Supplemental Digital Content is available in the text
Keywords: activities of daily living, meta-analysis, rehabilitation, stroke, subacute interventions, umbrella review
Abstract
Background:
Stroke is a leading cause of disabilities worldwide. One of the key disciplines in stroke rehabilitation is physical therapy which is primarily aimed at restoring and maintaining activities of daily living (ADL). Several meta-analyses have found different interventions improving functional capacity and reducing disability.
Objectives:
To systematically evaluate existing evidence, from published systematic reviews of meta-analyses, of subacute physical rehabilitation interventions in (ADLs) for stroke patients.
Methods:
Umbrella review on meta-analyses of RCTs ADLs in MEDLINE, Web of Science, Scopus, Cochrane, and Google Scholar up to April 2018. Two reviewers independently applied inclusion criteria to select potential systematic reviews of meta-analyses of randomized controlled trials (RCTs) of physical rehabilitation interventions (during subacute phase) reporting results in ADLs. Two reviewers independently extracted name of the 1st author, year of publication, physical intervention, outcome(s), total number of participants, and number of studies from each eligible meta-analysis. The number of subjects (intervention and control), ADL outcome, and effect sizes were extracted from each study.
Results:
Fifty-five meta-analyses on 21 subacute rehabilitation interventions presented in 30 different publications involving a total of 314 RCTs for 13,787 subjects were identified. Standardized mean differences (SMDs), 95% confidence intervals (fixed and random effects models), 95% prediction intervals, and statistical heterogeneity (I2 and Q test) were calculated. Virtual reality, constraint-induced movement, augmented exercises therapy, and transcranial direct current stimulation interventions resulted statistically significant (P < .05) with moderate improvements (0.5 ≤ SMD ≤ 0.8) and no heterogeneity (I2 = 0%). Moxibustion, Tai Chi, and acupuncture presented best improvements (SMD > 0.8) but with considerable heterogeneity (I2 > 75%). Only acupuncture reached “suggestive” level of evidence.
Conclusion:
Despite the range of interventions available for stroke rehabilitation in subacute phase, there is lack of high-quality evidence in meta-analyses, highlighting the need of further research reporting ADL outcomes.
1. Introduction
Stroke is the 2nd cause of mortality and the 3rd cause of long-term disability worldwide with 33 million stroke survivors.[1] Mortality is declining, yet prevalence is stable, meaning there are more survivors with long-term disability.[2] Using Global Burden of Disease incidence rates, between 2015 and 2035, it is projected that the number of stroke survivors in the European Union will rise from 3,718,785 in 2015 to 4,631,050 in 2035, an increase of 25%.[3] In the United States, approximately every 40 seconds someone experiences a stroke, as death rates have declined, stroke has become the leading cause of long-term disability.[4]
Interdisciplinary complex rehabilitation interventions are assumed to represent the mainstay of poststroke care.[5] One of the key disciplines in interdisciplinary stroke rehabilitation is physical therapy which is primarily aimed at restoring and maintaining activities of daily living (ADL).[6] In this work, we consider physical therapy as “therapeutic modalities frequently used in physical therapy specialty by physical therapists or physiotherapists to promote, maintain, or restore the physical and physiological well-being of an individual” (Mesh, MEDLINE Subject Heading). Although stroke patients at an early stage depend on a stroke unit in the acute hospital, their functional recovery and long-term health status are more affected by subacute (1–6 months) rehabilitation hospital.[7]
Several meta-analyses have found different interventions improving functional capacity and reducing disability.[7] A recent umbrella review[8] summarizes the effects of exercise therapy on functional capacity in patients considering 22 different chronic diseases. Eighty-five meta-analyses were included, nevertheless only 11 of them reported outcomes on stroke of which 5 refer to ADLs, in our work we aim to update (only 6 of the 11 studies are dated after 2013) and extend that body of knowledge.
Veerbeek and colleagues[9] retrieved randomized controlled trials (RCTs) regarding physical therapy in stroke. In their analysis, most database searches (90%) were performed by mid-2011. In this work, we aim to link interventions specifically to ADLs and include new therapeutic approaches in which physical exercise is combined with innovative treatments enhancing neuroplasticity, such as transcranial direct current stimulation[10] or repetitive peripheral magnetic stimulation.[11]
A nonspecific systemic inflammatory response occurs after both ischemic and hemorrhagic stroke, either as part of the process of brain damage or in response to complications such as deep venous thrombosis.[12] Inflammation may be important both before, in predisposing to a stroke, and afterwards, where it takes part in the mechanisms of cerebral injury and repair.[13,14] Clinically, the susceptibility of the patients to stroke and the subsequent prognosis are influenced by such inflammatory processes.[15–17] As stroke patients with systemic inflammation have been reported to exhibit clinically poorer outcomes,[18] in this work, we will report mentions to inflammatory processes in the identified meta-analyses.
To the best of our knowledge, no attempts of reviewing the existing literature through an umbrella review in stroke rehabilitation has been conducted. Umbrella review offers the possibility to analyze the strength of evidence and extent of potential biases in the association between physical interventions in subacute rehabilitation and ADL outcomes.
2. Methods
2.1. Literature search
According to the Joanna Briggs Institute Umbrella Review Methodology,[19] literature search was conducted independently by 2 reviewers (AGR, DSP) in MEDLINE (2000 through April 2018), Web of Science (through April 2018), Scopus (through April 2018), Cochrane database of systematic reviews, and Google Scholar (up to mid-April 2018). The search strategy included combinations of multiple search terms for 2 themes: Stroke and interventions (rehabilitation). The keywords used to search for studies for this review are listed in Appendix Search strategy. All meta-analyses registered in these databases that reported a systematic electronic search of literature for a defined period of time were included. Bibliographies of identified articles and manual search of relevant journals for additional references was conducted. The most updated or complete publication was used when more than an article was present for a single study. In addition, separate meta-analyses on multiple outcomes presented in a single article were assessed separately. Gray literature search was conducted using different internet search engines and websites: such as Google Scholar.
2.2. Inclusion and exclusion criteria
Studies were included if they met the following criteria, established by using the PICOS (Population, Intervention, Comparison, Outcome, Study Design)[19] strategy presented in Appendix - Supplementary Table 1.
