Abstract
The organs-on-a-chip technology has shown strong promise in mimicking the complexity of native tissues in vitro and ex vivo, and recently significant advances have been made in applying this technology to studies of the kidney and its diseases. Individual components of the nephron, including the glomerulus, proximal tubule, and distal tubule/medullary collecting duct, have been successfully mimicked using organs-on-a-chip technology and yielding strong promises in advancing the field of ex vivo drug toxicity testing and augmenting renal replacement therapies. Although these models show promise over 2-dimensional cell systems in recapitulating important nephron features in vitro, nephron functions, such as tubular secretion, intracellular metabolism, and renin and vitamin D production, as well as prostaglandin synthesis are still poorly recapitulated in on-chip models. Moreover, construction of multiple-renal-components-on-a-chip models, in which various structures and cells of the renal system interact with each other, has remained a challenge. Overall, on-chip models show promise in advancing models of normal and pathological renal physiology, in predicting nephrotoxicity, and in advancing treatment of chronic kidney diseases.
Keywords: kidney, microfluidics, microphysiological systems, organ-on-a-chip, tissue engineering
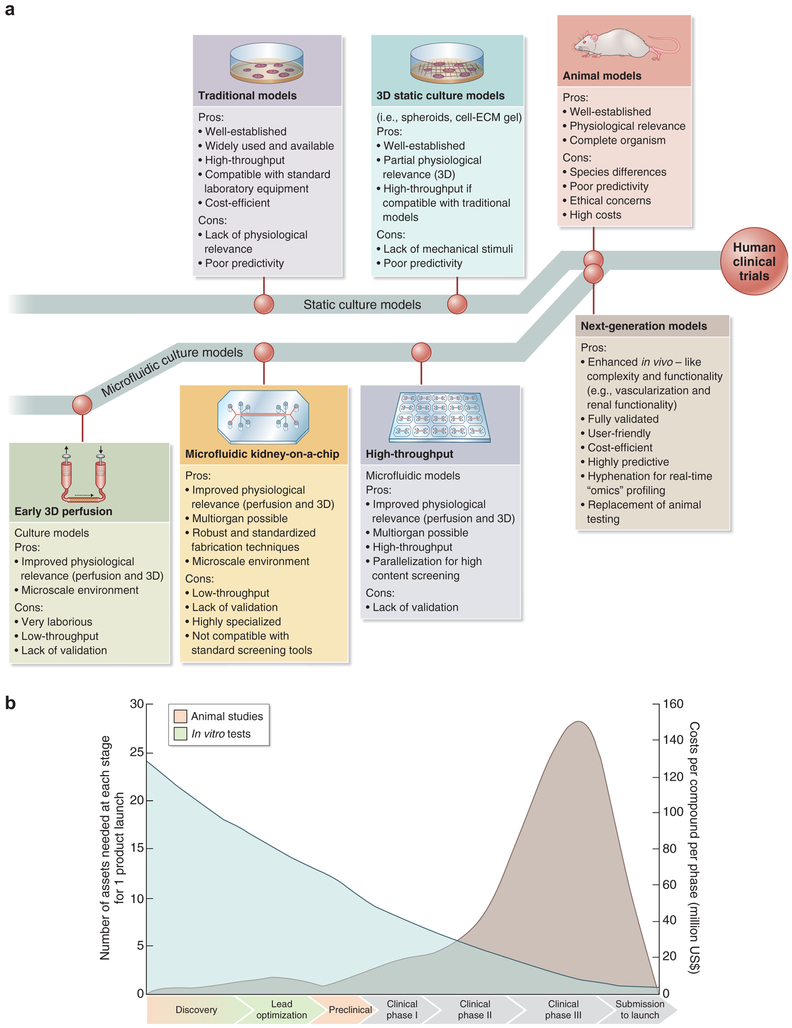
Using technology partially evolved from the bioreactor industry1 and adapted for tissue engineering, organ-on-a-chip technology has recently been created by culturing cells in microchannels and microfluidic systems, thus recapitulating important tissue- and organ-level functions in vitro and ex vivo. The addition of living renal proximal tubular cells to microchannels or hollow dialysis fibers improves waste excretion, glucose reabsorption, and middlesized molecule clearance.2 Metabolic and endocrine functions may be improved by culture of primary renal epithelial cell types found in normal renal tissue,2,3 and the use of flow in microfluidic systems recapitulates the renal tubular microenvironment ex vivo, stimulating cell polarization and augmenting function.4 To date, cell-based microphysiological systems have been applied to developing models of renal physiology, pathophysiology, and tissue pathology. These models may also be applied to testing drugs and chemicals for nephrotoxicity, circumventing limitations of 2-dimensional (2D) cell culture systems5–7 and animal models5 (Figure 1). In series with other organ-on-a-chip platforms, these models show promise in studies of systemic disease and secondary drug toxicities.10,11
Figure 1 ∣. Future of drug testing.
(a) Evolution of 3-dimensional (3D) physiologically relevant microfluidic models for nephrotoxicity screening. ECM, extracellular matrix. Adapted with permission from Wilmer MJ, Ng CP, Lanz HL, et al. Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol. 2016;34:156–170.8 (b) Drug testing challenge and dilemma. Reproduced from Dehne EM, Hasenberg T, Marx U. The ascendance of microphysiological systems to solve the drug testing dilemma. Future Sci OA. 2017;3:FSO185.9 This work is licensed under the Creative Commons Attribution 4.0 License.
This review discusses cell-based microsystems and their applications, highlighting untapped opportunities and state-of-the-art developments of various parts of kidney-on-a-chip platforms.4,7,12–14 The growing field of organ-on-a-chip technology is projected to have a global market of $6.13 billion by 202515 and will open new paths for multidisciplinary and translational research.
Cell-based microphysiological systems
Organ-on-a-chip.
The microfluidic technology was developed by integrating technologies developed in the fields of molecular analytics, molecular biology, biodefense research, and microelectronics.16 Living cells are then integrated into these lab-on-a-chip devices,17 creating microphysiological systems or organ-on-a-chip platforms.11,18 Organ-on-a-chip platforms integrate biophysicochemical cues, sensors, and precision control for accurate analysis of molecules and drugs.19,20 Using these platforms, fluid flow, pressure, and other parameters that modulate cell organization and function can be controlled at the microliter scale.16 Spatiotemporal chemical gradients and controlled dynamic mechanics influence cell differentiation and function, and coculture of varying cell types mimics the diversity and complexity of native tissues, overcoming limitations of conventional single cell type–dependent in vitro models. Several organ-on-a-chip devices can subsequently be linked to produce multi-organ-on-a-chip and body- or human-on-a-chip systems to investigate their interactions.10,21,22
Cells used for microphysiological systems.
Immortalized cell lines are most commonly used in developing cell-based microphysiological systems. These lines include canine (Madin-Darby canine kidney [MDCK]) tubular epithelial cells,23–25 porcine (Lilly Laboratories cell porcine kidney 1) tubular epithelial cells,26 opossum (opossum kidney) proximal tubular epithelial cells,27,28 and human (human kidney-2) proximal tubular epithelial cells.29 Unfortunately, none of these cell lines fully recapitulate the primary cell phenotype,6 nor do they display functional differentiation.7 The continued growth of immortalized cells may lead to blockage of hollow fibers,26 and species-dependent variations in tubular function may limit the usefulness of animal-derived cell lines in human disease models and therapeutic screening.
Primary human cells, such as human proximal tubule (PT) epithelial cells (hPTECs),30 circumvent these problems and are often used in in vitro models. However, it is often difficult to obtain sufficient numbers of primary PT cells that have a limited number of population doublings (a maximum of ~ 12 doublings).2 By using antisense nucleotides or RNA interference (small interfering RNA transfected to the tumor suppressor p53 or the cyclin-dependent kinase inhibitor p16INK4a), human PT cell doublings can be increased by 3- to 5-fold.2 When seeded into hollow fibers, these small interfering RNA–transfected cells form a confluent layer of cells that covered the inner surface of the hollow fibers within 1 week and well-developed microvilli were visible on the apical side of these cells. In comparison with membranes lacking this primary cell lining, leakage of creatinine and urea nitrogen decreases while water, sodium, and glucose reabsorption increase in PT cell-lined fibers.2
Embryonic stem cell-derived hPTEC-like cells are also promising but have not, to date, been sufficiently characterized in microfluidic systems.30 More recently, induced pluripotent stem cells (iPSCs) have been explored as a continuous source of primary cells. Musah et al. used human iPSC-derived terminally differentiated podocytes to successfully develop a glomerulus-on-a-chip.14,31,32 CRISPR-mutant kidney organoids33 have also shown promise in disease models and drug development studies.
Structural materials used in organ-on-a-chip systems.
