Abstract
South Africa recently implemented the ‘test and treat’ strategy for all HIV-infected individuals receiving diagnosis at the health facility level. However, the impact of this programme in terms of the prevention of HIV transmission, morbidity and mortality associated with HIV can only be maximized if patients are diagnosed early. This study determines the prevalence of late presentation among newly diagnosed HIV-infected individuals and also examines the socio-demographic and clinical determinants for late presentation in health facilities in the Eastern Cape Province, South Africa.
In this cross-sectional study, a total of 335 newly diagnosed patients were recruited consecutively between August 2016 and July 2017. Late presenter for HIV care was defined in accordance with the European Late Presenter Consensus working group as a patient who reports for care when the CD4 count is below 350 cells/μL and/or when there is an established AIDS-defining clinical condition, irrespective of CD4 count. Adjusted and unadjusted logistic regression analysis was used to examine the determinants of late HIV diagnosis.
Participants’ mean age was 33.6 (SD: 10.6). Almost 96% of the participants believed their route of HIV infection was heterosexual sex. Most newly diagnosed HIV-infected patients (60%) were late presenters (CD4+ count ≤350 cells/μL and/or having an AIDS-defining illness in World Health Organisation (WHO)-defined stage III/IV), with 35% presenting with Acquired Immune Deficiency Syndrome (AIDS)-related complications. In the adjusted model, only male sex (AOR: 2.81; CI: 1.51–5.23), no formal education (AOR: 5.63; CI: 1.68–18.85), and overweight body mass category (AOR: 2.45; CI: 1.04–5.75) were independently associated with late HIV diagnosis.
The majority of newly diagnosed HIV-infected individuals were late presenters. To maximize the impact of the ‘test and treat’ policy aimed at reducing new HIV transmissions and preventing the morbidity and mortality associated with HIV, there is a need for programmes to improve early detection of HIV in the study settings. This programme should target males and individuals with no formal education for maximum impact.
Keywords: AIDS, HIV-infected patient, late presentation for care
1. Introduction
Late presentation or detection of HIV-infected individuals remains a source of impediment to the eradication of infections and HIV-related mortality.[1] Accessing HIV care at a late stage of the disease has been shown to be detrimental to the health of infected persons and society at large.[2–4] Also, presenting late for HIV care does not only further HIV transmission,[5–8] but also drives AIDS-related deaths.[9,10] Several studies have documented the benefit of early and timely initiation of anti-retroviral therapy (ART) in HIV-infected individuals.[11–14] Recently, Cohen et al[12] have greatly influenced policies across the world by advocating for earlier initiation of ART to HIV-infected individuals, irrespective of their CD4 count.[15,16] Notably, the early use of ART depletes the size of the HIV reservoir[17,18] and ensures that patients who start ART do so with preserved immune function. The patient who presents late for HIV care is at risk of clinical complications. It is still debatable if the immune function ever returns to the pre-infective state of normal function, with studies of long-term cohorts indicating that patients who adhere to ART treatment have a normal life expectancy, similar to that of non-HIV infected persons.[19]
The late presentation of HIV infection was defined by the European Late Presenter Consensus working group as a patient who reports for care when the CD4 count is below 350 cells/μL or who has an established AIDS-defining clinical condition, irrespective of the CD4 count.[20] This definition is aimed at allowing for coordinated care and a uniform approach to the management of HIV disease to improve the standard of care of late HIV presenters. Another study proposed a definition of late presenters as patients who present with a CD4 count <200 cells/μL and/or have an AIDS-defining illness within a month after HIV diagnosis, or patients with a first-reported CD4 count <350 cells/μL or an AIDS-defining illness within a month after HIV diagnosis.[21] A common definition of late presentation of HIV infection was advocated to ensure uniformity of care.[22] However, no such standard definition exists in the South African context to describe the cohort of HIV-infected individuals who present late with HIV infection.