Study design: meta-analyses (quantitative analysis) of RCTs in subacute rehabilitation phase (1–6 months after onset).
Study population: stroke patients in subacute rehabilitation phase >18 years.
Outcomes: ADL outcome scales, regarded as continuous scaled, where usually higher scores indicate a good outcome, for example, functional independence measure,[20] Barthel index,[21] modified Rankin scale,[22] Frenchay activities index,[23] Rivermead ADL Assessment,[24] Katz index of independence in ADL,[25] motor activity log,[26] and modified Barthel index.[27] Eligible articles were required to be meta-analyses of RCTs, have outcome measures related to ADLs, compare physical therapy with no treatment or usual care, have adult participants, as defined by the Cochrane Collaboration,[28] and have stroke patients as defined by World Health Organization.[29] Meta-analyses were excluded if intervention did not clearly take place during subacute rehabilitation phase (considering the following criteria as in related research[30] acute: <1 month after stroke, subacute: 1 to 6 months after stroke and chronic: more than 6 months after stroke), they did not report the number of studies or participants in experimental or control groups, they assessed postsurgical recovery or site-specific musculoskeletal conditions such as patellofemoral pain syndrome (although physical therapy may be a standard treatment). We excluded meta-analyses that did not present study-specific data (effect size and 95% confidence intervals [CIs]).
2.3. Data extraction
We extracted the name of the 1st author, year of publication, physical intervention, ADL outcome(s), and number of studies from each eligible meta-analysis. From each individual study in a meta-analysis, we extracted the 1st author, year of publication, total number of subjects assigned to the intervention and to the control groups, mean value and standard deviation of ADL outcome, and maximally adjusted effect size measurements (mean difference [MD] or standardized MD [SMD]) along with the corresponding 95% CI. Two investigators (AGR, DSP) independently searched the literature, assessed the eligibility of the retrieved papers, and extracted the data using a standard pro-form. Disagreements were resolved by consensus with a third senior investigator (EOS or JMT).
2.4. Assessment of methodological quality of included studies
Both reviewers independently assessed the methodological quality of each review, using the AMSTAR (Assessment of Multiple Systematic Reviews) appraisal tool.[31] Disagreements were resolved by consensus with a 3rd senior investigator (EOS or JMT) (see Appendix Supplementary Table 3 for details).
2.5. Data analysis
Estimation of summary effect: The summary effect size and its CIs by 95% were estimated using both fixed effects and random effects models for each meta-analysis,[32] by using the Cochrane Collaboration's Review Manager V.5.3.
Assessment of heterogeneity: Heterogeneity between studies was assessed with Cochran Q test[33] and the I2 statistic.[34] For interpreting I2, we follow related research criteria[34]: I2 = 0% no heterogeneity, I2 = 25% low heterogeneity and when I2 exceeds 50% or 75%, the heterogeneity is considered substantial or considerable, respectively.
Estimation of prediction intervals: For the summary random effects, we estimated the 95% prediction interval (PI),[35] we therefore can report the range of effects across study settings, providing a more complete picture for clinical practice.
Grading the evidence: For each rehabilitation intervention, the data were analyzed qualitatively based on the SMDs of each included meta-analysis. The SMDs were evaluated using forest plot and graded as small (SMD < 0.5), moderate (SMD 0.5–0.8), and large (SMD > 0.8) effect sizes.[36] The significance of the results (P < .05) was judged based on the 95% CIs of the SMDs.
Publication bias: Studies published in peer-reviewed journals are much more likely to report statistically significant results than are studies that report a nonsignificant conclusion, especially for smaller studies. Publication bias is assessed checking for asymmetry in funnel plots.[37]
This study was approved by Institut Guttmann Local Ethics Committee.
2.6. Patient involvement
No patients were involved in setting the research question or outcome measures, nor were they involved in developing plans for design or implementation of the study.
3. Results
3.1. Literature review
The search retrieved 251 published systematic reviews evaluating rehabilitation interventions. Of these, 88 reviews met the abstract inclusion criteria and were selected for closer scrutiny. Full texts of these articles were retrieved and both reviewers performed the final selection. Overall, 11 reviews published in Cochrane Library database and 19 published in other academic journals were included. A PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) of the study selection process is provided in Figure 1.
Figure 1.
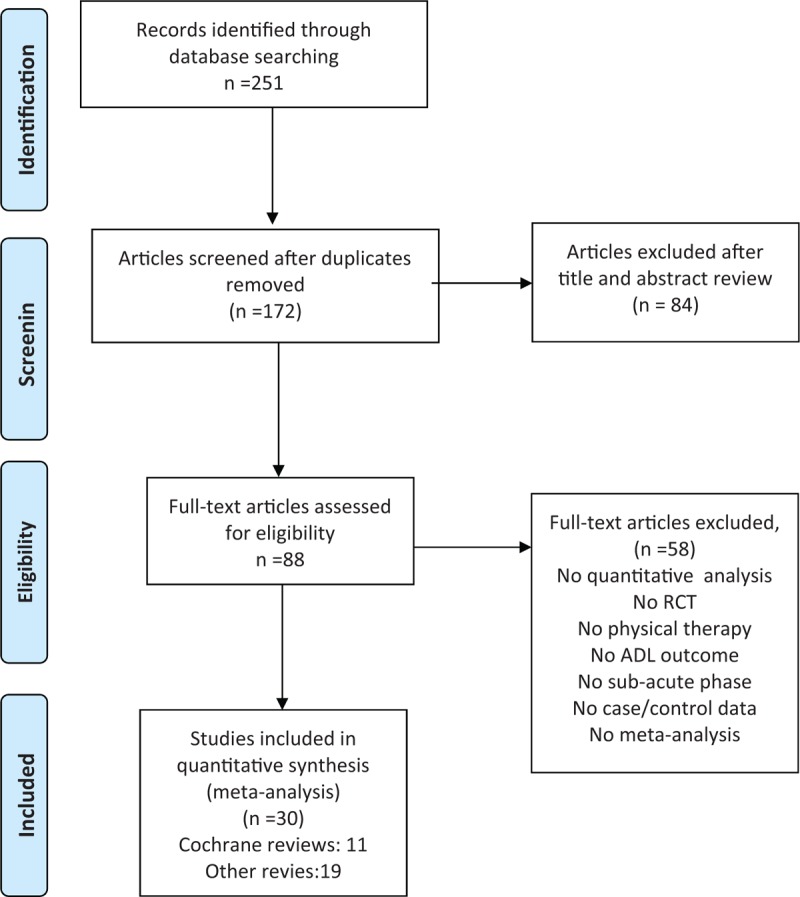
PRISMA Flow diagram of the study selection process.[19]
In Appendix Excluded, we present a list of a selection of excluded publications, all dated ≥2015 and most of them addressing interventions already included in the final selection that had to be left out for the reasons presented in Figure 1.
