Abstract
Background:
In this randomized, double-blind, parallel-group trial, we aimed to explore the effectiveness of trigger point dry needling in patients with chronic tension-type headache in reducing headache frequency, intensity and duration, and improvement of health-related quality of life.
Methods:
The 168 patients in 2 neurology clinics with chronic tension-type headache. The participants were randomly assigned to one of two treatment groups for dry needling or sham dry needling, delivered in 3 sessions a week for 2 weeks. The 160 patients fulfilled the study requirements. The dry needling was applied in active trigger points located in the musculature of the head and the neck. The patients received dry needling using sterile stainless-steel acupuncture needles of 0.25 × 40 mm and 0.25 × 25 mm dimensions. The sham dry needling procedure was applied into the adipose tissue located at any area where an active trigger point was absent. The primary outcome measurement was the headache intensity. Secondary outcomes were frequency and duration of headache, and quality of life, assessed by the Short Form-36. All outcomes were measured at baseline, at the end of 2-week, and 1-month follow-up period.
Results:
In the dry needling group, intensity, frequency and duration of headache, and the scores of Short Form-36 subscales were significantly improved after treatment (P < .05). In the dry needling group, all the effect sizes for headache variables were large.
Conclusions:
The results of this clinical trial suggest that trigger point dry needling in patients with chronic tension-type headache is effective and safe in reducing headache intensity, frequency and duration, and increasing health-related quality of life.
Trial registration:
Clinical Trials NCT03500861.
Keywords: pain, primary headache disorders, quality of life, sham treatment
1. Introduction
According to the 2013 Global Burden of Disease study, recurrent tension-type headache is the second most common chronic disease worldwide, with an age-standardized prevalence of 21.75%.[1] Although high prevalence of chronic tension-type headache (CTTH) has been reported in all world regions, it is also one of the most frequently neglected disorders, and it leads to headaches that are difficult to treat.[2] It receives much less attention from healthcare professionals and researchers than migraine does. The pathogenesis of CTTH is still unclear; peripheral myofascial mechanisms (myofascial nociception) and central mechanisms (sensitization and inadequate endogenous pain control) are implicated to have a potential relationship with the condition.[3,4] Myofascial pain may play an important etiologic role. It has been claimed that pain from the pericranial head, neck, and shoulder muscles is associated with the head and experienced as headache.[5,6] Simons et al described the referred pain pattern as different myofascial trigger points (TrPs) in the head and neck muscles, which produce pain characteristics that are usually found in patients. Active TrPs are a cause of referred pain, whereas latent TrPs may not be the source of pain. Within the cervical musculature, there are several head and neck muscles, for example, temporal, masseter, upper trapezius, sternocleidomastoid, temporalis, sub-occipital muscles, from which TrPs spread referred pain to the head.[6]
There are several pharmacological and non-pharmacological therapies for patients with CTTH. Non-pharmacological therapies include behavior treatments, physiotherapy interventions, and acupuncture. Physiotherapy is the most commonly used non-pharmacological treatment for CTTH. Methods such as postural control, relaxation, exercise programs, hot and cold packs, ultrasound, mobilization and manipulation, electromyographic biofeedback, and electrical stimulation are used for the management of patients with CTTH.[7–10] Although sports and orthopedic physiotherapists have used dry needling (DN) for a long time to address the pain and dysfunction associated with myofascial TrPs,[11] there is insufficient evidence to strongly advocate for use of DN for treatment of CTTH.[12] In this randomized, double-blind, parallel-group trial, we aimed to explore the effectiveness of trigger point dry needling in patients with CTTH in reducing headache intensity, frequency and duration, and improve health-related quality of life (HRQoL).
2. Materials and methods
2.1. Participants
This randomized, double-blind, parallel-group trial was carried out in a private clinic in Ankara, Turkey between April and August 2017 after the approval of the Eastern Mediterranean University BAYEK Health Ethics Subcommittee. A priori sample size calculation was performed using the G∗ Power software (version 3.1.9.2) considering the statistical tests to be used in the analyses and the conventional effect size values proposed by Cohen.[13] It was estimated that 67 subjects would be needed in both DN and sham dry needling (SDN) groups (α = 0.05, β = 0.20, and Cohen d = 0.5) in order to determine the statistically significant differences in study outcomes between the 2 groups. Considering the drop-out risk of the subjects, this initial sample size was increased by 25% in each group, and the final sample size was determined to be 84 subjects in each group.
