Abstract
Background:
Conflicting results have been reported on the prognostic significance of serum uric acid (SUA) in patients with acute heart failure (AHF). This meta-analysis aimed to determine the prognostic significance of SUA level in patients with AHF.
Methods:
We made a comprehensive literature search in Pubmed and Embase databases from inception to April 6, 2018. All available observational studies or post hoc analysis of randomized controlled trial that evaluated the prognostic value of SUA level in patients with AHF were eligible. Outcome of interests were all-cause mortality and the combined endpoint of death or readmission. Prognostic values of SUA level were summarized as higher vs lower SUA category or per 1 mg/ml SUA rise.
Results:
Ten studies involving 12,854 AHF patients were identified and analyzed. AHF patients with the highest SUA level had an increased risk of all-cause mortality (risk ratio [RR] 1.43; 95% confidence intervals [CI] 1.31–1.56) and combined endpoint of death or readmission (RR 1.68; 95% CI 1.33–2.13) after adjusting potential variables. In addition, per 1 mg/ml SUA rise significantly increased by 11% and 12% higher risk all-cause mortality and combined endpoint of death or readmission, respectively. A leave out 1 study sensitivity analysis confirmed the reliability of the pooling effect sizes.
Conclusion:
This meta-analysis indicates that elevated SUA level independently predicts all-cause mortality and the combined endpoint of death or readmission in AHF patients. Measurement of SUA level may improve risk stratification of adverse outcomes in these patients.
Keywords: all-cause mortality, major adverse cardiac events, meta-analysis, uric acid
1. Introduction
Heart failure is a worldwide public health concern. Acute heart failure (AHF) is a complex heterogeneous clinical syndrome.[1] Despite the advance of medical care, AHF is still associated with high death and readmission rate.[2] Therefore, early identification of AHF patients with poor prognosis is still an unmet need for better guide treatment. Accurate prediction of adverse outcomes of heart failure patients is an ongoing challenge.
Biomarkers can be used to refine the risk classification of AHF patients. Uric acid is a product of the metabolic breakdown of purine nucleotides.[3] Serum uric acid (SUA) is under investigation as prognostic biomarker in heart failure patients.[4,5] Previous 2 meta-analyses[6,7] have demonstrated that a higher level of SUA was a strong and independent predictor of all-cause mortality in patients with heart failure. However, this conclusion was mainly built on chronic heart failure patients and the strength of the prognostic value of SUA in AHF patients remains controversial. Nevertheless, magnitude of the reported prognostic value varied considerably.
Several new studies investigating the prognostic significance of SUA in AHF patients have been published, which instigated our efforts to conduct a focused meta-analysis. Therefore, we aimed to evaluate the prognostic value of SUA level among AHF patients in terms of all-cause mortality and the combined endpoint of death or readmission by performing a meta-analysis.
2. Materials and methods
2.1. Literature search
We made a comprehensive literature search in Pubmed and Embase databases from inception to April 6, 2018. Our search strategy included the following combined keywords:
“uric acid” OR “urate” OR “hyperuricemia” AND “acute heart failure” OR “congestive heart failure” AND “mortality” OR “death” OR “hospitalization” OR “major adverse cardiovascular events” AND “prognostic”. References of relevant articles were manually reviewed to ensure identification of any additional studies. No language restrictions were imposed. Ethical approval was not necessary due to the current study only applied the study-level data.
2.2. Study selection
Two authors independently selected the eligible studies according to the following criteria:
-
1.
Original full-text observational studies or post hoc analyses of randomized controlled trials;
-
2.
reported the prognostic value of all-cause mortality or death combined readmission associated with SUA level in AHF patients;
-
3.
provided multivariate adjusted hazard ratios (HR), risk ratio (RR) or odds ratios (OR) with their corresponding 95% confidence intervals (CI) for the prognostic measures;
-
4.
follow-up duration no less than 3 months.