The South African HIV guidelines have undergone numerous changes over the past few years, based on robust evidence supporting the treatment of all individuals diagnosed with HIV, irrespective of CD4 count[23] (the ‘test and treat’ strategy).[24] Preceding this change, immunological status (CD4 count) was crucial for ART initiation, which changed from 350 cells/uL to 500 cells/uL in 2015.[25] Of utmost importance is the adoption of a new HIV testing policy by both the World Health Organisation (WHO) and the South African Department of Health.[25]
In a study conducted in India, the percentage of patients who were detected with CD4 counts <200 and <350 cells/mm3 ranged from 46% to 68.7% respectively.[26] In developed countries, the prevalence of late presenters varies between 5% and 30%, and can rise up to 50%, depending on access to care in certain areas.[27] In South Africa, the prevalence of patients who present for HIV care with a CD4 count of less than 200 cells/uL is about 33.6%.[28] However, this figure was cited in a study conducted in 2012, when the CD4 threshold for enrolling into the ART programme was 350 cells/uL or less.[29]
Late presentation of HIV infection might be indicative of lack of knowledge about HIV, cultural and social stigmas, and/or socioeconomic barriers which limit access to healthcare.[28,30] Distances of more than 5 km between patients’ homes and health services was reported to have a substantial impact on timing of patients’ presentation at health care facilities.[28] Patients in rural areas in the United States of America were reported to present late for HIV care.[27] In China, the province where a patient resides has been reported to have a bearing on the time of presentation for HIV care.[31] Socioeconomic circumstances have also been found to cause a delay in early presentation for treatment. Lack of housing or not having a permanent place of abode contributes to late presentation.[31] This was corroborated in a similar study conducted in India.[26] The role of being a migrant significantly impacted on how early such migrant HIV patients access care.[32] Other socioeconomic factors which contributed to late presentation included lower educational attainment and non-business occupation.[33] Demographic factors such as age, gender and occupational status of patients have an impact on the timing of presentation for HIV care. Male gender, non-pregnancy state and older age were associated with late presentation for HIV care.[28,31–33]
Local epidemiological data on late presentation among newly diagnosed HIV-infected individuals could be important for policy advocacy and health managers. This valuable data is currently lacking in the Eastern Cape, South Africa. Also, the ‘test and treat’ policy could be a game-changer, given that treatments are freely available for everyone diagnosed with HIV in South Africa. The policy could have an impact at the population level if individuals living with HIV receive early diagnosis. Given the individual and public health benefits of early diagnosis and timely ART initiation,[30] it is crucial to gain an understanding of the extent of late presentation of HIV infection in the Eastern Cape, South Africa. This study examined the socio-demographic and clinical factors for late presentation amongst newly diagnosed HIV-infected individuals within the context of the ‘test and treat’ strategy in some health facilities in Buffalo City Municipality in the Eastern Cape.
2. Methods
2.1. Study areas and study population
All newly HIV-diagnosed patients in the HIV testing sites of Cecilia Makiwane Hospital and four randomly selected HCT sites at Buffalo City Municipality community health centers who consented to participate in the study were recruited into the study between August 2016 and July 2017. Cecilia Makiwane Hospital (CMH) is a regional hospital situated in Mdantsane, the second largest township in South Africa. The hospital serves as a major referral center for the central region of Eastern Cape. Various patients, regardless of ethnicity, geographical residence and socioeconomic status visit and are referred to the hospital. The HCT sites at the Buffalo District Municipality community health centers also cater for a wider region than only Buffalo City Municipality, and frequently serve as the first point of call for patients at primary health care level. The hospital and the community health centers are situated in Buffalo City Local Municipality, in Amathole District Municipality, where the prevalence of HIV infection, as recorded by the antenatal clinic attendance (ANC) survey, is 12.7%.[34] The required sample size of 335 was based on the 12.7% HIV-prevalence rate in the study setting, a 95% confidence level and a ±3.57% confidence interval.
Participants were recruited over a period of 1 year. Participants were eligible if they were 15 years old or over, newly diagnosed with HIV and had no prior exposure to ART. The target population were all newly established HIV-positive patients in the Eastern Cape Province.
2.2. Study procedure
All newly diagnosed patients in the study period who gave written consent were asked to complete a questionnaire by trained research nurses at the clinics. A pre-validated questionnaire was used to obtain relevant demographic characteristics: age, sex, place of residence, employment status, and level of education. Additional information on the probable route of transmission was elicited from the participants. The data instrument was initially piloted with ten participants, and findings were used to improve the questionnaire for the study. Information on WHO clinical stage, CD4 count and other clinical conditions was obtained from patients’ files and documented in the questionnaire. The research nurses measured the participants’ weight and height in accordance with standard protocols.
The study protocol was approved by the University of Fort Hare Postgraduate Ethical Committee (REC 270710028RA level 01) and the Eastern Cape Health Department (approval number: EC_2016RP22_139). All respondents provided written consent. The respondents’ rights to confidentiality and privacy were respected throughout the study.
2.3. Main outcome variable
Late presenter for HIV diagnoses and care was defined in accordance with the European Late Presenter Consensus working group as a patient who reports for care when the CD4 count is below 350 cells/ μL and/or when there is an established AIDS-defining clinical condition, irrespective of CD4 count. Patients were considered late presenters if their CD4 count was below 350 cells/μL or they had an established AIDS-defining clinical condition.
2.4. Data analysis
Data were coded and entered into the Statistical Package for Social Sciences (SPSS version 24, Chicago, IL). A simple frequency count was computed for all variables, and errors in data entries were corrected. Mean and standard deviations were estimated for age. Frequency counts and percentages were computed for categorical variables. Bivariate and multivariate analyses were used to examine the factors associated with late presentation. Adjusted and unadjusted logistic regression models were estimated at a 95% confidence interval. A P value of less than .05 was considered statistically significant.