3.2. Description of meta-analyses
A total of 55 meta-analyses on 21 subacute rehabilitation interventions presented in 30 different publications[1,10,11,38–65] (Table 1 ) involving a total of 314 RCTs were included in this umbrella review. Meta-analyses included a median of 5 studies (ranging from 1 to 19). Robotic training and CIM (constraint induced movement) are the interventions with the highest number of meta-analyses (10 each), followed by Acupuncture and transcranial direct current stimulation (tDCS) with 6 and 5, respectively, then modified CIM and Virtual Reality with 3, the rest of interventions have been studied in 1 or 2 meta analyses.
Table 1.
Summary effect calculations, studies, sample size (cases and controls), 95% prediction interval (PI) and heterogeneity.
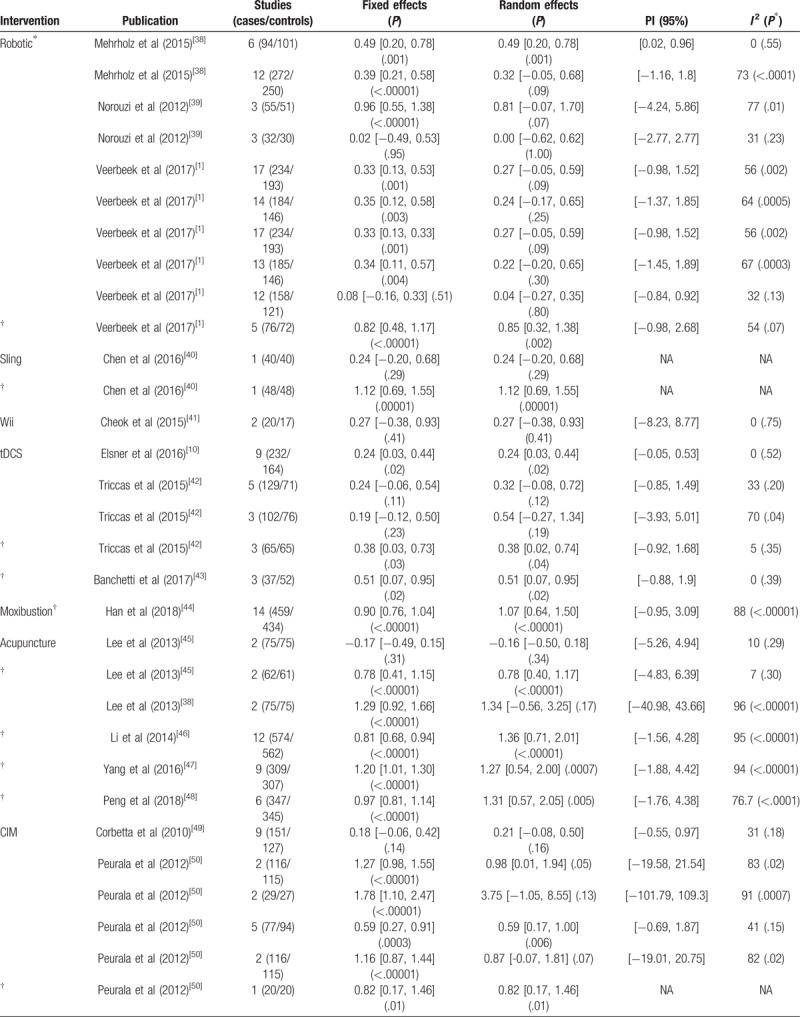
The total number of subjects in the included RCTs was 13,787 (7167 cases and 6,620 controls) and the median number of participants was 171 (range: 30–1136). In Appendix Supplementary Table 1, we present for each intervention the total number of cases and controls, almost 80% of subjects have participated in 7 of the 21 interventions (acupuncture, robotic, CIM, tDCS, moxibustion, repetitive task training [RTT], and mirror therapy).
For results that were not presented as SMD in the original meta-analysis, the Cochrane Collaboration's Review Manager V.5.3 was used to convert the outcomes to SMD to allow visual comparison of the results in a forest plot (presented in Supplementary Material along with forest plots for fixed and random effect sizes for all 55 meta-analyses).
3.3. Summary effect size
The evaluation of the level of significance for both random and fixed effect calculations, the number of studies, sample size (cases and controls), the 95% PI and heterogeneity (I2 and Q test level of significance) are reported in Table 1 for the resulting 55 meta-analyses, grouped by rehabilitation interventions. Thirty of the 55 meta-analyses (54%) reported nominally statistically significant findings (P < .05) in both fixed and random effect sizes denoted with † in Table 1 . Sixteen (77%) of the 21 interventions reported statistically significant results in both fixed and random effects in at least 1 meta-analysis.
Bilateral training, imagery training, repetitive transcranial magnetic stimulation (rTMS), repetitive peripheral magnetic stimulation (rPMS), and Nintendo Wii training did not report statistically significant results in any of their meta-analyses. Based only on the fixed effects model, 44 meta-analyses (80%) gave nominally statistically significant findings (P < .05). Wii, bilateral training, and rPMS did not report statistically significant results in any of their meta-analyses. Thirty-one meta-analyses (57%) gave a nominally significant findings based on the random effects model. Wii, bilateral training, imagery, rTMS, and rPMS did not report statistically significant results in any of their meta-analyses. Only Wii, bilateral training, and rPMS did not report statistically significant results neither in random nor in fixed effects models. At a stricter threshold of P < .001, 27 (49%) and 13 (24%) meta-analyses produced significant summary results using the fixed and random effects models, respectively. At P < 10−6, 23 (42%) and 6 (11%) meta-analyses were significant, respectively. In general, the magnitude of the observed summary estimates were small to moderate with 30% of the estimates yielding an effect size >0.8 as shown in Figure 2.
