Supplemental Digital Content is available in the text
Keywords: cohort studies, dementia, migraine
Abstract
The present study aimed to evaluate the association between migraines and dementia.
Data were collected from 11,438 dementia participants who were 1:4 matched by age, sex, income, region of residence, hypertension, diabetes, and dyslipidemia with 45,752 controls from the Korean National Health Insurance Service-National Sample Cohort from 2002 to 2013. Dementia was diagnosed using the International Classification of Disease-10 (ICD-10) codes (G30 or F00). For the integrity of diagnoses, we included only participants ≥60 years old who had been diagnosed with an ICD-10 code twice or more during ambulatory visits for the same episode. For migraine (ICD-10 code, G43), we included participants who had visited outpatient clinics twice or more for the same episode. In both dementia and control groups, a previous history of migraine was investigated.
Approximately 7.7% (881/11,438) of patients in the dementia group and 6.3% (2888/45,752) of those in the control group had a history of migraine (P < .001). The crude and adjusted odds ratios (ORs) for migraine with dementia was 1.22 (95% confidence interval [CI] = 1.13–1.32, P < .001) and 1.13 (95% CI = 1.05–1.23, P = .002), respectively. In the subgroup analyses according to age and sex, women demonstrated a significantly higher adjusted OR for migraine with dementia, whereas men did not exhibit an association between migraine and dementia.
In a nested case–control study using a national sample cohort, migraine increased the risk of dementia in women.
1. Introduction
Migraine is a neurological condition characterized by recurrent episodes of a pulsating headache that is most often unilateral and is sometimes accompanied by visual or sensory symptoms, namely, aura.[1] Migraine is a common type of headache disorder that affects millions of people of all ages worldwide, with an incidence ranging from 5% to 15%.[2] In Korea, the estimated incidence is 6.1%.[3] Migraine has been considered a disorder of the brain excitatory–inhibitory balance and trigeminovascular nociception.[4] Although the exact mechanism remains unclear, migraineurs often exhibit functional and structural changes in their brains,[5,6] which have been suggested to increase the risk of cognitive impairments. In addition to neural changes at the cortical level, white-matter abnormalities and silent infarcts may occur in relation to both migraine and dementia. Previously, a large population-based case–control study from Taiwan reported that relative to nonmigraine control participants, migraineurs had an increased risk of any kind of dementia.[7]
Dementia is a common neurodegenerative disease that primarily affects geriatric individuals. Various regions of the brain can deteriorate depending on the type and stage of dementia, resulting in broad cognitive impairments, such as memory loss and inability to perform daily activities.[8] Given that the number of people with dementia is expected to reach 75 million by 2030 and 131 million by 2050 as global average life expectancy increases,[9] the burden of dementia may have an increasing economic impact and impose a psychological strain upon society.[10] Thus, the identification of modifiable risk factors related to dementia may be key to prevent its development.
Despite increasing evidence of potential biological mechanisms linking migraine and cognitive decline,[11,12] few epidemiological studies investigating the association between migraine and dementia have been reported to date. Herein, we conducted a nested case–control study of a nationwide population cohort to ascertain the association between these 2 neurological conditions. In this study, the prevalence of prior migraines was evaluated in patients diagnosed with dementia and compared with that in control individuals matched by age, sex, income, region of residence, and past medical history of hypertension, diabetes, and dyslipidemia. Additionally, we performed subgroup analyses according to age and sex.
2. Materials and methods
2.1. Study population and data collection
The Ethics Committee of Hallym University (2014-I148) approved the use of the study data. Written informed consent was waived by the institutional review board.
