Abstract
This study aims to investigate the correlation between oxidative stress and intra-abdominal fat (IAF) in obese young and middle-aged males.
The present study included 136 male examinees in the Examination Center of the First Hospital of Qinhuangdao from October 10, 2015 to December 10, 2015. Then, clinical data, oxidative stress indices (8-iso-prostaglandin F2α [8-iso-PGF2α], malondialdehyde [MDA], and superoxide dismutase [SOD]), and IAF area were recorded. All subjects were assigned into 3 groups according to body mass index (BMI): obese group (BMI ≥ 28 kg/m2, 43 subjects), overweight group (24 ≤ BMI < 28 kg/m2, 46 subjects), and control group (BMI < 24 kg/m2, 47 subjects). Then, statistical analysis was performed.
There were significant differences in IAF area, leptin, adiponectin, 8-iso-PGF2α, MDA, SOD, fasting insulin (FINS), fasting blood glucose (FBG), and homeostasis model assessment-insulin resistance (HOMA-IR) among these 3 groups (P < .05). Male subjects in the obese group had higher leptin, 8-iso-PGF2α, MDA, FINS, and HOMA-IR levels, compared to subjects in the overweight and control groups. Furthermore, subjects in the overweight group had a larger IAF area and higher 8-iso-PGF2α, MDA, and FBG levels, when compared to controls. In addition, SOD was significantly lower in the obese and overweight groups than in the control group. However, there were no statistical differences in age, systolic and diastolic blood pressure, lipids, and islet β-cell secretion function (homeostasis model assessment-β) among these 3 groups (P ≥ .05). Moreover, the IAF area was positively correlated to 8-iso-PGF2α and MDA, and negatively correlated to SOD.
Oxidative stress is significantly associated with the IAF area in obese males, and abdominal obesity could increase oxidative stress level and insulin resistance.
Keywords: intra-abdominal fat, male, obesity, oxidative stress
1. Introduction
Obesity refers to the increase of adipose tissue in the body, and can also be defined as body weight exceeding the upper limit of physiologic needs caused by excessive fat accumulation.[1] There are brown and white fats in the human body. White fat contains fibroblasts, precursor adipocytes, mature adipocytes, and macrophages, which has obvious heterogeneity according to their distribution in visceral or subcutaneous locations. In addition, white fat is not only the energy storage tissue in the body, but also has endocrine, paracrine, and autocrine functions.[2] Research suggested that postoperative body weight, body mass index (BMI), arm circumference, waist circumference, hip circumference, and waist-to-height ratio index were significantly lower than preoperative indices.[3] The bioactive substances secreted by adipose tissues are called adipokines or adipocytokines, including leptin and adiponectin. These 2 can increase energy consumption, insulin sensitivity, and fatty acid oxidation, while leptin can inhibit the appetite and fat aggregation.[4] Furthermore, adipokines increase reactive oxygen species, and accordingly produce oxidative stress (OS).[5] Therefore, obesity is significantly correlated to the increase in OS markers.
Abdominal obesity is the external manifestation of intra-abdominal fat (IAF) accumulation, which is significantly correlated to insulin resistance and diabetes.[6–9] Magnetic resonance imaging (MRI) and computed tomography scanning can accurately measure the area of IAF, which are gold standards for evaluating the accumulation level of IAF.[10]
At present, few studies have investigated the correlation between IAF and OS in the male population. In the present study, 8-iso-prostaglandin F2α (8-iso-PGF2α), malondialdehyde (MDA), and superoxide dismutase (SOD) were used to evaluate the OS level, and investigate the correlation between OS and IAF in obese men.
2. Materials and methods
2.1. Study subjects
A total of 136 male subjects, who were examined in the Physical Examination Center of the First Hospital of Qinhuangdao from October 10, 2015 to December 10, 2015, were enrolled into the present study. The age of these male subjects ranged 22 to 58 years old (40.67 ± 9.89). The inclusion criteria were: young and middle-aged males, who had a normal glucose tolerance. The exclusion criteria were: significant hypertension, coronary heart disease, cerebral infarction, and diabetes; smoking and drinking habits; treated with the oral use of drugs or surgical treatment in the past 1 month. All subjects provided an informed consent prior to enrollment into the present study. This study was conducted in accordance with the declaration of Helsinki and approved by the Ethics Committee of our hospital.