Polydimethylsiloxane (PDMS) is a common material used for the fabrication of microfluidic devices, which combines the advantages of a long shelf life, excellent hemo- and biocompatibility profiles, high gas permeability, high chemical sensitivity, and transparency in a low-cost product.34,35 However, PDMS absorbs hydrophobic compounds, limiting its application in microfluidic devices used for drug testing.36–38 Thermoplastic materials composed of polycarbonate, polystyrene, and poly(methyl methacrylate), all of which have low absorption of hydrophobic compounds, are thus often preferred in biochips.39 Various natural and synthetic polymer-based hydrogels may be added to both PDMS- and non-PDMS-based microfluidic devices, allowing them to better mimic natural physiological scaffolds.40 The first such successful scaffold was developed when Ng et al., which demonstrated that hollow fibers containing an inner hydrogel core coated with fibrin displayed enhanced formation of hPTEC monolayers and increased fluid resorption from microchannels.41 Microsensors may be embedded in bioengineered scaffolds, improving real-time assessment of glomerular and tubular physiology in response to environmental changes. Ferrel et al. developed a microfluidic bioreactor from polycarbonate membranes coated with type IV collagen and seeded with human renal epithelial cells and MDCK cells. These bioreactors contained integrated electrodes for the measurement of transepithelial electrical resistance, renal epithelial cell growth, and the integrity of tight junctions.24 After a disruption in tight junctions, a decrease in transepithelial electrical resistance was noted, correlating with a change in the transport of inulin, which was used as a tracer in these studies. Later, renal tubule epithelial cells (opossum kidney cell line) were cultured in this microfluidic bioreactor to investigate the effects of shear stress on albumin handling.27,28 The finding that increased albumin uptake and/or degradation by renal cells associated with shear stress in this study suggested that embedded microsensors could be a useful monitoring device in microfluidic systems.
Models of kidney physiology and pathophysiology.
Multiple cell types and functional units comprise the human kidney. Thus, to be physiologically relevant, a biomimetic kidney-on-a-chip must integrate cell-cell interactions, such as those that exist between glomerular vascular endothelial cells and podocytes, transcellular electrochemical and osmotic pressure gradients, structural arrangement of renal tubular segments, fluid flow dynamics, and cellular metabolic and endocrine functions. To date, models of glomerular, proximal tubular, and distal tubular physiology have been developed using on-chip technology (Table 1). The integration of these components in the development of a true kidney-on-a-chip50 has yet to be achieved (Figure 2).
Table 1 ∣.
Summary of studies using microfluidics as a kidney microphysiological system or kidney-on-a-chip
| Kidney part | Cells | Objective | Findings | Implications | Reference |
|---|---|---|---|---|---|
| Tubular structure | MDCK cell line | Tissue engineering of a bioartificial renal tubule | Cells formed confluent monolayers with tight junctions and central cilia | Suggests that miniaturization of the existing bioartificial kidney will be feasible | Mackay et al.3 |
| MDCK cell line | Development of an in vitro renal tubule | MDCK cells attached and proliferated on PDMS biochips having microfluidic microchannels-dynamic culture | Baudoin et al.13 | ||
| MDCK cell line | In vitro tubule for pharmacokinetic and toxicity studies | Gentamicin toxicity demonstrated. Degree of toxicity was dependent on the administration regimen | Potential use of microfluidic cell culture models for pharmacokinetics and toxicity studies | Kim et al.25 | |
| HREC and MDCK cell lines | Measurement of TEER in renal epithelial cells | Decrease in TEER corresponded to a large increase in paracellular inulin transport | Meaningful flow conditions defined for evaluating cellular transport studies | Ferrell et al.24 | |
| OK cell line | Albumin resorption | FSS significantly enhances albumin uptake and/or degradation by PT cells | Ferrell et al.27 and Shen et al.28 | ||
| Primary hPT cells (transfected with siRNA to extend their life span) | Seeded in hollow fibers and perfused in a closed circuit | Cell with an extended life span showed good performances. Bioartificial renal tubule devices constructed with these cells showed reabsorption of water, sodium, and glucose | Sanechika et al.2 | ||
| MDCK cell line | Comparison of cell function in a microfluidic biochip and in plates (gene and protein expression) | Ion transporters and several genes involved in H transporters and pH regulation were upregulated in biochips. Various enzymes, multidrug resistance genes, and transporters were also upregulated in biochips | Snouber et al.42 | ||
| MDCK cell line | Analysis of the effect of ifosfamide on cells cultivated in microfluidic biochips | Modulation of the pathways related to cancer and inflammation in MDCK cells cultivated in biochips | Choucha Snouber et al.43 | ||
| MDCK cell line and pHUVECs | Functional coupling of renal tubular and vasa recta function | Formation of a vascular network in the hydrogel that mimicked passive diffusion | Mu et al.23 | ||
| Collecting duct | Primary rat IMCD cells | Development of a microfluidic device for efficient culture and analysis of renal tubular cells | Microfluidics enhanced cell polarization, cytoskeletal reorganization, and molecular transport | Jang and Suh4 | |
| Proximal Tubule | Primary hPTECs | PT-on-a-chip for drug transport and nephrotoxicity assessment | Cisplatin toxicity on a chip more closely mimic the in vivo responses | Jang et al.7 | |
| Primary hPTECs | Development of the PT, characterization, and in vitro transport | Cultured PT cells formed a confluent monolayer that was polarized and exhibited functional transport. Combination of hollow fiber and lab-on-a-chip technologies | Hollow fiber–based system and 3D culture model | Ng et al.41 | |
| Primary hPTECs | Creation of renal tubule | Flow shear stress induced polarization of cultured cells. For the first time, tubular ARPCs embedded into a microsystem to create a renal tubule with countercurrent flow | Proof of principle. Needs further characterization and validation | Sciancalepore et al.44 | |
| Primary hPTECs | Development of a cell-based approach for safety screening of kidney toxic compounds | Overexpression of the gene encoding HO-1 significantly correlated with increasing dose of 6 compounds | Adler et al.29 | ||
| Primary hPTECs | Development of a model for human PT function | Cells polarized and demonstrated characteristic renal cell morphology and function (i.e., ammoniagenesis and vitamin D biotransformation) | First demonstration of relevant basolateral solute transport, apical solute uptake, and intracellular enzymatic function by the hPTEC in vitro system | Weber et al.45 | |
| Primary hPTECs | Development of functional renal tubules | Secretory clearance of albumin-bound uremic toxins and albumin reabsorption across the developed renal tubule | Jansen et al.46 | ||
| Primary hPTECs | Functional coupling of jejunum, liver, kidney (PT), skeletal muscle, and neurovascular model | Absorption and metabolism of various drugs was consistent with clinical pharmacodynamics | Potential for studies of multiorgan toxicity and absorption, distribution, metabolism, and excretion | Vernetti et al.47 | |
| PTECs and fibroblasts | Development of a 3D-bioprinted PCT-on-a-chip | 3D PTs on a chip exhibit enhanced epithelial morphology and functional properties. Upon introducing cyclosporine A, the epithelial barrier was disrupted in a dose-dependent manner | 3D bioprinting of the PCT | Homan et al.12 | |
| Glomerulus | hiPSC-derived podocyte-like cells | Development of a glomerulus-on-a-chip | human iPSC-derived podocytes produced glomerular basement membrane collagen and recapitulated the natural tissue-tissue interface of the glomerulus, as well as the differential clearance of albumin and inulin, when cocultured with human glomerular endothelial cells. The device mimicked Adriamycin-induced podocyte injury | First use of hiPSC-derived podocyte-like cells | Musah et al.14 |
| Glomerular epithelia | Development of a multi-organ-on-a-chip comprising intestinal and glomerular epithelia | Digoxin absorption and nephrotoxicity were consistent with clinical pharmacodynamics | Li et al.48 | ||
| Glomerular epithelia | Development of a glomerular disease model (hypertensive nephropathy) | Physiological flow led to cytoskeletal rearrangement, demonstrating that mechanical forces play a role in the damage of cells and cellular junctions | Potential use in modeling disease states (i.e., hypertensive nephropathy) | Zhou et al.49 | |
| Nephron | – | Development of a nephron-on-a-chip | Model design | Weinberg et al.50 | |
| – | – | Use of MEMS to improve hemodialysis | Improved removal of uremic solutes during vascular flow | Kaazempur-Mofrad et al.51 |
3D, 3-dimensional; ARPC, adult renal progenitor cell; FSS, fluid shear stress; hiPSC, human induced pluripotent stem cell; HO-1, heme oxygenase-1; hPT, human proximal tubule; hPTEC, human proximal tubular epithelial cell; HREC, human renal epithelial cell; IMCD, inner medullary collecting duct, MDCK, Madin-Darby canine kidney; MEMS, microelectromechanical system; OK, opossum kidney; PCT, proximal convoluted tubule; PDMS, polydimethylsiloxane; pHUVEC, primary human umbilical vein endothelial cell; PT, proximal tubule; siRNA, small interfering RNA; TEER, transepithelial electrical resistance.