The inclusion criteria for the study were as follows:
-
(1)
being between 20 and 50 years of age,
-
(2)
having a diagnosis of CTTH based on the International Classification of Headache Disorders, 3rd edition (beta version) (ICHD-3 beta criteria),[14]
-
(3)
having at least one active TrP in given each muscle, and
-
(4)
having pain intensity greater than 2 cm on the Visual Analog Scale (VAS).
Seven subjects were excluded from the study because they did not meet the criteria for inclusion (Fig. 1).
Figure 1.
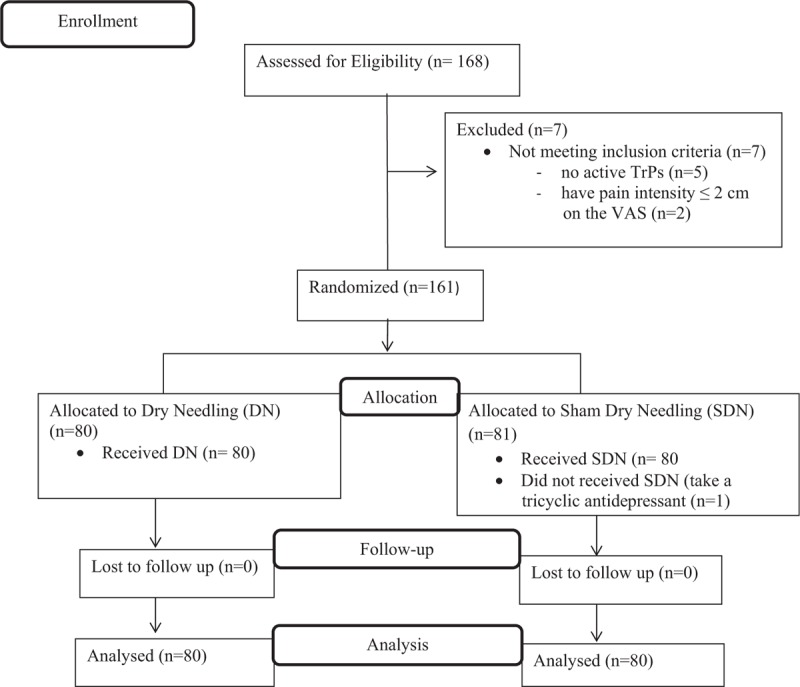
Flow diagram.
A one-block randomization procedure was carried out using the Random Allocation Software (version 1.0.0).[15] In this procedure, participants who were blinded to group allocation were divided as group ‘DN’ or ‘SDN’ by the second author (E.H.T.). The participants were selected consecutively from 2 neurology clinics located in Ankara, Turkey. All participants provided written informed consent prior to their participation in the study.
2.2. Materials
In this study, headache intensity was the primary outcome measure, and headache frequency, headache duration and quality of life were the secondary outcome measures. Pre-treatment, post-treatment, and 1-month follow-up assessments were performed by the same physiotherapist (G.E.) who was blinded to the allocation concealment. During the treatment and follow-up periods, the data related to the intensity, frequency and duration of headache were collected by a headache diary. The diary, along with the instructions, was given to the CTTH patients at their first examination at the clinic. In this diary, patients registered the frequency of headaches (days per week), headache intensity and duration of each headache attack (hours per day). Headache intensity was evaluated using a 10-cm horizontal Visual Analog Scale (VAS; range: 0 = no pain and 10 = maximum pain).[16]
The HRQoL assessments of patients were performed at their first examination and at the end of the follow-up period, using the Turkish version of Short Form-36 (SF-36). The Turkish version of SF-36 was previously validated.[17] It includes eight multi-item domains containing 2 to 10 items each, plus a single item to compare the current health of a person to their health one year ago (health transition).[18] The domains cover the dimensions of physical functioning (PF), role limitations due to physical health problems (RP), bodily pain (P), general health (GH), vitality (V), social functioning (SF), role limitations due to emotional problems (RE), and mental health (MH). All items pertaining to each domain (excluding health transition) are summed and transformed to form a domain from 0 to 100, where a higher score indicates a better state of health or well-being.
