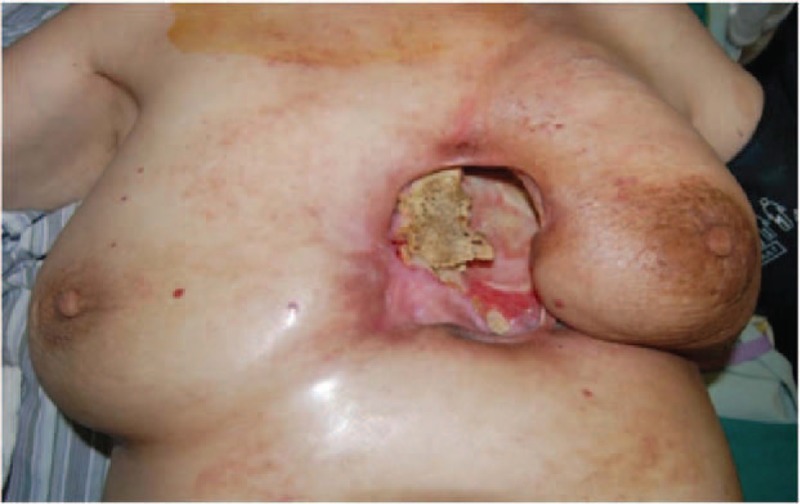
A 55-year-old patient from group B who had chronic radiation-induced ulcer for 3 years.
Figure 4.
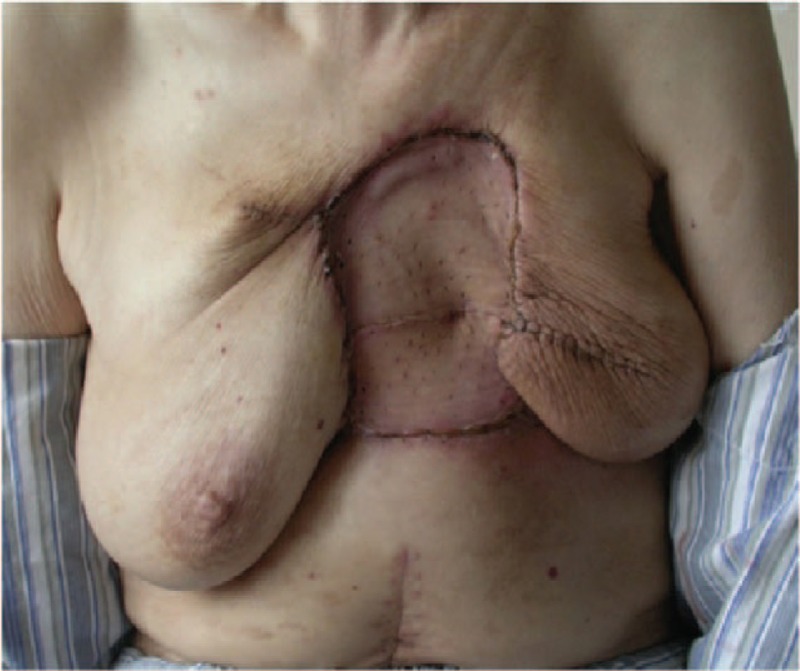
The 2nd stage of surgery: skin grafting.
Figure 2.