The exclusion criteria were:
-
1.
enrollment of chronic heart failure patients;
-
2.
reported the unadjusted risk estimates;
-
3.
3when multiple publications from the same studied patients, we selected the longest follow-up articles.
2.3. Data extraction and quality assessment
Two authors independently extracted the following data from each study: first author's surname, publication year, study design, study location, sample size, patient age and gender, cutoff value of SUA, event number, duration of follow-up, multivariate adjusted risk estimate for prognostic outcomes, and adjustment for variables. The study quality of the selected studies was assessed using the Newcastle–Ottawa Scale for the cohort studies.[8] Studies with 7 or more points were considered as good quality. Any discrepancies in data extraction and quality assessment were resolved by discussion.
2.4. Statistical analysis
All the meta-analyses were conducted using STATA12.0 software (Stata Corporation, College Station, TX). Data analyses were performed using the most fully adjusted risk estimate for the higher vs the lower SUA level or each 1 mg/ml SUA rise. For analyzing SUA as continuous value, we recalculated risk estimate by 1 mg/ml SUA rise using the following formula: RR1 = exp(ln (RRSD)/SD). The pooled summary was expressed as RR and 95%CI. To explore the heterogeneity across studies, we applied the Cochran Q statistic (significance level of P < .10) and I2 statistics (significance level of 50%). A random effects model was used when the significant heterogeneity among studies was found. Otherwise, we selected a fixed-effect model. Publication bias was assessed using the Begg rank correlation[9] and Egger linear regression test[10] when the number of analyzed studies was more than ten.[11] To investigate the influence of any single study on the pooling summary, sensitivity analysis was performed by removing individual study at each turn.
3. Results
3.1. Search results and studies characteristics
Figure 1 shows the study selection process. The electronic literature search produced 732 potential citations. Of these, 246 records were screened after duplicated articles removed. We excluded 192 obvious irrelevant articles after reviewed the titles and abstracts thus retrieved 54 full-text articles for a detailed assessment. Forty-three studies were further removed mainly because the studied populations were not restricted in AHF patients. No additional studies were identified from the hand search. Thus, 10 studies[12–21] finally met our predefined inclusion criteria.
Figure 1.
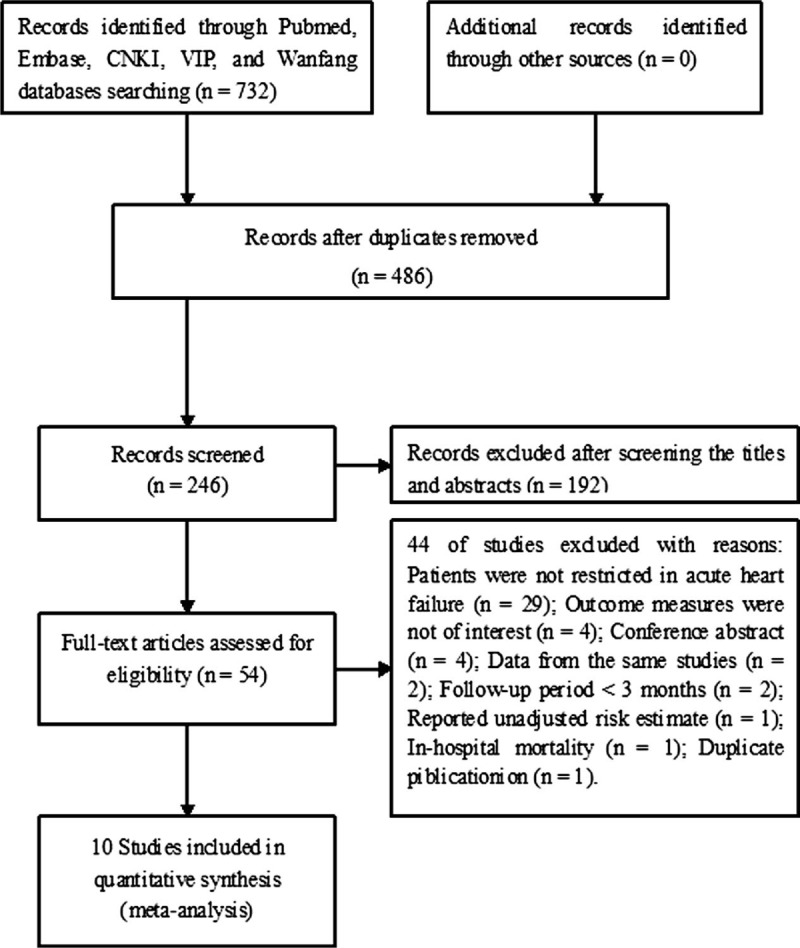
Flow chart of studies selection process.