2.3. Procedure
The trigger point DN procedure was performed by a certified physiotherapist (S.G.) who was not blinded to the group allocation. There are 2 types of TrPs which can be come across during manual examination. The points located in the palpable taut band, which produce referred pain, local twitch response and spontaneous pain are defined as active TrPs. Only active TrPs were included in this study so the second type, latent TrPs defined as foci of hyperirritability in a taut band of muscle, which are clinically associated with a local twitch response, tenderness and/or referred pain upon manual examination were not included. Active TrPs were discriminated from latent TrPs by applying pressure to several points and comparing them.
In terms of palpation methods, the pincer palpation method was used for upper trapezius muscle while the flat palpation method was used for other muscles (masseter, temporalis, frontalis, splenius cervicis and capitis, and sub-occipital). A pressure was applied on the all selected muscles for 10 seconds elicited referred pain. Within the selection criterion of active TrPs, a usual and/or familiar pain was recognized by the patient when the referred pain elicited during examination reproduced at least part of the TTH pain pattern. As a result, these active TrPs which are most commonly seen in the population who has CTTH were then selected for this study.
While the patient was sitting, the therapist firstly cleaned the area with alcohol. Then, DN was applied into the active TrPs in masseter, temporalis, frontalis, splenius cervicis and capitis, upper trapezius and sub-occipital (rectus capitis posterior major and minor, as well as obliquus capitis inferior and superior) muscles on the basis of the technique described by Hong.[19] The needle remained in the trigger points for 20 minutes. Upon removal of the needle, the area was compressed firmly with a cotton swab for 60 secs. The DN procedure used sterile stainless-steel acupuncture needles of 0.25 × 40 mm and 0.25 × 25 mm dimensions (Hua Long ). DN was applied three times a week for 2 weeks, in previously diagnosed active trigger points located in the musculature of the head and the neck. Since weekly calculation of the headache index was aimed in the study, DN was applied until the end of the treatment sessions even if the active TrPs became latent TrPs in the other sessions. In the SDN group, three times a week for 2 weeks, the SDN procedure was applied into the adipose tissue located at any area where an active TrP was absent. The patients in both groups were requested not to use any analgesic medication during the treatment and follow-up periods.
2.4. Statistical analysis
All statistical analyses were carried out with the IBM SPSS Statistics software version 20.0. Before the statistical tests were conducted, we checked potential outliers and missing data. While deciding to use parametric or nonparametric tests, normal distribution assumptions of the data were checked with Shapiro-Wilk test. We derived a weekly headache severity index (HSI) using the data on the headache diary for use in evaluations in the treatment and follow-up periods: HSI = (frequency (day / week)) × (duration (total headache hours in a week / frequency)) × (intensity (total headache intensity in a week / frequency)). We calculated the headache index at the end of the week. For multiple comparisons, we used Friedman test. We used post-hoc Wilcoxon Signed-Rank test for the pairwise comparisons, when Friedman test showed there were statistically significant differences between the measurements. We used Mann-Whitney U test to compare 2 different sample means. Chi-Square test was used to compare the categorical variables. Significance level was set at P < .05. In the case of a significant difference of pre-treatment measurements between groups, we used the General Linear Model (GLM) for comparison of post-treatment measurements. The data were presented both with a point estimate and 95% confidence interval (CI) estimate.[20] Statistical analyses were interpreted along with P values and 95% CIs, as proposed by Andrea Knezevic.[21] To estimate the size of treatment effects, we calculated Cohen d’ effect size using the following formulae: where spooled = √[(s12 + s22) / 2] (d = Cohen's d; x = mean; s = Standard deviation).[22] Effect size was interpreted as small (d = 0.2), medium (d = 0.5) and large (d = 0.8) based on the benchmarks suggested by Cohen.[13]
3. Results
Eight patients were treated with neither DN nor SDN protocols due to the exclusion criteria of the trial. There were no active TrPs in 5 of them. Two patients had pain intensity lesser than 2 cm on the VAS. In the SDN group, 1 patient was not included in the statistical analysis because he reported taking tricyclic antidepressant medication during the second week of treatment. Thus, the statistical analyses were conducted on the data collected from 160 subjects, including 80 subjects in each group (Fig. 1).
As shown in Table 1, the demographic and pre-treatment clinical characteristics of the subjects were similar in both groups (all P values > .05). In the pre-treatment assessment, the subjects in the SDN group reported poorer health in comparison to the subjects in the DN group for the GH, SF, RE and MH domains (Table 2).