2.2. Methods
The age, height, weight, systolic blood pressure (SBP), diastolic blood pressure (DBP), levels of leptin, adiponectin, 8-iso-PGF2α, MDA, SOD, triglyceride, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, fasting insulin (FINS), and fasting blood glucose (FBG), and the area of IAF measured by MRI at the L4/5 level of study subjects were recorded. Then, the homeostasis model assessment-insulin resistance index (HOMA-IR = FINS × FBG/22.5) and islet β-cell secretion function (HOMA-β = 20 × FINS [FBG-3.5]) were calculated.
According to the Guidelines for Prevention and Control of Overweight and Obesity in Chinese Adults for BMI, all subjects were divided into 3 groups: obesity group (BMI ≥ 28 kg/m2, n = 43), overweight group (24 ≤ BMI < 28 kg/m2, n = 46), and control group (BMI < 24 kg/m2, n = 47). This measure is commonly used in China. Then, statistical analysis was performed.
2.3. Statistical analysis
Data were statistically analyzed using statistical software SPSS 13.0. Values were expressed as mean ± standard deviation. The comparison of the means of multiple samples was conducted using 1-way analysis of variance (ANOVA). Inter-group comparison was conducted using Student–Newman–Keuls test, and Pearson correlation coefficient was used to measure the strength of the association between 2 variables. P < .05 was considered statistically significant.
3. Results
3.1. Comparison of the basic clinical data in the different groups
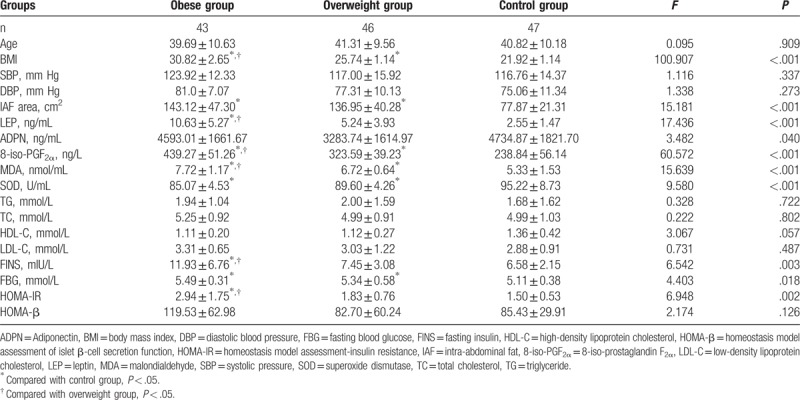
The differences in IAF area, and levels of leptin, adiponectin, 8-iso-PGF2α, MDA, SOD, FINS, FBG, and HOMA-IR among the 3 groups were statistically significant (P < .05). Furthermore, the levels of leptin, 8-iso-PGF2α, MDA, FINS and HOMA-IR were significantly higher in the obesity group than in the overweight group and control group, while the area of IAF was significantly larger and the level of FBG was significantly higher in the obesity group than in the control group. In addition, the area of IAF was significantly larger and the levels of 8-iso-PGF2α, MDA and FBG were significantly higher in the overweight group than in the control group. Moreover, the SOD level was significantly lower in the obesity and overweight groups than in the control group. However, the differences in age, SBP, DBP, and levels blood lipid and HOMA-β between these groups were not statistically significant (P ≥ .05) (Table 1).
Table 1.
Comparison of the basic clinical data of men in the different groups.
3.2. Correlation analysis of the OS indexes and IAF area
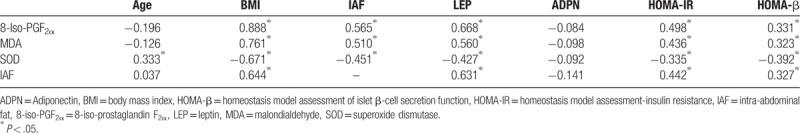
In healthy men, the OS indexes (8-iso-PGF2α, MDA, and SOD) and IAF level were significantly correlated to BMI, leptin, HOMA-IR, and HOMA-β. In addition, 8-iso-PGF2α and MDA were positively correlated to IAF, while SOD was negatively correlated to IAF (Table 2).