3. Results
3.1. Sociodemographic
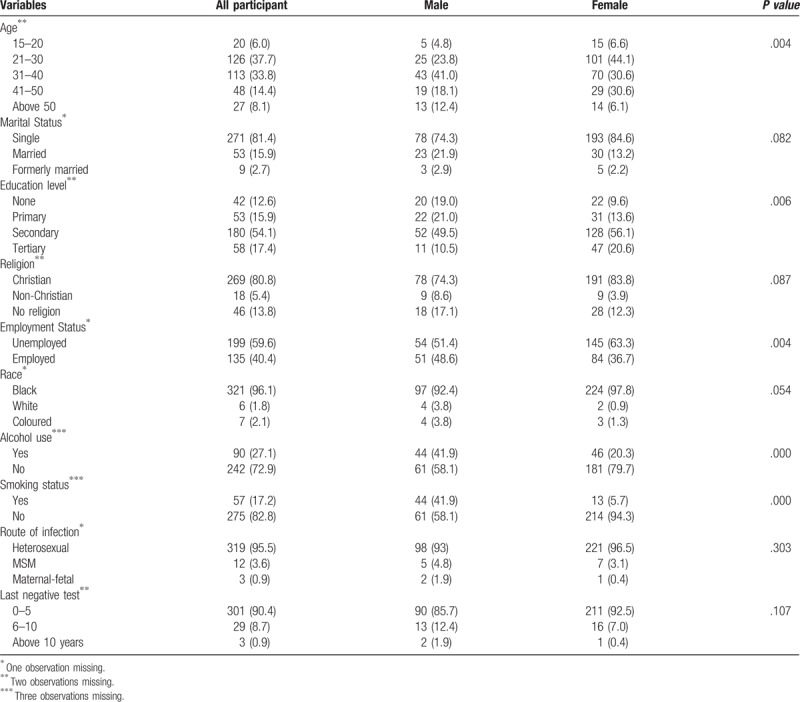
Of the 335 participants, 105 (31%) were men and 230 (69%) women. The mean age of the patients was 33.6 (SD: 10.6). The majority were single (81.4%), had a secondary education (54%) and were unemployed (60%). Concerning the pattern of infection, almost 96% of the participants believed they had acquired the HIV infection through heterosexual sex, while 3.6% of the participants had acquired the HIV infection through partners of the same sex. With regard to frequency of HIV testing, 90% of the study participants had undergone an HIV test within the last 5 years ().
Table 1.
Characteristics and proportion of late presentation of HIV infection among newly diagnosed patients.
3.2. Clinical parameters
3.2.1. Stages of presentation
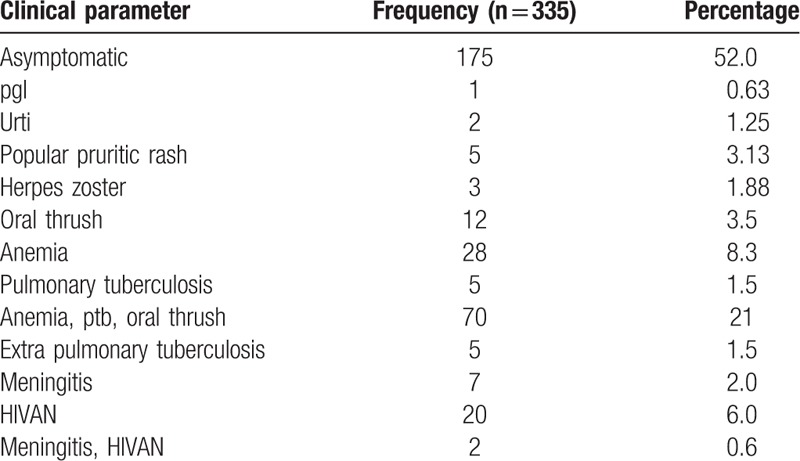
As shown in Figure 1, 60% of patients were late presenters, with 35% presenting with advanced disease. Over half the patients were asymptomatic. Most late presenters had developed an AIDS-defining condition such as tuberculosis (3%), oral candidiasis (1.5%), anemia (8.3%), meningitis (2%), and HIVAN (6%), with 21% presenting with oral candidiasis, anemia and tuberculosis (Table 2). In terms of WHO clinical staging, 51.4% were in clinical stage 1, 3% in stage 2, 36.6% in stage 3 and 9.1% in stage 4.
Figure 1.