This national cohort study relied on data from the Korean National Health Insurance Service (NHIS)-National Sample Cohort. The Korean NHIS selects samples directly from the entire population database to minimize nonsampling errors. Approximately 2% of the samples (1 million) were selected from the entire Korean population (50 million). These data can be classified according to 1476 levels (age [18 categories], sex [2 categories], and income level [41 categories]) using randomized stratified systematic sampling methods via proportional allocation to represent the entire population. After data selection, the appropriateness of the sample was verified in a previous study.[13] The details of the methods used to perform these procedures have been provided by the National Health Insurance Sharing Service.[14] This cohort database included
-
(1)
personal information,
-
(2)
health insurance claim codes (procedures and prescriptions),
-
(3)
diagnostic codes using the International Classification of Disease-10 (ICD-10),
-
(4)
death records from the Korean National Statistical Office (using the Korean Standard Classification of disease),
-
(5)
socioeconomic data (residence and income), and
-
(6)
medical examination data for each participant over a period ranging from 2002 to 2013.
Because all Korean citizens are recognized by a 13-digit resident registration number from birth to death, exact population statistics can be determined using this database. It is mandatory for all Koreans to enroll in the NHIS. All Korean hospitals and clinics use these 13-digit resident registration numbers to register individual patients in the medical insurance system. Therefore, the risk of overlapping medical records is minimal, even if a patient changes residences. Moreover, all medical treatments in Korea can be tracked without exception using the Health Insurance Review and Assessment system. In Korea, a notice of death must be provided to an administrative entity before a funeral can be held. The cause and date of death are recorded by medical doctors on a death certificate.
2.2. Participant selection
We designed a nested case–control study to ascertain the association of migraine with dementia. From a total of 1,125,691 patients with 114,369,638 medical claim codes, we selected participants who were diagnosed with dementia. Dementia was defined as diagnoses of Alzheimer disease (G30) or dementia in Alzheimer disease (F00). To reduce the inclusion of patients with incorrect diagnoses, we only selected participants who were treated ≥2 times. The reliability of the diagnosis of dementia is described in the supplement (S1).
Migraine was diagnosed using the ICD-10 code G43. We included participants with a migraine who had visited an outpatient clinic twice or more for the same episode (n = 45,587).
The dementia participants were matched 1:4 with control participants (control group) who had never been diagnosed with dementia from 2002 to 2013 from this cohort. The control group participants were selected from the mother population (n = 1,112,589). They were matched by age, sex, income, region of residence, and past medical history (hypertension, diabetes, and dyslipidemia). To prevent selection bias when selecting matched participants, the control group participants were sorted using randomly generated numbers and then selected in numerical order. It was assumed that each matched control participant was involved at the same time as each matched dementia participant (index date). Accordingly, control group participants who died before the index date were excluded. Dementia participants for whom we could not identify enough matching participants were also excluded (n = 1152). We excluded participants who were younger 60 years old because of the rare incidence of dementia in younger populations (n = 512); furthermore, in young populations, dementia often has a specific underlying etiology. Finally, 1:4 matching resulted in the inclusion of 11,438 dementia participants and 45,752 control participants. However, the participants were not matched for ischemic heart disease or cerebral stroke history because such strict matching increased the exclusion of dementia participants due to a lack of control participants. After matching, we analyzed previous histories of migraine in both dementia and control groups (Fig. 1).
Figure 1.
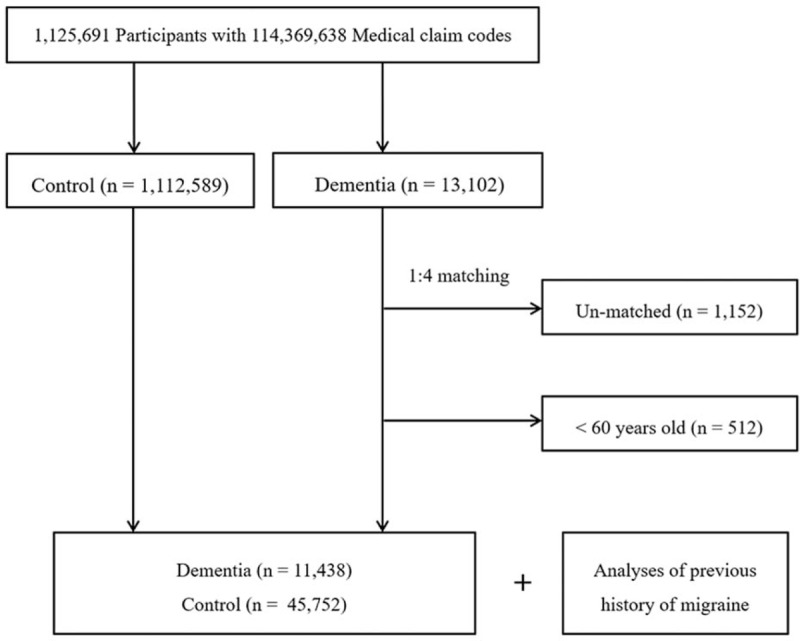
Schematic illustration of the participant selection process in the present study. Out of a total of 1,125,691 participants, 11,438 dementia participants were matched with 45,752 control participants by age, sex, income, region of residence, and past medical history.