Figure 2.

Association of the meta-analysis summary random effects estimate with the inverse of the standard error.
Negative effects summaries were reported in 4 meta-analyses from 3 interventions (acupuncture [2], CIM [1], and rPMS [1]).
3.4. Heterogeneity between studies
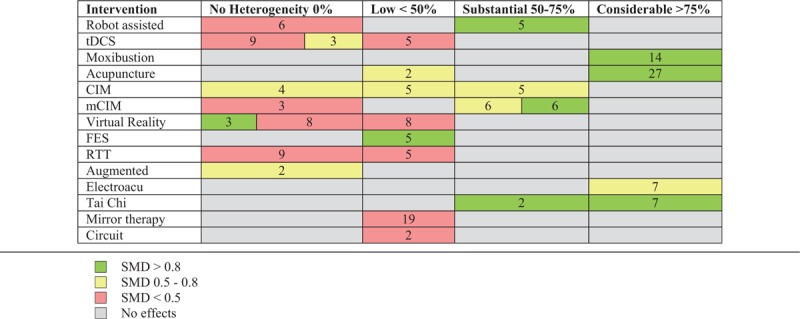
Q test was significant at P ≤ 0.10 in 24 of the 55 meta-analyses (44%). Eleven meta-analyses (20%) showed no heterogeneity (I2 = 0%) for several interventions (robotic, Wii, tDCS, CIM, mCIM, virtual reality, RTT, bilateral, and augmented). Thirteen meta-analyses (24%) showed low heterogeneity (I2 < 50%) in robotic, tDCS, acupuncture, CIM, virtual reality, functional electrical stimulation (FES), RTT, mirror therapy, and circuit interventions. Substantial heterogeneity (50% ≤ I2 ≤ 75%) is present in 11 (20%) meta-analyses addressing 5 interventions (robotic, tDCS, CIM, mCIM, Tai Chi). Considerable heterogeneity (I2 > 75) is shown in 15 (27%) meta-analyses in 8 interventions (robotic, moxibustion, acupuncture, CIM, imagery, ElectroAcu, Tai Chi, rTMS). Table 2 shows the number of statistical significant studies at the 4 levels of heterogeneity of small (SMD < 0.5), moderate (SMD 0.5–0.8), and large (SMD > 0.8) effect sizes.
Table 1 (Continued).
Summary effect calculations, studies, sample size (cases and controls), 95% prediction interval (PI) and heterogeneity.
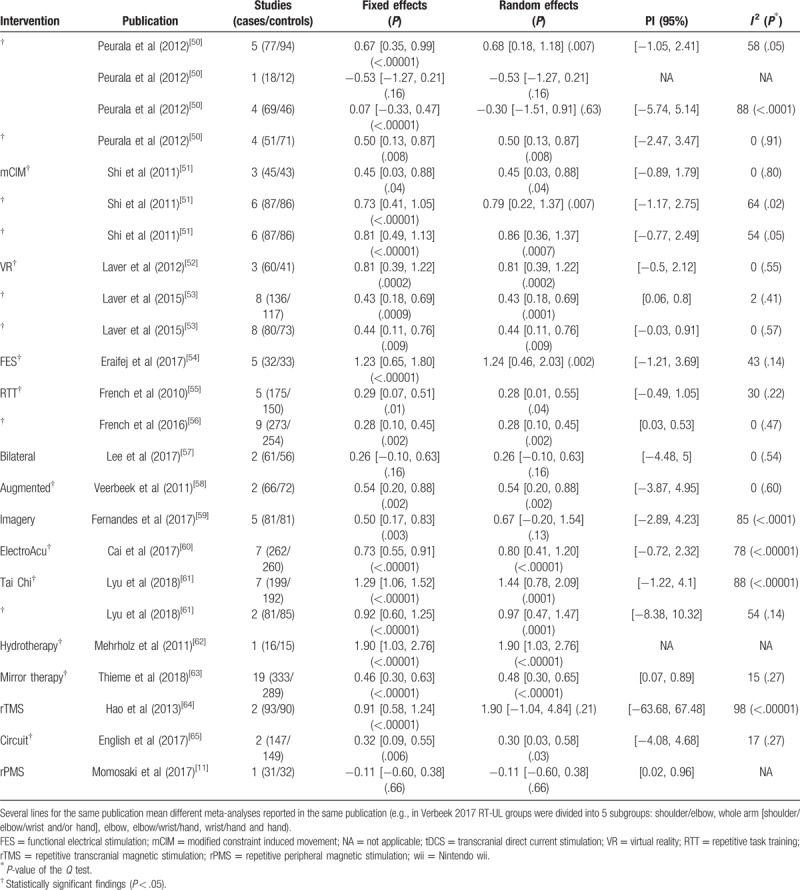
Table 2.
Summary of the number of studies for small, moderate, and large effect sizes grouped by heterogeneity level for the different intervention.
3.5. Assessment of methodological quality of included studies (AMSTAR)
Twenty-nine (94%) of the included publications are rated high quality (mean value 9.61, see Appendix Supplementary Table 3). All meta-analyses provided an “a priori” design, performed a literature search in at least 2 electronic databases, reported duplicate study selection and made it possible to replicate the literature search, but 20% did not report the inclusion of grey literature, 7% did not report conflict of interest, and 20% did not assess the scientific quality of the included studies. Appendix Supplementary Table 4, shows top rated interventions: circuit, FES, hydrotherapy, mirror therapy, rPMS, rTMS, tCDS, and virtual reality.
3.6. Prediction intervals
We calculated 95% PI, as presented in Table 1 ; the null value was excluded in only 10 meta-analyses (from a total of 55).