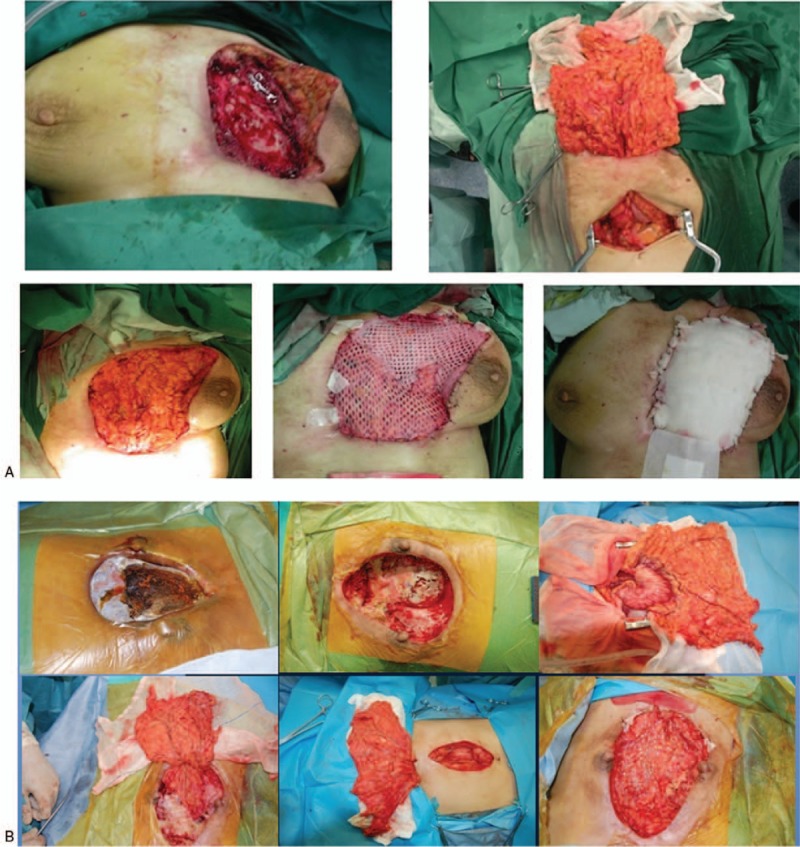
(A) The 1st stage of surgery: debridement and omentum majus flap transplantation. (B) The 1st stage of surgery in another patient.
Figure 3.
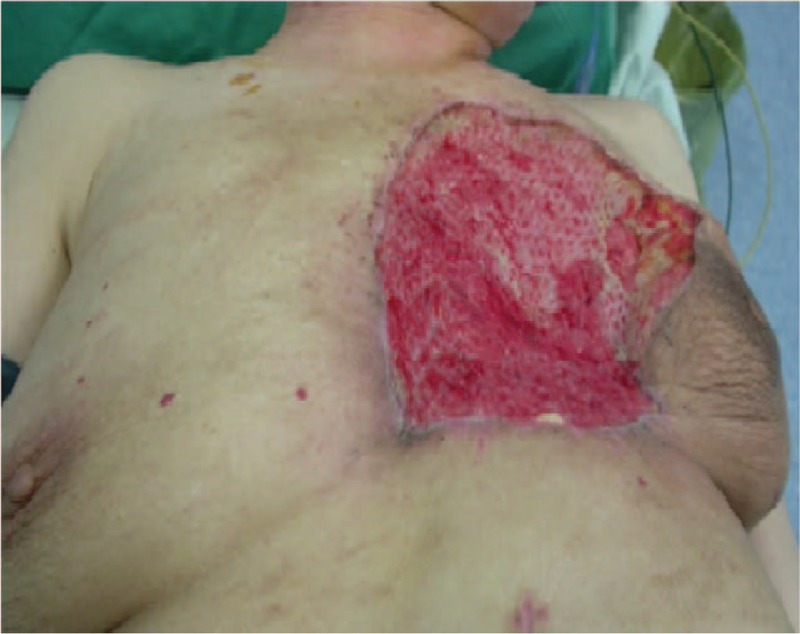
Two weeks later, the omentum majus flap was well–vascularized.
2.6. Follow-up
All patients were followed up for 10 years. The clinical data of patients from our center during the 10-year period were collected and reviewed to evaluate for complications, surgical outcomes, and short-term and long-term prognoses. Data obtained from regular 3-month follow-up visits and phone calls were combined. Furthermore, the duration and cost of hospitalization between these 2 groups were compared.
2.7. Statistical analysis
Statistical analyses were performed using SPSS 19.0 (SPSS, Chicago, IL). The results were expressed as mean ± standard error. Two-sided P-values were calculated, and a probability level of .05 was chosen for statistical significance. The Logistic regression analysis were used to determine which factor is more important for prognosis.
