Supplemental Digital Content is available in the text
Keywords: cardiovascular surgery, fluid therapy, outcome, perioperative period, renal insufficiency
Abstract
We assessed whether perioperative fluid management with balanced solutions and a limited volume of hydroxyethyl starch (renal-protective fluid management [RPF] strategy) could improve renal outcomes after cardiovascular surgery.
For this retrospective observational study, we evaluated 2613 patients who underwent cardiovascular surgery from January 1, 2010 to December 31, 2013. The control group were given intravenous fluids with saline-based solutions and unlimited volumes of hydroxyethyl starch solutions and the RPF group were given intravenous fluids with RPF. The primary outcome was the incidence of acute kidney injury (AKI) and chronic dialysis within 12 months after cardiovascular surgery. Multivariable regression and propensity analyses were performed to evaluate the association between perioperative fluid management strategy and postoperative renal outcomes.
Postoperative AKI and chronic dialysis occurred in 213 (21.2%) and 5 (0.5%) patients in the RPF group compared with 696 (43.2%) and 38 (2.4%) patients in the control group, respectively. After adjustment, the RPF group was linked to a decreased risk of postoperative AKI, severe AKI, persistent AKI, use of renal replacement therapy, chronic kidney disease, chronic dialysis, and a shorter postoperative extubation time and intensive care unit, and hospital stay duration.
The perioperative fluid management strategy with balanced solutions and a limited volume of hydroxyethyl starch was related to improved acute and 1-year renal and clinical outcomes after cardiovascular surgery. These findings indicate the need for further definitive clinical trials on perioperative fluid management strategy.
1. Introduction
The administration of intravenous fluids is an essential part of the prevention and treatment of hypovolemia during the perioperative period, but the choice of replacement fluid is still debated.[1–4] Although there are many available intravenous fluids with various biological and physiochemical properties, 0.9% saline and hydroxyethyl starch (HES) solution are the most frequently used crystalloid and colloid solutions, respectively.[5] Recently, several studies demonstrated that the choice of intravenous fluids administered could affect patient outcomes, especially renal outcomes.[2,6–10] Colloids have potential advantages over crystalloids, including more efficient volume expansion, improved microcirculation, decreased edema, and better preserved lung function. However, recent studies demonstrated that volume resuscitation using HES solutions was related to an increased risk of mortality and acute kidney injury (AKI), and higher doses of HES solution were related to a higher incidence of these adverse effects.[4,6,7,9,11,12] Additionally, excessive use of 0.9% saline and saline-based colloids containing supraphysiological concentrations of chloride for resuscitation can lead to hyperchloremic metabolic acidosis, which could be associated with altered inflammatory response, impaired coagulation, and deteriorated cardiovascular function.[8,13,14] Moreover, infusion of hyperchloremic solutions can cause renal vasoconstriction and consequently lead to reductions in renal perfusion and glomerular filtration rate (GFR).[15–17] Recent observational studies have suggested that intravenous fluid replacement with a balanced solution may be associated with a decreased risk of renal dysfunction, morbidities, and mortality in critically ill or surgical patients.[2,8,10,14]
Given the high risk of AKI in cardiovascular surgical patients and the growing concern regarding the potential adverse effects of HES and saline-based solutions, perioperative fluid therapy using balanced solution and a limited amount of HES solution (renal-protective intravenous fluid management, RPF) may have beneficial effects on renal outcomes after cardiovascular surgery. Recently, we have reported that this RPF strategy is related to a decreased risk of AKI after elective off-pump coronary artery bypass graft surgery.[18] However, the ability to generalize across studies is limited, because testing occurred in highly preselected patient populations. Therefore, we conducted this study to further evaluate the effect of a RPF management strategy on the postoperative immediate and 1-year renal function and clinical outcomes in more a heterogeneous population with preoperative normal renal function undergoing cardiovascular surgery.
2. Methods
2.1. Study population
We performed a retrospective observational study including all patients aged ≥20 years who underwent cardiovascular surgery (coronary bypass, valve surgery, aortic surgery, or combined surgery) at our institution between January 1, 2010 and December 31, 2013. All clinical data for all patients were acquired from the Asan Medical Center Cardiovascular Surgery and Anesthesia Database and from a retrospective review of the computerized patient record system (Asan Medical Center Information System Electronic Medical Record).
This retrospective study included 2 cohorts that were defined according to the type and amount of fluid received during the perioperative period (i.e., during and within 48 hours after surgery). On July 5, 2012, we changed the institutional perioperative fluid management strategy from a saline-based solution and unlimited amount of HES solution to a balanced solution and limited amount (perioperative cumulative amount <30 mL/kg body weight) of HES solution.[18] We defined the control group as all patients who underwent cardiovascular surgery at our institution during the control period (January 1, 2010–July 4, 2012) and the RPF group as all patients who underwent cardiovascular surgery during the RPF period (July 23, 2012–December 31, 2013). This control period was chosen as the immediately prior 2 years to minimize the effects of other perioperative management practice patterns. There were no significant changes in the surgical and perioperative care protocol between the control and the RPF period except the perioperative intravenous fluid administration strategy.