2.3. Variables
Six age groups were established: 60 to 64…, and 85+ years old. The income groups were initially divided into 41 classes (1 health aid class, 20 self-employment health insurance classes, and 20 employment health insurance classes). These groups were recategorized into 5 classes (class 1 [lowest income]–5 [highest income]). Participants’ regions of residence were divided into 16 areas according to administrative districts. These regions were regrouped into urban (Seoul, Busan, Daegu, Incheon, Gwangju, Daejeon, and Ulsan) and rural (Gyeonggi, Gangwon, Chungcheongbuk, Chungcheongnam, Jeollabuk, Jeollanam, Gyeongsangbuk, Gyeongsangnam, and Jeju) areas.
Participants’ prior medical histories were evaluated using ICD-10 codes. To ensure the accuracy of the diagnoses, hypertension (I10 and I15), diabetes (E10-E14), or dyslipidemia (E78) was regarded as present if a participant was treated for the condition ≥2 times. Ischemic heart disease (I24 and I25) or cerebral stroke (I60-I66) was regarded as present if a participant was treated ≥1 time.
2.4. Statistical analyses
Chi-square tests were used to compare the rates of general characteristics between dementia and control groups.
To analyze the odds ratio (OR) for migraine with dementia, conditional logistic regression analyses were used. Crude (simple) and adjusted (ischemic heart disease, and stroke) models were constructed, and 95% confidence intervals (CIs) were