Abstract
Bullous pemphigoid is an autoimmune subepidermal blistering skin disease immunologically defined by autoantibodies directed against basement membrane zone antigens, the main of which is BP180. Laboratory tests are essential for diagnosis and include direct immunofluorescence and serologic assessments with indirect immunofluorescence and ELISA. Serology may be performed on blister fluid, in alternative to blood serum. This study investigated the use of a Biochip-based indirect immunofluorescence approach for the serum diagnosis of bullous pemphigoid on blister fluid. We compared the results using the Biochip-method with the ELISA detection of bullous pemphigoid-180 autoantibodies in blister fluid and observed a perfect correlation between these 2 methods in our group of 13 patients with clinical and direct immunofluorescence diagnosis of bullous pemphigoid. The Biochip is a simple, standardized and inexpensive diagnostic tool and its use on blister fluid may facilitate the diagnosis of this and other autoimmune bullous disorders. Our results suggest that the Biochip assay on serum of bullae is a non-invasive screening technique for the early diagnosis of bullous pemphigoid that is practical for fragile elderly patients and achievable even in small laboratory settings.
Keywords: bullous disorders, pemphigoid, skin immunology
1. Introduction
Bullous pemphigoid (BP) is the most common bullous autoimmune disease and is recognized as the prototype of subepidermal autoimmune blistering disorders (SABD). BP, like pemphigus vulgaris, occurs in older adults more commonly than in younger individuals with typical onset between 60 and 80 years of age.[1]
Tense blisters are a characteristic feature of SABD, owing to basement membrane zone disruption and consequent splitting of the skin. SABD are immunologically characterized by the presence of autoantibodies directed against basement membrane zone antigens and BP is defined by IgG antibodies against bullous pemphigoid antigen 180 (BP180), the most common antigenic target in the disease, and bullous pemphigoid antigen 230 (BP230).
When BP is suspected, an accurate diagnostic approach is essential to rule out differential diagnoses, as blistering disorders share clinical presentation. Laboratory diagnosis relies on direct immunofluorescence (DIF), which examines linear antibody or complement deposition at the basement-membrane zone of the skin on a biopsy sample, and serologic tests, namely indirect immunofluorescence (IIF) studies and ELISA, for the detection of circulating antibodies on the serum.
DIF is considered the gold standard for diagnosis and should be performed on a skin biopsy from perilesional tissue.[2] Serologic studies provide additional information that is useful for diagnosis and therapeutic management in most patients.[3]
Furthermore, serum testing combining the 2 techniques of IIF and ELISA supports a clinical diagnosis of BP when DIF is negative in a patient with clinical and histopathologic findings that are consistent with bullous pemphigoid.[4]
BP is prevalent in elderly adults and, according to the experience of the authors, is most common in very old people. Such a subset of patients stands out for a high prevalence of comorbidities that contraindicate surgical biopsy procedures. In these cases, serum testing procedures are ultimately essential when adequate biopsy tissue may not be obtained.[2]
It is accepted that serum testing in SABD may be performed on serum centrifuged from blood samples as well as on blister fluid. Autoantibodies, and other inflammatory mediators including interleukins and cytokines, are detected in blister fluid, a finding consistent with a localized inflammatory process.[5–7]
In 2004, Daneshpazhooh et al performed IIF on blister fluid to compare antibody titers with those of serum in patients with SABD.[8] The authors conducted serum testing on salt-split skin to enhance sensitivity to the test.[9,10] 88% (22 out of 25) BP patients were positive for IgG in both serum and blister fluid, with an equivalent IgG titer in 16 out of 22 patients and 1 or 2 dilutions lesser in the remaining patients. No significant difference between serum and blister fluid antibody titers (P > .05) emerged and the authors concluded that IIF sensitivity on blister fluid is no more than that on serum and that the blister fluid of patients with SABD can be used for diagnosis with IIF.
Antibody titer in blister fluid is equivalent or lesser than in serum since antibody production in BP takes place systemically and, subsequently, immunoglobulins diffuse locally to blister fluid. The performance of IIF on blister fluid as an alternative to serum has been previously proposed for the diagnosis of SABD[11] in patients with poor venous access, a feature common in the very elderly adults that are most frequently affected by BP.
The purpose of this study was to detect BP180 autoantibody in blister fluid from BP patients with poor venous access, not allowing for adequate blood samples, using Biochip-based IIF. To assess the performance of our Biochip assay, the results were compared to those obtained from ELISA.
A Biochip-based indirect immunofluorescence technique for the determination of BP180 autoantibodies has been recently described,[12] however, there are no studies concerning the use of blister fluid as substrate for this novel approach.