Table 2.
Correlation analysis of the oxidative stress indexes and IAF area.
4. Discussion
Obesity has become a real disease that can cause a series of diseases, such as diabetes and hypertension.[11] Allison et al revealed that as the body weight decreased by 1 standard deviation, the mortality rate increased by 30%, while as the fat content decreased by 1 standard deviation, the mortality rate decreased by 15%.[12] Abdominal obesity can increase the level of OS, and promote platelet adhesion activation[4] and lipid peroxidation, which has clearer clinical significance, when compared with subcutaneous fat accumulation. In the present study, with IAF measured by MRI as an indicator of abdominal obesity, and 8-iso-PGF2α, MDA, and SOD as the evaluation indexes of OS, an investigation was conducted in a healthy male population.
White adipose tissues can secrete leptin, and leptin plays a vital role in energy homeostasis. Patients with leptin deficiency present with excess appetite and obesity.[13] These individuals can achieve complete relief after leptin treatment.[14,15] However, in the normal population, high concentrations of leptin cannot decrease body weight.[16] A previous small-scale study on a healthy obese population revealed that leptin level was not significantly correlated to the area of IAF. However, in the area of IAF measured by ultrasonography,[17] the data obtained were not the gold standard. According to the results of the present study, the level of leptin was significantly correlated to the area of IAF (r = 0.631). Furthermore, the data measured by MRI were more accurate than the ultrasound results. A study on the long-acting recombinant human methionyl Fc-leptin molecule (Leptin A-200) revealed that the area of IAF decreased by 23.5% in obese subjects treated with Leptin A-200, and this decreased by only 1% in the placebo group.[18] There were also studies that explained the effect of leptin on human metabolism from another perspective. Studies suggested that leptin might also be involved in modulating the set point of the thyroid axis under physiologic conditions and the stimulatory effect of leptin on thyrotropin release was probably due to positive regulation of thyrotropin-releasing hormone production and release by leptin.[19,20] These are further supporting results of the present study.
Adiponectin is mainly secreted from adipose tissues into the blood. A previous study revealed that decreased adiponectin levels were correlated to metabolic diseases, arteriosclerotic diseases, sleep apnea, nonalcoholic fatty liver disease, gastroesophageal reflux, and tumors, but was negatively correlated to the area of IAF.[21] Furthermore, adiponectin level was significantly increased in women with abdominal obesity.[22] In the present study, the difference in adiponectin level among healthy men with different obesity levels was statistically significant. However, the inter-group comparison revealed no statistical differences. In the perspective of numerical values, the adiponectin level was highest in the control group, lowest in the overweight group, and moderate in the obesity group. In addition, leptin level was not significantly correlated to the area of IAF. This suggests that there may be no negative correlation between adiponectin level and the IAF area in men, and that there may be a difference in adiponectin secretion and mechanism between different gender populations.
For glycometabolism, hypertrophic adipocytes have a lower density of insulin receptors and a higher density of β-3 adrenergic receptors, which accordingly helps monocytes exude into the visceral adipose matrix, and initiate the inflammatory response cycle of adipocyte-monocytes.[4] In the present study, the level of insulin resistance was significantly increased in the obesity group, and the secretion function of pancreatic β-cells was slightly increased (the difference was not statistically significant). In addition, the levels of FINS and FBG were significantly higher, when compared to the control group, and IAF was significantly correlated to HOMA-IR and HOMA-β levels. This suggests that the major glycometabolism abnormality in obese men is insulin resistance, instead of the decrease in secretion function of insulin β-cells.