Table 1.
Demographic and pre-treatment clinical characteristics of the subjects who participated into the study, (95% CI).
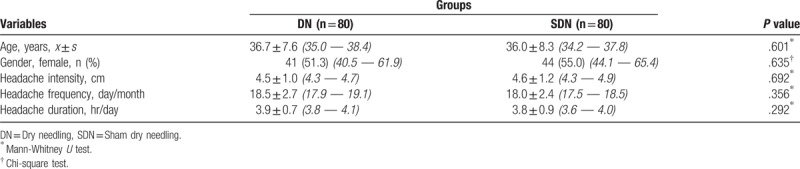
Table 2.
Short Form-36 subscales’ scores of the subjects who participated into the study at the pre-treatment period, x ± s, (95% CI).
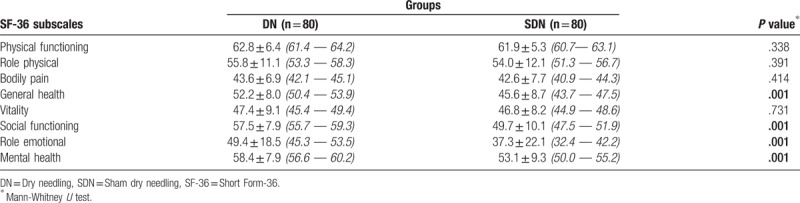
Table 3 shows the comparisons of headache intensity, frequency, and headache duration prior to treatment, at the end of therapy and at a 1-month follow-up. The Friedman test revealed statistically significant differences between measurements in both groups (all P values < .05). In the DN group, pairwise comparisons which were made with Wilcoxon Signed-Rank test revealed that there were statistically significant differences for all headache variables (all P values < .05), with the exception of headache duration in the period from post-treatment to follow-up (P = .089). For the variables of headache intensity and duration, the 95% CI of the difference between post-treatment and follow-up measurement covered the value of zero. In the SDN group, pairwise comparisons made with Wilcoxon Signed-Rank test revealed that there were statistically significant differences for all headache variables (all P values < .05). There were no overlaps in the 95% CIs of the pre-treatment, post treatment, and the follow-up measurements for the variable of headache frequency, with the exception of all other measurements. The 95% CI of the difference between the post-treatment and follow-up measurements on the variable of headache intensity did not cover the value of zero. The 95% CI of the difference between pre-treatment and follow-up measurements on the variable of headache duration did not cover the value of zero. Comparison of weekly headache index trends in the DN and SDN groups revealed significant differences between the groups (Fig. 2). In the DN group, the Friedman test and post-hoc Wilcoxon Signed-Rank test revealed that headache indices in the first two weeks were significantly higher than the ones in the other weeks (all P values < .05).
Table 3.
Comparisons of headache intensity, frequency and headache duration at prior to treatment, at the end of therapy and at a 1-month follow-up, x ± s, (95% CI).

Figure 2.
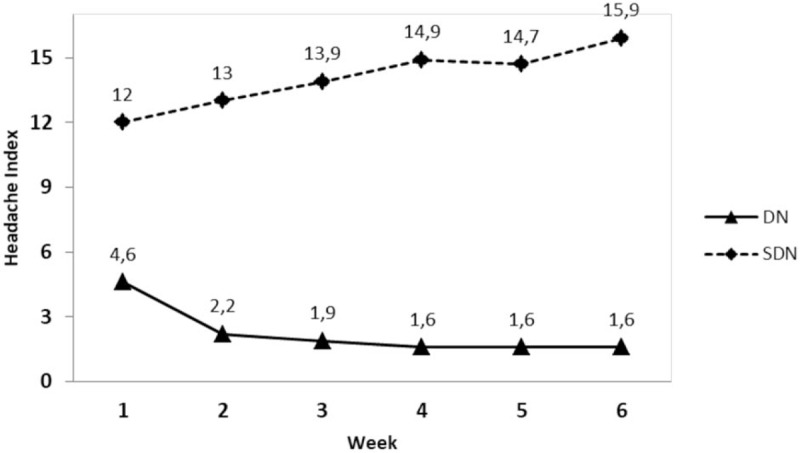
Comparison of weekly headache index trends in the DN and SDN groups.