Commercially available ELISA antigen-specific serologic testing for BP are widely employed for the detection of circulating IgG against BP180 and BP230 in blood serum and their role in blister fluid detection of these autoantibodies has been demonstrated, with reported sensitivity of 61,5% for anti BP180 ELISA.[13] IgG BP180 antibody levels have been demonstrated to correlate with disease activity in BP,[14] and several clinicians also measure response to therapy with ELISA. Finally, assessing the reliability of testing on blister fluid may avoid consecutive blood withdrawals in fragile patients with poor venous access.
2. Methods
This pilot study included 13 patients of Caucasian ethnicity, 4 females and 9 males. The diagnosis of BP was established by positive DIF in patients with typical clinical phenotype and for which adequate venous access could not be obtained. Blister fluid was collected from each of the 13 individuals before initiating treatment and all sera were anonymized prior to testing. Analyses were conducted by experienced staff members of our Dermatology Unit, using both the Biochip antibody detection technology and target antigen-specific ELISA assay.
2.1. Biochip indirect immunofluorescence
Biochip assay is a test devised for in vitro detection of human antibodies and investigates, like other indirect immunofluorescence techniques, the presence of basement membrane zone antibodies in the serum of patients with BP. Unlike traditional indirect immunofluorescence, where components of an epithelial specimen that are not from the patient are applied to microscope slides, Biochip substrates are applied to thin glass slides that are mechanically cut into millimeter-sized fragments in a fashion comparable to the manufacture of microchips in the electronics industry. Such production method yields large standardized batches of substrate, with minimal variations in quality. Biochips are then glued side by side onto microscope slides employing automated assembly equipment. The small scale of Biochip fragments is suitable for the assembly of multiparametric Biochip mosaics for the simultaneous incubation of a single fluid sample on the required number of substrates and may be customized to include frozen tissue sections of human 1 M salt-split skin or monkey esophagus, desmoglein-1 and desmoglein-3 expressing cells, BP230-gC-expressing cells and recombinant tetrameric BP180-NC16A spots.[15]
Slides mounting the Biochips are first incubated with patient samples at 1:10 dilution for 30 minutes at room temperature, then rinsed and immersed in wash buffer solution for 5 minutes. In a second incubation step, fluorescein isothiocyanate (FITC)-conjugated goat anti-human IgA/G antibodies to detect bound antibodies are applied for 30 minutes at room temperature, followed by washing as before. Results are visually examined through conventional fluorescence microscopy. A positive biochip reaction occurs when specific antibodies attach to the tested antigens and subsequently become stained with fluorescein-labelled anti-human immunoglobulins (Euroimmun Italy, Padua, Italy)
The substrate for the detection of BP180 autoantibodies on blister fluid used in this study was recombinant tetrameric BP180-NC16A spotted on cover glass then fragmented into Biochips and was supplied by Euroimmun Italy (Fig. 1).
Figure 1.

Detection of BP180 autoantibodies using recombinant tetrameric BP180-NC16A. spotted on cover glass. A positive reactivity is characterized by rhomboidal-shaped fluorescent microdrops.
The extracellular membrane-proximal domain of BP180, which is designated as NC16A, has been recognized, using in vitro and in vivo models, to harbor target antigen sites of pathogenic autoantibodies of BP and is considered an appropriate substrate for the detection of BP180 antibodies.[16–18]
2.2. ELISA enzyme-linked immunosorbent assay
ELISA is currently the preferred technique for assessing basal membrane zone antibodies in the diagnosis of bullous pemphigoid and other blistering disorders.[19] ELISAs for BP antibodies are currently commercially available and detect circulating IgG against BP180, which is the most common antigenic target.
In this study, ELISA for the detection of anti-BP180 antibodies employed monomeric recombinant NC16A domain expressed as a GST fusion protein and was performed according to the manufacturer's instructions (“MBL Medical & Biological Laboratories Co., Ltd.”). The cut-off value for a positive result was set at 9 U/mL.[20]
The anti-BP180 ELISA test is a validated diagnostic tool, with reported sensitivity and specificity of 89.0% and 94.8%, respectively, and is also used to support treatment decisions as antibody levels correlate with disease activity, especially in patients who are free from skin lesions. In 2007 a new ELISA system using recombinant tetrameric NC16A domains was developed, showing comparable results, with 89.8% sensibility and 97.8% specificity.[21]
This was a retrospective archive study hence we did not seek ethical approval.
3. Results
Biochip detected autoantibodies reactive to BP180 in the blister fluid of 10 out of 13 patients with clinical suspect of bullous pemphigoid. The results paralleled those obtained by means of ELISA assay. Negative results with Biochip were observed in 3 cases out of 13 and correlated to low antibody titers on ELISA. The 2 alternative assays scored equivalent specificity of 76,9% and no discordant result was observed (Tables 1 and 2).
Table 1.
Results at the time of diagnosis. ELISA values are expressed as Unit/mL.