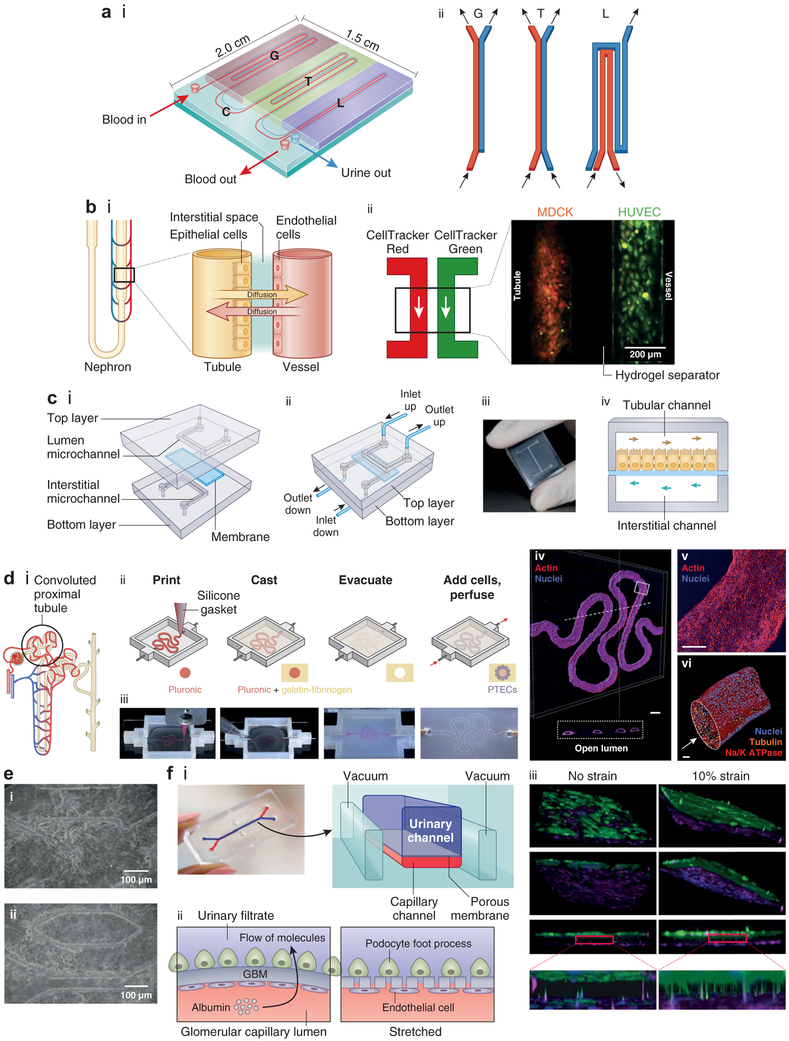
Figure 2 ∣. Biomimetic nephron and its components.
(a) Design of a nephron-on-a-chip model composed of an inlet for blood, an outlet for blood, and an outlet for urine. (i) Schematic showing the glomerulus (G) part, renal tubule (T) part, loop of Henle (L) part, and a connector (C) part. (ii) Schematic showing the arrangement of channels in different parts of the nephron model. Adapted from Weinberg E, Kaazempur-Mofrad M, Borenstein J. Concept and computational design for a bioartificial nephron-on-a-chip. Int J Artif Organs. 2008;31:508.50 (b) Biomimetic nephron (nephron-on-a-chip) using renal epithelial cells and endothelial cells (ECs) cultured in a microtubular system resembling the nephron (proximal tubule and neighboring vessel). (i) Schematic of normal structures of the nephron. Adapted from Mu X, Zheng W, Xiao L, et al. Engineering a 3D vascular network in hydrogel for mimicking a nephron. Lab Chip. 2013;13:1612, with permission of The Royal Society of Chemistry.23 (ii) Madin-Darby canine kidney (MDCK) cells and human renal epithelial cells (HUVECs) seeded into hydrogel microchannels as a way of mimicking the tubular and vascular proximity. Each microchannel is perfused with dye (red for MDCK and green for HUVEC). Reproduced with permission from Mu X, Zheng W, Xiao L, et al. Engineering a 3D vascular network in hydrogel for mimicking a nephron. Lab Chip. 2013;13:1612.23 (c) Multilayered chip, resembling renal proximal tubule (PT) composed of cells cultured on a porous membrane that separates microchannels, simulating the tubular lumen and the interstitium. The membrane is coated with either fibronectin, laminin, or Matrigel. (i) Two overlapped polydimethylsiloxane (PDMS) layers are engraved with microchannels. The apical aspect of the cells is exposed (upper channel), and the interstitium is in contact with basolateral membranes (lower channel). The channels are continuously fed in a countercurrent manner. (ii) Once assembled, the device presents 2 inlets and 2 outlet ports. (iii) Photograph of the device. (i–iii) Reproduced with permission from Sciancalepore AG, Sallustio F, Girardo S, et al. A bioartificial renal tubule device embedding human renal stem/progenitor cells. PLoS One. 2014;9:e87496.44 Copyright © Sciancalepore et al. (iv) Schematic illustrating the 2 channels with countercurrent flow in the 2 microchannels separated by the membrane. Adapted with permission from PLoS One.44 (d) (i) Schematic of the proximal convoluted tubule (PCT). (ii, iii) Fabrication of PCT steps in which a fugitive ink is printed on a gelatin-fibrinogen extracellular matrix (ECM), an additional ECM is cast around, the fugitive ink is evacuated to create a tubule, and PT cells are seeded within the tubule. (iv) A 3-dimensional (3D) rendering of the printed PCT acquired by confocal microscopy (actin is in red and nuclei in blue). A cross-sectional view is shown below, where PT cells circumscribe the open lumens in 3D. Bar = 500 μm. (v) Higher-magnification view of the region in (iv) denoted by the white rectangle. Bar = 200 μm. (vi) 3D rendering of the PCT, where an open lumen circumscribed with an epithelial lining is directionally perfused on a chip (Na/K ATPase is in red, acetylated tubulin is orange [highlighting the primary cilia], and nuclei are in blue). Bar = 50 μm. Reproduced with permission from Homan KA, Kolesky DB, Skylar-Scott MA, et al. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep. 2016;6:34845.12 Copyright © Nature Publishing Group. (e) Fibronectin-coated microchannels in PDMS chips having MDCK cells cultured inside, after 72 hours of perfusion, corresponding to 96 hours of culture (i, ii). Cells formed 3D-like tissue structures covering the microchannels. Bar = 100 μm. Reproduced with permission from Baudoin R, Griscom L, Monge M, et al. Development of a renal microchip for in vitro distal tubule models. Biotechnol Progr. 2007;23:1245–1253.13 Copyright © 2007 American Institute of Chemical Engineers (AIChE). (f) Images of a glomerulus-on-a-chip and schematic of the device, (i) in which terminally differentiated podocytes derived from human induced pluripotent stem cells are cultured on one side of a laminin-coated membrane and ECs on the other side in a microchannel microfluidic system, showing movement of molecules and pores appearing in the membrane upon stretching (ii). (iii) 3D reconstructed views of the tissue-tissue interface formed by podocytes (top: green) and ECs (bottom: magenta) showing that the cyclic application of 10% strain enhances the extension of podocyte cell processes through the pores of the flexible ECM-coated PDMS membrane so that they insert into the abluminal surface of the underlying EC (insets). Bar = 100 μm. Reproduced by permission from Macmillan Publishers Ltd: Nature Biomedical Enginerring, Musah S, Mammoto A, Ferrante TC, et al. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat Biomed Eng. 2017;1:0069.14 Copyright © 2017. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
Glomerulus on-a-chip.
The development of a functional glomerulus-on-a-chip was stymied by a lack of functional podocytes until recent work by Musah et al.,14 who obtained terminally differentiated podocytes from human iPSCs. These podocytes were cocultured with endothelial cells on opposite sides of a laminin-coated membrane (Figure 2f) in a system with separate microfluidic channels mimicking urinary and blood flow. Production of basement membrane collagen and a tissue-tissue interface were observed as was a physiological differential clearance of albumin and inulin. Adriamycincaused podocyte disruption, loss of function, and cell death in this system mimicking the toxicity of Adriamycin in vivo were observed.