3. Results
The age of patients in the entire study cohort ranged within 45 to 70 years old (mean: 55 years old). The area of the ulcer ranged from 3 × 5 cm2 to 8 × 10 cm2. These patients were followed up for 48 to 55 months (mean: 50 months; Table 1). There were no significant differences between groups A and B in terms of general patient characteristics such as age. One patient in group A died of septicemia, 5 days after the operation. Overall survival was 98%. There were no patients suffered the malignant transformations and local recurrences. The average length of hospital stay was significantly shorter in group A (12.5 days) than in group B (21.7 days) (P = .001). Similarly, the average hospitalisation cost (in US dollars) was lower in group A than in group B (8465 ± 138 vs 14,710 ± 598; P = .02). There were 70% patients received full thickness resections and 30% received partial thickness resections. There were no postoperative problems related to thoracic stability such as paradoxical breathing and expiratory dyspnea in either of the groups. In group A, 2 patients developed heart failure after the operation, and 1 patient had respiratory insufficiency, which required ventilator support for 1 week. Many bacterial species were detected in the wound tissue samples from both groups, including methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa. Antibiotics were prescribed based on the results of the culture and sensitivity tests. The incidence of subcutaneous hydrops due to infection was higher in group A than in group B (10/25 vs 2/25; P = .001). Six patients in group A developed flap infection. Among these patients, 4 patients progressed to flap necrosis (6/25 vs 0/25; P = .000). These 4 patients required reoperation to change the flap (Table 1). We built the Logistic regression model and put many factors such as operation stages, types of flaps, age, and cooperation into together. The result showed that the operation-stage is the most important factor to the prognosis.
4. Discussion
This study compared the outcomes of 1-stage vs 2-stage reconstruction surgery, in order to determine the optimal reconstruction method for breast cancer patients who developed chronic radiation-induced ulcers of the chest wall. The findings revealed that the 2-stage procedure was associated with significantly better outcomes and few complications, compared to the 1-stage procedure.
Radionecrosis of the chest wall is a severe delayed complication of radiotherapy, and is most commonly found in patients undergoing irradiation following mastectomy. This condition sometimes presents as an intractable infectious ulcer complicated with the degeneration of the chest wall, including the skin, soft tissue and bony thorax.[11–13] Chronic radiation-induced ulcers of the chest wall secondary to radiotherapy for breast cancer, lymphoma, primary tumors of the chest wall, or other malignant tumors are invariably challenging to treat. When a patient develops a wound in an irradiated lesion, biopsy and histological examination must be performed immediately to rule out a recurrent tumor. Patients with radiation-induced ulcers should initially be treated with narcotic analgesic agents, antibiotics, debridement and local care. However, these conservative therapies are often ineffective. Recently, due to technological advancements in radiotherapy, the incidence of radiation-induced chest wall necrosis has decreased. However, chronic ulcers due to radiation-induced damage are still encountered in clinical practice.[14] Moreover, these patients are usually infected, resulting in poor prognosis.
Many surgical procedures have been used to reconstruct the chest wall.[15,16] Several years ago, negative pressure wound therapy was used to cure those ulcers, which was limited to the area and locations. Of course, the hospital stay was longer. Some authors have repaired radiation-induced ulcers of the chest by inserting tissue expanders in non-irradiated adjacent skin. The advantage of this approach is to avoid extensive surgery and functional defects that result from flap harvesting. Furthermore, the expanded skin is expected to provide well-vascularized tissues for irradiated wounds with complications such as flap necrosis.[17,18] However, complications continue to occur, including necrosis of the skin and surgical failure. Generally, the reconstruction of radiation-induced ulcers includes both bone and soft tissue repair. However, in the present study, none of these patients required osseous reconstruction.
Operation could be considered the best method by surgeons. Many doctors prefer to debride and repair the defect simultaneously in a single-stage procedure.[11,19–22] Mansour et al[11] reported that 1-stage reconstruction is adequate for a variety of major chest wall defects. However, their study did not include patients with radiation-induced ulcers with necrosis and infection. The advantages of a single-stage operation include lower hospitalisation costs and duration, and the avoidance of repeat anesthesia induction and reoperation. However, in the present study, single-stage reconstruction was associated with a significantly higher rate of postoperative complications, compared to 2-stage reconstruction (Table 1). The likelihood of operation failure and complications such as infection and flap necrosis were higher in group A than in group B. Due to inadequate preoperative preparation and failure to control the infection, one patient died of septicemia. In addition, 4 patients in group A developed flap necrosis, which necessitated reoperation. Thus, it was considered that a 2-stage operation is the optimal treatment choice for radiation-induced ulcers. The therapy for chronic ulcers differs from general chest wall tumor resection, which tends to be performed as a single-stage procedure. Unlike chest wall tumors, chronic ulcers are often accompanied by severe infection, immune suppression and poor general condition. Therefore, in the case of chronic ulcers, it is not advisable to perform reconstruction early when active infection may still be present. Furthermore, the investigators considered that it is not necessary to complete the operation in 1 stage for patients with chronic radiation-induced ulcers. In the experience of the investigators, debridement and construction should be performed in a staged manner. The aim of the 1st stage is meticulous debridement, which ensures the complete removal of necrotic tissues, infection control and nutritional support. After the infection has been controlled and the wound surface has healed satisfactorily, the 2nd stage of reconstruction can commence.