calculated. In these analyses, age, sex, income, region of residence, hypertension, diabetes, and dyslipidemia were stratified. For the subgroup analyses, we divided the participants by age (<70 years old and ≥70 years) and sex (male and female).
Two-tailed analyses were conducted, and P values less than .05 were considered to indicate significance. The results were statistically analyzed using SPSS v. 22.0 (IBM, Armonk, NY).
3. Results
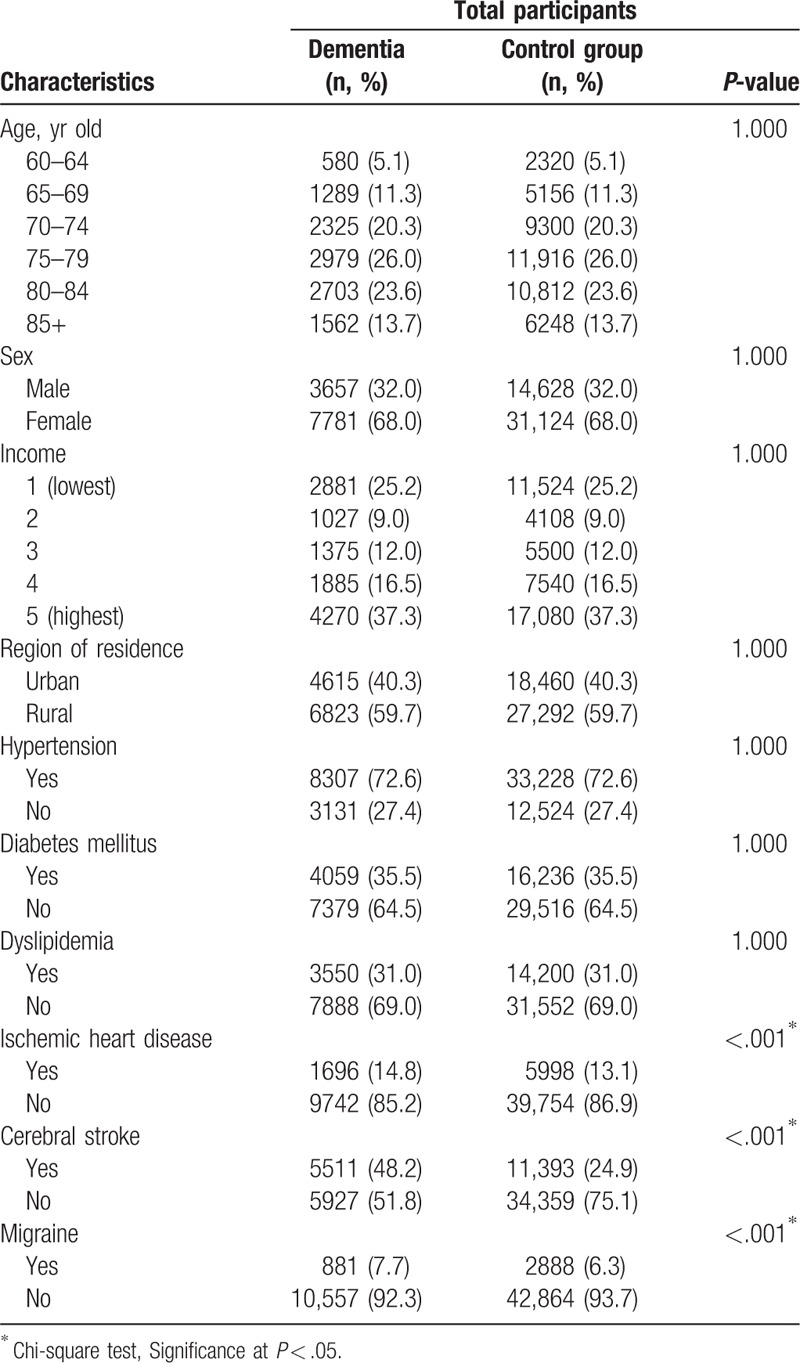
The mean duration from migraine to index date was 58.6 months (standard deviation [SD] = 37.1) in dementia participants, and 60.8 months (SD = 36.4) in the control group. The rate of prior migraine in the dementia group (7.7% [881/11,438]) was higher than that in the control group (6.3% [2888/45,752], P < .001, Table 1). The general characteristics (age, sex, income, region of residence, and history of hypertension, diabetes, or dyslipidemia) of the participants were the same between the 2 groups due to the matching procedure (P = 1.000). The rates of ischemic heart disease and cerebral stroke were higher in the dementia group than in the control group (each P < .05).
Table 1.
General characteristics of participants.
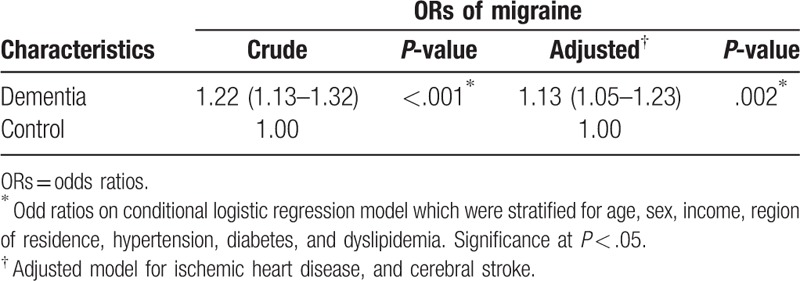
The crude and adjusted ORs for migraine were 1.22 times (95% CI = 1.13–1.32) and 1.13 times (95% CI = 1.05–1.23) higher, respectively, in the dementia group than in the control group (each P < .05, Table 2).