We excluded patients with missing serum creatinine (sCr), those with preoperative dialysis or an eGFR <60 mL/min/1.73 m2, those with a preoperative intra-aortic balloon pump or mechanical circulatory assist devices, those who underwent endovascular surgery, those with past history of organ transplantation or nephrectomy, and those receiving combinations of saline-based and balanced solutions during surgery. We also excluded patients admitted during the approximate 2-week period (duration of the combined use of saline-based solutions and balanced solution) between the 2 fluid management strategy periods and who received cumulative amounts >30 mL/kg body weight of HES solution during the RPF period. This study was approved by the Institutional Review Board of our institution (AMC-IRB 2015–0103) and informed consent was waived by the board. This study was conducted in accordance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.[19]
For fluid management, all patients in the control group received intravenous fluids with saline-based solutions and an unlimited amount of HES solution during surgery and the immediate postoperative period. The saline-based solutions included 0.9% saline (chloride concentration: 150 mmol/L; JW Pharmaceutical Co., Seoul, South Korea) and 6% HES 130/0.4 (chloride concentration: 154 mmol/L, Voluven; Fresenius-Kabi, Bad Homburg, Germany). In contrast, all patients in the RPF group received intravenous fluids with balanced solutions during surgery and a limited amount of HES solution during surgery and in the immediate postoperative period (cumulative amount <30 mL/kg body weight). The balanced solutions included Plasma Solution-A (chloride concentration: 98 mmol/L; CJ HealthCare Co., Seoul, South Korea) and 6% HES 130/0.4 (chloride concentration: 110 mmol/L, Volulyte; Fresenius-Kabi). Although patients in the RPF group were given a totally balanced solution during surgery, they received balanced HES and 0.9% saline for volume replacement during the immediate postoperative period. Administration of the cumulative amount of HES solution during the perioperative period was limited to 30 mL/kg, and, when requiring higher than the maximum cumulative dose of HES solution, crystalloid, or a blood product was used as needed.
Our cardiovascular surgery and perioperative management strategies have been previously described in detail.[20] During both study periods, the goals of hemodynamic management during perioperative period were to maintain hemodynamic stability (mean arterial pressure >65 mmHg, heart rate >60 beats/min, central venous pressure of 8–12 mmHg, pulmonary capillary wedge pressure of 12–15 mmHg, urine output >0.5 mL/kg/h, cardiac index >2.0 L/min/m2, and mixed venous oxygen saturation >70%). Standard hemodynamic management for cardiovascular surgery included mainly fluid administration to achieve normovolemia and preset hemodynamic goal in addition to vasopressors or inotropes to prevent excessive fluid administration (see Expanded Methods, Supplemental Content, which describes our cardiovascular surgery and perioperative management strategies).
2.2. Outcome variables and definitions
The primary endpoint was the incidence of AKI and chronic dialysis within 12 months after cardiovascular surgery. Postoperative AKI was defined and staged by the Kidney Disease Improving Global Outcomes (KDIGO) criteria using a change in sCr (an increase in sCr by ≥0.3 mg/dL within 48 hours of surgery; or an increase in sCr to ≥1.5 times baseline within 7 days of surgery).[21] Chronic dialysis was defined as ongoing dialysis support beyond 90 days after initiation within 12 months of cardiovascular surgery, with the start date defined as the date of the first of these treatments.
Secondary endpoints were the incidence of severe AKI (≥KDIGO stage 2), requirement for renal replacement therapy (RRT) in the hospital, renal outcome at the time of discharge, chronic kidney disease (CKD) within 12 months after surgery, time to extubation following surgery, length of intensive care unit (ICU) and hospital stay after surgery, and death within 12 months after surgery. Mortality was defined as death from any cause within 12 months of cardiovascular surgery. Data regarding renal outcomes and mortality were obtained during visiting outpatient clinics, and by a detailed review of all medical records, by telephone interviews, or from the National Population Registry of the Korean National Statistical Office. For chronic renal outcomes, an individual who was surviving at 3 months after surgery was censored at death or at the end of the study period (see Expanded Methods, Supplemental Content, which describes our outcome variables and definitions).
2.3. Statistical analysis
The primary objective of this study is to assess the effect of an RPF strategy on the postoperative AKI and chronic dialysis. The secondary objective is to assess the effect of an RPF strategy on other renal and clinical outcomes after cardiovascular surgery.