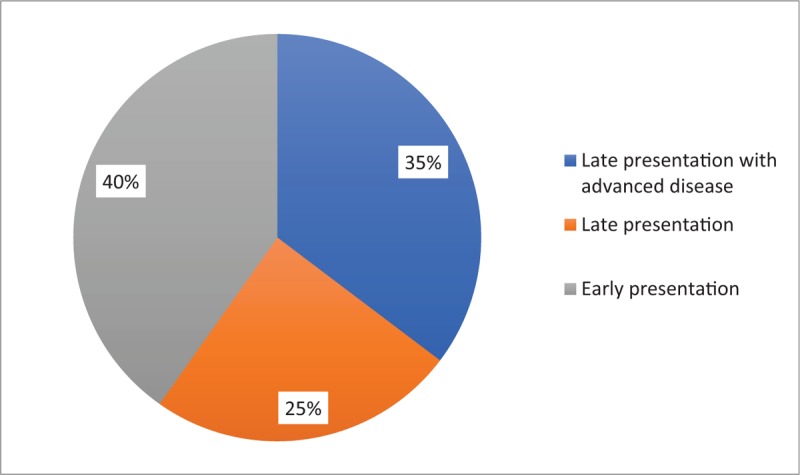
Stages of presentation for care.
Table 2.
Clinical parameters at presentation.
3.2.2. Determinants of late presentation among newly diagnosed patients
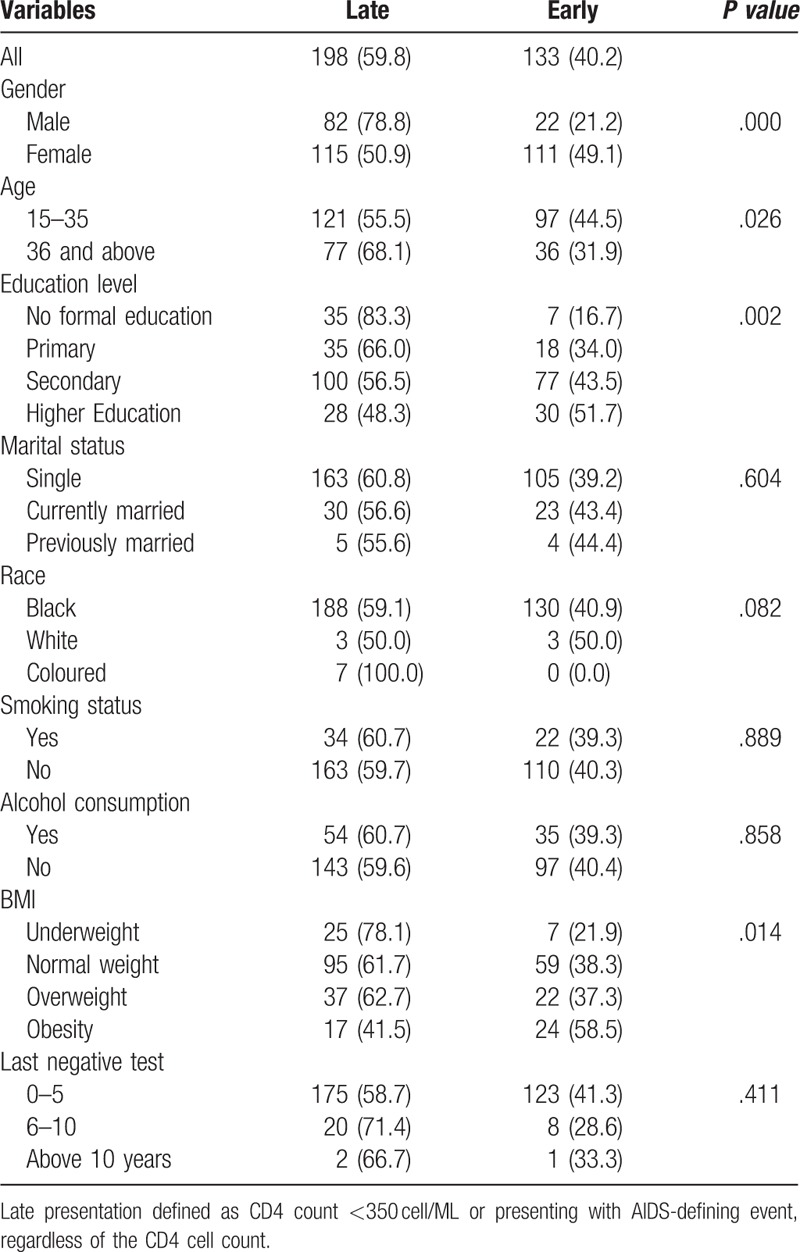
The results of the bivariate analysis are presented in Table 3. The proportion of late presenters varies significantly by age, sex, educational level, and body mass index. Males (78.8%) were significantly more likely to be late presenters than females (50.9%). Similarly, individuals with no formal education were significantly more likely to be late presenters than those with higher education. Also, patients aged 36 years or over were significantly more likely to present late for treatment than those who were under 36 years old. Alcohol consumption, race, marital status and smoking did not significantly influence timing of presentation.
Table 3.
Relationship between demographic variables and late presentation.