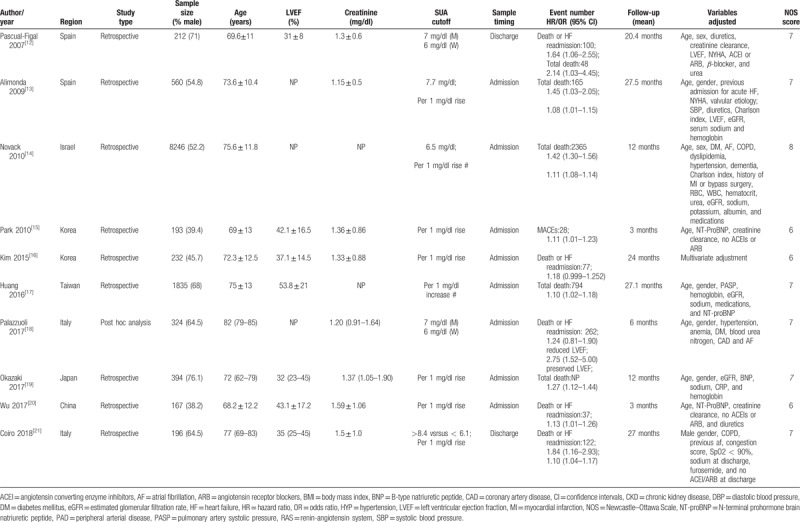
Baseline characteristics of these 10 studies are presented in Table 1. A total of 12,854 AHF patients were identified and analyzed. The mean/median age of the patients ranged from 68.2 to 82 years. All the selected studies were retrospective analysis. The sample size of the included studies ranged between 167 and 8246. The duration of follow-up ranged from 3 to 27.5 months. Of these studies, 2 reported the risk estimate by both categorical and continuous SUA level, 2 reported the risk estimate by categorical SUA level, and 5 reported data as continuous SUA level. For methodological quality assessment, the NOS of these studies ranged from 5 to 7 points.
Table 1.
Baseline characteristics of the included studies.
3.2. All-cause mortality
Three studies[12–14] reported the all-cause mortality outcome by categorical SUA level and 4 studies[13,14,17,22] provided data by continuous SUA level. As shown in Figure 2A, elevated SUA level was associated with an increased risk of all-cause mortality (RR 1.43; 95% CI 1.31–1.56) in a fixed-effect model. There was no significant heterogeneity across studies (I2 = 0%; P = .550). In addition, each 1 mg/dl rise in SUA level, the pooled RR of all-cause mortality was 1.11 (95%CI 1.08–1.14; Fig. 2B). Heterogeneity was not significant among studies (I2 = 41.8%; P = .161). Sensitivity analysis suggested that individual study could not significantly influence the overall pooled risk summary (data not shown).
Figure 2.