Categorical variables are expressed as numbers (percentages), and continuous variables as mean ± standard deviation or median with interquartile range. In the unmatched cohort, between-group differences in preoperative and intraoperative characteristics and postoperative outcomes were compared using the chi-squared test or Fisher exact test for categorical variables and the Student t test or Mann–Whitney rank-sum test for continuous variables when appropriate.
To reduce the effect of treatment-selection bias and potential confounding due to systematic differences between the study groups and to determine the effect of perioperative intravenous fluid administration strategy alone on postoperative renal function, propensity score matching was conducted. Multivariable logistic regression analyses and multivariable Cox proportional hazards regression analyses were also conducted to assess the independent relationships between the perioperative intravenous fluid administration strategy and postoperative acute and 1-yr renal outcomes. All predictor variables in Table 1 were assessed independently, and variables with a P value <.20 in the univariate analyses were selected to be entered into the multivariable analyses. A backward elimination process with a P value cut-off of .05 was used to develop the final multivariable models. Adjusted odds ratio (OR) with 95% confidence interval (CI) for the logistic regression and adjusted hazard ratio (HR) with 95% CIs for the Cox proportional hazards regression were calculated. Cumulative chronic dialysis-free 1-year survival curves in the entire and propensity matched cohorts were drawn using Kaplan–Meier analyses, and log-rank tests were used to determine the statistical significance of the differences.
Table 1.
Baseline and operative characteristics of the study patients.
To evaluate the robustness of our findings, several sensitivity analyses were also performed. Because death could be a competing risk for chronic renal outcomes, we also performed competing risk sensitivity analyses using the Fine and Gray method.[22] An additional weighted logistic regression and weighted Cox proportional hazards regression analyses with an inverse probability of treatment weighting was conducted (see Expanded Methods, Supplemental Content, which demonstrates detailed descriptions of the propensity score matching, inverse probability of treatment weighting and sensitivity analyses).
All the reported P values are 2-sided, and P values <.05 were considered statistically significant. All data manipulations and statistical analyses were conducted using SAS Version 9.1 (SAS Institute Inc., Cary, NC) software and SPSS 20.0 for Windows (IBM Corp., Armonk, NY).
3. Results
Between January 1, 2010 and December 31, 2013, a total of 3389 patients underwent cardiovascular surgery at our institution. After excluding patients who met any of the exclusion criteria (n = 776), a total of 2613 patients with cardiovascular surgery remained, 1610 patients in the control group, and 1003 patients in the RPF group (Fig. 1).
Figure 1.
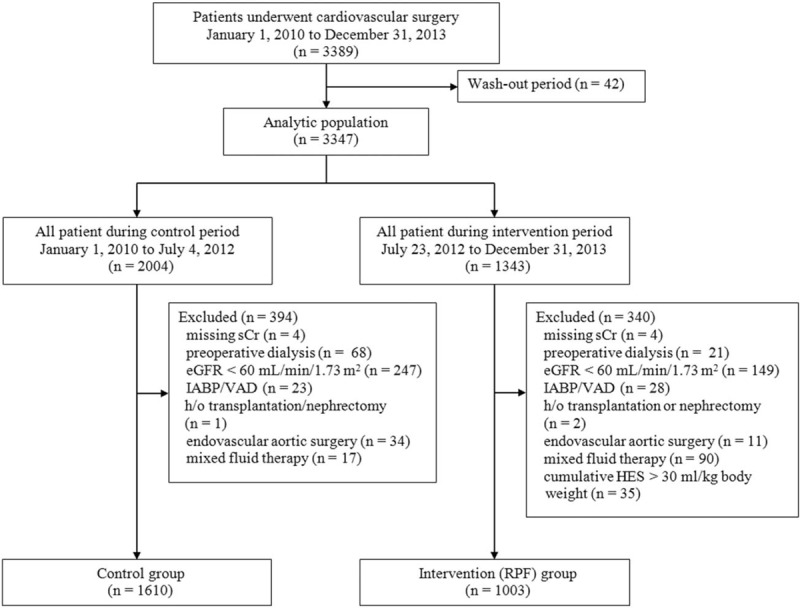
Study flow diagram. Cr = creatinine, eGFR = estimated glomerular filtration rate, HES = hydroxyethyl starch, IABP = intraaortic balloon pump, RPF = renal-protective intravenous fluid management, VAD = ventricular assist device.