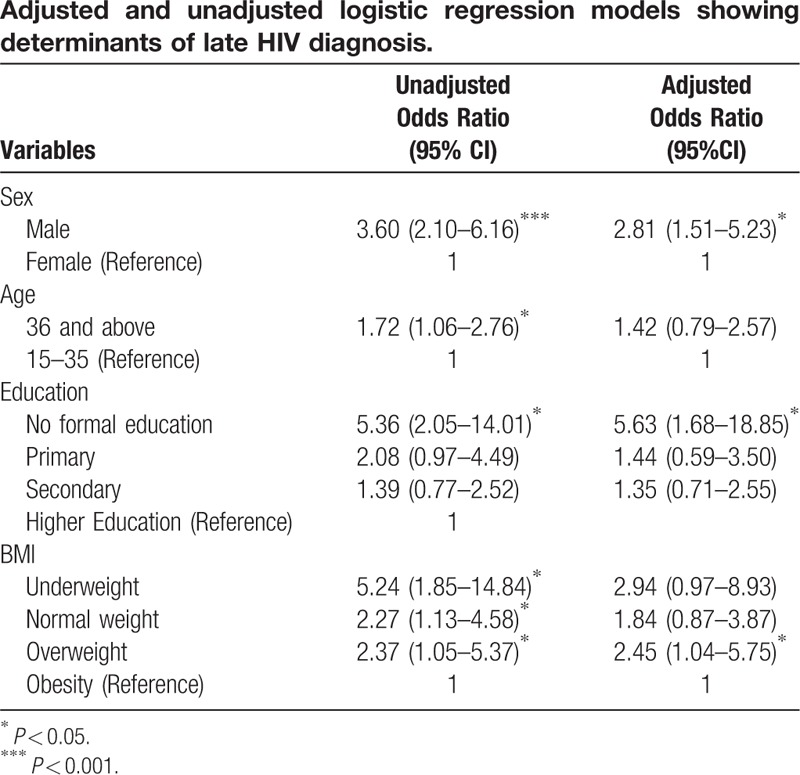
The results of adjusted and unadjusted logistic regression models are presented in Table 4. Male sex, age 36 or over, no formal education and underweight were independently associated with late presentation among newly diagnosed HIV-infected patients. In the adjusted model, only male sex, no formal education, and overweight body mass category were independently associated with late HIV diagnosis. Males were about three times more likely to present late than females. Patients who had no formal education were about 6 times more likely to be diagnosed late compared to those with tertiary levels of education. Compared to patients who were obese, overweight patients were more than twice as likely to present late for HIV diagnosis.
Table 4.
Adjusted and unadjusted logistic regression models showing determinants of late HIV diagnosis.
4. Discussion
To our knowledge, this is the first study to examine the prevalence of late presentation and explore the determinants of late presentation among newly diagnosed HIV-infected individuals in the Eastern Cape Province, South Africa. Our study shows that most of the newly diagnosed patients presented late, with CD4 counts below 350 cells/ μL and advanced stages of disease. A retrospective study of 8138 newly diagnosed HIV-positive individuals in 35 clinics in South Africa reported a higher rate of late diagnosis, however, their definition of late presentation (CD4 count below 500 cells/ μL) differed from the definition used in this study.[35] Another South African study reported a lower prevalence of late HIV diagnosis; however, they included only patients with CD4 counts below 100 cells/ μL.[28]
The current study adds to the existing body of knowledge on the proportion of patients with delayed presentation for HIV care. Our finding provides recent data on late presentation and shows that late presentation is still a major challenge in the study setting. Late presentation for HIV diagnosis and care remains common practice, despite efforts to get patients to present early and access care. The goal of such efforts is obviously to prevent the morbidity and mortality associated with the late initiation of ART, and to ensure continuity of HIV care. The current study did not examine the trends in late presentation for HIV diagnosis over a period of time. As such, we are unable to determine whether there has been an improvement or a decline in the area of late presentation for HIV diagnosis in the study setting. A Spanish study shows that late presentation for HIV diagnosis and care have decreased substantially since 2001.[36] There is, therefore, a need for trend analysis studies on late presentation among newly diagnosed HIV patients in South Africa.