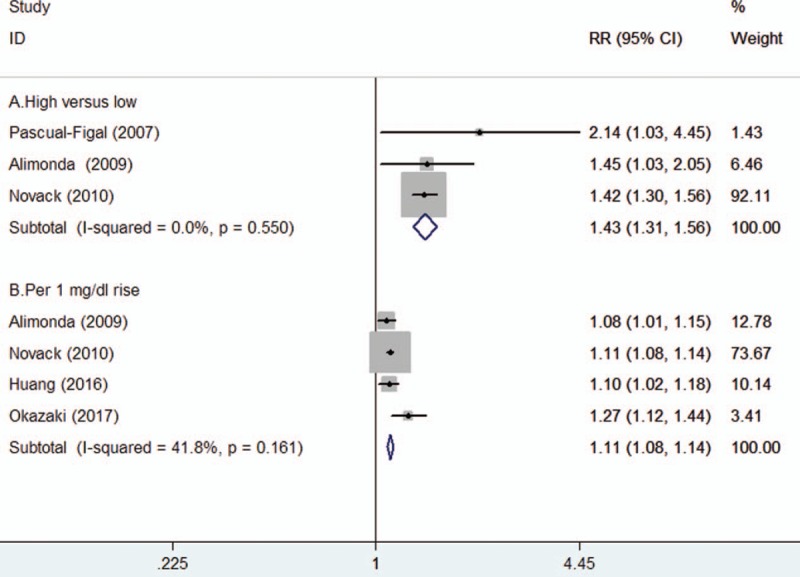
Forest plots showing pooled RR with 95% CI of all-cause mortality for the higher vs lower serum uric acid level (A) and per 1 mg/dl serum uric acid rise (B).
3.3. Combined endpoint of death or readmission
Three studies[12,18,21] reported the combined endpoint of death or readmission events by categorical SUA level analysis and four studies[15,16,20,21] reported this outcome by continuous SUA level analysis. As shown in Figure 3A, elevated SUA level was associated with an increased risk of the combined endpoint of death or readmission (RR 1.68; 95% CI 1.33–2.13) in a fixed-effect model, without statistical significant heterogeneity among studies (I2 = 36.7%; P = .192). Moreover, each 1 mg/dl rise in SUA level increased by 12% (RR 1.12; 95% CI 1.07–1.17; Fig. 3B) risk of the combined endpoint of death or readmission. No statistically significant heterogeneity (I2 = 0%; P = .747) was found across these studies. Sensitivity analysis by removal of 2 studies[15,20] with short-term (3months) following duration, the pooled RR of the combined endpoint of death or readmission was 1.12 (95% CI 1.06–1.18) for each 1 mg/dl rise in SUA level.
Figure 3.
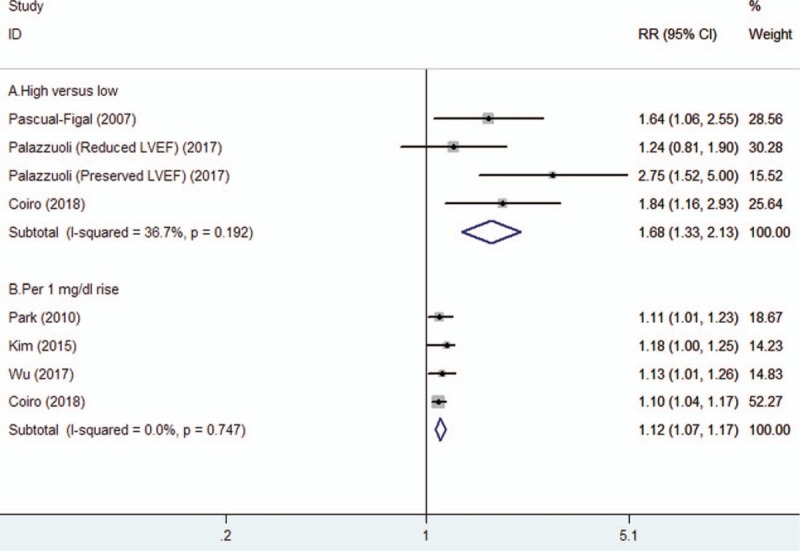
Forest plots showing pooled RR with 95% CI of the combined endpoint of death or readmission for the higher vs lower serum uric acid level (A) and per 1 mg/dl serum uric acid rise (B).