Figure 3 shows the forest plot of the meta-analyses with significant (P < .05) summary fixed and random effects with random effects 95% PI excluding null value, along with the number of studies (treatments and controls) implemented in forest plot R package.[66]
Figure 3.
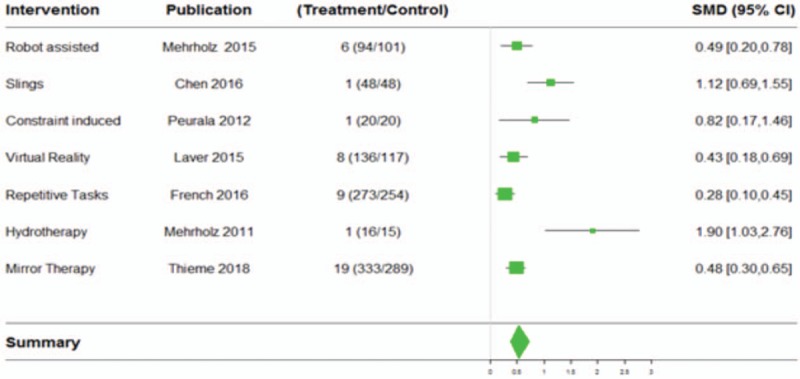
Forest plot for meta-analysis with significant (P < .05) summary fixed and random effects with random effects 95% prediction interval excluding null value.
In Appendix Supplementary Figure 1, we included the funnel plots (all of them presenting visually acceptable symmetry) for the 4 meta-analyses with more than 1 study from Figure 3 to visually assess symmetry (these funnel plots are extracted from Supplementary Material, where we present all funnel plots for all selected meta-analyses).
For publications with P < .05 and number of studies ≥9,
we performed regression analysis with meta R package[67] on the number of participants in experimental groups and the year of publication covariates, as in similar previous umbrella reviews.[68] We identified 2 studies [47,63] with P-value <.05 for the total participants in experimental group covariate. As shown in Appendix Supplementary Table 5, 2.28% and 8.21%, respectively, of effect size is explained by the number of participants in the experimental group.
we performed sensitivity analysis with meta R package[67] to explain high heterogeneity (publications with P < .05 and number of studies ≥9). Therefore, we analyzed Han et al[44] (89%), Li et al[46] (95%), and Yang et al[47] (94%). We obtained our best results for Li et al,[46] when omitting Wu study (2011), heterogeneity declines from 95% to 89%. In the other 2 publications, best reductions are only 1% to 2%.
-
we performed subgroup analysis (by using the Cochrane Collaboration's Review Manager V.5.3) on those publications with P < .05 and number of studies ≥9 grouping by year of publication. For example, in Thieme et al,[63] we performed subgroup analysis with 3 groups:
-
∘
Group 1: 2008 to 2012 (6 studies)
-
∘
Group 2: 2013 to 2014 (7 studies)
-
∘
Group 3: 2015 to 2018 (6 studies)
-
∘
Differences could be identified within the 3 periods because of technology evolution in the administration of mirror therapy interventions (e.g., virtual reality technologies). But as shown in page 83 of Supplementary Material, no significant subgroup differences were identified. We proceeded similarly with other publications[44,47] with no significant differences either.
Details of meta-regression, sensitivity, and subgroups analysis are presented in Supplementary Material.
In Appendix Supplementary Figure 2, we included a work design diagram showing our whole analysis process, taking as starting point the outcome of PRISMA flow diagram of the study selection process presented in Figure 1.
4. Discussion
It is recommended[69] that the 95% PI should be routinely reported to allow more informative inferences in meta-analyses. It presents the expected range of true effects in similar studies, therefore in 95% of cases the true effect of a new study (assessing the impact of a physical intervention in ADLs) will fall within the PI values. In our case, most PIs contain null values, leading us to consider that although therapists’ interventions are effective on average to increase performance in ADLs most of them may not be effective because the null value was excluded in only 10 (with 5 of them NA) of the 55 meta-analyses.
Considering recent umbrella reviews in medical field (e.g., Cancer or Parkinson disease)[70] “highly convincing” evidence requires >1000 cases, P < 10–6 by random effects, 95% PI excluding the null and not large heterogeneity (I2 < 50%). “Suggestive” evidence requires >1000 cases and P < .001 by random effects. Only acupuncture meta-analysis reached such thresholds, leaving the rest of studies included in this work at the “weak” level, pointing out the need of new RCTs assessing ADLs.
After full-text review of the 30 included studies, we did not find mentions to inflammatory process. Clinical outcomes after stroke are highly variable, and reasons for these variations are often unexplained.[71] Recovery after ischemic or hemorrhagic stroke begins immediately after acute onset, and several different levels of biological responses are involved. Genetic factors also influence many different aspects of brain function and repair, as well as recurrent stroke risk and response to interventions, and can account for unexplained variation in stroke recovery.[71] Up to 30% of ischemic strokes remain unexplained after thorough investigation.[72] Cryptogenic stroke is more common in patients with stroke occurring at a young age, defined as age 55 or less. For example, it has been reported that approximately 1% to 2% of young stroke patients are demonstrated with Fabry disease,[72] a rare inherited disorder of the metabolism, associated with renal, cardiac, and cerebrovascular complications.[73,74] With such a multitude of molecular events being related to recovery, not surprisingly a number of genes have been suggested as important to variability in stroke recovery. Genetic variation in any of these components might influence each individual's capacity for brain plasticity and could explain the variability in motor rehabilitation efficacy.
4.1. Study limitations
We did not publish a protocol for this study. We only included systematic reviews with meta-analysis (we took this approach as in recent reviews related to health).[75]
5. Conclusion
Moxibustion, acupuncture, and Tai Chi not only show large SMD values and large number of participants, but also the highest values of heterogeneity (I2 > 75), when interventions are grouped according to the four levels of heterogeneity of small (SMD < 0.5), moderate (SMD 0.5–0.8), and large (SMD > 0.8) effect sizes considering the significance of the results (P < .05) based on the 95% CIs of the SMDs for both fixed and random effects.