Consistent with previous studies, most newly diagnosed patients are female.[28,31–33] This is in contrast to studies in Spain and Italy which reported a higher male preponderance of HIV infection.[36,37] The higher proportion of females in our study could be attributable to the health-seeking behavior disparity between the 2 genders. Also, gender differences in HIV prevalence rate have been reported, with females having a disproportionately higher HIV-prevalence rate than males.[34] The inference from our study is that the majority of patients who were newly diagnosed as HIV positive were women. This pattern was also found in three high-burden districts in South Africa between 2014 and 2015, where 69% of the study population were women. Another study showed an eight-fold higher rate of HIV infection amongst adolescent girls and young women aged 15 to 24 years when compared to their male peers in Sub-Saharan Africa.[38]
In this study, male gender was found to be an independent predictor of late presentation for HIV diagnosis. This finding is consistent with previous studies,[26,39] although an Ethiopian study reported a contrasting result, with females significantly more likely to be late presenters.[40] A study in China found no sex differences in the CD4 counts of newly diagnosed HIV-infected patients.[31] Females have far more opportunities to test for HIV than males do because of pregnancy and childbearing. The prevention of mother-to-child transmission of HIV programme (PMTCT) in South Africa, which made it mandatory for testing of all pregnant women for HIV during antenatal visits, provides opportunities for HIV diagnosis in women. Perhaps couples testing, especially during antenatal care, could be implemented in the study setting. This might not only strengthen the PMTCT programme in South Africa, but also increase the opportunities for men to get tested for HIV.
Our study shows that lack of formal education is significantly associated with late HIV diagnosis. This finding is in contrast to a study conducted in Denmark, which reported that no association existed between level of education and late presentation for HIV care.[39] Considering that Denmark is among countries with the highest levels of literacy, that finding is not comparable with the finding in this study. In addition, lower level of education was found to be associated with late presentation among newly diagnosed patients in other studies.[41,42] It is plausible that individuals with no formal education are less knowledgeable about HIV. Since this is the case, an intervention targeting these individuals is imperative.
Our study also shows that older age is associated with late diagnosis of HIV, which is consistent with findings in previous studies,[36,39,40] although it contrasts with findings in a study conducted in China.[31] Even though the effect of age on late presentation disappeared after controlling for other covariates, the consistency in the direction of the effect, as shown in the literature, suggests older age is a risk factor for late presentation for HIV diagnosis and care. Intervention that targets older people may have clinical benefits.
Our study shows that body mass index is associated with late diagnosis of HIV. It is plausible that patients seek care for rapid weight loss, which is considered a severe symptom and thus influences patients’ choice of seeking care in health facilities. The health belief model asserts that illness perception influences health-seeking behavior. Individuals who consider their symptoms to be less severe may not seek care in clinics and may prefer self-medication. Further studies are needed to establish the link between body mass index and late presentation for HIV care.
Even though this study contributes to the literature on late presentation for HIV care, the findings should be interpreted within its limitations. In this cross-sectional survey, we were not able to ascertain causality of associations with delayed presentation. The study also did not entirely explore the role of distance to health care facilities, health system-related factors and impact of patient-related factors such as stigma on late presentation for HIV care. Future studies could examine trends in late presentation and the effect of distance to health care facilities, health-system factors and stigma perceptions on late presentation for HIV care.
5. Conclusion
Late presentation for HIV care remains a major challenge in the study setting. To maximize the impact of the ‘test and treat’ policy aimed at reducing morbidity and mortality associated with HIV, there is a need for programmes to improve early detection of HIV in the study settings. This programme should target males and individuals with no formal education for maximum impact. Also, efforts are needed to encourage men to access and utilize existing health care facilities as well as improve access to formal education.
Acknowledgments
The Authors acknowledge the help of the staff of the Department of Health at the respective facility used and also the study respondents. The authors also acknowledge the Discovery Foundation for funding this study.
Author contributions
OOS developed the protocol, collected the data and drafted the manuscript, DTG and BCI reviewed the data and reviewed the manuscript, AAI conducted the analysis of the quantitative data, AI, LCO and UN and provided intellectual content on the draft of the manuscript. All authors read and agreed with the final draft of the manuscript.
Conceptualization: Olufunso O Sogbanmu, Daniel T Goon, Larry C. Obi, Ben C. Iweriebor, Uchechukwu Nwodo, Anthony I. Okoh.
Data curation: Olufunso O Sogbanmu, Larry C. Obi, Anthony I. Okoh.
Formal analysis: Anthony Idowu Ajayi.
Funding acquisition: Olufunso O Sogbanmu.
Investigation: Olufunso O Sogbanmu, Daniel T Goon, Larry C. Obi, Ben C. Iweriebor, Uchechukwu Nwodo, Anthony Idowu Ajayi, Anthony I. Okoh.
Methodology: Olufunso O Sogbanmu, Daniel T Goon, Larry C. Obi, Ben C. Iweriebor, Uchechukwu Nwodo, Anthony Idowu Ajayi, Anthony I. Okoh.
Project administration: Olufunso O Sogbanmu, Anthony Idowu Ajayi.
Resources: Olufunso O Sogbanmu, Ben C. Iweriebor, Anthony Idowu Ajayi, Anthony I. Okoh.
Software: Anthony Idowu Ajayi.
Supervision: Olufunso O Sogbanmu, Daniel T Goon, Larry C. Obi, Ben C. Iweriebor, Uchechukwu Nwodo, Anthony I. Okoh.
Validation: Anthony Idowu Ajayi.