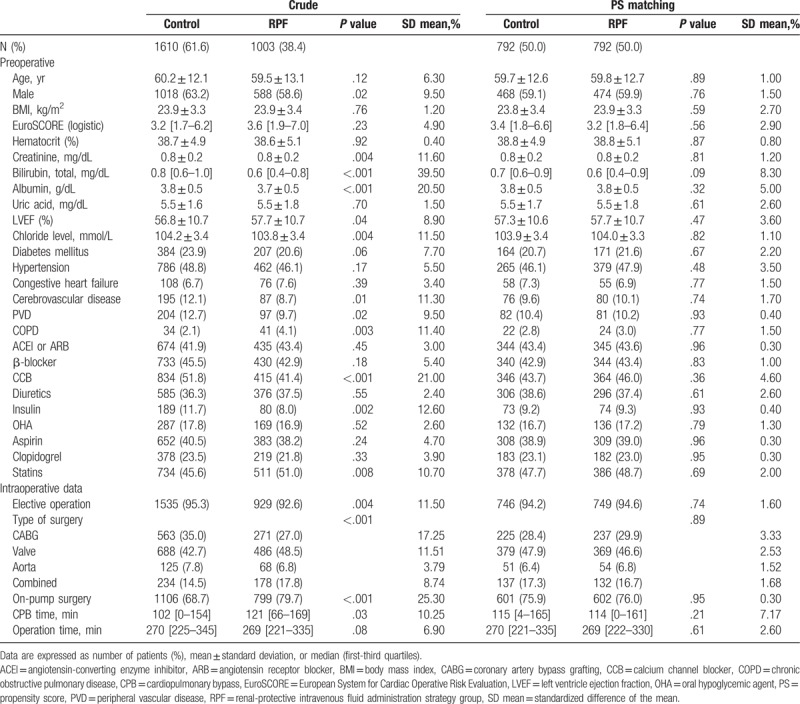
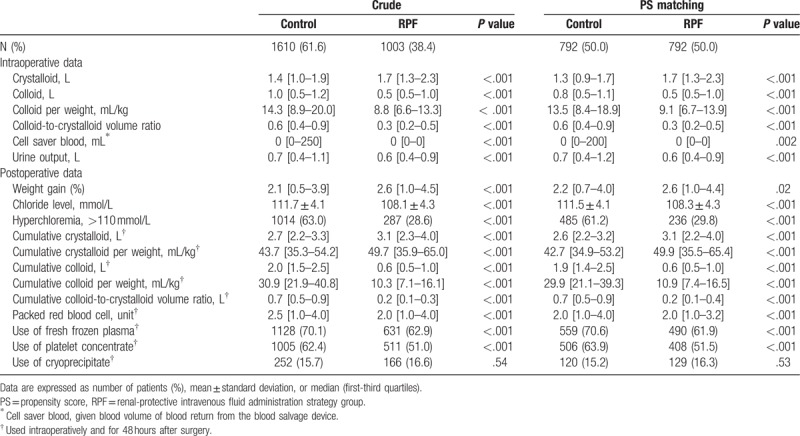
The baseline and operative characteristics in the entire and the propensity matched cohorts are shown in Table 1. During surgery, the RPF group received more crystalloid solutions and less colloid solutions than the control group. The mean colloid/crystalloid ratio was 0.4:1 and 0.7:1 in the RPF and the control groups, respectively. In the RPF group, the infused salvaged blood volumes and urine output were lower and the postoperative body weight gain was higher. During surgery and the immediate postoperative period, less packed red blood cells were used in the RPF group, and less patients in the RPF group received fresh frozen plasma and platelet concentrate. The preoperative serum chloride level was slightly but significantly decreased in the RPF group, but the serum chloride level after surgery was significantly less increased (4.3 ± 4.1 mmol/L vs 7.5 ± 4.2 mmol/L, P < .001) and the incidence of immediate postoperative hyperchloremia was significantly lower in the RPF group compared with the control group (Table 2).
Table 2.
Perioperative fluid administration in the study patients.
In the entire and propensity score matched cohorts, extubation time and ICU and hospital stay were shorter and the incidence of reoperation for bleeding was lower in the RPF group compared with the control group, but 30-day and 1-year mortality were similar between the 2 groups (Table 3). Renal outcomes after surgery were shown in Table 4. In the entire and propensity score matched cohort, the incidence of AKI, severe AKI, in-hospital RRT, persistent AKI at discharge, 1-year CKD, and 1-year chronic dialysis were lower in the RPF group than in the control group. These results were consistent with the results of the inverse probability of treatment weighting analyses (see Table S2 and Table S3, Supplemental Content, which illustrates the perioperative outcome data in the inverse probability of treatment weighting populations).
Table 3.
Postoperative outcomes in the study groups.
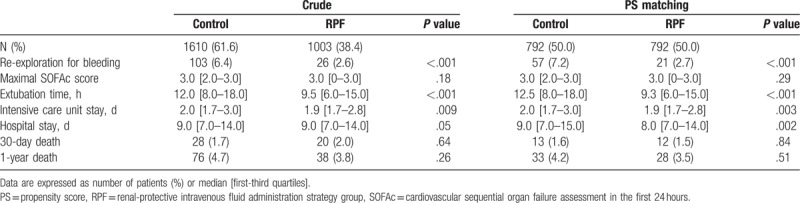
Table 4.
Impact of renal-protective intravenous fluid administration strategy on renal outcomes.