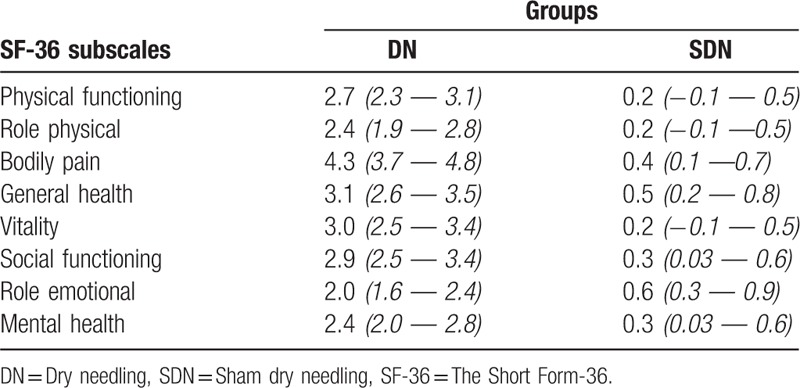
Table 4 shows the comparisons of the SF-36 subscale scores of the groups at the 1-month follow-up. The Mann-Whitney U test revealed statistically significant differences between the groups on the subscales of SF, RF, P, and V. GLM revealed statistically significant differences between the groups on the GH, SF, RE, and MH subscales after controlling for the differences of the initial measurements. Table 5 shows the estimated Cohen d effect sizes for headache intensity, frequency, and headache duration at the end of therapy (T1) and at the 1-month follow-up (T2). In the DN group, all effect sizes for the headache variables were large. A large effect was found in the SDN group only for the headache frequency for T1 (Table 5). Table 6 shows the estimated Cohen d effect sizes for the Short Form-36 domains at the 1-month follow-up.
Table 4.
Comparisons of the Short Form-36 subscales’ scores of groups at the 1-month follow-up, x ± s, (95% CI).
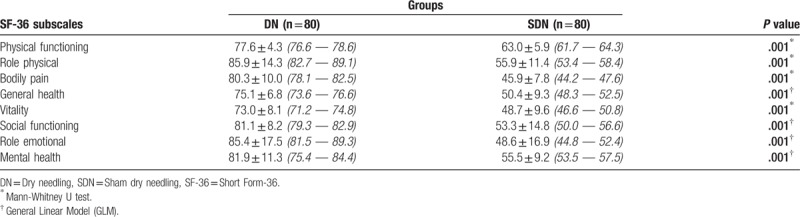
Table 5.
Estimated Cohen d's effect sizes for headache intensity, frequency and headache duration at the end of therapy and at a 1-month follow-up, (95% CI).
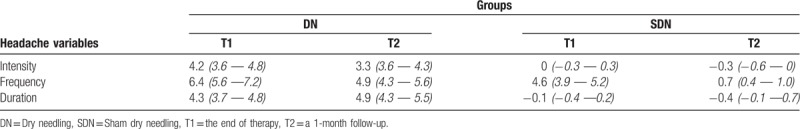
Table 6.
Estimated Cohen d's effect sizes for the Short Form-36 domains at the 1-month follow-up, (95% CI).
3.1. Adverse effects
Five of the patients in each group experienced pain and fear during the procedure.
4. Discussion
Based on the statistical significance and clinical effectiveness, the results of this randomized, parallel group, sham-controlled, double-blind, single center clinical trial suggest that trigger point dry needling in patients with CTTH is effective and safe in reducing headache frequency, intensity and duration, and increasing health-related quality of life. Effectiveness of treatment begins in the first week of therapy and continues throughout the second week and follow-up periods.
DN refers to insertion of thin monofilament needles without using a chemical agent. It is a new treatment modality used by physicians and physical therapists as a part of complex treatment of chronic musculoskeletal pain.[23] DN of myofascial TrPs in patients with CTTH is becoming an increasingly common therapeutic approach despite the scarcity of research-based evidence supporting its use. A recent systematic review suggested that further research with a stronger methodological design is required because of insufficient evidence.[12] In our double-blind randomized study conducted based on the criticisms on this systematic review, insertion of a dry needle into the active TrPs resulted in a significant decline in the mean headache index scores in comparison to sham therapy, where needles were inserted into incorrect points. This decline started at the end of the first week and continued in the second week. The headache index scores were stable in the follow-up period. This finding suggests that DN treatment in 3 sessions per week for 2 weeks is an effective intervention in management of CTTH. Based on the calculated effect sizes, we may conclude that DN is effective especially in reduction of pain intensity and duration. Interestingly, headache frequency was significantly lower in the post-treatment and follow-up periods than the pre-treatment in the SDN group. However, the clinical effectiveness for this outcome, expressed as effect size measurement, did not continue at the end of the follow-up period. So, from a statistical point of view, this finding may be explained partly by the placebo effect or the Hawthorne effect. On the other hand, it should be emphasized that effectiveness of interventions for headaches should be based on not only frequency, but also duration and intensity, as well as the ultimate goal of improving quality of life.