Zhou et al. used the glomerulus-on-a-chip concept to develop a model of hypertensive glomerulopathy.49 Using conditionally immortalized glomerular endothelial cells and mouse podocyte precursor (mouse podocyte clone 5) cells, 2 microfluidic channels were lined by closely approximated layers of glomerular endothelial cells and podocytes. Fluid flow in the channels affected cytoskeletal rearrangement in the adjacent cells, resulting in cellular damage and glomerular leakage. Wang et al.52 subsequently reported the use of a glomerulus-on-a-chip microdevice for studying early-stage diabetic nephropathy, providing further proof of principle that these devices could serve as disease models of glomerulopathy.
PT-on-a-chip.
Several investigators have successfully cultured renal proximal tubular cells in tunable hollow fibers, providing immunoprotection during extracapillary blood flow. Ng et al. seeded hPTECs on the inner surface of fibers composed of fibrin-coated hydrogel and noted the successful formation of a monolayer that had an enormous transport potential for glucose (Figure 3).41 Jang et al. cultured hPTECs on microchannels composed of collagen type IV-coated polyester membranes.7 Compared to control systems not exposed to fluid shear stress, tubular cells in microfluidic systems regained normal columnar shape, polarity, and primary cilia. They also displayed an increase in cellular uptake of albumin and an increased recovery from cisplatin-induced damage.7 Sciancalepore et al. cultured human renal progenitor cells (hPCs) on porous polycarbonate membranes coated with either fibronectin, laminin, or Matrigel that separated PDMS microchannels.44 In this study, fibronectin was associated with the highest number of metabolically active cells (Figure 2c) and flow shear stress-induced polarization of cultured cells. Jansen et al.46 developed functional renal tubules by using hPTEC-biofunctionalized hollow fibers and demonstrated secretory clearance of albumin-bound uremic toxins and albumin reabsorption across the tubule. Most recently, Weber et al. used hPTECs in a previously developed microfluidic chamber filled with the extracellular matrix surrounding tubular channels and noted excellent cell viability (>95%) for up to 4 weeks in culture.45 Importantly, these cells produced a collagen matrix, formed tubular structures, and maintained renal epithelial differentiation, morphology, and polarization. They also showed evidence of ammoniagenesis and vitamin D activation, thus demonstrating, for the first time, basolateral solute transport, apical solute uptake, and intracellular enzymatic function. Microfluidic systems thus appear to mimic physiological conditions and maintain PT cells in a more differentiated state in vitro than do conventional culture systems.
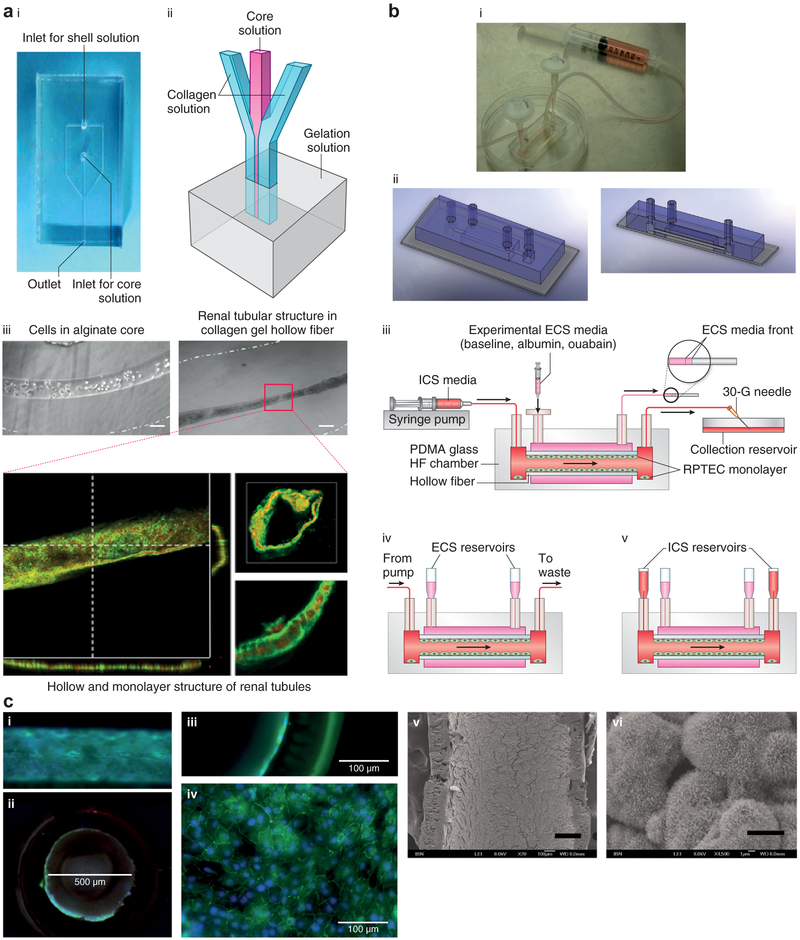
Figure 3 ∣. Hollow fibers (HFs) and lab-on-a-chip.
(a) Image (i) and schematic (ii) of the collagen gel HF microfluidic device. (iii) Human kidney-2 cell-laden collagen HF with Ca-alginate core having a diameter of 100 μm (left) and HF having an inner diameter of 50 μm (right) after 10 days of culture. Confocal images of renal tubules in HF are shown at the bottom, which depicts renal tubules with a cross-sectional view (bar = 50 μm) (lower left); 3D reconstructed confocal image (bar = 50 μm) (upper right); and magnified view showing the cell-cell connection (bar = 20 μm) (lower right). Reproduced with permission from Shen C, Zhang G, Wang Q, Meng Q. Fabrication of collagen gel hollow fibers by covalent cross-linking for construction of bioengineering renal tubules. ACS Appl Mater Interf. 2015;7:19789.28 (b) Combining polyethersulfone-polyvinyl pyrrolidone HF and lab-on-a-chip technology. Human proximal tubular epithelial cells are cultured on the coarse inner surface of hollow fibers coated with fibrin that is embedded in a polydimethylsiloxane-glass chamber. (i) Image and (ii) schematic of the lab-on-a-chip HF bioreactor. (iii) Setup for inulin recovery perfusion studies, (iv) normal perfusion culture, and (v) static configuration for urea, creatinine, and glucose transport studies. ECS, extracapillary space surrounding the hollow fibers; ICS, intracapillary of the hollow fibers; PDMA, poly(N,N-dimethylacrylamide); RPTEC, renal proximal tubule epithelial cells. (c) Immunofluorescence and electron microscopic images of the cell-laden HF: (i) transverse section and (ii) cross-section (bar = 500 μm). Close-up views of the (iii) cross-section and (iv) transverse section (confocal image) revealing a cell monolayer in the HF (bar = 100 μm). (v) Scanning electron microscopic image of the cell-laden HF (bar = 200 μm), with (vi) the magnified view showing microvilli expression on the cell surface (bar = 5 μm). (b,c) Reproduced with permission from Ng CP, Zhuang Y, Lin AWH, Teo JCM. A fibrin-based tissue-engineered renal proximal tubule for bioartificial kidney devices: development, characterization and in vitro transport study. Int J Tissue Eng. 2013; article ID 319476.41 To optimize viewing of this image, please see the online version of this article at http://www.kidney-international.org.
Recent advances in 3-dimensional (3D) bioprinting have allowed the development of increasingly complex structures. Homan et al. printed a fugitive pluronic ink on a gelatinfibrinogen extracellular matrix and cast an additional extracellular matrix hydrogel around the bioprinted material.12 Convoluted microchannels were created in the hydrogel by evacuating the pluronic ink and seeded with hPTECs (hPTEC-TERT1 cell line). Perfused cells in this system demonstrated greater albumin uptake and morphological properties more similar to human proximal tubular cells than did cells in classical 2D culture systems (Figure 2d). In this study, system functionality was maintained for >2 months, suggesting that bioprinting may allow the creation of increasingly complex structures with improved cellular functionality and longevity.
Distal tubule-/collecting duct-on-a-chip.
In contrast to PT cells, few studies have examined the physiology of distal tubular and cortical collecting duct cells cultured on biomimetic platforms. In 2007, Baudoin et al. developed a microchip by using fibronectin-coated microchannels in a PDMS device. MDCK (distal tubular) cells were cultured in microchannels. These cells were able to attach, proliferate, and form 3D tissue-like structures covering the microchannels (Figure 2e). However, fluid rates of 50 μl/min resulted in impaired cell proliferation and increased cell death in this system, suggesting that MDCK cells are not accustomed to the flow rates observed in the PT.13 In 2010, Jang and Suh cultured primary rat renal collecting duct cells on a multilayer microfluidic device created from thin fibronectin-coated porous polyster membranes situated in a PDMS microchamber.4 Shear stress was maintained at 1 dyn/cm2 (0.1 Pa) by accounting for fluid flow rates, viscosity, and channel size in this system. At this stress level, cytoskeletal rearrangement, number of cell junctions, and cell polarity increased. Higher and denser cell monolayers were formed in collagen hollow fibers as compared with thinner, less dense cells in monolayers formed on flat collagen membranes. In addition to morphological changes, cells in this system displayed an increased capacity for sodium and water transport and an increased expression of brush border enzymes of alkaline phosphatase and γ-glutamyltransferase (Figure 3).27,28 Cell functionality was related to fiber diameter, with smaller-diameter fibers resulting in increasingly functional cells (normal renal tubules have a small diameter of ~50 μm53). These findings suggested that cells could sense structural and spatial cues and that surface curvature might have a positive effect on distal tubular cell function.27,28,54 They further suggested that a diameter of 50 μm (i.e., the size of native renal tubules) should be used in bioengineered distal tubules to mimic the size of native tubules and result in optimal functionality.