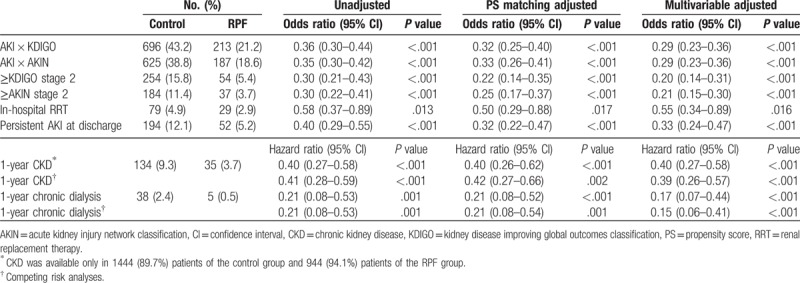
After adjusting by the multivariable analyses, the RPF group was independently related to a lower risk of postoperative AKI and 1-yr chronic dialysis (see Table S4, Supplemental Content, which illustrates the other risk factors related to the postoperative AKI). The RPF group was also independently associated with a lower risk of severe AKI, in-hospital RRT, persistent AKI at discharge, and 1-year CKD. These relationships between RPF and chronic renal outcomes were consistent with the results of the competing risk analyses. Kaplan–Meier survival analyses stratified by the perioperative intravenous fluid administration strategy showed that compared with patients in the control group, patients in the RPF group had a significantly higher likelihood of a chronic dialysis-free survival rate in the total cohort (P < .001, Fig. 2A) and the propensity matched cohort (P < .001, Fig. 2B). The association between the RPF strategy and postoperative AKI was preserved across the sensitivity analyses (see Table S5, Supplemental Content, which illustrates the results of the sensitivity analyses).
Figure 2.
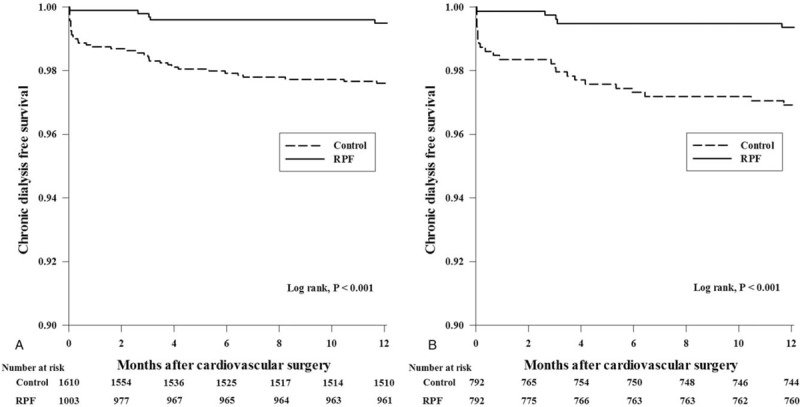
Kaplan–Meier curves for the time to chronic dialysis after cardiovascular surgery by perioperative fluid management strategy in the total cohort (A) and the propensity matched cohort (B).
4. Discussion
In this observational study of patients who underwent cardiovascular surgery, we found that the RPF strategy was associated with a significant decrease in the incidence of AKI, severe AKI, in-hospital RRT, persistent AKI at discharge, CKD, and chronic dialysis within 1 year after cardiovascular surgery. In addition, we found that this strategy was also related to a shorter extubation time, ICU stay, and hospital stay. Furthermore, these findings remained significant after adjustment for baseline variables and were supported by several sensitivity analyses.