3.4. Publication bias
The number of analyzed studies in the outcomes was small; therefore, we did not perform a funnel plot, Begg rank or Egger test to examine publication bias.
4. Discussion
The main findings of this meta-analysis suggested that high SUA level independently predicted all-cause mortality and the combined endpoint of death or readmission in AHF patients. AHF patients with hyperuricemia were associated with a 43% and 68% higher risk of all-cause mortality and the combined endpoint of death or readmission. Furthermore, per 1 mg/ml SUA rise significantly increased by 11% all-cause mortality and 12% combined endpoint of death or readmission risk.
The prognostic significance of hyperuricemia on mortality and readmission has been widely investigated both in patients with AHF and chronic heart failure. In line with our meta-analysis, 2 previous meta-analyses have summarized that hyperuricaemia (SUA > 6.5 mg/dl) was associated with approximately 2.1-fold higher risk of all-cause mortality in both acute and chronic heart failure patients. However, most of the selected studies enrolling chronic heart failure patients. By contrast, our meta-analysis focused on AHF patients who required intensive care. AHF patients with hyperuricemia were associated with a 43% greater risk of all-cause mortality. Uric acid level at admission was an independent predictor of readmission or all-cause death during a 30 day period after discharge in acutely decompensated heart failure.[23] In addition, hyperuricemia was associated with increased all-cause mortality and the composite endpoint in patients hospitalized for worsening chronic heart failure.[24]
Left ventricular ejection fraction (LVEF) of AHF may be an important confounder for the prognostic value of SUA. In patients with preserved LVEF, concomitant hyperuricemia was significantly associated with the combined endpoint of death or readmission but not in those with reduced LVEF.[18] The reduced LVEF reflects a severe condition of heart failure. The impact of hyperuricemia on the prognosis was more pronounced in preserved LVEF than reduced LVEF. However, due to insufficient data, we could not conduct subgroup analysis according to the LVEF.
Patients admitted to hospital with heart failure commonly have some degree of renal dysfunction.[25] Worsening renal function is one of the most important prognostic variables in heart failure patients.[26] The impaired renal clearance and upregulation of xanthine oxygenase can be responsible for elevated uric acid level.[27] Ischemic renal dysfunction rather than xanthine oxidoreductase activity may be the predominant factor for elevated uric acid level in AHF.[15]
Despite the marked reduction of uric acid level, xanthine oxidase inhibitors (XOI) had no clear effect on improving clinical outcomes in the OPT–CHF study[28] and EXACT-HF trial.[29] However, post hoc analysis of the OPT–CHF study[28] revealed that benefits occurred in patients with increased SUA level in a manner correlating with the degree of SUA reduction. A recently published meta-panalysis[30] showed that use of XOI could reduce total cardiovascular events but not all-cause mortality and worsening heart failure. However, this well-designed meta-analysis enrolled heterogeneous patients but not focused on the AHF patients. Therefore, the benefits of uric acid lowering therapy should be further investigated in the specific subgroup of heart failure patients.
Our meta-analysis had a number of potential limitations. First, our meta-analysis analyzed the retrospective study-level data but not individual patients's data and the inherent limitation of the original studies could not be avoided. Second, the number of the analyzed study in individual outcomes was small, which prevented us to conduct the subgroup analysis. In addition, different follow-up duration also may make the results less reliable. Third, majority of patients enrolled in the analysis were elderly population, therefore, generalizability of the present findings to the younger patients should be with caution. Fourth, the included studies reported the various cut-off value of SUA level and we could not determine the optimal cut-off value of hyperuricemia. Finally, use of diuresis is an important prognostic factor about both hospitalization and mortality in patients with heart failure. However, this variable was not clearly defined in the individual studies. Lack of adjustment of patients’ past medical history of gout, use of XOI or diuresis in the statistical model may have affected the prognostic significance of SUA.