Robot assisted, virtual reality, tDCS, and RTT present small and moderate effect sizes but without heterogeneity (I2 = 0). The total number of participants in the included RCTs was 13,787 and the median number was 171 with almost 80% of subjects participating in only 7 of the 21 interventions (acupuncture, robotic, CIM, tDCS, moxibustion, RTT, and mirror therapy), meaning that 80% of participants are recruited in only 30% of interventions. Besides, when categorizing our evidence according to state-of-the art thresholds only weak levels of evidence are reached in almost all of the included studies.
Acupuncture as a complementary therapy has increased worldwide and has become widely applied to stroke rehabilitation.[76] Similarly, Tai Chi has been applied in stroke rehabilitation for over 10 years worldwide.[61] Moxibustion has been less studied, being noninvasive it might be more applicable in other cultures.[44] Our results encourage further RCTs into them.
Future research could analyze the excess significance bias, whether observed number of studies with statistically significant results is different from the expected number of them[77] and extend subgroup analyses considering gender and/or age differences.
Acknowledgments
The authors thank Olga Araujo from Institute Guttmann's Centre de Documentació en Neurorehabilitació Santi Beso Arnalot for her continuous support in publications retrieval.
Author contributions
Conceptualization: Eloy Opisso Salleras, Josep Maria Tormos.
Data curation: Alejandro Garcia-Rudolph, David Sanchez-Pinsach.
Formal analysis: Alejandro Garcia-Rudolph.
Funding acquisition: Josep Maria Tormos.
Investigation: Alejandro Garcia-Rudolph.
Methodology: Alejandro Garcia-Rudolph.
Project administration: Eloy Opisso Salleras.
Resources: Eloy Opisso Salleras.
Software: Alejandro Garcia-Rudolph, David Sanchez-Pinsach.
Supervision: Josep Maria Tormos.
Writing – original draft: Alejandro Garcia-Rudolph.
Writing – review & editing: Alejandro Garcia-Rudolph, Eloy Opisso Salleras, Josep Maria Tormos.
Supplementary Material
Footnotes
Abbreviations: ADLs = activities of daily living, AMSTAR = assessment of multiple systematic reviews, CI = confidence interval, CIM = constraint-induced movement, FES = functional electrical stimulation, GBD = global burden of disease, MD = mean difference, PI = prediction interval, PICOS = population, intervention, comparison, outcome, study design, RCT = randomized controlled trial, rPMS = repetitive peripheral magnetic stimulation, rTMS = repetitive transcranial magnetic stimulation, RTT = repetitive task training, SMD = standard mean difference, tDCS = transcranial direct current stimulation.
This research was partially funded by EU H2020 PRECISE4Q - Personalized Medicine by Predictive Modeling in Stroke for better Quality of Life (Grant Agreement 777107 – Research and Innovation Action).
The authors have no conflicts of interest to disclose.
References
- [1].Veerbeek JM, Langbroek-Amersfoort AC, van Wegen EE, et al. Effects of robot-assisted therapy for the upper limb after stroke. Neurorehabil Neural Repair 2017;31:107–21. [DOI] [PubMed] [Google Scholar]
- [2].Wattchow KA, McDonnell MN, Hillier SL. Rehabilitation interventions for upper limb function in the first four weeks following stroke: a systematic review and meta-analysis of the evidence. Arch Phys Med Rehabil 2018;99:367–82. [DOI] [PubMed] [Google Scholar]
- [3].The Burden of Stroke in Europe Report. Overview of stroke burden and care in each EU and SAFE member country (2017). ISBN 978-1-5272-0857-5 Stroke Alliance for Europe -Stroke Association House 240 City Road, London. [Google Scholar]
- [4].Benjamin E. Heart disease and stroke statistics—2017 update a report from the American Heart Association. Circulation 2017;135:e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Langhorne P, Legg L. Evidence behind stroke rehabilitation. J Neurol Neurosurg Psychiatry 2003;74Suppl 4:iv18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet 2011;377:1693–702. [DOI] [PubMed] [Google Scholar]
- [7].Lee SH. (ed), Stroke Revisited: Diagnosis and Treatment of Ischemic Stroke, Stroke Revisited, Rehabilitation in Sub-acute and Chronic Stage After Stroke Han-Young Jung (2011). [Google Scholar]
- [8].Pasanen T, Tolvanen S, Heinonen A, et al. Exercise therapy for functional capacity in chronic diseases: an overview of meta-analyses of randomised controlled trials. Br J Sports Med 2017;51:1459–65. [DOI] [PubMed] [Google Scholar]
- [9].Veerbeek JM, van Wegen E, van Peppen R, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One 2014;9:e87987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Elsner B, Kugler J, Pohl M, et al. Transcranial direct current stimulation (tDCS) for improving activities of daily living, and physical and cognitive functioning, in people after stroke. Cochrane Database of Syst Rev 2016, Issue 3. Art. No.: CD009645. DOI: 10.1002/14651858. CD009645.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Momosaki R, Yamada N, Ota E, et al. Repetitive peripheral magnetic stimulation for activities of daily living and functional ability in people after stroke. Cochrane Database of Syst Rev 2017, Issue 6. Art. No.: CD011968. doi: 10.1002/14651858.CD011968.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].De Simoni MG, Milia P, Barba M, et al. The inflammatory response in cerebral ischemia: focus on cytokines in stroke patients. Clin Exp Hypertens 2002;24:535–42. [DOI] [PubMed] [Google Scholar]
- [13].Hedley CA, Emsley, Pippa J. Tyrrell Inflammation and Infection in Clinical Stroke Journal of Cerebral Blood Flow & Metabolism 22:1399-1419.© 2002 The International Society for Cerebral Blood Flow and Metabolism. [DOI] [PubMed] [Google Scholar]
- [14].Tuttolomondo A, Di Sciacca R, Di Raimondo D, et al. Effects of clinical and laboratory variables and of pretreatment with cardiovascular drugs in acute ischaemic stroke: a retrospective chart review from the GIFA study. Int J Cardiol 2011;151:318–22. [DOI] [PubMed] [Google Scholar]