Table 2.
Crude and adjusted odds ratio (95% confidence interval) for migraine in dementia.
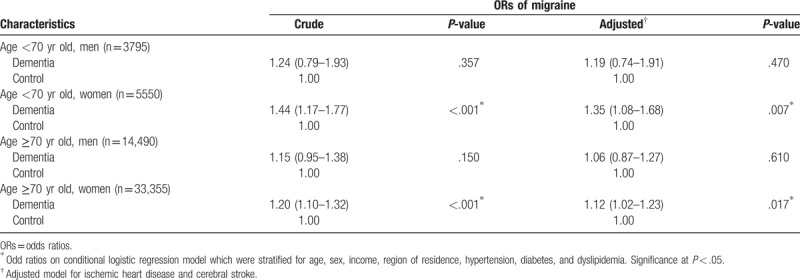
In the subgroup analyses performed according to age and sex, the crude and adjusted ORs for migraine with dementia were higher for women than for men in both the <70-year-old and ≥70-year-old age groups (each P < .05, Table 3). The adjusted OR was 1.35 (95% CI = 1.08–1.68) for women <70 years old and 1.12 (95% CI = 1.02–1.23) for women ≥70 years old. However, the crude and adjusted ORs for migraine with dementia did not reach statistical significance in men.
Table 3.
Crude and adjusted odds ratio (95% confidence interval) for migraine in dementia in subgroup analysis according to age and sex.
4. Discussion
In this study, the risk of dementia increased in patients with a history of migraine even after matching and adjusting for age, sex, income, region of residence, and past medical history of hypertension, diabetes, and dyslipidemia. The subgroup analyses according to age and sex showed a significantly increased risk of dementia among female patients with migraine. Interestingly, women <70 years old demonstrated the highest association between migraine and dementia, with an adjusted OR of 1.40.
Dementia, which is characterized by progressive cognitive decline, is a complex disorder influenced by the interplay between genetic and environmental factors[15]; therefore, multifactorial intervention has been proposed for the prevention of dementia.[16] In addition to lifestyle modifications and pharmacological treatments, the management of comorbidities may be crucial for reducing the dementia-related socioeconomic burden.[17] Given that migraines are prevalent in Korea, our findings may provide a novel way to prevent dementia by managing migraines, particularly in women >60 years old. In accordance with the findings of the present study, a previous epidemiologic study in Taiwan reported an association between migraine and dementia (hazard ratio: 1.33, 95% CI = 1.22–1.46).[7] Notably, pretreatment with traditional Chinese medicine that exerts neuroprotective and antioxidant effects in patients with migraine significantly decreased the risk of dementia in patients aged 70 to 79 years, suggesting a causal relationship between migraine and dementia.[18]
Although there have been incongruent results with respect to cognitive decline during migraine attacks,[19,20] a recent systematic review demonstrated a consistent pattern of neuropsychological impairments, including abnormal executive function, language, and psychomotor activities.[21] Cognitive deterioration has been shown to be associated with the duration, severity, and frequency of migraines[20]; thus, repeated migraine attacks associated with chronic migraine are likely to lead to an increased risk of dementia. The neural changes related to cognitive impairment during migraine events have also been shown to be associated with migraine and dementia in neuroimaging studies.[22]
Recently, white-matter hyperintensities in patients with migraines with aura have been found to be related to alterations in resting cerebral blood flow.[5] A population-based magnetic resonance imaging study and a meta-analysis revealed that structural brain changes, including white-matter abnormalities and silent infarct lesions, were more common in migraineurs than in control participants.[23,24] Given these observations, the neurophysiological mechanisms of migraine, such as inflammation and reduced cerebral blood flow, are presumed to be the underlying causes of dementia. Long-lasting migraines can lead to significant permanent neurological damage as a result of biological cascades involving increased oxidative stress, neurodegeneration and, eventually, cell death.[11,25] Additionally, mitochondrial dysfunction-induced metabolic changes in brain cells may contribute to the development of dementia.[12]
In this study, there was a significant association between migraine and dementia overall; however, the subgroup analyses revealed an association in women but not in men. There is growing evidence of differences in brain structure and function between men and women referred to as dimorphic neurology.[26] Several studies have suggested that a higher incidence rate of dementia in women than in men.[27] Given the longer life expectancy of women than men, women have a higher rate of dementia at any given age.[28] In particular, the apolipoprotein E genotype, which is associated with hippocampal pathology and memory performance, has been shown to exert a significant impact on women.[26] A meta-analysis revealed that the apolipoprotein E genotype is significantly associated with headache, including migraine and tension-type headache.[29] Moreover, systemic inflammatory mediators, such as hypertension, diabetes, and hyperlipidemia, are more prevalent in older women than in similarly aged men, which results in an increased risk of migraine in women.[27] In addition, in female populations, common psychiatric diseases such as depression that often co-occur with migraine and dementia may play roles.[30,31] Given these observations, the sex-based difference in the association between migraine and dementia may be due to dimorphic neurology or the longer life expectancy in women than in men.
