Supplemental Digital Content is available in the text
Keywords: dementia, meta-analysis, proton pump inhibitors, risk
Abstract
Background:
Proton pump inhibitors (PPIs) are an established kind of drugs used to the treatment of most acid-related diseases. Some prospective studies have noticed that PPI use was associated with increased dementia risk. However, the results of those studies were inconsistent and controversial. This meta-analysis aims to determine the association of PPI use and risk of dementia among older people.
Methods:
Relevant articles were systematically identified by searching the PubMed, EMBASE, and Cochrane Library databases from inception to February 2018. Cohort studies that reported the risk of dementia or Alzheimer's disease (AD) among PPI users compared with non-PPI users were included. The quality of studies was assessed using the Newcastle-Ottawa Scale (NOS). The publication bias was detected by a funnel plot and Egger test. The meta-analysis will abstract risk estimates including relative risks (RRs), hazard ratios (HRs), and odds ratios (ORs) with a 95% confidence interval (CI) for the associations between PPI use and dementia or Alzheimer's risk. Study-specific results were pooled using a random-effects model.
Results:
Six cohort studies were selected finally. The pooled RRs of dementia and AD were 1.23 (95% CI: 0.90–1.67) and 1.01 (95% CI: 0.78–1.32), respectively, compared with those of non-PPI use. The Egger test and funnel plot showed no existence of publication bias. Overall, there was no statistically significant association between PPI use and risk of dementia or AD (P >.05).
Conclusions:
This meta-analysis suggests that there was no statistical association between PPIs use and increased risk of dementia or AD.
1. Introduction
Proton pump inhibitors (PPIs) which can suppress the secretion of gastric acid by inhibiting the H+/K+ ATPase present on the plasma membrane of the gastric parietal cells are widely prescribed class of medications for treatment of acid-related disorders such as gastroesophageal reflux and peptic ulcer.[1] Almost 30 years ago, it was first introduced as prescription drugs in the United States.[2] Considering its safety and relatively lower incidence of adverse effects, the use of PPI has significantly increased over the last decades.[3] However, some observational studies have shown that there are 40% to 60% of PPI prescriptions may pose a risk to the elderly persons.[4,5] According to a primary care database study, nearly 60 percent of patients who received long-term PPI therapy had no attempt to stop or reduce their dosage.[6] In addition, mounting studies in recent years have pointed out that there are many potential adverse events may be linked to PPI use, such as hip fractures,[7,8] community-acquired pneumonia,[9] hypomagnesemia,[10] and kidney disease.[11] Some evidence suggested that PPI use might affect cognition.[12,13] A recent study[14] have found that PPI use was also associated with an increased risk of incident dementia.
Dementia is a chronic, progressive, multifactorial syndrome characterized by a decline in cognitive function and capacity for independent living.[15] The number of people with dementia in 2012 was 35.6 million, and it is expected to double by 2030.[16] The World Health Organization estimated that the proportion of the world's population over 60 years will increase to 22% by 2050, and 25% to 30% of people aged 85 or older will experience a certain degree of cognitive decline.[17] Although numbers of factors have been ascertained as important risk factors of dementia, such as depression, diabetes, hypertension, smoking, and physical or cognitive decline.[18] The exploration of other risk factors is still ongoing, especially for those factors that are controversial. Gomm et al[14] reported that there are significant associations between PPI use and the incidence of dementia. However, other studies[19,20] suggested that PPI use was not related to dementia risk. Additionally, a case-control study of 23912 objects from a database of general practice medical records in Germany conducted by Booker et al[21] showing a statistically significant reduction in the risk of dementia with PPI use (P = .0008). In concluding the relationship between PPI use and risk of dementia is still inconclusive.
There had been published a similar meta-analysis conducted by Wijarnpreecha et al.[22] However it included only 4 articles (2 cohort studies, 1 cross-sectional study and 1 case–control study). Although these included studies were high quality, findings of 2 medical registry-based studies possibly inaccuracy and incompleteness may affect the overall results. Further, another similar study conducted by Batchelor et al[23] only made a systematic review using composite end points, including dementia, and cognitive impairment. Considering the uncertainty of causality and potential bias from cross-sectional and case–control studies, the cohort design would be considered the most robust approach. Therefore, we conducted this systematic review which includes only cohort studies to determine whether the use of PPIs increases the risk of dementia and to gain a better understanding of whether PPI use potentially influences the risk of dementia or AD.