5. Conclusions
AHF patients with a higher level of SUA significantly increase risk of all-cause mortality and the combined endpoint of death or readmission, even after adjustment for conventional confounding factors. Determination of SUA level may improve risk stratification in patients with AHF. Future large randomized controlled trials are needed to evaluate whether use of xanthine oxidase-inhibitor can improve the prognosis.
Author contributions
Conceptualization: Gang Huang.
Data curation: Juan Qin, Xuejun Deng, Mei Zhang.
Formal analysis: Guiquan Luo, Dongmei Yu.
Investigation: Juan Qin, Xuejun Deng, Mei Zhang.
Methodology: Gang Huang, Juan Qin, Xuejun Deng, Guiquan Luo, Lei Wang.
Project administration: Gang Huang.
Resources: Juan Qin, Xuejun Deng, Mei Zhang.
Software: Xuejun Deng.
Supervision: Gang Huang.
Validation: Gang Huang, Guiquan Luo, Dongmei Yu.
Visualization: Guiquan Luo, Dongmei Yu, Lei Wang.
Writing – original draft: Juan Qin.
Writing – review & editing: Gang Huang, Shiheng Zhou.
Gang Huang orcid: 0000-0003-1627-2169.
Footnotes
Abbreviations: AHF = acute heart failure, CI = confidence intervals, NOS = Newcastle–Ottawa Scale, RR = risk ratios, SUA = serum uric acid.
The authors have no conflicts of interest to disclose.
References
- [1].Mentz RJ, O’Connor CM. Pathophysiology and clinical evaluation of acute heart failure. Nat Rev Cardiol 2016;13:28–35. [DOI] [PubMed] [Google Scholar]
- [2].Chen J, Normand SL, Wang Y, et al. National and regional trends in heart failure hospitalization and mortality rates for medicare beneficiaries, 1998–2008. JAMA 2011;306:1669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med 2008;359:1811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kaufman M, Guglin M. Uric acid in heart failure: a biomarker or therapeutic target? Heart Fail Rev 2013;18:177–86. [DOI] [PubMed] [Google Scholar]
- [5].Doehner W, Jankowska EA, Springer J, et al. Uric acid and xanthine oxidase in heart failure—emerging data and therapeutic implications. Int J Cardiol 2016;213:15–9. [DOI] [PubMed] [Google Scholar]
- [6].Tamariz L, Harzand A, Palacio A, et al. Uric acid as a predictor of all-cause mortality in heart failure: a meta-analysis. Congest Heart Fail 2011;17:25–30. [DOI] [PubMed] [Google Scholar]
- [7].Huang H, Huang B, Li Y, et al. Uric acid and risk of heart failure: a systematic review and meta-analysis. Eur J Heart Fail 2014;16:15–24. [DOI] [PubMed] [Google Scholar]
- [8].Wells G, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. XXX XXX;http://www.ohri.ca/programs/clinical_epidemiology/oxford.asphttp://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [assess June 15, 2018]. [Google Scholar]
- [9].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [10].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lau J, Ioannidis JP, Terrin N, et al. The case of the misleading funnel plot. BMJ 2006;333:597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pascual-Figal DA, Hurtado-Martinez JA, Redondo B, et al. Hyperuricaemia and long-term outcome after hospital discharge in acute heart failure patients. Eur J Heart Fail 2007;9:518–24. [DOI] [PubMed] [Google Scholar]
- [13].Alimonda AL, Nunez J, Nunez E, et al. Hyperuricemia in acute heart failure. More than a simple spectator? Eur J Intern Med 2009;20:74–9. [DOI] [PubMed] [Google Scholar]