Renal dysfunction occurs frequently after cardiovascular surgery and is associated with a high morbidity and mortality rate.[23,24] Because there are currently no effective treatment options, optimizing perioperative care for the prevention of postoperative renal dysfunction would be beneficial. Therefore, the appropriate use of intravenous fluids during this period could be important. However, to date, despite extensive studies assessing the risks and benefits of the types and volume of fluids, the ideal resuscitation fluid or combination of fluids during the perioperative period remains an ongoing controversy. To our knowledge, this is the first study to assess the impact of perioperative intravenous fluid administration strategy on acute and chronic renal outcomes in patients undergoing cardiovascular surgery. However, our findings are in line with previous observations in different clinical settings suggesting that a large volume infusion of HES solution and saline solutions with supraphysiological concentrations of chloride may have detrimental renal effects.[2,4,8,9,12,25]
Although the exact mechanisms remain unclear, a large volume infusion of colloid and saline solution has been suggested to affect renal function adversely. Although 0.9% saline is the most frequently used intravenous solution during the perioperative period, there is a concern about the adverse effects on renal function of an intravenous solution containing supraphysiological concentrations of chloride. Our study shows that administration of saline-based solutions during surgery causes an immediate postoperative hyperchloremia, consistent with previous studies showing that 0.9% saline results in significantly more hyperchloremia and acidosis.[8,14] Earlier animal and human studies showed that hyperchloremic solutions could induce renal vasoconstriction and reduce renal blood flow, renal cortical tissue perfusion, and GFR.[15–17] Recent observational studies demonstrated that acute postoperative hyperchloremia was associated with a poor postoperative outcome, and large infusions of chloride-liberal fluids including 0.9% saline was linked to an increased risk of major postoperative complications, including renal dysfunction in non-cardiac surgery patients.[8,25,26]
Additionally, HES solutions are effective volume expanders and have been used widely for intravascular volume replacement during the perioperative period. Recently, a number of clinical trials and meta-analyses have questioned the safety of using a HES solution for fluid resuscitation due to concerns about adverse effects on mortality and increased renal dysfunction, especially in septic or critically ill patients.[4,6,7] However, different underlying pathophysiologic conditions, which could lead to different efficacy and safety profiles of HES solutions, complicates the generalization of the safety concerns from the critical care setting to the perioperative setting. Moreover, the biological effects of the newer 6% HES 130/0.4 significantly differ from older-generation starches, and these compounds are believed to be less likely to be nephrotoxic and to cause coagulopathy in the perioperative setting.[3,27] Thus, to date, no specific guidelines for the perioperative use of new-generation 6% HES 130/0.4 are available. However, a recent observational study in cardiac surgery patients demonstrated that perioperative fluid therapy with the newer 6% HES 130/0.4 was related to a higher risk of renal failure and greater use of RRT.[9]
These findings from previous studies suggest that perioperative volume replacement with chloride-restricted balanced solutions and low volume HES could result in better postoperative renal outcomes. We have recently reported in 783 patients that a perioperative fluid management strategy affected renal and clinical outcomes after off-pump coronary artery bypass graft surgery.[18] These results were confirmed in the present more heterogeneous and larger cohort. Furthermore, our results show that a RPF strategy is associated with better chronic renal outcomes as well as acute renal outcomes. These results strongly support the concept that perioperative fluid management strategies can affect long-term outcomes beyond the immediate postoperative period.
Our findings suggest that higher cumulative doses of new-generation 6% HES 130/0.4 during the perioperative period could also be related to a higher risk of renal impairment and blood transfusion in patients undergoing cardiovascular surgery. Furthermore, the mean cumulative amount of HES solution during the control period was 33.3 mg/kg, which was well below the manufacturer's recommended daily dose limit of 50 mg/kg for 6% HES 130/0.4. These observations are in accordance with the findings of the previous studies suggesting that the adverse effects of HES solutions may be linked to dosage[9,11] and support the European Society of Intensive Care Medicine task force recommendation to reassess the existing dose limits for newer tetrastarches.[28]
Another finding from our study was that patients in the RPF group had a decreased need for perioperative blood products and reoperation for bleeding. This finding is in agreement with previous studies that saline-based solution and HES could have negative effects on blood coagulation and could aggravate the cardiopulmonary bypass-related effects on the hemostatic system.[8,29–31] On the other hand, beneficial effects of the RPF strategy on renal outcomes may be due to the reduction in perioperative blood transfusion rather than the RPF strategy itself. Indeed, several studies have demonstrated that perioperative blood transfusion may be independently linked to an increased risk of AKI after cardiovascular surgery.[32,33] Thus, we cannot completely exclude the possibility that differences in the amount of perioperative blood transfusion could have influenced our results.
The present study has several limitations. First, the 2 study groups were from 2 different time periods (i.e., before-and-after design), thus creating the possibility of related confounding factors. For example, increasing concerns about AKI in the RPF period might have encouraged physicians to implement interventions, resulting in improved outcomes. Thus, we cannot exclude the possibility that observed differences in outcomes between the 2 groups may be due to secular changes over time as a result of general improvements in the care of patients undergoing cardiovascular surgery rather than the RPF strategy itself. Moreover, because this was an observational study using data collected retrospectively in the before and after periods, the limitations of a nonrandomized retrospective observational study remain. Although we performed a multivariable analysis and propensity analyses with many variables to control bias and obtain reliable results, it is still possible that residual confounding due to unmeasured variables which cannot be controlled by the statistical analysis may have influenced the results. In other words, it is possible that any differences between the 2 groups in renal outcome may not have resulted from the intervention but might have been due to differences in unmeasured baseline characteristics or just due to random noise. Therefore, despite its strong relationships with a high magnitude of statistical significance, our findings should be regarded as a hypothesis generation step, and a causal relationship between the perioperative fluid management strategy and risk of postoperative renal dysfunction could not be determined. Third, our findings could be attributed to the net effect of both of 2 protective measures (i.e., an intraoperative use of balanced solutions and a perioperative limited amount of HES solution). However, dissecting the relative contributions of these 2 factors is difficult. Moreover, this was a study to assess the effects of implementing a care bundle comprised of 2 components of perioperative intravenous fluid administration strategy on renal outcomes after cardiovascular surgery. Therefore, as with all studies evaluating a “bundle of process,” we are not able to identify which component of our intervention (restricting chloride, giving less sodium, using a limited amount of HES solution during the perioperative period, using balanced solutions containing acetate and gluconate, delivering more potassium or magnesium, or any combination of these) may have contributed to the changes in outcomes observed. Thus, our results should be interpreted with caution, and further prospective randomized studies will be needed to increase our understanding of this association. Fourth, our results from patients with preoperatively normal renal function should not be extrapolated to patients with renal dysfunction. Fifth, intraoperative hemodynamic instability could have a negative impact on postoperative renal outcomes. Because our database unfortunately contains no information on the intraoperative hemodynamic data, we could not report the effect of intraoperative hemodynamic status on renal outcomes, however. Finally, there is wide variability across institutions in the practice of fluid administered in the perioperative period. Because this was a single-center study conducted at a tertiary care academic medical center, caution should be taken when generalizing these results to centers with different patient profiles and different practices of fluid therapy.