Writing – original draft: Olufunso O Sogbanmu, Daniel T Goon, Larry C. Obi, Ben C. Iweriebor, Uchechukwu Nwodo, Anthony Idowu Ajayi, Anthony I. Okoh.
Writing – review & editing: Olufunso O Sogbanmu, Daniel T Goon, Larry C. Obi, Ben C. Iweriebor, Uchechukwu Nwodo, Anthony Idowu Ajayi, Anthony I. Okoh.
Footnotes
Abbreviations: AIDS = acquired immune deficiency syndrome, ART = antiretroviral therapy, ARV = anti-retroviral, HCT = HIV counselling and testing, HIV = human immunodeficiency-virus, HIVAN = HIV-associated nephropathy, PLHIV = national department of health, people living with HIV, PMTCT = prevention of mother to child transmission of HIV, VCT = voluntary counselling and testing center, WHO = World Health Organisation.
University of Fort Hare Postgraduate Ethical Committee REC 270710028RA level 01. Eastern Cape Health Department approval number: EC_2016RP22_139. Written informed consent was obtained from all the participants in the study.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding for study was obtained from the Discovery Foundation towards the conduct of the study. Grant number: 034557. Further funding in support of this study was received by Anthony I. Okoh from the South Africa Medical Research Council (# SAMRC/UFH/P790)
The Author OO Sogbanmu received grant from the Discovery Foundation towards the conduct of the study. Grant number: 034557. The Discovery foundation had no input in any part of the research.
The authors report no conflicts of interest.
References
- [1].Lin K-Y, Cheng C-Y, Li C-W, et al. Trends and outcomes of late initiation of combination antiretroviral therapy driven by late presentation among HIV-positive Taiwanese patients in the era of treatment scale-up. PloS One 2017;12:e0179870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Valdiserri RO. Late HIV diagnosis: bad medicine and worse public health. PLoS Med 2007;4:e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Limb M. More routine HIV testing is needed to reduce late diagnoses, says public health agency. BMJ: Br Med J (Online) 2011. 343. [DOI] [PubMed] [Google Scholar]
- [4].Mocroft A, Lundgren JD, Sabin ML, et al. Risk factors and outcomes for late presentation for HIV-positive persons in Europe: results from the Collaboration of Observational HIV Epidemiological Research Europe Study (COHERE). PLoS Med 2013;10:e1001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sanders GD, Bayoumi AM, Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med 2005;352:570–85. [DOI] [PubMed] [Google Scholar]
- [6].Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS 2006;20:1447–50. [DOI] [PubMed] [Google Scholar]
- [7].Fisher M. Late diagnosis of HIV infection: major consequences and missed opportunities. Curr Opin Infect Dis 2008;21:1–3. [DOI] [PubMed] [Google Scholar]
- [8].Hall HI, Holtgrave DR, Maulsby C. HIV transmission rates from persons living with HIV who are aware and unaware of their infection. AIDS 2012;26:893–6. [DOI] [PubMed] [Google Scholar]
- [9].Castelnuovo B, Chiesa E, Rusconi S, et al. Declining incidence of AIDS and increasing prevalence of AIDS presenters among AIDS patients in Italy. Eur J Clin Microbiol Infect Dis 2003;22:663–9. [DOI] [PubMed] [Google Scholar]
- [10].Brännström J, Åkerlund B, Arneborn M, et al. Patients unaware of their HIV infection until AIDS diagnosis in Sweden 1996–2002–a remaining problem in the highly active antiretroviral therapy era. Int J STD AIDS 2005;16: [DOI] [PubMed] [Google Scholar]
- [11].Cohen MS, Smith MK, Muessig KE, et al. Antiretroviral treatment of HIV-1 prevents transmission of HIV-1: where do we go from here? Lancet 2013;382:1515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016;375:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lee SA, Deeks SG. The benefits of early antiretroviral therapy for HIV infection: how early is early enough? EBioMedicine 2016;11:7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Deeks SG, Lewin SR, Bekker L-G. The end of HIV: still a very long way to go, but progress continues. PLoS Med 2017;14:e1002466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].World Health Organization. Global health observatory data repository 2013. Available at: who.int/gho/data/node.mainA,2013:364. Accessed September 22, 2018. [Google Scholar]
- [16].Meintjes G, Black J, Conradie F, et al. Southern African HIV Clinicians Society adult antiretroviral therapy guidelines: Update on when to initiate antiretroviral therapy. Southern African J HIV Med 2015;16:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jain V, Hartogensis W, Bacchetti P, et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis 2013;208:1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chéret A, Bacchus-Souffan C, Avettand-Fenoël V, et al. Combined ART started during acute HIV infection protects central memory CD4+ T cells and can induce remission. J Antimicrob Chemother 2015;70:2108–20. [DOI] [PubMed] [Google Scholar]