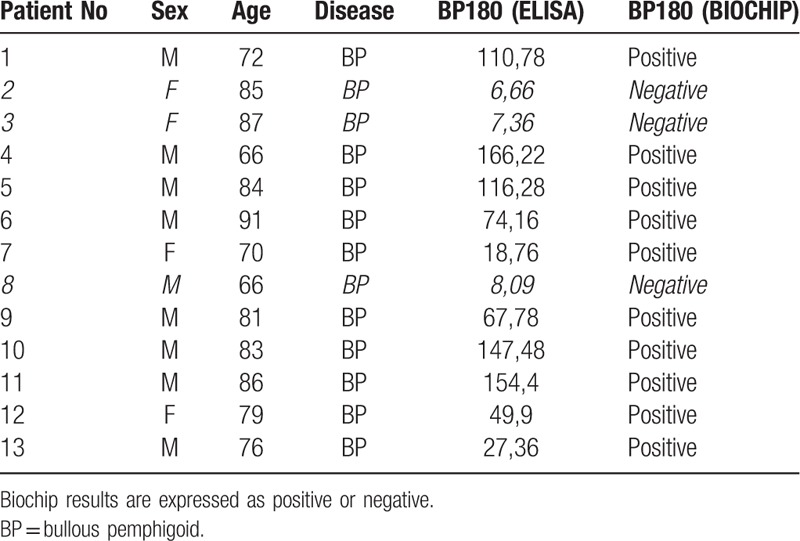
Table 2.
Summary of results in BP patients.

4. Discussion
The aim of this pilot study was to evaluate the potential diagnostic value of testing BP patient blister fluid with a Biochip-based indirect immunofluorescence technique for the detection of autoantibodies against BP180 and to assess its performance in relation to ELISA test results on the same samples. Blister fluid is a biologic sample that may be easily obtained, once or consecutively, from patients with BP and other cutaneous autoimmune blistering disorders. Serum components, which are produced systemically in SABD and enter blood circulation, diffuse to local sites of the inflammatory process so that autoantibodies and other inflammatory mediators may be detected in skin lesions.[5–7] This constitutes the basis for performing serum testing in SABD on centrifuged blister fluid, an alternative to blood samples.
We propose a non-invasive technique as a safe and tolerable test in elderly patients with poor venous access and for which a biopsy procedure may not be performed.
The use of biochip-based indirect immunofluorescence for the determination of BP180 autoantibodies has been previously described and the technique is proven to be a specific and sensitive alternative to ELISA testing in the serum diagnosis of BP.[12,22]
A previous study evaluated blister fluid from BP patients as substrate for IIF in comparison to serum diagnosis on blood samples,[8] but our investigation is the first in literature to use a Biochip approach on blister fluid samples. We also used the same biofluid samples to perform a direct comparison between the Biochip and ELISA, showing a perfect correlation of the results for BP180 autoantibodies in our group of 13 patients.
ELISA is currently the preferred technique for detecting basement membrane zone antibodies in SABD, as it holds numerous advantages: it is a readily reproducible method that allows simultaneous analysis of multiple samples in limited time and, notably, provides quantitative results. Levels of IgG BP180 NC16A antibody are known to correlate with disease activity and current clinical practice relies on ELISA not only to support diagnosis but also to measure response to therapy.[14] A drawback to the test is its high cost and false negative results due to the recombinant protein not containing all epitopes present in vivo.
Up to 10% of patients with BP are thought to bear antibodies to epitopes of BP180 and BP230 that are not recognized by commercially available ELISA tests. In support to this hypothesis is the observation of strong reactivity by immunoblot to BP180 epitopes outside of NC16A domain in patients testing negative to ELISA for BP180 NC16A.[23] This may explain the negative results scored by 3 patients of our study with ELISA and Biochip, being detection limited to antibody specificities within the NC16A domain.
Biochip technique may outdo the limitations of ELISA offering an alternative affordable even for a small laboratory, as the test is about 50% cheaper than ELISA and the execution is simple and fast, taking about 100 minutes. Incubation steps all take place at room temperature, reagent and serum consumption is 50 μL per test field and only standard fluorescence microscopy equipment is required for the visual interpretation of results.
Furthermore, unlike ELISA commercial kits that are specific for a single parameter, the Biochip approach is devised for the simultaneous detection of different antibody specificities on a panel of substrates in a single incubation procedure and a custom Biochip mosaic may be tailored according to requirements. For this reason, the test is suitable for initial screening in the diagnostic workup of BP and other SABDs, reserving ELISA to confirm doubtful results.
The main limitation of the Biochip approach is that it does not provide a quantitative result, although a semi-quantitative analysis may be performed measuring the highest of serial dilutions of the tested serum that still produces immunofluorescence.