5. Conclusion
This retrospective, single-center observational study found that a perioperative intravenous fluid administration strategy using an intraoperative total balanced solution and a perioperative limited amount of HES solution was associated with better acute and 1-year renal outcomes after cardiovascular surgery. These findings indicate an urgent need for further definitive clinical trials in this field.
Author contributions
Conceptualization: Eun-Ho Lee, Jun-Young Jo, Dae-Kee Choi, In-Cheol Choi.
Data curation: Eun-Ho Lee, Sung-Cheol Yun, Ye-Ji Lim, Jun-Young Jo, Dae-Kee Choi.
Formal analysis: Eun-Ho Lee, Sung-Cheol Yun.
Investigation: Eun-Ho Lee, Ye-Ji Lim, Jun-Young Jo, Dae-Kee Choi, In-Cheol Choi.
Methodology: Eun-Ho Lee, Sung-Cheol Yun, Dae-Kee Choi.
Project administration: In-Cheol Choi.
Resources: Eun-Ho Lee, In-Cheol Choi.
Software: Ye-Ji Lim.
Supervision: In-Cheol Choi.
Validation: Dae-Kee Choi, In-Cheol Choi.
Visualization: Eun-Ho Lee, Jun-Young Jo.
Writing – original draft: Eun-Ho Lee, Ye-Ji Lim, Jun-Young Jo, Dae-Kee Choi.
Writing – review & editing: Sung-Cheol Yun, In-Cheol Choi.
Eun-Ho Lee orcid: 0000-0002-6369-7429.
Supplementary Material
Footnotes
Abbreviations: AKI = acute kidney injury, CI = confidence interval, CKD = chronic kidney disease, GFR = glomerular filtration rate, HES = hydroxyethyl starch, HR = hazard ratio, ICU = intensive care unit, KDIGO = kidney disease improving global outcomes, OR = odds ratio, RPF = renal-protective fluid management, RRT = renal replacement therapy, sCr = serum creatinine.
E-HL and S-CY have contributed equally to this work.
Financial Disclosures: none.
The authors have declared that no competing interest exists.
Supplemental Digital Content is available for this article.
References
- [1].Habicher M, Perrino A, Jr, Spies CD, et al. Contemporary fluid management in cardiac anesthesia. J Cardiothorac Vasc Anesth 2011;25:1141–53. [DOI] [PubMed] [Google Scholar]
- [2].Yunos NM, Bellomo R, Hegarty C, et al. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 2012;308:1566–72. [DOI] [PubMed] [Google Scholar]
- [3].Martin C, Jacob M, Vicaut E, et al. Effect of waxy maize-derived hydroxyethyl starch 130/0.4 on renal function in surgical patients. Anesthesiology 2013;118:387–94. [DOI] [PubMed] [Google Scholar]
- [4].Zarychanski R, Abou-Setta AM, Turgeon AF, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA 2013;309:678–88. [DOI] [PubMed] [Google Scholar]
- [5].Finfer S, Liu B, Taylor C, et al. Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care 2010;14:R185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012;367:1901–11. [DOI] [PubMed] [Google Scholar]
- [7].Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med 2012;367:124–34. [DOI] [PubMed] [Google Scholar]
- [8].Shaw AD, Bagshaw SM, Goldstein SL, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg 2012;255:821–9. [DOI] [PubMed] [Google Scholar]
- [9].Bayer O, Schwarzkopf D, Doenst T, et al. Perioperative fluid therapy with tetrastarch and gelatin in cardiac surgery–a prospective sequential analysis∗. Crit Care Med 2013;41:2532–42. [DOI] [PubMed] [Google Scholar]
- [10].Raghunathan K, Shaw A, Nathanson B, et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis∗. Crit Care Med 2014;42:1585–91. [DOI] [PubMed] [Google Scholar]
- [11].Schabinski F, Oishi J, Tuche F, et al. Effects of a predominantly hydroxyethyl starch (HES)-based and a predominantly non HES-based fluid therapy on renal function in surgical ICU patients. Intensive Care Med 2009;35:1539–47. [DOI] [PubMed] [Google Scholar]
- [12].Kashy BK, Podolyak A, Makarova N, et al. Effect of hydroxyethyl starch on postoperative kidney function in patients having noncardiac surgery. Anesthesiology 2014;121:730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Base EM, Standl T, Lassnigg A, et al. Efficacy and safety of hydroxyethyl starch 6% 130/0.4 in a balanced electrolyte solution (Volulyte) during cardiac surgery. J Cardiothorac Vasc Anesth 2011;25:407–14. [DOI] [PubMed] [Google Scholar]