Furthermore, 8-iso-PGF2α can promote platelet adhesion and aggregation, which is one of the indexes of lipid peroxidation in the body. This is often used as the gold standard for evaluating the OS level in the body, which has been proven to be one of the independent risk factors of coronary heart disease.[23] Furthermore, the 8-iso-PGF2α levels are significantly increased in patients with abdominal obesity and metabolic syndrome.[22,24] At 1 week after patients were treated with Roux-en-Y gastric bypass, the plasma level of 8-iso-PGF2α decreased by more than 50%, while 8-iso-PGF2α levels decreased by more than 1/3 in subcutaneous adipose tissues.[5] This suggests that abdominal obesity can increase OS level, and lead to platelet adhesion activation. This is consistent with the results of the present study. SOD is an oxygen-free radical scavenger, which can inhibit the formation of 8-iso-PGF2α, which decreases with the increase in BMI.
The MDA is often used as one of the important indicators for evaluating lipid OS. Serum MDA level is significantly increased in obese mice.[10] Both high-fluoride and high-fat diets can increase MDA levels in rabbits.[25] Studies have revealed that MDA increases with the increase in BMI[26] and insulin resistance level.[27] Furthermore, the increase in level of MDA was more significant in an obese population with abdominal fat accumulation.[26] Moreover, even obesity in mothers can increase the MDA level in newborns.[28] The present study also revealed that the MDA level was significantly correlated to abdominal obesity and insulin resistance. However, no correlation between MDA and adiponectin was found in obese men. This conclusion needs to be verified through more large-scale prospective randomized controlled studies.
In summary, OS level was significantly correlated to IVF in obese men, and abdominal obesity can increase the level of OS and insulin resistance.
Author contributions
Conceptualization: Xiao-Jiao Jia.
Data curation: Lan-Xiang Liu.
Formal analysis: Yi-Ming Tian.
Resources: Rui Wang.
Supervision: Qiang Lu.
Writing – original draft: Xiao-Jiao Jia, Qiang Lu.
Writing – review & editing: Xiao-Jiao Jia, Qiang Lu.
Footnotes
Abbreviations: ANOVA = analysis of variance, BMI = body mass index, DBP = diastolic blood pressure, FBG = fasting blood glucose, FINS = fasting insulin, HOMA-IR = homeostasis model assessment-insulin resistance, IAF = intra-abdominal fat, 8-iso-PGF2α = 8-iso-prostaglandin F2α, MDA = malondialdehyde, MRI = magnetic resonance imaging, OS = oxidative stress, SBP = systolic blood pressure, SOD = superoxide dismutase, .
The authors have no funding and conflicts of interest to disclose.
References
- [1].Polyzos SA, Margioris AN. Sarcopenic obesity. Hormones (Athens) 2018;17:321–31. [DOI] [PubMed] [Google Scholar]
- [2].Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci 2014;15:6184–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rojhani-Shirazi Z, Amini M, Meftahi N, et al. Comparison of anthropometric measures in people with and without short-and long-term complications after laparoscopic sleeve gastrectomy. Comp Clin Pathol 2017;26:1375–9. [Google Scholar]
- [4].Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci 2010;1212:E1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ueda Y, Hajri T, Peng D, et al. Reduction of 8-iso-prostaglandin F2α in the first week after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring) 2011;19:1663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pouliot MC, Després JP, Nadeau A, et al. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes 1992;41:826–34. [DOI] [PubMed] [Google Scholar]
- [7].Brisson D, Perron P, Guay SP, et al. The “hypertriglyceridemic waist” phenotype and glucose intolerance in pregnancy. CMAJ 2010;182:E722–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Egeland GM, Cao Z, Young TK. Hypertriglyceridemic-waist phenotype and glucose intolerance among Canadian Inuit: the International Polar Year Inuit Health Survey for Adults 2007–2008. CMAJ 2011;183:E553–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Du T, Sun X, Huo R, et al. Visceral adiposity index, hypertriglyceridemic waist and risk of diabetes: the China Health and Nutrition Survey 2009. Int J Obes (Lond) 2014;38:840–7. [DOI] [PubMed] [Google Scholar]