- [19].Johnson LF, Mossong J, Dorrington RE, et al. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med 2013;10:e1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Antinori A, Coenen T, Costagiola D, et al. Late presentation of HIV infection: a consensus definition. HIV Med 2011;12:61–4. [DOI] [PubMed] [Google Scholar]
- [21].Jiang H, Yin J, Fan Y, et al. Gender difference in advanced HIV disease and late presentation according to European consensus definitions. Sci Rep 2015;5:14543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kozak M, Zinski A, Leeper C, et al. Late diagnosis, delayed presentation and late presentation in HIV: proposed definitions, methodological considerations and health implications. Antivir Ther 2013;18:17–23. [DOI] [PubMed] [Google Scholar]
- [23].National Department of Health South Africa. South African National Strategic Plan for HIV, STIs and TB 2017–2022. National Department of Health Pretoria; 2017. [Google Scholar]
- [24].Venter F, Majam M, Jankelowitz L, et al. South African HIV self-testing policy and guidance considerations. South African J HIV Med 2017;18:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].National Department of Health South Africa. The South African antiretroviral treatment guidelines. National Department of Health Pretoria; 2015. [Google Scholar]
- [26].Alvarez-Uria G, Midde M, Pakam R, et al. Factors associated with late presentation of HIV and estimation of antiretroviral treatment need according to CD4 lymphocyte count in a resource-limited setting: data from an HIV cohort study in India. Interdiscip Perspect Infect Dis 2012;2012:293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Battegay M, Fluckiger U, Hirschel B, Furrer H. Late presentation of HIV-infected individuals. Antivir Ther 2007;12:841–51. [PubMed] [Google Scholar]
- [28].Drain PK, Losina E, Parker G, et al. Risk factors for late-stage HIV disease presentation at initial HIV diagnosis in Durban, South Africa. PloS One 2013;8:e55305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].National Department of Health South Africa. The South African antiretroviral treatment guidelines. National Department of Health Pretoria; 2013. [Google Scholar]
- [30].Cheng W, Tang W, Han Z, et al. Late presentation of HIV infection: prevalence, trends, and the role of HIV testing strategies in Guangzhou, China, 2008–2013. BioMed Res Int 2016;2016: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shen Y, Lu H, Wang Z, et al. Analysis of the immunologic status of a newly diagnosed HIV positive population in China. BMC Infect Dis 2013;13:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lanoy E, Mary-Krause M, Tattevin P, et al. Frequency, determinants and consequences of delayed access to care for HIV infection in France. Antivir Ther 2007;12:89. [DOI] [PubMed] [Google Scholar]
- [33].Kigozi IM, Dobkin LM, Martin JN, et al. Late disease stage at presentation to an HIV clinic in the era of free antiretroviral therapy in sub-Saharan Africa. J Acquir Immune Defic Syndr (1999) 2009;52:280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shisana O, Rehle T, Simbayi LC, et al. South African national HIV prevalence, incidence and behaviour survey, 2012. Cape Town: HSRC Press; 2014. [Google Scholar]
- [35].Fomundam H, Tesfay A, Mushipe S, et al. Prevalence and predictors of late presentation for HIV care in South Africa. S Afr Med J 2017;107:1058–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].de Olalla PG, Manzardo C, Sambeat MA, et al. Epidemiological characteristics and predictors of late presentation of HIV infection in Barcelona (Spain) during the period 2001–2009. AIDS Res Ther 2011;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Girardi E, Aloisi MS, Arici C, et al. Delayed presentation and late testing for HIV: demographic and behavioral risk factors in a multicenter study in Italy. J Acquir Immune Defic Syndr 2004;36:951–9. [DOI] [PubMed] [Google Scholar]
- [38].Kharsany AB, Karim QA. HIV infection and AIDS in Sub-Saharan Africa: current status, challenges and opportunities. The Open AIDS J 2016;10:34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Darcis G, Lambert I, Sauvage A-S, et al. Factors associated with late presentation for HIV care in a single Belgian reference center: 2006–2017. Sci Rep 2018;8:8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gesesew HA, Ward P, Woldemichael K, Mwanri L. Late presentation for HIV care in Southwest Ethiopia in 2003-2015: prevalence, trend, outcomes and risk factors. BMC Infect Dis 2018;18:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wilson KdA, Dray-Spira R, Aubrière C, et al. Frequency and correlates of late presentation for HIV infection in France: older adults are a risk group–results from the ANRS-VESPA2 Study, France. AIDS Care 2014;26(Supp 1):S83–93. [DOI] [PubMed] [Google Scholar]
- [42].Beyene MB, Beyene HB. Predictors of late HIV diagnosis among adult people living with HIV/AIDS who undertake an initial CD4 T cell evaluation, Northern Ethiopia: a case-control study. PloS One 2015;10:e0140004. [DOI] [PMC free article] [PubMed] [Google Scholar]