- [14].Novack V, Pencina M, Zahger D, et al. Routine laboratory results and thirty day and one-year mortality risk following hospitalization with acute decompensated heart failure. PloS One 2010;5:e12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Park HS, Kim H, Sohn JH, et al. Combination of uric acid and NT-ProBNP: a more useful prognostic marker for short-term clinical outcomes in patients with acute heart failure. Korean J Intern Med 2010;25:253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim TH, Kim H, Kim IC. The potential of cystatin-C to evaluate the prognosis of acute heart failure: a comparative study. Acute Cardiac Care 2015;17:72–6. [DOI] [PubMed] [Google Scholar]
- [17].Huang WM, Hsu PF, Cheng HM, et al. Determinants and prognostic impact of hyperuricemia in hospitalization for acute heart failure. Circ J 2016;80:404–10. [DOI] [PubMed] [Google Scholar]
- [18].Palazzuoli A, Ruocco G, De Vivo O, et al. Prevalence of hyperuricemia in patients with acute heart failure with either reduced or preserved ejection fraction. Am J Cardiol 2017;120:1146–50. [DOI] [PubMed] [Google Scholar]
- [19].Okazaki H, Shirakabe A, Kobayashi N, et al. The prognostic impact of uric acid in patients with severely decompensated acute heart failure. J Cardiol 2016;68:384–91. [DOI] [PubMed] [Google Scholar]
- [20].Wu HL, Xiao JM. Uric acid and N-terminal pro brain natriuretic peptide combination for predicting short-term clinical outcomes in acute heart failure patients. Jilin Med J 2017;38:1689–91. [Google Scholar]
- [21].Coiro S, Carluccio E, Biagioli P, et al. Elevated serum uric acid concentration at discharge confers additive prognostic value in elderly patients with acute heart failure. Nutr Metab Cardiovasc Dis 2018;28:361–8. [DOI] [PubMed] [Google Scholar]
- [22].Okazaki H, Shirakabe A, Kobayashi N, et al. Are atherosclerotic risk factors associated with a poor prognosis in patients with hyperuricemic acute heart failure? The evaluation of the causal dependence of acute heart failure and hyperuricemia. Heart Vessels 2017;32:436–45. [DOI] [PubMed] [Google Scholar]
- [23].Amin A, Chitsazan M, Shiukhi Ahmad Abad F, et al. On admission serum sodium and uric acid levels predict 30 day rehospitalization or death in patients with acute decompensated heart failure. ESC Heart Fail 2017;4:162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vaduganathan M, Greene SJ, Ambrosy AP, et al. Relation of serum uric acid levels and outcomes among patients hospitalized for worsening heart failure with reduced ejection fraction (from the efficacy of vasopressin antagonism in heart failure outcome study with tolvaptan trial). Am J Cardiol 2014;114:1713–21. [DOI] [PubMed] [Google Scholar]
- [25].Bielecka-Dabrowa A, Godoy B, Schefold JC, et al. Decompensated heart failure and renal failure: what is the current evidence? Curr Heart Fail Rep 2018;doi: 10.1007/s11897-018-0397-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [26].Llauger L, Jacob J, Miro O. Renal function and acute heart failure outcome. Med Clin 2018;151:281–90. [DOI] [PubMed] [Google Scholar]
- [27].Hare JM, Johnson RJ. Uric acid predicts clinical outcomes in heart failure: insights regarding the role of xanthine oxidase and uric acid in disease pathophysiology. Circulation 2003;107:1951–3. [DOI] [PubMed] [Google Scholar]
- [28].Hare JM, Mangal B, Brown J, et al. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J Am Coll Cardiol 2008;51:2301–9. [DOI] [PubMed] [Google Scholar]
- [29].Givertz MM, Anstrom KJ, Redfield MM, et al. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: the xanthine oxidase inhibition for hyperuricemic heart failure patients (EXACT-HF) study. Circulation 2015;131:1763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bredemeier M, Lopes LM, Eisenreich MA, et al. Xanthine oxidase inhibitors for prevention of cardiovascular events: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord 2018;18:24. [DOI] [PMC free article] [PubMed] [Google Scholar]