2. Materials and methods
We performed this systematic review and meta-analysis according to MOOSE (Meta-Analysis of Observational Studies in Epidemiology) guidelines.[24] The review protocol was registered on PROSPERO (http://www.crd.york.ac.uk/prospero/) with the identification number CRD42017073686.
2.1. Search strategy
We mainly searched the databases of PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL). The following terms were applied: “proton pump inhibitors”, “PPI”, “lansoprazole”, “dexlansoprazole”, “kapidex”, “prevacid”, “omeprazole”, “esomeprazole”, “nexium”, “prilosec”, “pantoprazole”, “protonix”, “rabeprazole”, “aciphex”, “dexrabeprazole”, “pariet” and “dementia”, “Alzheimer disease”, “cognitive decline”, “cognitive impairment” and “Prospective Studies”, “Cohort Studies”, “Longitudinal Studies”, and “Follow-Up Studies” were applied. There present full electronic search strategy of 1 database (Supplemental Digital Content 1).
2.2. Study selection
Studies were considered eligible if they met the following criteria:
-
(1)
cohort study design;
-
(2)
PPI use was 1 exposure-outcome of interest;
-
(3)
dementia or AD represented 1 outcome of interest;
-
(4)
patients aged ≧55 years, controls were age- and sex-matched to the study subjects;
-
(5)
RRs, odds ratios (ORs), HRs, and the corresponding 95% confidence interval (CI) (or data to calculate them) were reported.
Studies were excluded if they met the following criteria:
-
(1)
repeated publications;
-
(2)
inability to obtain full text or data; and
-
(3)
reviews, cross-sectional studies, commentaries, and letters.
Two review authors independently screened studies against the inclusion and exclusion criteria. The third author adjudicated any discordance in assessments by discussion with this 2 review authors.
2.3. Data extraction
Two reviewers independently and respectively extracted data from the included studies using a standardized data-collection form. The information included author's last name, publication year, source of study, participants’ characteristics, definition, and ascertainment of PPI use and dementia, sample size, potential confounder adjustment, and the dementia risk estimated with 95% CI. Studies for inclusion were also identified by screening references of included articles. Any data discrepancy was resolved by discussing.
2.4. Risk of bias (quality) assessment
The quality of the included studies was assessed by 2 independent review authors using the Newcastle-Ottawa Scale (NOS)[25] as recommended by the Cochrane Non-Randomized Studies Methods Working Group. This instrument was developed to assess the quality of non-randomized studies, specifically cohort and case-control studies. The scale awards a maximum of 9 stars to each study: 4 stars for the adequate selection of cohort participants, 2 stars for the comparability of cohort participants on the basis of study design and analysis, and 3 stars for the adequate ascertainment of outcomes. Studies that received stars equal to or more than 7 were considered to be high quality.
2.5. Statistical analysis
Data analysis was performed using Review Manager 5.3 software from the Cochrane Collaboration (London, UK). To explore the association between PPI use and the risk of dementia, we will abstract risk estimates (RRs, HRs, and ORs) with 95% CI, and the HRs were directly considered as RRs. Taking a conservative approach, we used a random-effects model to calculate the pooled RR and 95% CI for all articles. Relative to fixed-effects models, random-effects models were more appropriate for the present study because test statistics showed evidence of heterogeneity among these included studies. The significance of the pooled RR will be determined by the Z test and a P value of less than .05 will be considered significant. The heterogeneity among studies will be confirmed by Cochran Q test and I2 statistic. A value of I2 of 0% to 25% represents insignificant heterogeneity, more than 25% but less than or equal to 50% represents low heterogeneity, more than 50% but less than or equal to 75% represents moderate heterogeneity, and more than 75% represents high heterogeneity.[26] In addition, 1 study[19] separately reported the risk of dementia in 3 groups based on specific levels of cumulative exposure. We combined these 3 groups into a single group and calculated a combined hazard ratio (HR) using a fixed-effects model for the main analysis.[27] Besides, we excluded every single study in turn to test the influence of a single study on the overall risk estimate. The sensitivity analysis was conducted for dementia only since there were few studies on other outcomes. A funnel plot was used to visually evaluate the publication bias. Egger test (significance level<0.05) was conducted to quantitatively explore the possible publication bias using the Stata software package (version 12.0; StataCorp LP, College Station, TX).