It has been commonly considered that auto-grafts are the best material for body repair. Hence, surgical reconstruction should be performed with the patient's own tissue as far as possible. For patients with infections, the application of artificial materials to cover the wound tends to result in surgical failure and more complications, and is thereby not recommended.[23] The investigators consider that the best choice of graft material in patients with radiation-induced ulcers is the omentum majus flap. The omentum majus performs many functions such as bacterial and foreign-body phagocytosis, resistance to infection, vascularization, lymph drainage and the promotion of wound healing. The investigators recommend that chronic radiation-induced ulcers be reconstructed using omental flaps to repair the wound in the 1st stage. However, in these patients, necrotic tissue may persist even after debridement, facilitating bacterial growth. Therefore, the omentum majus flap should be used to fill in the wound and eliminate the dead space. Approximately 2 weeks after debridement and omentum majus flap transplantation, skin grafting or another flap transplantation should be performed to cover the wound (Figs. 1–4).
The ultimate outcome of surgery depends on several factors. At present, chest wall resection and reconstruction is nearly always performed by thoracic surgeons. However, thoracic surgeons may lack the experience in selecting and applying tissue flaps. Hence, the investigators collaborated with plastic surgeons throughout the patient-management process, from preoperative consultation to surgical treatment. In the present study, debridement was mainly performed by thoracic surgeons, while reconstruction was mainly performed by plastic surgeons, which resulted to satisfactory surgical results. So, the confounding factors could influence the prognosis of patients. For example, the different flaps or flap-rotation technique could affect the result. From the Logistic regression analysis, we found 2-stage operation still was the most important factor.
In conclusion, it was considered that 2-stage reconstruction is the optimal surgical method for patients with radiation-induced ulcers. The aims of the 1st stage are debridement, infection control and improvement of the patient's general condition. Reconstruction should be performed in the 2nd stage.
Author contributions
Data curation: Yaodong Zhou.
Formal analysis: Yaodong Zhou.
Investigation: Yaodong Zhou.
Methodology: Yixin Zhang.
Project administration: Yixin Zhang.
Resources: Yaodong Zhou, Yixin Zhang.
Supervision: Yixin Zhang.
Writing – original draft: Yaodong Zhou.
Writing – review & editing: Yaodong Zhou, Yixin Zhang.
Footnotes
Abbreviations: CT = computed tomography, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, SPSS = Statistic Package for Social Science.
YZ and DG contributed equally to this work and should be considered co-first authors.
Ethical approval: All procedures performed in this study complied with the ethical standards of the National Research Committee, and with the tenets of the 1964 Helsinki Declaration and its later amendments.
Informed consent: Informed consent was obtained from all individual participants included in the study.
This study was supported in part by funding from Shanghai Municipal Education Commission Gaofeng Clinical Medicine Grant Support: Economy and Synthetic Treatment Strategy Study of Pathological Scars (#20152227) and the Foundation of Medical Engineering Cross-work (#YG2016QN07).
The authors have no conflicts of interest to disclose.