- [10].van der Kooy K, Seidell JC. Techniques for the measurement of visceral fat: a practical guide. Int J Obes Relat Metab Disord 1993;17:187–96. [PubMed] [Google Scholar]
- [11].Wu P, Zhang F, Dai Y, et al. Serum TNF-α, GTH and MDA of high-fat diet-induced obesity and obesity resistant rats. Saudi Pharm J 2016;24:333–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Allison DB, Zannolli R, Faith MS, et al. Weight loss increases and fat loss decreases all-cause mortality rate: results from two independent cohort studies. Int J Obes Relat Metab Disord 1999;23:603–11. [DOI] [PubMed] [Google Scholar]
- [13].Farr OM, Gavrieli A, Mantzoros CS. Leptin applications in 2015: what have we learned about leptin and obesity? Curr Opin Endocrinol Diabetes Obes 2015;22:353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 1999;341:879–84. [DOI] [PubMed] [Google Scholar]
- [15].Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 2002;110:1093–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 1999;282:1568–75. [DOI] [PubMed] [Google Scholar]
- [17].Ugrinska A, Miladinova D, Trajkovska M, et al. Correlation of serum leptin with anthropometric parameters and abdominal fat depots determined by ultrasonography in overweight and obese women. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2013;34:115–9. [PubMed] [Google Scholar]
- [18].Liu AG, Smith SR, Fujioka K, et al. The effect of leptin, caffeine/ephedrine, and their combination upon visceral fat mass and weight loss. Obesity (Silver Spring) 2013;21:1991–6. [DOI] [PubMed] [Google Scholar]
- [19].Legradi G, Emerson CH, Ahima RS, et al. Leptin prevents fasting-induced suppression of prothyrotropin releasing hormone messenger ribonucleic acid in neurons of th hypothalamic paraventricular nucleus. Endocrinol 1997;138:2569–76. [DOI] [PubMed] [Google Scholar]
- [20].Nazifi S, Saeb M, Sepehrimanesh M, et al. The effects of wild pistachio oil on serum leptin, thyroid hormones, and lipid profile in female rats with experimental hypothyroidism. Comp Clin Pathol 2012;21:851–7. [Google Scholar]
- [21].Kishida K, Funahashi T, Shimomura I. Adiponectin as a routine clinical biomarker. Best Pract Res Clin Endocrinol Metab 2014;28:119–30. [DOI] [PubMed] [Google Scholar]
- [22].Vazzana N, Guagnano MT, Cuccurullo C, et al. Endogenous secretory RAGE in obese women: association with platelet activation and oxidative stress. J Clin Endocrinol Metab 2012;97:E1726–30. [DOI] [PubMed] [Google Scholar]
- [23].Vasudevan A, Bottiglieri T, Tecson KM, et al. Residual thromboxane activity and oxidative stress: influence on mortality in patients with stable coronary artery disease. Coron Artery Dis 2017;28:287–93. [DOI] [PubMed] [Google Scholar]
- [24].Mure K, Yoshimura N, Hashimoto M, et al. Urinary 8-iso-prostaglandin F2α as a marker of metabolic risks in the general Japanese population: The ROAD study. Obesity (Silver Spring) 2015;23:1517–24. [DOI] [PubMed] [Google Scholar]
- [25].Sun L, Gao Y, Zhang W, et al. Effect of high fluoride and high fat on serum lipid levels and oxidative stress in rabbits. Environ Toxicol Pharmacol 2014;38:1000–6. [DOI] [PubMed] [Google Scholar]
- [26].Abbasian M, Delvarianzadeh M, Ebrahimi H, et al. Relationship between serum levels of oxidative stress and metabolic syndrome components. Diabetes Metab Syndr 2018;12:497–500. [DOI] [PubMed] [Google Scholar]
- [27].Codoner-Franch P, Navarro-Ruiz A, Fernandez-Ferri M, et al. A matter of fat: insulin resistance and oxidative stress. Pediatr Diabetes 2012;13:392–9. [DOI] [PubMed] [Google Scholar]
- [28].Gallardo JM, Gomez-Lopez J, Medina-Bravo P, et al. Maternal obesity increases oxidative stress in the newborn. Obesity (Silver Spring) 2015;23:1650–4. [DOI] [PubMed] [Google Scholar]