Notably, patients <70 years old had a higher adjusted risk than did those ≥70 years old. This finding is in accordance with the previous study that reported a stronger association between dementia and migraine in young people than in old people.[7] This phenomenon implies that migraine-related excitotoxicity or vascular and thrombotic properties may impact the development of dementia in younger patients.[32] In previous studies, younger patients with migraine appeared to have a higher incidence of stroke or silent infarct lesions, which are associated risk factors for dementia and cognitive impairment, than did older patients.[33,34]
A strength of the present study is its use of NHIS data, which includes all Korean citizens without exception; thus, this cohort is highly representative of the general population, and there were no missing participants. Moreover, the participants were individuals ≥60 years old who were more likely than younger individuals to develop dementia,[35] increasing the integrity of the analysis. Additionally, the control group was randomly selected and subsequently matched to minimize the impacts of confounders including age, sex, income, region of residence, and medical history.
Although we demonstrated a significant association between migraine and dementia, our study is limited by several factors that should be controlled for in further investigations. First, we identified an association between migraine and dementia; however, we did not identify a causal link between these 2 clinical disorders. Further studies with longitudinal follow-up to evaluate changes in the rate of dementia after migraine treatment may be necessary to confirm the causal association. Second, analyses according to dementia subtype were not performed in this study. In an epidemiological study performed in Canada, individuals with a history of migraine had an increased risk of Alzheimer disease, whereas no significant association was found between migraine and vascular dementia despite the vascular mechanisms involved in migraine biology.[36] Thus, future studies including different types of dementia are warranted to determine the associations. Third, chronic migraine appears to be associated with comorbidities such as hypertension, diabetes, coronary arterial disease, head injuries, and psychological diseases.[7] These comorbidities may be involved in the mechanism underlying the association between migraine and dementia.[5] Some medications for migraine, either prophylactic or abortive, may induce mitochondrial damage, resulting in neurodegeneration.[37] The association between migraine and dementia might have become clearer in the present study if we had matched the participants for confounding factors such as depression or anxiety. However, despite the large size of this population-based cohort study, we were unable to match the participants for ischemic heart disease, cerebral stroke, and depression because such strict matching led to the exclusion of dementia subjects due to a lack of control participants and low statistical power. Moreover, the NHIS does not include detailed information regarding several suspected confounding factors, such as smoking, alcohol consumption, and socioeconomic status.[38] Furthermore, the insurance data are anonymous; thus, relevant clinical variables including participant blood pressure, laboratory test results, and imaging findings were not available for analysis.
5. Conclusion
A history of migraine appears to increase the risk of dementia. In particular, migraine increased the risk of dementia in women ≥60 years.
Author contributions
Conceptualization: Jae-Sung Lim, Hyo Geun Choi.
Data curation: Hyo Geun Choi.
Formal analysis: Hyo Geun Choi.
Funding acquisition: Hyo Geun Choi.