References
- [1].Arnold PG, Pairolero PC. Reconstruction of the radiation-damaged chest wall. Surg Clin North Am 1989;69:1081. [DOI] [PubMed] [Google Scholar]
- [2].Feldmeier JJ. Hyperbaric oxygen therapy and delayed radiation injuries (soft tissue and bony necrosis): 2012 update. Undersea Hyperb Med 2012;39:1121–39. [PubMed] [Google Scholar]
- [3].Futran ND, Trotti A, Gwede C. Pentoxifylline in the treatment of radiation-related soft tissue injury: preliminary observations. Laryngoscope 1997;107:391. [DOI] [PubMed] [Google Scholar]
- [4].Enomoto M, Yagishita K, Okuma K, et al. Hyperbaric oxygen therapy for a refractory skin ulcer after radical mastectomy and radiation therapy: a case report. J Med Case Rep 2017 Jan 4;11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hultman CS, Culbertson JH, Jones GE, et al. Thoracic reconstruction with the momentum: indications, complications, and results. Ann Plast Surg 2001;46:242–9. [DOI] [PubMed] [Google Scholar]
- [6].Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5:v8–30. [DOI] [PubMed] [Google Scholar]
- [7].Harashina T, Takayama S, Ikuta Y, et al. Reconstruction of chest-wall radiation ulcer with free latissimus dorsi muscle flap and meshed skin graft. Plast Reconstr Surg 1983;71:805. [DOI] [PubMed] [Google Scholar]
- [8].Strawberry CW, Jacobs JS, McCraw JB. Reconstruction for cervical irradiation ulcers with myocutaneous flaps. Head Neck Surg 1984;6:836. [DOI] [PubMed] [Google Scholar]
- [9].Rudolph R, Arganese T, Woodward M. The ultrastructure and etiology of chronic radiotherapy damage in human skin. Ann Plast Surg 1982;9:282. [DOI] [PubMed] [Google Scholar]
- [10].Ryan JL. Ionizing radiation: the good, the bad, and the ugly. J Invest Dermatol 2012;132:985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mansour KA1, Thourani VH, Losken A, et al. Chest wall resections and reconstruction: a 25-year experience. Ann Thorac Surg 2002 Jun;73:1720–5. [DOI] [PubMed] [Google Scholar]
- [12].Seyfer AE. Radiation-associated lesions of the chest wall. Surg Gynec Obst 1988;167:129–31. [PubMed] [Google Scholar]
- [13].Parker RG, Berry HC. Late effects of therapeutic irradiation on the skeleton and bone marrow. Cancer 1976;37:1162–71. [DOI] [PubMed] [Google Scholar]
- [14].Kagkiouzis J1, Platoni K, Kantzou I, et al. Review of the three-field techniques in breast cancer radiotherapy. J BUON 2017 May-Jun;22:599–605. [PubMed] [Google Scholar]
- [15].Harenberg PS, Viol AW, D’Amico TA, et al. Thoracic wall defect reconstruction and dead space obliteration with an intra-/extrathoracic free flap. Chirurg 2009;80:641–4. [DOI] [PubMed] [Google Scholar]
- [16].Kaur S, Pawar M, Banerjee N. Evaluation of the efficacy of hyperbaric oxygen therapy in the management of chronic nonhealing ulcer and role of periwound transcutaneous oximetry as a predictor of wound healing response: a randomized prospective controlled trial. J Anaesthesiol Clin Pharmacol 2012;28:70–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].MacMillan RW, Arias JD, Stayman JW. 3rd: Management of radiation necrosis of the chest wall following mastectomy: a new treatment option. Plast Reconstr Surg 1986;77:832. [DOI] [PubMed] [Google Scholar]
- [18].Sander AL, Henrich D, Muth CM, et al. In vivo effect of hyperbaric oxygen on wound angiogenesis and epithelialization. Wound Repair Regen 2009;17:179–84. [DOI] [PubMed] [Google Scholar]
- [19].Chang RR, Mehrara BJ, Hu QY, et al. Reconstruction of complex oncologic chest wall defects: a 10-year experience. Ann Plast Surg 2004;52:471–9. discussion 479. [DOI] [PubMed] [Google Scholar]
- [20].Yoshino N, Yamauchi S, Akimoto M, et al. A case report on a full-thickness chest wall reconstruction with polypropylene mesh and stainless steel mesh concurrently using a transverse rectus abdominis myocutaneous flap. Ann Thorac Cardiovasc Surg 2006;12:445–8. [PubMed] [Google Scholar]
- [21].Ito T, Ito K, Okada T, et al. Full-thickness chest-wall resection followed by thorax reconstruction for recurrent malignant phyllodes tumor. Int J Clin Oncol 2011 Apr;16:156–60. [DOI] [PubMed] [Google Scholar]
- [22].Itano H, Andou A, Date H, et al. Chest wall reconstruction with perforator flaps after wide full-thickness resection. J Thorac Cardiovasc Surg 2006;132:e13–14. [DOI] [PubMed] [Google Scholar]
- [23].Bury TF, Reece GP, Janjan NA, et al. Closure of massive chest wall defects after full-thickness chest wall resection. Ann Plast Surg 1995;34:409–14. [DOI] [PubMed] [Google Scholar]