Chronic headaches reduce the quality of life for those who suffer from them and adversely affect the patient's family, as well as the society.[24] For people with CTTH, HRQoL as measured with SF-36, seems to be as low as it is for migraineurs.[25] Holroyd et al reported that CTTH patients had lower scores on Short Form-20 in comparison to controls, while their emotional well-being, sleep and energy levels were significantly impaired.[26] Our study revealed that, in the DN group, all domains of quality of life measured on SF-36 improved significantly from pre-treatment to 1-month follow-up. Based on this finding, we suggest that DN is effective not only on the physical health of CTTH patients but also on their both mental and social health.
In our study, long-term effects of DN were not investigated. This is the main limitation of the study. A longer follow-up period would be required to determine how long the effects would last.
These results suggest that DN is effective and safe in reducing headache frequency, intensity and duration, and increasing HRQoL in patients with CTTH. However, our results may not be generalized to the population of all people diagnosed with CTTH. Further trials, particularly those comparing DN to other treatment modalities, are needed.
Acknowledgment
We thank Prof. Zeki Gökçil, the neurologists of Gazi University and Ministry of Health Dişkapi Yildirim Beyazit Training and Research Hospital for their referral of patients for our study.
Author contributions
Conceptualization: Sila Gildir, Emine Handan Tüzün, Goncagül Eroğlu, Levent Eker.
Data curation: Emine Handan Tüzün, Levent Eker.
Formal analysis: Levent Eker.
Investigation: Sila Gildir, Goncagül Eroğlu.
Methodology: Sila Gildir, Emine Handan Tüzün, Goncagül Eroğlu, Levent Eker.
Supervision: Emine Handan Tüzün.
Writing – original draft: Sila Gildir, Emine Handan Tüzün, Goncagül Eroğlu, Levent Eker.
Writing – review & editing: Sila Gildir, Emine Handan Tüzün, Levent Eker.
Emine Handan Tüzün orcid: 0000-0001-6989-6675.
Footnotes
Abbreviations: CI = confidence interval, CTTH = chronic tension-type headache, DN = dry needling, GH = general health, GLM = general linear model, HRQoL = health-related quality of life, HSI = headache severity index, ICHD-3 beta = the international classification of headache disorders, 3rd edition (beta version), MH = mental health, P = bodily pain, PF = physical functioning, RE = role limitations due to emotional problems, RP = role limitations due to physical health problems, s = standard deviation, SDN = sham dry needling, SF = social functioning, SF-36 = short form-36, T1 = the end of therapy, T2 = a 1-month follow-up, TrPs = trigger points, V = vitality, VAS = visual analog scale, x = mean.