Investigation: Dong Jun Oh, Hyo Geun Choi.
Methodology: Sang-Yeon Lee, Jae-Sung Lim, Dong Jun Oh, Hyo Geun Choi.
Project administration: Hyo Geun Choi.
Resources: Hyo Geun Choi.
Software: Hyo Geun Choi.
Supervision: Il Gyu Kong, Hyo Geun Choi.
Validation: Jae-Sung Lim, Hyo Geun Choi.
Visualization: Hyo Geun Choi.
Writing – original draft: Sang-Yeon Lee.
Writing – review and editing: Il Gyu Kong.
Hyo Geun Choi orcid: 0000-0003-1655-9549.
Supplementary Material
Footnotes
Abbreviations: CIs = confidence intervals, HR = hazard ratio, ICD-10 = International Classification of Disease-10, NHIS = National Health Insurance Service, OR = odds ratio.
This work was supported in part by a research grant (NRF-2015-R1D1A1A01060860) from the National Research Foundation (NRF) of Korea. The manuscript was edited for the proper English language, grammar, punctuation, spelling, and overall style by the highly qualified native English-speaking editors at American Journal Experts (3E5E-BB5C-CE9B-4339-D96P).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Friedman BW. Managing migraine. Ann Emerg Med 2017;69:202–7. [DOI] [PubMed] [Google Scholar]
- [2].Lipton RB, Scher A, Kolodner K, et al. Migraine in the United States epidemiology and patterns of health care use. Neurology 2002;58:885–94. [DOI] [PubMed] [Google Scholar]
- [3].Lee MJ, Lee C, Chung C-S. The migraine–stroke connection. J Stroke 2016;18:146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vecchia D, Pietrobon D. Migraine: a disorder of brain excitatory–inhibitory balance? Trends Neurosci 2012;35:507–20. [DOI] [PubMed] [Google Scholar]
- [5].Bashir A, Lipton RB, Ashina S, et al. Migraine and structural changes in the brain: a systematic review and meta-analysis. Neurology 2013;81:1260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Amin FM, Hougaard A, Magon S, et al. Change in brain network connectivity during PACAP38-induced migraine attacks: a resting-state functional MRI study. Neurology 2016;86:180–7. [DOI] [PubMed] [Google Scholar]
- [7].Chuang CS, Lin CL, Lin MC, et al. Migraine and risk of dementia: a nationwide retrospective cohort study. Neuroepidemiology 2013;41:139–45. [DOI] [PubMed] [Google Scholar]
- [8].Hughes CP, Berg L, Danziger W, et al. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566–72. [DOI] [PubMed] [Google Scholar]
- [9].Winblad B, Amouyel P, Andrieu S, et al. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol 2016;15:455–532. [DOI] [PubMed] [Google Scholar]
- [10].Prince MJ, Wu F, Guo Y, et al. The burden of disease in older people and implications for health policy and practice. Lancet 2015;385:549–62. [DOI] [PubMed] [Google Scholar]
- [11].Peers C, Dallas ML, Boycott HE, et al. Hypoxia and neurodegeneration. Ann N Y Acad Sci 2009;1177:169–77. [DOI] [PubMed] [Google Scholar]
- [12].Sas K, Pardutz A, Toldi J, et al. Dementia, stroke and migraine – some common pathological mechanisms. J Neurol Sci 2010;299:55–65. [DOI] [PubMed] [Google Scholar]
- [13].Lee J, Lee JS, Park SH, et al. Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol 2017;46:e15. [DOI] [PubMed] [Google Scholar]
- [14]. Available at: http://nhiss.nhis.or.kr/ inception date: 2016.10.24. [Google Scholar]
- [15].van der Flier WM, Scheltens P. Epidemiology and risk factors of dementia. J Neurol Neurosurg Psychiatry 2005;76Suppl 5:v2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Koczy P, Klie T, Kron M, et al. Effectiveness of a multifactorial intervention to reduce physical restraints in nursing home residents with dementia. Z Gerontol Geriatr 2005;38:33–9. [DOI] [PubMed] [Google Scholar]