- [15].Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol 2008;7:341–53. [DOI] [PubMed] [Google Scholar]
- [16].McColl BW, Allan SM, Rothwell NJ. Systemic infection, inflammation and acute ischemic stroke. Neuroscience 2009;158:1049–61. [DOI] [PubMed] [Google Scholar]
- [17].Di Raimondo D, Tuttolomondo A, Buttà C, et al. Effects of ACE-inhibitors and angiotensin receptor blockers on inflammation. Curr Pharm Des 2012;18:4385–413. [DOI] [PubMed] [Google Scholar]
- [18].Elkind MS, Cheng J, Rundek T, et al. Leukocyte count predicts outcome after ischemic stroke: the Northern Manhattan Stroke Study. J Stroke Cerebrovasc Dis 2004;13:220–7. [DOI] [PubMed] [Google Scholar]
- [19].Aromataris E, Fernandez R, Godfrey C, et al. Methodology for JBI umbrella reviews. Joanna Briggs Institute Reviewers’ Manual: 2014 edition/Supplement ( 1-34). Australia: The Joanna Briggs Institute. [Google Scholar]
- [20].Hamilton BB, Laughlin JA, Fiedler RC, et al. Interrater reliability of the 7-level functional independence measure (FIM). Scand J Rehabil Med 1994;26:115–9. [PubMed] [Google Scholar]
- [21].Wade DT, Hewer RL. Functional abilities after stroke: measurement, natural history and prognosis. J Neurol Neurosurg Psychiatry 1987;50:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bonita R, Beaglehole R. Modification of Rankin scale: recovery of motor function after stroke. Stroke 1988;19:1497–500. [DOI] [PubMed] [Google Scholar]
- [23].Schuling J, De Haan R, Limburg M, et al. The Frenchay activities index: assessment of functional status in stroke patients. Stroke 1993;24:1173–7. [DOI] [PubMed] [Google Scholar]
- [24].Whiting S, Lincoln N. An ADL assessment for stroke patients. Br J Occup Ther 1980;43:44–6. [Google Scholar]
- [25].Hartigan I. A comparative review of the Katz ADL and the Barthel Index in assessing the activities of daily living of older people. Int J Older People Nurs 2007;2:204–12. [DOI] [PubMed] [Google Scholar]
- [26].Uswatte G, Taub E, Morris D, et al. Reliability and validity of the upper-extremity motor activity log-14 for measuring real-world arm use. Stroke 2005;36:2493–6. [DOI] [PubMed] [Google Scholar]
- [27].Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol 1989;42:703–9. [DOI] [PubMed] [Google Scholar]
- [28].Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011], 2011. www.handbook.cochrane.org Accessed April 2018. [Google Scholar]
- [29].Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ 1976;54:541–53. [PMC free article] [PubMed] [Google Scholar]
- [30].Peurala SH, Karttunen AH, Sjögren T, et al. Evidence for the effectiveness of walking training on walking and self-care after stroke: a systematic review and meta-analysis of randomized controlled trials. J Rehabil Med 2014;46:387–99. [DOI] [PubMed] [Google Scholar]
- [31].Shea BJ, Bouter LM, Peterson J, et al. External validation of a measurement tool to assess systematic reviews (AMSTAR). PLoS One 2007;2:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [33].Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101–29. [Google Scholar]
- [34].Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- [36].Cohen J. Statistical Power Analysis for the Behavioral Sciences. London: Academic Press; 1988. [Google Scholar]
- [37].Mlinarić A, Horvat M, Šupak Smolčić V. Dealing with the positive publication bias: why you should really publish your negative results. Biochem Med 2017;27: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mehrholz J, Pohl M, Platz T, et al. Electromechanical and robot-assisted armtraining for improving activities of daily living, armfunction, and armmuscle strength after stroke. Cochrane Database of Systematic Reviews 2015, Issue 11. Art. No.: CD006876. doi: 10.1002/14651858.CD006876.pub4. [Google Scholar]
- [39].Norouzi-Gheidari N, Archambault PS, Fung J. Effects of robot-assisted therapy on stroke rehabilitation in upper limbs: systematic review and meta-analysis of the literature. J Rehabil Res Dev 2012;49:479–96. [DOI] [PubMed] [Google Scholar]
- [40].Chen L, Chen J, Peng Q, et al. Effect of sling exercise training on balance in patients with stroke: a meta-analysis. PLoS One 2016;11:e0163351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cheok G, Tan DM, Low A, et al. Is nintendo wii an effective intervention for individuals with stroke? A systematic review and meta-analysis. Journal of the American Medical Directors Association 2015;1611:923–32. [DOI] [PubMed] [Google Scholar]
- [42].Tedesco Triccas L, Burridge JH, Hughes AM, et al. Multiple sessions of transcranial direct current stimulation and upper extremity rehabilitation in stroke: a review and meta-analysis. Clin Neurophysiol 2016;127:946–55. [DOI] [PubMed] [Google Scholar]
- [43].Banchetti PA, Marini C. Efficacy of transcranial direct current stimulation as support for neurorehabilitation therapy: a metaanalysis of available studies. Int J Phys Med Rehabil 2017;5:417. [Google Scholar]
- [44].Han C-H, Ma JN, An N, et al. Moxibustion for stroke: systematic review, meta-analysis, and GRADE-based recommendations. Eur J Integrative Med 2018;20:115–25. [Google Scholar]
- [45].Lee S-J, Shina B-C, Lee MS, et al. Scalp acupuncture for stroke recovery: a systematic review and meta-analysis of randomized controlled trials. Eur J Integr Med 2012;5:87–99. [Google Scholar]
- [46].Li L, Zhang H, Meng S-q, et al. An updated meta-analysis of the efficacy and safety of acupuncture treatment for cerebral infarction. PLoS One 2014;9:e114057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yang A, Wu HM, Tang JL, et al. Acupuncture for stroke rehabilitation. Cochrane Database of Systematic Reviews 2016, Issue 8. Art. No.: CD004131. doi: 10.1002/14651858.CD004131.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Peng L, Zhang C, Zhou L, et al. Traditional manual acupuncture combined with rehabilitation therapy for shoulder hand syndrome after stroke within the Chinese healthcare system: a systematic review and meta-analysis. Clin Rehabil 2018;32:429–39. [DOI] [PubMed] [Google Scholar]