2.6. Ethical approval
This systematic review does not require ethical approval or patient consent because it was a secondary analysis of human subject data published in the public domain.
3. Results
3.1. Literature search and study characteristics
The search strategy yielded 1583 unique relevant articles (19 articles from the Cochrane Library; 249 articles from PubMed; and 1315 articles from EMBASE). After the removal of 184 duplicate articles, there were 1399 articles screened by 2 independent reviewers. Of the 1319, articles were excluded after the first screening based on abstracts or titles mainly because they clearly did not fulfill the inclusion criteria, leaving 80 articles for a full-length article review. After a second screening based on the full texts, the majority were excluded for being the following: reviews and comments (n = 25); letters (n = 12); a non-PPI therapy or no non-PPI control group (n = 20); unable to obtain full text (n = 2); unable to obtain full data (n = 2); not a cohort study (n = 7); and a duplicate abstract of published studies (n = 6). Finally, 6 articles met the inclusion criteria and were included in our meta-analysis.[14,19,20,28–30] A flow chart showing the study selection is presented in (Fig. 1).
Figure 1.
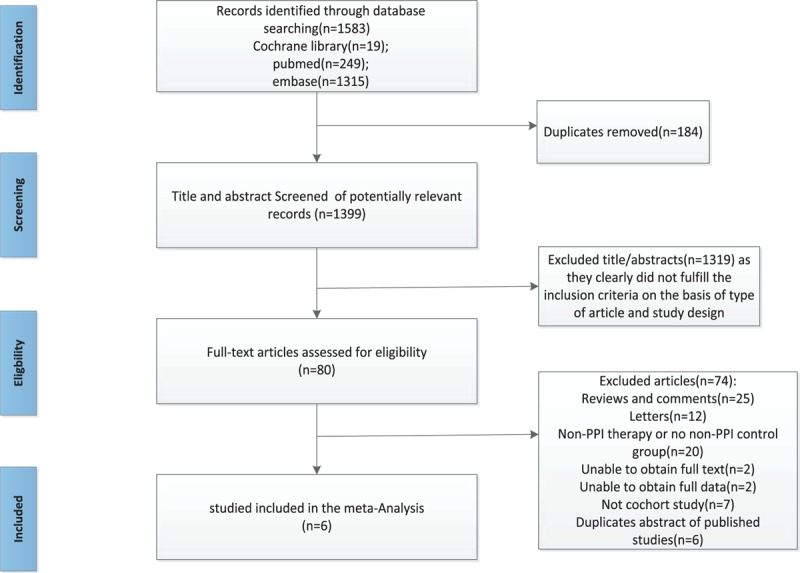
Flow chart showing literature search for include cohort studies.
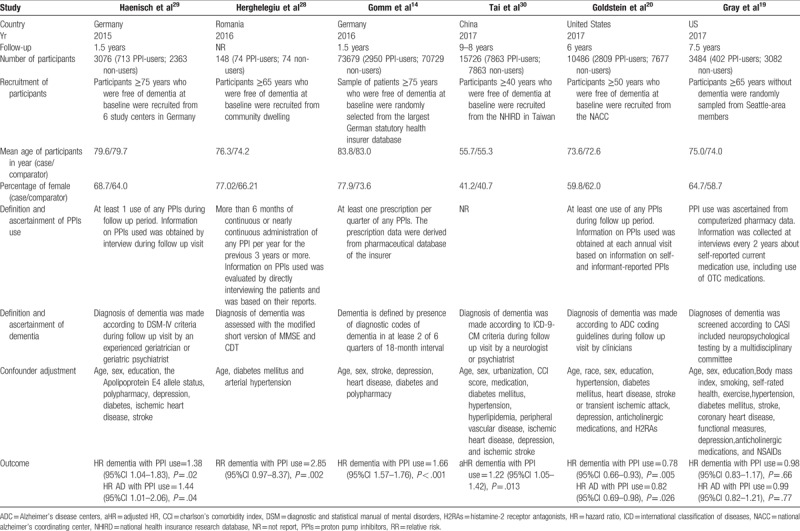
The characteristics of the 6 cohort studies are presented in Table 1. The length of follow-up ranged from 0.67 to 9 years. The sizes of the cohort study ranged from 148 to 73679. The definition of PPI use varies from study to study mostly based on medicine records or self-reports of patients. Outcome assessments were from a variety of sources, including medical records, self-reports, and hospital databases. One of included study[30] failed to give the definition and ascertainment of PPI use. Another study[28] adjusted for age, diabetes mellitus, and arterial hypertension, whereas others controlled a group of conventional risk factors for dementia, such as age, sex, polypharmacy, diabetes, and depression.