Toward kidney-on-a-chip.
In 2008, Weinberg et al. proposed a design for replicating a nephron, with a system containing 4 parts: glomerulus, PT, loop of Henle, and connector (Figure 2a).50 To date, the PT has been best studied in model systems; early work on glomerular and distal tubule physiology is promising. However, other portions of the nephron, including the thick ascending limb, the interstitium, and the cortical collecting duct, have been less well-characterized in vitro. In addition, the structural-functional arrangements of different nephron segments, the specialized structures resulting from these arrangements, the physiology of countercurrent exchange, and the diffusion of nutrients, waste, hormones, and drugs through the interstitium of the native kidney22 make the development of a functional kidney-on-a-chip more than just the sum of individual nephron components. Although higher-order structures have not yet been modeled in vitro, new developments hold promise for surmounting some of these challenges. In 2013, Mu et al. cultured MDCK renal epithelial cells and primary human umbilical vein endothelial cells on opposite sides of an alginate-collagen type I composite hydrogel in a microtubular 3D system (Figure 2b).23 This organization represented the first step toward creating a system that mimics the interaction between blood vessels and tubular cells, as occurs in the juxtaglomerular apparatus and in countercurrent exchange. Moreover, current technologies allow the creation of chemical, oxygen, and nutritional gradients in on-chip platforms and current data suggest that efficient chemotaxis can be achieved with much shallower gradients in these systems than was previously achieved with 2D in vitro systems.22 Thus, more complex physiological in vitro systems of renal physiology may be on the horizon.
Clinical applications
Drug development and testing.
New drugs must be evaluated for efficacy and safety before clinical use, and preliminary tests for nephrotoxicity are typically performed in animals. However, animal studies are expensive and slow. They are often species-specific, failing to predict human nephrotoxicity; and they are often associated with ethical concerns.5,55 The in vitro use of cell lines avoids ethical concerns; however, many cell lines are derived from animals and both human- and animal-derived immortalized cell lines do not fully recapitulate the structure and function of their native counterparts. As a result, nephrotoxicity is often not discovered until testing is initiated in humans and many cases of nephrotoxicity are not identified until phase III and phase IV trials.56,57
Because of their high energy needs, PT cells are particularly susceptible to drug toxicity,7,58,59 making the development of in vitro models of proximal tubular function attractive to drug developers. High resorption and molecule transport rates allow drugs and chemicals to accumulate in PT cells and in the intercellular space.12 Multiple 2D PT cell culture models have been useful in defining the nephrotoxic pathophysiology of drugs such as cisplatin, which accumulate in proximal tubular cells.60,61 These models have also been useful in studying cimetidine-mediated recovery from cisplatin-induced proximal tubular dysfunction.7 However, 2D cell culture models fail to identify toxicity of drugs and chemicals with flow-dependent tubular actions. In an attempt to more faithfully recapitulate in vivo physiology, multicellular systems including static 3D culture and organoid models were developed. Unfortunately, organoids lack accessible inlets and outlets, limiting their usefulness 12 and static 3D culture models lack natural flow dynamics necessary for the development of cell polarity and function.4 Recent attention has thus been directed to integrating microfluidic technology 62 into 3D models. In one study aimed at developing a reliable cell-based nephrotoxicity screening system, primary hPTECs were cultured on tubules embedded in type I collagen.29 In this system, heme oxygenase-1 RNA and protein expression in cell lysates were found to be sensitive and specific markers of nephrotoxicity. In a bioprinted system of the human PT, PTEC-TERT1 cells exposed to cyclosporine A demonstrated a dose-dependent disruption in function, suggesting that this system could also be used for qualitative and quantitative studies of drug-induced proximal tubular dysfunction.12
Drugs that disturb renal blood flow have traditionally proved challenging for in vitro nephrotoxicity screens. Vasoconstrictors such as catecholamines and calcineurin inhibitors reduce oxygen delivery, resulting in injury to the renal medulla.63,64 However, recently developed blood vessel-on-a-chip devices65 have demonstrated that vasoconstrictive behaviors can be evaluated in organ-on-chip models, raising the possibility that on-chip models might soon be available to model vasoconstrictive nephrotoxicity.
Evaluation of many types of toxicities in vitro requires systems to integrate multiple cell types from different organs,66 which can help to evaluate systemic10 and secondary drug toxicities resulting from drug metabolism11 and to evaluate inflammatory responses that may not always be detected during direct testing of the drug on the target organ. In 2017, Vernetti et al. combined on-chip systems representing absorption (human jejunal enteroids), metabolism (primary human hepatocytes), and clearance (primary human proximal tubular epithelial cells) with skeletal muscle (primary human myocytes) and neurovascular (iPSC-derived human neurons and astrocytes) models, in which media were transferred sequentially from one organ to the other.47 Multi-organ-on-a-chip models comprising intestine (primary human small intestinal epithelial cells), liver (primary human hepatic stellate cells combined with immortalized HepaRG cells), skin (human biopsy tissue), and human renal PT cell line (RPTEC/TERT1) were similarly developed by Maschmeyer et al.67 to assess drug absorption, distribution, metabolism, and excretion as well as multiorgan toxicity. Li et al. further developed a system that linked intestine colorectal adenocarcinoma cell line with rat primary glomerular endothelial cells in a compartmentalized microchamber of the microfluidic system.48 When tested for digoxin, this system demonstrated that digoxin induced glomerular endothelial toxicity, manifested by increased permeability and cell death, consistent with clinical digoxin nephrotoxicity.
In sum, present data suggest that on-chip systems may be useful tools for predicting in vivo proximal tubular toxicity. Recent advances in modeling vascular systems hold promise for vasoconstrictive nephropathy screening and multi-organ-on-a-chip models hold promise for improving prediction of secondary toxicities. However, on-chip technologies for testing distal tubular drug toxicities (as occurs with medications such as amphotericin), tubular obstruction (i.e., acyclovir toxicity), and interstitial nephritis (as occurs with medications such as nonsteroidal anti-inflammatory drugs and cephalosporins) remain limited.
Renal replacement therapies.
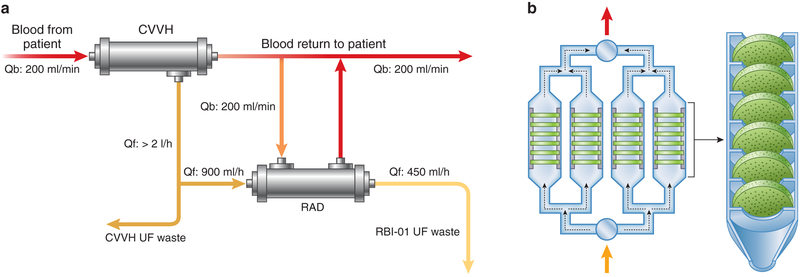
In addition to microscopic on-chip technologies, the integration of cells with microfluidics has therapeutic potential at macroscale. Although lifesustaining for many, current hemodialysis therapies offer only a primitive approximation of renal function and mortality rates in dialysis have not changed in decades. In contrast to the functioning nephron, in which toxins are filtered and secreted while vital electrolytes and small molecules are reabsorbed, clearance of small- and mid-sized molecules by hemodialysis relies on 1-way diffusion and/or convection across a relatively nonselective semipermeable membrane. Efforts have been made to modify traditional dialysis filters to more accurately approximate true nephron function. MacKay et al. first demonstrated in 1998 that MDCK renal epithelial cells could be cultured on hollow fibers, growing into confluent monolayers with functional transport, metabolic, and endocrine capabilities.3 Subsequently, Kaazempur-Mofrad et al. suggested that PT cells might be combined with microelectromechanical systems in hemodialysis systems to allow electrolyte and small- and mid-sized molecule reabsorption and secretion.51 Useful molecules that may escape during dialysis conducted with current methods may thus be reabsorbed by PT cells in these hybrid systems. A renal tubule assist device (RAD) was subsequently developed for use with continuous venovenous hemofiltration (Figure 4a). RAD was associated with improved survival in an animal model of septic shock,70 and an initial phase II multicenter study suggested a survival advantage of RAD over conventional hemofiltration.68 Although a subsequent clinical trial of 134 patients with acute kidney injury in the intensive care unit randomized to continuous renal replacement therapy alone or to continuous renal replacement therapy and RAD showed no overall benefit of RAD,71 a subgroup analysis demonstrated lower mortality rates in patients with a postfilter ionized calcium level of ≤0.4 mmol/l who received RAD compared to conventional continuous renal replacement therapy. Although these initial results appeared promising for improving outcomes in certain clinical settings, advances in RAD technology have been hindered by slow production rates and by expensive and delicate storage requirements.69,70 New designs and manufacturing techniques, including the development of niobium-coated carbon disks housed in a perfusable polycarbonate chamber and seeded with human epithelial cells (Figure 4b), will enable simplified and increased production rates.69 Limitations in cell sourcing (i.e., maintaining primary cells in adequate numbers and for sufficient times without reverting to progenitor cells) may in future be addressed by using patient-derived iPSCs and/or allogenic cells that are immune evasive from the extracorporeal circuit.69
Figure 4 ∣. Cell-based renal tubule assist device (RAD).