- [17].Zhu CW, Cosentino S, Ornstein KA, et al. Interactive effects of dementia severity and comorbidities on medicare expenditures. J Alzheimers Dis 2017;57:305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu CT, Wu BY, Hung YC, et al. Decreased risk of dementia in migraine patients with traditional Chinese medicine use: a population-based cohort study. Oncotarget 2017;8:79680–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bonavita V, De Simone R, Ranieri A. Pain cognition in migraine: from basic neurophysiology to a behavioral paradigm. Neurol Sci 2018;39Suppl 1:3–9. [DOI] [PubMed] [Google Scholar]
- [20].Cady R, Farmer K. Migraine and cognition. Headache 2013;53:587–8. [DOI] [PubMed] [Google Scholar]
- [21].Gil-Gouveia R, Oliveira AG, Martins IP. Assessment of cognitive dysfunction during migraine attacks: a systematic review. J Neurol 2015;262:654–65. [DOI] [PubMed] [Google Scholar]
- [22].Sprenger T, Borsook D. Migraine changes the brain: neuroimaging makes its mark. Curr Opin Neurol 2012;25:252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang Q, Datta R, Detre JA, et al. White matter lesion burden in migraine with aura may be associated with reduced cerebral blood flow. Cephalalgia 2017;37:517–24. [DOI] [PubMed] [Google Scholar]
- [24].Kruit MC, van Buchem MA, Launer LJ, et al. Migraine is associated with an increased risk of deep white matter lesions, subclinical posterior circulation infarcts and brain iron accumulation: the population-based MRI CAMERA study. Cephalalgia 2010;30:129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Moskowitz M, Macfarlane R. Neurovascular and molecular mechanisms in migraine headaches. Cerebrovasc Brain Metab Rev 1993;5:159–77. [PubMed] [Google Scholar]
- [26].Rocca WA, Mielke MM, Vemuri P, et al. Sex and gender differences in the causes of dementia: a narrative review. Maturitas 2014;79:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Azad NA, Al Bugami M, Loy-English I. Gender differences in dementia risk factors. Gend Med 2007;4:120–9. [DOI] [PubMed] [Google Scholar]
- [28].Rasmussen KL, Tybjaerg-Hansen A, Nordestgaard BG, et al. Absolute 10-year risk of dementia by age, sex and APOE genotype: a population-based cohort study. CMAJ 2018;190:E1033–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Miao J, Wang F, Zheng W, et al. Association of the Apolipoprotein E polymorphism with migraine: a meta-analysis. BMC Neurol 2015;15:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gudmundsson LS, Scher AI, Sigurdsson S, et al. Migraine, depression, and brain volume: the AGES-Reykjavik Study. Neurology 2013;80:2138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hesdorffer DC. Comorbidity between neurological illness and psychiatric disorders. CNS Spectr 2016;21:230–8. [DOI] [PubMed] [Google Scholar]
- [32].Trauninger A, Leél-Őssy E, Kamson DO, et al. Risk factors of migraine-related brain white matter hyperintensities: an investigation of 186 patients. J Headache Pain 2011;12:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2672–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].T O’Brien J, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol 2003;2:89–98. [DOI] [PubMed] [Google Scholar]
- [35].Snowden J. Mild Cognitive Impairment: Aging to Alzheimer's Disease. Brain 2004;127:231–8. [Google Scholar]
- [36].Morton R. Does a History of Migraines Increase the Risk of Late-life Cognitive Health Outcomes? Waterloo, Ontario, Canada: University of Waterloo; 2011. [Google Scholar]
- [37].Neustadt J, Pieczenik SR. Medication-induced mitochondrial damage and disease. Mol Nutr Food Res 2008;52:780–8. [DOI] [PubMed] [Google Scholar]
- [38].Tzeng NS, Chung CH, Lin FH, et al. Headaches and risk of dementia. Am J Med Sci 2017;353:197–206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