Table 1.
Main characteristics of the studies included in this meta-analysis of the association between PPIs and dementia.
3.2. Risk of bias (quality) assessment
In this meta-analysis, the assessment of risk of quality showed that all studies had high quality.[14,19,20,28–30] Details are shown in (Supplemental Digital Content 2).
3.3. Primary Outcome
Six studies[14,19,20,28–30] were available to assess the association between PPI use and the risk of dementia. (Fig. 2) illustrates the results via the random-effects model of the pooled RRs for dementia. Among the 6 studies, 4 studies showed a significantly positive association between PPI use and risk of dementia. However, the RRs of the association ranged from 0.78 to 2.85 across studies. The outcome demonstrated that PPI use had a mildly increased risk of dementia compared with that of non-PPI use, with the pooled RR of 1.23 (95% CI: 1.05–1.42), though it was not statistically significant (P = .19). Substantial heterogeneity was observed (P < .00001, I2 = 95%).
Figure 2.
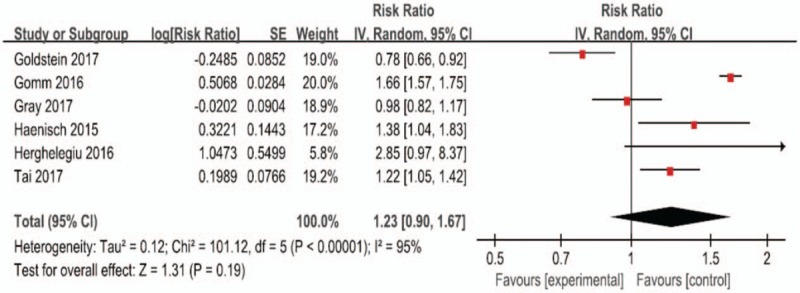
Forest plot of the included studies of the associations between PPIs use and risk of dementia. PPI = proton pump inhibitor.
Only 3 studies[19,20,29] reported the association between PPI use and risk of AD. (Fig. 3) indicates the results from random-effects model combining the RRs for AD. Although there were only 3 articles that mentioned the association between PPI use and risk of AD, the results were relatively consistent to the risk of dementia. Overall, compared with non-PPI use, the risk of AD caused by using PPIs was not statistically significant (P = .93). Therefore, the statistical heterogeneity was high (P = .02, I2 = 76%).
Figure 3.
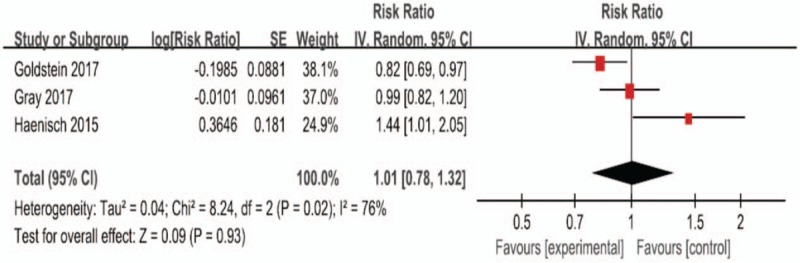
Forest plot of the included studies of the associations between PPIs use and risk of Alzheimer's disease. PPI = proton pump inhibitor.