- [14].Potura E, Lindner G, Biesenbach P, et al. An acetate-buffered balanced crystalloid versus 0.9% saline in patients with end-stage renal disease undergoing cadaveric renal transplantation: a prospective randomized controlled trial. Anesth Analg 2015;120:123–9. [DOI] [PubMed] [Google Scholar]
- [15].Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest 1983;71:726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chowdhury AH, Cox EF, Francis ST, et al. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte(R) 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg 2012;256:18–24. [DOI] [PubMed] [Google Scholar]
- [17].Chowdhury AH, Cox EF, Francis ST, et al. A randomized, controlled, double-blind crossover study on the effects of 1-L infusions of 6% hydroxyethyl starch suspended in 0.9% saline (voluven) and a balanced solution (Plasma Volume Redibag) on blood volume, renal blood flow velocity, and renal cortical tissue perfusion in healthy volunteers. Ann Surg 2014;259:881–7. [DOI] [PubMed] [Google Scholar]
- [18].Kim JY, Joung KW, Kim KM, et al. Relationship between a perioperative intravenous fluid administration strategy and acute kidney injury following off-pump coronary artery bypass surgery: an observational study. Crit Care 2015;19:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology 2007;18:805–35. [DOI] [PubMed] [Google Scholar]
- [20].Joung KW, Jo JY, Kim WJ, et al. Association of preoperative uric acid and acute kidney injury following cardiovascular surgery. J Cardiothorac Vasc Anesth 2014;28:1440–7. [DOI] [PubMed] [Google Scholar]
- [21].Okusa MD, Davenport A. Reading between the (guide)lines–the KDIGO practice guideline on acute kidney injury in the individual patient. Kidney Int 2014;85:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- [23].Mangano CM, Diamondstone LS, Ramsay JG, et al. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med 1998;128:194–203. [DOI] [PubMed] [Google Scholar]
- [24].Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 2009;119:2444–53. [DOI] [PubMed] [Google Scholar]
- [25].Nadeem A, Salahuddin N, ElHazmi A, et al. Chloride-liberal fluids are associated with acute kidney injury after liver transplantation. Crit Care 2014;18:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McCluskey SA, Karkouti K, Wijeysundera D, et al. Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: a propensity-matched cohort study. Anesth Analg 2013;117:412–21. [DOI] [PubMed] [Google Scholar]
- [27].Jacob M, Fellahi JL, Chappell D, et al. The impact of hydroxyethyl starches in cardiac surgery: a meta-analysis. Crit Care 2014;18:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Reinhart K, Perner A, Sprung CL, et al. Consensus statement of the ESICM task force on colloid volume therapy in critically ill patients. Intensive Care Med 2012;38:368–83. [DOI] [PubMed] [Google Scholar]
- [29].Roche AM, James MF, Bennett-Guerrero E, et al. A head-to-head comparison of the in vitro coagulation effects of saline-based and balanced electrolyte crystalloid and colloid intravenous fluids. Anesth Analg 2006;102:1274–9. [DOI] [PubMed] [Google Scholar]
- [30].Navickis RJ, Haynes GR, Wilkes MM. Effect of hydroxyethyl starch on bleeding after cardiopulmonary bypass: a meta-analysis of randomized trials. J Thorac Cardiovasc Surg 2012;144:223–30. [DOI] [PubMed] [Google Scholar]
- [31].Rasmussen KC, Johansson PI, Hojskov M, et al. Hydroxyethyl starch reduces coagulation competence and increases blood loss during major surgery: results from a randomized controlled trial. Ann Surg 2014;259:249–54. [DOI] [PubMed] [Google Scholar]
- [32].Haase M, Bellomo R, Story D, et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol Dial Transplant 2012;27:153–60. [DOI] [PubMed] [Google Scholar]
- [33].Karkouti K. Transfusion and risk of acute kidney injury in cardiac surgery. Br J Anaesth 2012;109suppl:i29–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