Registration number and name of trial registry: NCT03500861 and Trigger Point Dry Needling for Chronic Tension-Type Headache.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bendtsen L, Jensen R. Tension type headache: the most common, but also the most neglected headache disorder. Curr Opin Neurol 2006;5:342–6. [DOI] [PubMed] [Google Scholar]
- [3].Arendt-Nielsen L, Castaldo M, Mechelli F, et al. Muscle triggers as a possible source of pain in a subgroup of tension-type headache patients? Clin J Pain 2016;32:711–8. [DOI] [PubMed] [Google Scholar]
- [4].Yu S, Han X. Update of chronic tension-type headache. Curr Pain Headache Rep 2015;19:469. [DOI] [PubMed] [Google Scholar]
- [5].Gerwin R. Ferguson L, Gerwin R. Headache. Clinical Mastery in the Treatment of Myofascial Pain. Philadelphia, PA: Lippincott Williams &Wilkins; 2005. 1–24. [Google Scholar]
- [6].Simons DG, Travell JG, Simons LS. Myofascial pain and dysfunction: the trigger point manual. In: Upper Half of Body. Volume 1, 2nd ed., Baltimore: Williams, Wilkins; 1999. [Google Scholar]
- [7].Jensen R, Roth JM. Olesen J, Goadsby PJ, Ramadan N, Peer Pfelt-Hansen K, Welch Michael A. Physiotherapy of Tension-Type Headaches. The Headaches 3rd ed.Philadelphia: Lippincott Williams Wilkins; 2005. 721–6. [Google Scholar]
- [8].Fernandez-De-Las-Penas C. Physical therapy and exercise in headache. Cephalalgia 2008;(1 suppl):36–8. [DOI] [PubMed] [Google Scholar]
- [9].Ohlsen BA. Combination of acupuncture and spinal manipulative therapy: management of a 32-year-old patient with chronic tension-type headache and migraine. J Chiropr Med 2012;11:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Espí-López GV, Arnal-Gómez A, Arbós-Berenguer T, et al. Effectiveness of physical therapy in patients with tension-type headache: literature review. J Jpn Phys Ther Assoc 2014;17:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Unverzagt C, Berglund K, Thomas JJ. Dry needling for myofascial trigger point pain: a clinical commentary. Int J Sports Phys Ther 2015;10:402–18. [PMC free article] [PubMed] [Google Scholar]
- [12].France S, Bown J, Nowosilskyj M, et al. Evidence for the use of dry needling and physiotherapy in the management of cervicogenic or tension-type headache: a systematic review. Cephalalgia 2014;34:994–1003. [DOI] [PubMed] [Google Scholar]
- [13].Cohen J. Statistical Power Analysis for the Behavioural Sciences. San Diego, CA: Academic Press; 1988. [Google Scholar]
- [14].Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd ed. (beta version). Cephalalgia 2013;33:629–808. [DOI] [PubMed] [Google Scholar]
- [15].Saghaei M. Random allocation software for parallel group randomized trials. BMC Med Res Methodol 2004;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jensen MP, Turbner JA, Romano JM, et al. Comparative reliability and validity of chronic pain intensity measures. Pain 1999;83:157–62. [DOI] [PubMed] [Google Scholar]
- [17].Kocyigit H, Aydemir O, Fisek G, et al. Reliability and validity of the Turkish version of short form-36 (SF-36): a study in a group of patients will rheumatic diseases. Turk J Drugs Ther 1999;12:102–6. (in Turkish). [Google Scholar]
- [18].Ware JE, Jr, Gandek B. Overview of the SF-36 health survey and the International Quality of Life Assessment (IQOLA) project. J Clin Epidemiol 1998;51:903–12. [DOI] [PubMed] [Google Scholar]
- [19].Hong CZ. Lidocaine injection versus dry needling to myofascial trigger point. The importance of the local twitch response. Am J Phys Med Rehabil 1994;73:256–63. [DOI] [PubMed] [Google Scholar]
- [20].Herbert R. 2016 Confidence Interval Calculator. Available at: https://www.pedro.org.au/english/downloads/confidence-interval-calculator/ [access date August 10, 2017]. [Google Scholar]
- [21].Knezevic A. StatNews # 73: Overlapping Confidence Intervals and Statistical Significance. Available at: https://www.cscu.cornell.edu/news/statnews/stnews73.pdf [access date August 10, 2017]. [Google Scholar]
- [22].Becker LA. Effect Size Calculators. Available at: https://www.uccs.edu/lbecker/index.html [access date August 10, 2017]. [Google Scholar]
- [23].Kalichman L, Vulfsons S. Dry needling in the management of musculoskeletal pain. JABFM 2010;23:640–6. [DOI] [PubMed] [Google Scholar]
- [24].Solomon GD, Skobieranda FG, Gragg LA. Quality of and well-being of headache patients: measurement by the medical outcomes study instrument. Headache 1993;33:351–8. [DOI] [PubMed] [Google Scholar]
- [25].Wang SJ, Fuh JL, Lu SR, et al. Quality of life differs among headache diagnoses: analysis of SF-36 survey in 901 headache patients. Pain 2001;89:285–92. [DOI] [PubMed] [Google Scholar]
- [26].Holroyd KA, Stensland M, Lipchik GL, et al. Psychosocial correlates and impact of chronic tension-type headaches. Headache 2000;40:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]