3.4. Subgroup analyses
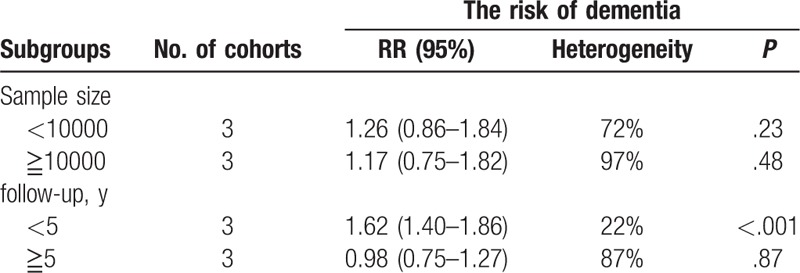
To make our results more meaningful, we performed subgroup analyses according to sample size and follow-up time of included studies. We found the estimated RR of studies with shorter follow-up time (<5 years) was 1.62 (95% CI: 1.40–1.86), which was inverse to the overall combined RR. We considered this may because these 3 studies show positive results, and 2 of them come from the same region. When studies with longer follow-up time (≧5 years) were pooled, we found there was no statistically significant association between PPIs use and risk of dementia 0.98 (95% CI: 0.75–1.27). In addition, neither small sample studies (sample size <10000) with the pooled RR of 1.26 (95% CI: 0.86–1.84) nor large sample studies (sample size ≧ 10000) with the pooled RR of 1.17 (95% CI: 0.75–1.82) changed the overall combined RR. Details are illustrated in Table 2.
Table 2.
Subgroup analyses for PPIs use and risk of dementia.
3.5. Sensitivity analyses
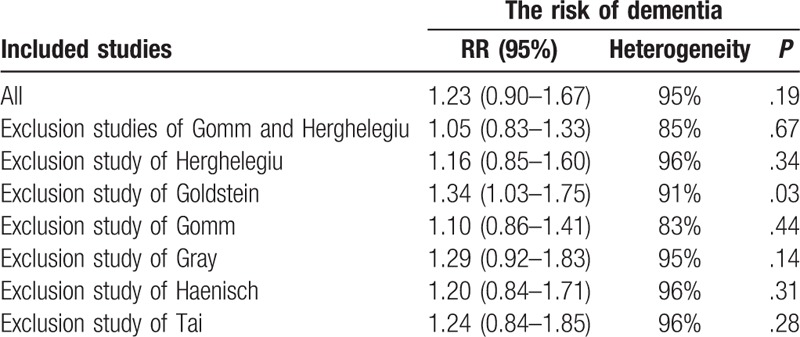
Exclusion of 2 studies[14,28] with too large or small sample size did not change the overall risk estimate (RR: 1.05[95% CI: 0.83–1.33]; P = .67), and the heterogeneity was still high (I2 = 85%). The further exclusion of any single study at a time did not materially alter the overall combined RR except Goldstein et al,[20] with a range from 1.10 (95% CI: 0.85–1.41; P = .44) to 1.29 (95% CI: 0.92–1.83; P = .14). Details are illustrated in Table 3.
Table 3.
Sensitivity analyses of studies.
3.6. Publication bias
The roughly symmetrical funnel plot shown in Figure 4 indicates no existence of publication bias in this meta-analysis. Egger test (P >.05) further confirmed this result.
Figure 4.
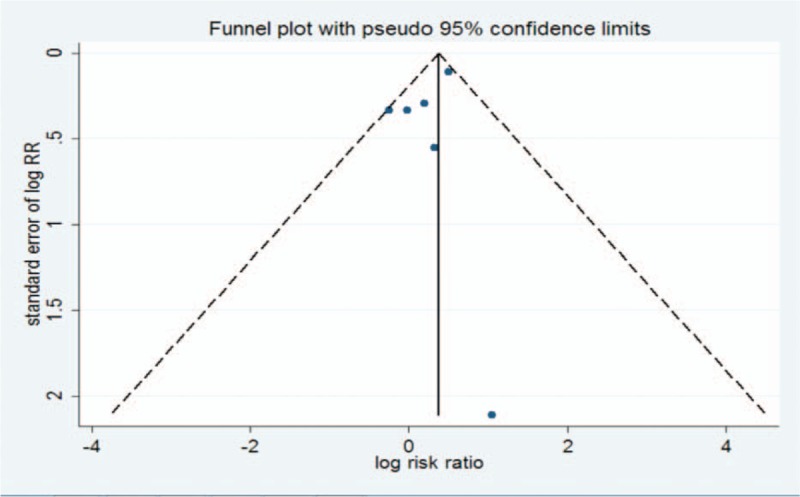
Funnel plot for all of this meta-analysis.