- [49].Corbetta D, Sirtori V, Moja L, et al. Constraint-induced movement therapy in stroke patients: systematic review and meta-analysis. Eur J Phys Rehabil Med 2010;46:537–44. [PubMed] [Google Scholar]
- [50].Peurala SH, Kantanen MP, Sjögren T, et al. Effectiveness of constraint-induced movement therapy on activity and participation after stroke: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil 2012;26:209–23. [DOI] [PubMed] [Google Scholar]
- [51].Shi YX, Tian JH, Yang KH, et al. Modified constraint-induced movement therapy versus traditional rehabilitation in patients with upper-extremity dysfunction after stroke: a systematic review and meta-analysis. Arch Phys Med Rehabil 2011;92:972–82. [DOI] [PubMed] [Google Scholar]
- [52].Laver K, George S, Thomas S, et al. Cochrane review: virtual reality for stroke rehabilitation. Eur J Phys Rehabil Med 2012;48:523–30. [PubMed] [Google Scholar]
- [53].Laver KE, George S, Thomas S. Virtual reality for stroke rehabilitation. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No.: CD008349. doi: 10.1002/14651858.CD008349.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Eraifej J, Clark W, France B, et al. Effectiveness of upper limb functional electrical stimulation after stroke for the improvement of activities of daily living and motor function: a systematic review and meta-analysis. Syst Rev 2017;6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].French B, Thomas LH, Leathley M, et al. Does repetitive task training improve functional activity after stroke? A Cochrane systematic review and meta-analysis. J Rehabilit Med 2010;42:9–14. [DOI] [PubMed] [Google Scholar]
- [56].French B, Thomas LH, Coupe J, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No.: CD006073. doi: 10.1002/14651858.CD006073.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lee Y, Kim MY, Park JH, et al. Comparison of the effects of bilateral and unilateral training after stroke: a meta-analysis. NeuroRehabilitation 2017;40:301–13. [DOI] [PubMed] [Google Scholar]
- [58].Veerbeek JM, Koolstra M, Ket JC, et al. Effects of augmented exercise therapy on outcome of gait and gait-related activities in the first 6 months after stroke: a meta-analysis. Stroke 2011;42:3311–5. [DOI] [PubMed] [Google Scholar]
- [59].Fernandez Guerra Z, Lucchetti ALG, Lucchetti G. Motor imagery training after stroke: a systematic review and meta-analysis of randomized controlled trials. J Neurol Phys Ther 2017;41:205–14. [DOI] [PubMed] [Google Scholar]
- [60].Cai, Zhang CS, Liu S et al. Electroacupuncture for poststroke spasticity: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2017; 98(12):2578-2589.e4. doi: 10.1016/j.apmr.2017.03.023. Epub 2017 Apr 25. [DOI] [PubMed] [Google Scholar]
- [61].Lyu D, Lyu X, Zhang Y, et al. Tai Chi for stroke rehabilitation: a systematic review and meta-analysis of randomized controlled trials. Front Physiol 2018;9:983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Mehrholz J, Kugler J, Pohl M. Water-based exercises for improving activities of daily living after stroke. Cochrane Database of Systematic Reviews 2011, Issue 1. Art. No.: CD008186. doi: 10.1002/14651858.CD008186.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Thieme H, Morkisch N, Mehrholz J, Pohl M, Behrens J, Borgetto B, Dohle C. Mirror therapy for improving motor function after stroke. Cochrane Database of Systematic Reviews 2018, Issue 7. Art. No.: CD008449. doi: 10.1002/14651858.CD008449.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hao Z, Wang D, Zeng Y, Liu M. Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Database of Systematic Reviews 2013, Issue 5. Art. No.: CD008862. doi: 10.1002/14651858.CD008862.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].English C, Hillier SL, Lynch EA. Circuit class therapy for improving mobility after stroke. Cochrane Database of Systematic Reviews 2017, Issue 6. Art. No.: CD007513. doi: 10.1002/14651858.CD007513.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gordon M, Lumley T. Advanced Forest Plot Using ‘grid’ Graphics. Available at: https://cran.r-project.org/web/packages/forestplot/forestplot.pdf Accessed August 20, 2018. [Google Scholar]
- [67].Schwarzer G. meta: An R Package for Meta-Analysis Available at: https://cran.rproject.org/web/packages/meta/meta.pdf Accessed August 20, 2018. [Google Scholar]
- [68].O'Sullivan JW, Muntinga T, Grigg S, et al. Prevalence and outcomes of incidental imaging findings: umbrella review. BMJ 2018;361:k2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].IntHout J, J.P.A., Rovers MM, et al. Plea for routinely presenting prediction intervals in meta-analysis BMJ Open 2016;6:e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Bellou V, Belbasis L, Tzoulaki I, et al. Environmental risk factors and Parkinson's disease: an umbrella review of meta-analyses. Parkinsonism Relat Disord 2016;23:1–9. [DOI] [PubMed] [Google Scholar]
- [71].Pearson-Fuhrhop KM, Burke E, Cramer SC. The influence of genetic factors on brain plasticity and recovery after neural injury. Curr Opin Neurol 2012;25:682–8. [DOI] [PubMed] [Google Scholar]
- [72].Feldt-Rasmussen U. Fabry disease and early stroke. Stroke Res Treat 2011;2011:615218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Tuttolomondo A, Pecoraro R, Simonetta I, et al. Neurological complications of Anderson-Fabry disease. Curr Pharm Des 2013;19:6014–30. Review. [DOI] [PubMed] [Google Scholar]
- [74].Tuttolomondo A, Pecoraro R, Simonetta I, et al. Anderson-Fabry disease: a multiorgan disease. Curr Pharm Des 2013;19:5974–96. [DOI] [PubMed] [Google Scholar]
- [75].Dinu M, Pagliai G, Casini A, et al. Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. Eur J Clin Nutr 2018;72:30–43. [DOI] [PubMed] [Google Scholar]
- [76].Gu J, Wang Q, Wang XG, et al. Assessment of registration information on methodological design of acupuncture RCTs: a review of 453 registration records retrieved from WHO International Clinical Trials Registry Platform. Evid Based Complement Alternat Med 2014;2014:614850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials 2007;4:245–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