(a) Schematic of the extracorporeal perfusion circuit for cell-based RAD composed of a hemofilter perfusion pump system (continuous venovenous hemofiltration [CVVH]), a RAD perfusion system i.v. pump for the pre-RAD ultrafiltrate (UF) line, and a blood pump for the post-RAD blood line. Qb, blood flow; Qf, rate of fluid filtration. Adapted from Tumlin J. Efficacy and safety of renal tubule cell therapy for acute renal failure. J Am Soc Nephrol. 2008;19:1034–1040.68 (b) Bioartificial renal epithelial cell system composed of housing pieces and 4 porous disk columns, each consisting of six 2-mm-thick disks.
Opportunities and challenges.
The use of microfluidic systems allows coculture of different cell types, which is essential for studying cell-cell interactions, signaling, and cell recruitment in both health and disease states.8 Moreover, the ability to integrate sensors into microfluidic systems72,73 permits continuous measurement of cellular behavior.74 In addition to the use of electrodes in monitoring epithelial barrier integrity through transepithelial electrical resistance,75 the integration of mass spectrometric analysis to on-chip platforms76 allows the analysis of genetic, proteomic, and metabolomic signatures in response to specific compounds and stimuli.8,77 Linking on-chip systems with data communication tools is anticipated to further improve cellular monitoring as well as prediction of toxicity models. Moreover, although animal-derived cell lines with poor translation to human physiology have been traditionally used in microfluidic models, iPSC-derived cells from humans have been increasingly used in recent years to model terminally differentiated human cells. Combining stimuli-responsive materials78,79 and biosensors80 with advances in stem cell biology in deriving and propagating terminally different cell types14 is predicted to further improve the ability of on-chip technology to provide useful models of renal physiology, renal disease, and drug toxicity screening.
However, several challenges persist in the pursuit of true kidney-on-a-chip devices. One major obstacle is the limited life span of cells in these devices.81 This limitation is particularly acute when primary—as opposed to immortalized—cells are used in on-chip systems. The appropriateness of current cell lines in faithfully mimicking in vivo biology also limits the application of these model systems. To date, all on-chip systems of drug toxicity have been hampered by inadequate in vitro modeling of tubular drug transporters and metabolic enzymes. Current models only partially reproduce normal physiology. Furthermore, challenges persist in optimizing biomaterials used in microfluidic systems. For clinical use, these materials need to be biocompatible, inert (so as not to absorb chemicals), and nonleaching (to avoid interference of compounds from the material itself).8 Thus, although preliminary data suggest that integrated systems can be used to assess drug absorption, distribution, metabolism, and excretion characteristics in vitro, system validation, design standardization, and integration with standard laboratory tools will be essential before these systems can be used on a large scale.8 To date, no single on-chip system has gained widespread acceptance, and all are limited by a lack of tissue-tissue interactions and lack of integration with lymphatic and nervous systems. As a result, reliable extrapolation of drug toxicity data obtained from in vitro testing to in vivo biology remains limited.9
Advances in engineering and cell biology already provide potential solutions to these limitations. Microfluidics-based systems allow the precise control of cell microenvironments in both space and time82 and also allow the direct delivery of therapeutics to cells (i.e., stem cell transfection with modified mRNA).83 In turn, advances in stem cell technology, such as those that have been demonstrated in the integration of iPSC-derived podocytes into microfluidic devices,14 have helped to overcome limitations of modeling terminally differentiated cell types in vitro. The 3D bioprinting technology enables precise production of complex and multiplexed structures84,85 and ensures reproducibility in manufacturing.86 The use of nanotechnology will further improve precision at the level of cellular diagnosis and therapy. Recently, 4-dimensional bioprinting has been developed87–89 in which biomimetic and dynamic88 tissues can potentially be integrated into microfluidic platforms.
To date, disease models have been developed with the help of microfluidics in several organ systems, offering promise that on-chip models may, in the future, be used to study kidney-specific diseases. In 2015, Huh developed a lung-on-a-chip in which alveolar epithelial cell and pulmonary microvascular endothelial cells culture on opposite sides of a 10-μm microporous membrane. In this model, interleukin-2 induced vascular leakage, providing a novel in vitro model of chemotherapy-induced pulmonary edema90 that could be useful in developing models of renal basement membrane permeability, such as that occurs in diabetic nephropathy or nephrotic syndrome. More recently, Banaeiyan et al. observed 3D tissue-like structures and bile canaliculi network formation when coculturing human hepatocellular carcinoma cell line (HepG2) with human iPSC-derived hepatocytes in hexagonal chambers. Albumin secretion and urea synthesis were observed in the microfluidic devices of this liver-on-a-chip model, demonstrating that structural configuration and exposure to continuous fluid flow increase cell functionality.91 The effects of physiological and pathological strain on vascular aging have been evaluated in an on-chip model of Hutchinson-Gilford progeria syndrome in which patient-specific iPSC-derived endothelial cells were cultured in microfluidic systems.92 The effective use of these iPCS-derived cells in microfluidic systems suggest that patient-specific genetic renal disease (i.e., Alport syndrome and cystic kidney diseases) may, in future, be successfully modeled in vitro. Finally, the bioprinted vascular endothelium has recently been used to study the physiology and pathophysiology of thrombosis93 and tunable microfluidic structures that allow the antibody capture and release of circulating tumor cells have been used in cancer research.94,95 These improved printing technologies and advanced materials hold promise for the future of studying the role of vascular damage and immune-mediated disease in conditions such as hemolytic uremic syndrome/thrombotic microangiopathy and of evaluating the mechanisms of renal transplantation rejection and tolerance.
Conclusions
Over the last 20 years, research in bioengineered materials and microfluidics, starting with the development of renal cell-seeded hollow fiber systems and progressing to models representing more diverse kidney functions, has led to the development of on-chip platforms that may prove useful adjuncts to predicting drug toxicity and to modeling of human kidney disease in vitro. It is hoped that many patients may be saved from the morbidity and mortality associated with unpredicted adverse drug reactions and drug development costs may be substantially reduced. It is hoped that these technologies will facilitate research into the pathogenesis and treatment of renal diseases that contribute to considerable human and societal costs worldwide.