4. Discussion
Our meta-analysis included 6 cohort studies that explored the association of PPI use with the risk of dementia. To our knowledge, this study is the first meta-analysis to include only cohort studies assessing the associations of the use of PPI and the incidence of dementia. There is substantial heterogeneity among included studies, which was not surprising given the differences in characteristics of participants, ascertainment of dementia and PPIs exposure, and adjustment for confounding factors. Considering the Herghelegiu et al[28] and the Gomm et al[14] study may have little or a great impact on the outcome, we exclude both of them in sensitivity analysis. And the finding did not alter the overall combined RR. However, our sensitivity analysis showed that the exclusion of Goldstein et al[20] had a significant influence on the final result, the heterogeneity did not change substantially. Compared to other included studies, the Goldstein et al[20] had a more rigorous ascertainment of dementia, which relied on diagnoses of cognitive status by a team of experienced clinicians in academic medical centers. This could lead to a lower correlation between PPI use and the risk of dementia. In fact, the study showed negative results with evidently smaller RR than other studies. Since the study had a low risk of bias according to the risk of bias assessment, the results possibly due to the large sample size too (more than 10000).
Our outcomes are remarkably similar with Wijarnpreecha et al,[22] which showed an increased risk of dementia among PPI users but it did not reach statistical significance. Recently, a German prospective cohort study including 73679 participants over 75 and free of dementia at baseline showed that PPI users had an increased risk of developing dementia.[14] Subsequently, Haenisch et al[29] discovered that PPI use was associated with a significantly increased risk of developing dementia among primary care patients aged 75 years and over as well. However, these results were questioned. Some studies from healthcare databases, such as the Korean Healthcare Database[31] and the National Alzheimer's Coordinating Center (NACC) database,[20] do not support the claim that PPI use is associated with a greater risk of dementia. Their findings are in line with those of a Finnish nationwide nested case–control study conducted by Taipale et al,[32] which assessed the impact of the lag window between exposure and outcome, finding no clinically meaningful relation between PPI use and the risk of AD. Furthermore, a case–control study with following up 10 years by Booker et al[21] found that using PPIs can even reduce the risk of dementia.(HR 0.94; 95% CI: 0.90–0.97).
Although the association between PPI use and risk of dementia is contradictory, there are some possible mechanisms that PPI use may contribute to dementia. First, PPIs may affect amyloid-beta (Aβ) metabolism, one of the pathological markers of Alzheimer's disease (AD).[33] There is evidence that PPIs could cross the blood-brain barrier, enhance Aβ levels in the brain and decrease Aβ degradation.[13,34] Strooper et al[35] reported that the precipitation of Aβ peptide in the central nervous system could conceivably increase the possibility of dementia. Second, another possible mechanism of PPI-induced dementia could relate to vitamin B-12 deficiency. Long-term use of PPI will decrease the secretion of gastric acid, resulting in a reduction in the release of the protein used to bind to vitamin B12, causing damage to vitamin B12 absorption.[36] Furthermore, decreased gastric acid increases the PH in the small bowel, which allows bacterial overgrowth and competition for uptake of vitamin B12, further reducing vitamin B12 availability.[37] In a population-based sample, Lam et al[5] pointed out that vitamin B12 deficiency negatively affects cognitive function.
Though these mechanisms demonstrated that PPI use may contribute to dementia, but “correlation does not prove causation”. In other words, the increased risk of dementia found in PPI users may be independent of PPI themselves. Additionally, PPIs were used widely in older people who inherently are at increased risk of dementia. In our meta-analysis, included studies controlled a group of conventional risk factors for dementia, but these controlled factors are varying from study to study. There are numerous confounding factors that can contribute to the increased risk of dementia including many conditions that may increase the risk, such as diabetes and depression. Maybe those people who took PPIs also smoked, drank, and had pre-existing dementia-related conditions or taking medications that may be at risk for dementia. Furthermore, evidence suggested that a prevalence of polypharmacy in the elderly is increased with age.[38,39] A meta-analysis conducted by Nattawut et al[40] indicated that polypharmacy was associated with dementia. In fact, Gomm et al[14] found that people who took 5 or more medications other than PPIs also had an increased likelihood of developing dementia by approximately 15%.[1] Besides, there may have the possibility that the increased risk of dementia in patients taking PPIs was also somehow related to their other medical diagnosis. Perhaps those patients who took multiple medications experienced more health care encounters which could have increased the likelihood of diagnosing dementia. In summary, whether PPI use actually causes an increase in the risk of dementia is, therefore, an important question requiring further evaluation.