ACKNOWLEDGMENTS
AH acknowledges GCC-2017-005 grant from Qatar University under the GCC research program and the NPRP grant (NPRP 9-144-2-021) from the Qatar National Research Fund (a member of The Qatar Foundation). The statements made herein are solely the responsibility of the authors. YSZ acknowledges funding from the National Institutes of Health (K99CA201603, R21EB025270) and the New England Anti-Vivisectio Society. NA acknowledges funds received from the Biotechnology Research Center, Libyan Authority of Research Science and Technology and UNESCO. The authors thank Mr. Mohammed Xohdy for producing some of illustrations in this paper.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.Hegab HM, Elmekawy A, Stakenborg T. Review of microfluidic microbioreactor technology for high-throughput submerged microbiological cultivation. Biomicrofluidics. 2013;7:21502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanechika N, Sawada K, Usui Y, et al. Development of bioartificial renal tubule devices with lifespan-extended human renal proximal tubular epithelial cells. Nephrol Dial Transplant. 2011;26:2761–2769. [DOI] [PubMed] [Google Scholar]
- 3.Mackay S, Funke A, Buffington D, Humes H. Tissue engineering of a bioartificial renal tubule. ASAIO J. 1998;44:179–183. [DOI] [PubMed] [Google Scholar]
- 4.Jang K, Suh K. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip. 2010;10:36–42. [DOI] [PubMed] [Google Scholar]
- 5.Hartung T. Toxicology for the twenty-first century. Nature. 2009;460:208–212. [DOI] [PubMed] [Google Scholar]
- 6.Jenkinson S, Chung G, van Loon E, et al. The limitations of renal epithelial cell line HK-2 as a model of drug transporter expression and function in the proximal tubule. Pflugers Arch. 2012;464:601–611. [DOI] [PubMed] [Google Scholar]
- 7.Jang KJ, Mehr AP, Hamilton GA, et al. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol (Camb). 2013;5:1119–1129. [DOI] [PubMed] [Google Scholar]
- 8.Wilmer MJ, Ng CP, Lanz HL, et al. Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol. 2016;34:156–170. [DOI] [PubMed] [Google Scholar]
- 9.Dehne EM, Hasenberg T, Marx U. The ascendance of microphysiological systems to solve the drug testing dilemma. Future Sci OA. 2017;3:FSO185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oleaga C, Bernabini C, Smith AST, et al. Multi-organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci Rep. 2016;6:20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webb S Cells in the third dimension. Biotechniques. 2017;62:93–98. [DOI] [PubMed] [Google Scholar]
- 12.Homan KA, Kolesky DB, Skylar-Scott MA, et al. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep. 2016;6:34845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baudoin R, Griscom L, Monge M, et al. Development of a renal microchip for in vitro distal tubule models. Biotechnol Progr. 2007;23:1245–1253. [DOI] [PubMed] [Google Scholar]
- 14.Musah S, Mammoto A, Ferrante TC, et al. Mature induced-pluripotentstem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat Biomed Eng. 2017;1. article number: 0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Organ-on-chip market analysis & trends—organ (heart-on-chip, human-on-chip, intestine-on-chip, kidney-on-chip, liver-on-chip, lung-on-chip), application—forecast to 2025. PR Newswire; January 18, 2017. Available at: https://www.prnewswire.com/news-releases/organ-on-chip-market-analysis-trends—organ-heart-on-chip-human-on-chip-intestine-on-chip-kidney-on-chip-liver-on-chip-lung-on-chip-application—forecast-to-2025-300393217.html. [Google Scholar]
- 16.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–373. [DOI] [PubMed] [Google Scholar]
- 17.Ugolini GS, Visone R, Redaelli A, et al. Generating multicompartmental 3D biological constructs interfaced through sequential injections in microfluidic devices. Adv Healthc Mater. 2017;6(10). [DOI] [PubMed] [Google Scholar]
- 18.Lind JU, Busbee TA, Valentine AD, et al. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat Mater. 2017;16:303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volpatti LR, Yetisen AK. Commercialization of microfluidic devices. Trends Biotechnol. 2014;32:347. [DOI] [PubMed] [Google Scholar]
- 20.Yang Q, Lian Q, Xu F. Perspective: fabrication of integrated organ-on-a-chip via bioprinting. Biomicrofluidics. 2017;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang C, Zhao Z, Abdul Rahim NA, et al. Towards a human-on-chip: culturing multiple cell types on a chip with compartmentalized microenvironments. Lab Chip. 2009;9:3185. [DOI] [PubMed] [Google Scholar]
- 22.Huh D, Torisawa YS, Hamilton GA, et al. Microengineered physiological biomimicry: organs-on-chips. Lab Chip. 2012;12:2156–2164. [DOI] [PubMed] [Google Scholar]
- 23.Mu X, Zheng W, Xiao L, et al. Engineering a 3D vascular network in hydrogel for mimicking a nephron. Lab Chip. 2013;13:1612. [DOI] [PubMed] [Google Scholar]
- 24.Ferrell N, Desai RR, Fleischman AJ, et al. A microfluidic bioreactor with integrated transepithelial electrical resistance (TEER) measurement electrodes for evaluation of renal epithelial cells. Biotechnol Bioeng. 2010;107:707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S, LesherPerez SC, Kim BC, et al. Pharmacokinetic profile that reduces nephrotoxicity of gentamicin in a perfused kidney-on-a-chip. Biofabrication. 2016;8:015021. [DOI] [PubMed] [Google Scholar]
- 26.Ozgen N, Terashima M, Aung T, et al. Evaluation of long-term transport ability of a bioartificial renal tubule device using LLC-PK1 cells. Nephrol Dial Transplant. 2004;19:2198–2207. [DOI] [PubMed] [Google Scholar]
- 27.Ferrell N, Ricci KB, Groszek J, et al. Albumin handling by renal tubular epithelial cells in a microfluidic bioreactor. Biotechnol Bioeng. 2012;109:797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen C, Zhang G, Wang Q, Meng Q. Fabrication of collagen gel hollow fibers by covalent cross-linking for construction of bioengineering renal tubules. ACS Appl Mater Interfaces. 2015;7:19789. [DOI] [PubMed] [Google Scholar]
- 29.Adler M, Ramm S, Hafner M, et al. A quantitative approach to screen for nephrotoxic compounds in vitro. J Am Soc Nephrol. 2016;27:1015–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narayanan K, Schumacher KM, Tasnim F, et al. Human embryonic stem cells differentiate into functional renal proximal tubular-like cells. Kidney Int. 2013;83:593–603. [DOI] [PubMed] [Google Scholar]
- 31.Ashammakhi N, Elkhammas E, Hasan A. Glomerulus-on-a-chip: life up. Transplantation. 2017;101:e343–e344. [DOI] [PubMed] [Google Scholar]
- 32.Allison SJ. Bioengineering: kidney glomerulus-on-a-chip. Nat Rev Nephrol. 2017;13:382. [DOI] [PubMed] [Google Scholar]
- 33.Freedman BS, Brooks CR, Lam AQ, et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun. 2015;6:8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mata A, Fleischman A, Roy S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed Microdevices. 2005;7:281–293. [DOI] [PubMed] [Google Scholar]
- 35.van Poll ML, Zhou F, Ramstedt M, et al. A self-assembly approach to chemical micropatterning of poly(dimethylsiloxane). Angew Chem Int ed. 2007;46:6634–6637. [DOI] [PubMed] [Google Scholar]
- 36.Toepke MW, Beebe DJ. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip. 2006;6:1484–1486. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Douville N, Takayama S, ElSayed M. Quantitative analysis of molecular absorption into PDMS microfluidic channels. Ann Biomed Eng. 2012;40:1862–1873. [DOI] [PubMed] [Google Scholar]
- 38.Halldorsson S, Lucumi E, Gómez-Sjöberg R, Fleming RMT. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens Bioelectron. 2015;63:218–231. [DOI] [PubMed] [Google Scholar]
- 39.Nge PN, Rogers CI, Woolley AT. Advances in microfluidic materials: functions, integration, and applications. Chem Rev. 2013;113:2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasan A, Paul A, Memic A, Khademhosseini A. A multilayered microfluidic blood vessel-like structure. Biomed Microdevices. 2015;17:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng CP, Zhuang Y, Lin AWH, Teo JCM. A fibrin-based tissue-engineered renal proximal tubule for bioartificial kidney devices: development, characterization and in vitro transport study. Int J Tissue Eng. 2013; article ID 319476. [Google Scholar]
- 42.Snouber LC, Letourneur F, Chafey P, et al. Analysis of transcriptomic and proteomic profiles demonstrates improved Madin-Darby canine kidney cell function in a renal microfluidic biochip. Biotechnol Progr. 2012;28:474–484. [DOI] [PubMed] [Google Scholar]
- 43.Choucha Snouber L, Jacques S, Monge M, et al. Transcriptomic analysis of the effect of ifosfamide on MDCK cells cultivated in microfluidic biochips. Genomics. 2012;100:27–34. [DOI] [PubMed] [Google Scholar]