There is some strength in our article. First, we reviewed current published literature on PPI use and risk of dementia by a comprehensive and systematic approach. Second, all the included original studies used a cohort design without other epidemiological observational studies. Third, most of them are of high quality with a large sample size, a long follow-up period and reliable exposure and outcome assessment.
However, the limitations of this meta-analysis must be considered as well. First, we only included cohort studies and the number of cohort studies on the association between PPI use and risk of dementia is relatively limited, so the inherent biases and selection bias cannot be avoided. Second, observational studies cannot prove causality. Finally, there is substantial heterogeneity among our studies, and we considered the reason to be because of variations in the methods of assessment of PPI use and dementia, cohort type, study population, follow-up time and adjustment variables across the included studies. In summary, further scientific study is required to define the actual relationship between PPI use and risk of dementia.
5. Conclusions
In conclusion, this meta-analysis showed that the use of PPIs was not significantly associated with risk of dementia or AD. Though there are some limitations in this meta-analysis, the results may be clinically significant and useful in discussing potential risks of PPI therapy with patients.
Author contributions
Data curation: Sisi Yu.
Formal analysis: Min Li.
Methodology: Min Li.
Software: Zheng Luo.
Visualization: Sisi Yu.
Writing – original draft: Min Li, Zheng Luo, Sisi Yu.
Writing – review & editing: Zhenyu Tang.
Zhenyu Tang orcid: 0000-0001-6164-2312.
Supplementary Material
Footnotes
Abbreviations: Aβ = amyloid-beta, AD = Alzheimer's disease, CI = confidence interval, HRs = hazard ratios, ORs = odds ratios, PPIs = proton pump inhibitors, RRs = relative risks.
ML and ZL contributed equally to this work.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Namazi MR, F Jowkar MD. A succinct review of the general and immunological pharmacologic effects of proton pump inhibitors. J Clin Pharm Ther 2008;33:215–7. [DOI] [PubMed] [Google Scholar]
- [2].Simonson W. Do proton pump inhibitors cause dementia. Geriatric Nursing 2016;37:228–9. [DOI] [PubMed] [Google Scholar]
- [3].Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors. JAMA Intern Med 2016;176:172–4. [DOI] [PubMed] [Google Scholar]
- [4].Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care 2010;16:228–34. [PubMed] [Google Scholar]
- [5].Pasina L, Nobili A, Tettamanti M, et al. Prevalence and appropriateness of drug prescriptions for peptic ulcer and gastro-esophageal reflux disease in a cohort of hospitalized elderly. Eur J Intern Med 2011;22:205–10. [DOI] [PubMed] [Google Scholar]
- [6].Othman F, Card TR, Crooks CJ. Proton pump inhibitor prescribing patterns in the UK: a primary care database study. Pharmacoepidemiol Drug Saf 2016;25:1079–87. [DOI] [PubMed] [Google Scholar]
- [7].Khalili H, Huang ES, Jacobson BC, et al. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMJ 2012;344:129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yang YX, Lewis JD, Epstein S, et al. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006;296:2947–53. [DOI] [PubMed] [Google Scholar]
- [9].Gulmez SE, Holm A, Frederiksen H, et al. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007;167:950–5. [DOI] [PubMed] [Google Scholar]
- [10].Park CH, Kim EH, Roh YH, et al. The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta-analysis. PLoS One 2014;9:e112558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 2016;176:238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lam JR, Schneider JL, Zhao W, et al. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA J Am Med Assoc 2017;152:821–9. [DOI] [PubMed] [Google Scholar]
- [13].Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One 2013;8:e58837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gomm W, Von HK, Thomã© F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016;73:410–6. [DOI] [PubMed] [Google Scholar]
- [15].Sosaortiz AL, Acostacastillo I, Prince MJ. Epidemiology of dementias and Alzheimer's disease. Arch Med Res 2012;43:600–8. [DOI] [PubMed] [Google Scholar]
- [16].Catindig JA, Venketasubramanian N, Ikram MK, et al. Epidemiology of dementia in Asia: insights on prevalence, trends and novel risk factors. J Neurol Sci 2012;321:11–6. [DOI] [PubMed] [Google Scholar]
- [17].World Health Organization. Ageing and life course, Facts about ageing, World Health Organization. Available at: http://www.who.int/ageing/about/facts/en/ [accessed date September 30, 2014.]. [Google Scholar]
- [18].Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 2011;10:819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gray SL, Walker RL, Dublin S, et al. Proton pump inhibitor use and dementia risk: prospective population-based study. J Am Geriatr Soc 2018;66:247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Goldstein FC, Steenland K, Zhao L, et al. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc 2017;65:1969–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Booker A, Jacob LE, Rapp M, et al. Risk factors for dementia diagnosis in German primary care practices. Value Health J Int Soc Pharm Outcomes Res 2015;18:1–7. [DOI] [PubMed] [Google Scholar]
- [22].Wijarnpreecha K, Thongprayoon C, Panjawatanan P, et al. Proton pump inhibitors and risk of dementia. Ann Transl Med 2016;4:240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Batchelor R, Gilmartin JF, Kemp W, et al. Dementia, cognitive impairment and proton pump inhibitor therapy—a systematic review. J Gastroenterol Hepatol 2017;32: [DOI] [PubMed] [Google Scholar]
- [24].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. J Am Med Assoc 2008;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [25].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [26].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [27].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Herghelegiu AMGP. Prolonged use of proton pump inhibitors and congnitive function in older adults. FARMACIA 2016;64:262–7. [Google Scholar]
- [29].Haenisch B, Von HK, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci 2015;265:419–28. [DOI] [PubMed] [Google Scholar]
- [30].Tai SY, Chien CY, Wu DC, et al. Risk of dementia from proton pump inhibitor use in Asian population: a nationwide cohort study in Taiwan. PLoS One 2017;12:e0171006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Park SK, Baek YH, Pratt N, et al. The uncertainty of the association between proton pump inhibitor use and the risk of dementia: prescription sequence symmetry analysis using a Korean healthcare database between 2002 and 2013. Drug Saf 2013;2018:1–0. [DOI] [PubMed] [Google Scholar]
- [32].Taipale H, Tolppanen AM, Tiihonen M, et al. No association between proton pump inhibitor use and risk of Alzheimer's disease. Am J Gastroenterol 2017;112:1802–8. [DOI] [PubMed] [Google Scholar]
- [33].Querfurth HW, Laferla FM. Alzheimer's disease. New Engl J Med 2010;362:329. [DOI] [PubMed] [Google Scholar]
- [34].Kuller LH. Do proton pump inhibitors increase the risk of dementia. JAMA Neurol 2016;73:379–81. [DOI] [PubMed] [Google Scholar]
- [35].De SB, Annaert W. Proteolytic processing and cell biological functions of the amyloid precursor protein. J Cell Sci 2000;113:1857–70. [DOI] [PubMed] [Google Scholar]
- [36].Jung SB, Nagaraja V, Kapur A, et al. The association between vitamin B12 deficiency and long-term use of acid lowering agents: a systematic review and meta-analysis. Intern Med J 2015;45:409–16. [DOI] [PubMed] [Google Scholar]
- [37].Wolters M, Ströhle A, Hahn A. Cobalamin: a critical vitamin in the elderly. Prevent Med 2004;39:1256–66. [DOI] [PubMed] [Google Scholar]
- [38].Kaufman DW, Kelly JP, Rosenberg L, et al. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA 2002;287:337–44. [DOI] [PubMed] [Google Scholar]
- [39].Hovstadius B, Hovstadius K, Astrand B, et al. Increasing polypharmacy-an individual-based study of the Swedish population. BMC Clin Pharmacol 2010;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Leelakanok N, D’Cunha RR. Association between polypharmacy and dementia-A systematic review and meta-analysis. Aging Ment Health 2018;10:1–0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