- 44.Sciancalepore AG, Sallustio F, Girardo S, et al. A bioartificial renal tubule device embedding human renal stem/progenitor cells. PLoS One. 2014;9:e87496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weber EJ, Chapron A, Chapron BD, et al. Development of a microphysiological model of human kidney proximal tubule function. Kidney Int. 2016;90:627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jansen J, Fedecostante M, Wilmer MJ, et al. Bioengineered kidney tubules efficiently excrete uremic toxins. Sci Rep. 2016;6:26715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vernetti L, Gough A, Baetz N, et al. Functional coupling of human microphysiology systems: intestine, liver, kidney proximal tubule, bloodbrain barrier and skeletal muscle. Sci Rep. 2017;7:42296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Z, Su W, Zhu Y, et al. Drug absorption related nephrotoxicity assessment on an intestine-kidney chip. Biomicrofluidics. 2017;11:034114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou M, Zhang X, Wen X, et al. Development of a functional glomerulus at the organ level on a chip to mimic hypertensive nephropathy. Sci Rep. 2016;6:31771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinberg E, Kaazempur-Mofrad M, Borenstein J. Concept and computational design for a bioartificial nephron-on-a-chip. Int J Artif Organs. 2008;31:508. [DOI] [PubMed] [Google Scholar]
- 51.Kaazempur-Mofrad MR, Vacanti JP, Krebs NJ, Borenstein JT. A MEMS based renal replacement system. Paper presented at: Solid-State Sensor, Actuator, and Microsystems Workshop June 6–10, 2004; Hilton Head Island, SC. [Google Scholar]
- 52.Wang L, Tao T, Su W, et al. A disease model of diabetic nephropathy in a glomerulus-on-a-chip microdevice. Lab Chip. 2017;17:1749–1760. [DOI] [PubMed] [Google Scholar]
- 53.Jameson JL, Loscalzo J. Harrison’s Nephrology and Acid-Base Disorders. New York: McGraw-Hill; 2010. [Google Scholar]
- 54.Shen C, Meng Q, Zhang G. Increased curvature of hollow fiber membranes could up-regulate differential functions of renal tubular cell layers. Biotechnol Bioeng. 2013;110:2173–2183. [DOI] [PubMed] [Google Scholar]
- 55.Wang B, Gray G. Concordance of noncarcinogenic endpoints in rodent chemical bioassays. Risk Anal. 2015;35:1154–1166. [DOI] [PubMed] [Google Scholar]
- 56.Redfern WS, Ewart L, Hammond TG, et al. Impact and frequency of different toxicities throughout the pharmaceutical life cycle. Toxicologist. 2010;114:1081. [Google Scholar]
- 57.Naughton CA. Drug-induced nephrotoxicity. Am Fam Phys. 2008;78:743–750. [PubMed] [Google Scholar]
- 58.Tiong HY, Huang P, Xiong S, et al. Drug-induced nephrotoxicity: clinical impact and preclinical in vitro models. Mol Pharm. 2014;11:1933–1948. [DOI] [PubMed] [Google Scholar]
- 59.Perazella MA. Renal vulnerability to drug toxicity. Clin J Am Soc Nephrol. 2009;4:1275–1283. [DOI] [PubMed] [Google Scholar]
- 60.Schetz M, Dasta J, Goldstein S, Golper T. Drug induced acute kidney injury. Curr Opin Crit Care. 2005;11:555–565. [DOI] [PubMed] [Google Scholar]
- 61.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334:115–124. [DOI] [PubMed] [Google Scholar]
- 62.Kimura H, Sakai Y, Fujii T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab Pharmacokinet. 2018;33:43–48. [DOI] [PubMed] [Google Scholar]
- 63.Hansell P, Welch WJ, Blantz RC, Palm F. Determinants of kidney oxygen consumption and their relationship to tissue oxygen tension in diabetes and hypertension. Clin Exp Pharmacol Physiol. 2013;40:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Evans RG, Gardiner BS, Smith DW, O’Connor PM. Intrarenal oxygenation: unique challenges and the biophysical basis of homeostasis. Am Physiol Renal Physiol. 2008;295:1259–1270. [DOI] [PubMed] [Google Scholar]
- 65.Yasotharan S, Pinto S, Sled JG, et al. Artery-on-a-chip platform for automated, multimodal assessment of cerebral blood vessel structure and function. Lab Chip. 2015;15:2660–2669. [DOI] [PubMed] [Google Scholar]
- 66.Cho S, Yoon J. Organ-on-a-chip for assessing environmental toxicants. Curr Opin Biotechnol. 2017;45:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maschmeyer I, Lorenz AK, Schimek K, et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip. 2015;15:2688–2699. [DOI] [PubMed] [Google Scholar]
- 68.Tumlin J Efficacy and safety of renal tubule cell therapy for acute renal failure. J Am Soc Nephrol. 2008;19:1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pino C, Westover A, Buffington D, Humes H. Bioengineered renal cell therapy device for clinical translation. ASAIO J. 2017;63:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Westover AJ, Buffington DA, Johnston KA, et al. A bio-artificial renal epithelial cell system conveys survival advantage in a porcine model of septic shock. J Tissue Eng Regen Med. 2017;11:649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tumlin JA, Galphin CM, Tolwani AJ, et al. A multi-center, randomized, controlled, pivotal study to assess the safety and efficacy of a selective cytopheretic device in patients with acute kidney injury. PLoS One. 2015;10:e0132482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anna SL. Droplets and bubbles in microfluidic devices. Ann Rev Fluid Mech. 2016;48:285–309. [Google Scholar]
- 73.Stone HA, Stroock AD, Ajdari A. Engineering flows in small devices: microfluidic toward a lab-on-a-chip. Ann Rev Fluid Mech. 2004;36:381. [Google Scholar]
- 74.Shin SR, Kilic T, Zhang YS, et al. Label-free and regenerative electrochemical microfluidic biosensors for continual monitoring of cell secretomes. Adv Sci (Weinh). 2017;4:1600522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Henry OYF, Villenave R, Cronce MJ, et al. Organs-on-chips with integrated electrodes for trans-epithelial electrical resistance (TEER) measurements of human epithelial barrier function. Lab Chip. 2017;17:2264–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oedit A, Vulto P, Ramautar R, et al. Lab-on-a-chip hyphenation with mass spectrometry: strategies for bioanalytical applications. Curr Opin Biotechnol. 2015;31:79–85. [DOI] [PubMed] [Google Scholar]
- 77.Maoz BM, Herland A, Henry OYF, et al. Organs-on-chips with combined multi-electrode array and transepithelial electrical resistance measurement capabilities. Lab Chip. 2017;17:2294–2302. [DOI] [PubMed] [Google Scholar]
- 78.Ashammakhi N, Kaarela O. Stimuli-responsive biomaterials: next wave. J Craniofac Surg. 2017;28:1647–1648. [DOI] [PubMed] [Google Scholar]
- 79.Lu Y, Aimetti AA, Langer R, Gu Z. Bioresponsive materials. Nat Rev Mater. 2016;2:16075. [Google Scholar]
- 80.Hasan A, Nurunnabi M, Morshed M, et al. Recent advances in application of biosensors in tissue engineering. Biomed Res Int. 2014;2014:307519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wikswo JP. Looking to the future of organs-on-chips: interview with Professor John Wikswo. Future Sci OA. 2017;3:FSO163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang J, Rodriguez V, Xu F, Li XJ. Stem cell culture and differentiation in microfluidic devices towards organ-on-a-chip. Future Sci OA. 2017;3:FSO187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luni C, Giulitti S, Serena E, et al. High-efficiency cellular reprogramming with microfluidics. Nat Methods. 2016;13:446–452. [DOI] [PubMed] [Google Scholar]
- 84.Ma X, Qu X, Zhu W, et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc Nat Acad Sci. 2016;113:2206–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu J, Hwang HH, Wang P, et al. Direct 3D-printing of cell-laden constructs in microfluidic architectures. Lab Chip. 2016;16:1430–1438. [DOI] [PubMed] [Google Scholar]
- 86.Tumbleston JR, Shirvanyants D, Ermoshkin N, et al. Additive manufacturing: continuous liquid interface production of 3D objects. Science. 2015;347:1349. [DOI] [PubMed] [Google Scholar]
- 87.Tibbits S 4D printing: multi-material shape change. Arch Design. 2014;84:116–121. [Google Scholar]
- 88.Li YC, Zhang YC, Akpek YS, et al. 4D bioprinting: the next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication. 2016;9:012001. [DOI] [PubMed] [Google Scholar]
- 89.Gladman AS, Matsumoto EA, Nuzzo RG, et al. Biomimetic 4D printing. Nat Mater. 2016;15:413. [DOI] [PubMed] [Google Scholar]
- 90.Huh DD. A human breathing lung-on-a-chip. Ann Am Thorac Soc. 2015;12(suppl 1):S42–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Banaeiyan AA, Theobald J, Paukštyte J, et al. Design and fabrication of a scalable liver-lobule-on-a-chip microphysiological platform. Biofabrication. 2017;9:015014. [DOI] [PubMed] [Google Scholar]
- 92.Ribas J, Zhang YS, Pitrez PR, et al. Biomechanical strain exacerbates inflammation on a progeria-on-a-chip model. Small. 2017;13(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang YS, Davoudi F, Walch P, et al. Bioprinted thrombosis-on-a-chip. Lab Chip. 2016;16:4097–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu Z, Li E, Guo Z, et al. Design and construction of a multi-organ microfluidic chip mimicking the in vivo microenvironment of lung cancer metastasis. ACS Appl Mater Interfaces. 2016;8:25840–25847. [DOI] [PubMed] [Google Scholar]
- 95.Yoon HJ, Shanker A, Wang Y, et al. Tunable thermal-sensitive polymergraphene oxide composite for efficient capture and release of viable circulating tumor cells. Adv Mater. 2016;28:4891–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]