5. Conclusions
In conclusion, our study suggests that a Biochip-based test on blister fluid is an appropriate initial approach to the diagnostic workup of patients with suspected BP. Our results, though the size of our sample was limited, support the use of blister fluid as an alternative to serum for IIF in fragile elderly patients with poor venous access. The Biochip IIF technique is proposed as a simple standardized approach to be readily implemented even in small labs. Finally, future immunologic studies testing Biochip mosaics on the bulla, the primary skin lesion of SABD, will provide new insight into the pathogenesis of bullous disorders.
Author contributions
Conceptualization: Mauro Alaibac.
Data curation: Alvise Sernicola, Irene Russo, Mauro Alaibac.
Formal analysis: Mauro Alaibac.
Funding acquisition: Mauro Alaibac.
Investigation: Alvise Sernicola, Andrea Saponeri, Mauro Alaibac.
Methodology: Alvise Sernicola, Irene Russo, Andrea Saponeri, Mauro Alaibac.
Supervision: Mauro Alaibac.
Validation: Irene Russo, Mauro Alaibac.
Writing – original draft: Alvise Sernicola, Irene Russo, Mauro Alaibac.
Footnotes
Abbreviations: BP = bullous pemphigoid, BP180 = bullous pemphigoid antigen 180, BP230 = bullous pemphigoid antigen 230, DIF = direct immunofluorescence, IIF = indirect immunofluorescence, SABD = subepidermal autoimmune blistering disorders.
The authors have no conflicts of interest to disclose.
References
- [1].Wolff K, Johnson RA, Saveedra AP. Sydor A, Davis K. Genetic acquired bullous diseases. Fitzpatrick's color atlas and synopsis of clinical dermatology 7th EditionNew York, NY, USA: McGraw-Hill Education, LLC; 2013. 94–115. [Google Scholar]
- [2].Sardy M, Kostaki D, Varga R, et al. Comparative study of direct and indirect immunofluorescence and of bullous pemphigoid 180 and 230 enzyme-linked immunosorbent assays for diagnosis of bullous pemphigoid. J Am Acad Dermatol 2013;69:748–53. [DOI] [PubMed] [Google Scholar]
- [3].Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev 2010;10:84–9. [DOI] [PubMed] [Google Scholar]
- [4].Chan YC, Sun YJ, Ng PPL, et al. Comparison of immunofluorescence microscopy, immunoblotting and enzyme-linked immunosorbent assay methods in the laboratory diagnosis of bullous pemphigoid. Clin Exp Dermatol 2003;28:651–6. [DOI] [PubMed] [Google Scholar]
- [5].Ameglio F, D’Auria L, Bonifati C, et al. Cytokine pattern in blister fluid and serum of patients with bullous pemphigoid: Relationships with disease intensity. Br J Dermatol 1998;138:611–4. [DOI] [PubMed] [Google Scholar]
- [6].Bhol KC, Rojas AI, Khan IU, et al. Presence of interleukin 10 in the serum and blister fluid of patients with pemphigus vulgaris and pemphigoid. Cytokine 2000;12:1076–83. [DOI] [PubMed] [Google Scholar]
- [7].Sun CC, Wu J, Wong TT, et al. High levels of interleukin-8, soluble CD4 and soluble CD8 in bullous pemphigoid blister fluid. The relationship between local cytokine production and lesional T-cell activities. Br J Dermatol 2000;143:1235–40. [DOI] [PubMed] [Google Scholar]
- [8].Daneshpazhooh M, Shahdi M, Aghaeepoor M, et al. A comparative study of antibody titers of blister fluid and serum in patients with subepidermal immunobullous diseases. Int J Dermatol 2004;43:348–51. [DOI] [PubMed] [Google Scholar]
- [9].Kelly SE, Wojnarowska F. The use of chemically split tissue in the detection of circulating anti-basement membrane zone antibodies in bullous pemphigoid and cicatricial pemphigoid. Br J Dermatol 1988;118:31–40. [DOI] [PubMed] [Google Scholar]
- [10].Gammon WR, Briggaman RA, Inman AO, et al. Differentiating anti-lamina lucida and anti-sublamina densa anti-BMZ antibodies by indirect immunofluorescence on 1.0 M sodium chloride-separated skin. J Invest Dermatol 1984;82:139–44. [DOI] [PubMed] [Google Scholar]
- [11].Zhou S, Wakelin SH, Allen J, et al. Blister fluid for the diagnosis of subepidermal immunobullous diseases: a comparative study of basement membrane zone autoantibodies detected in blister fluid and serum. Br J Dermatol 1998;139:27–32. [DOI] [PubMed] [Google Scholar]
- [12].Zarian H, Saponeri A, Michelotto A, et al. Biochip Technology for the Serological Diagnosis of Bullous Pemphigoid. ISRN Dermatol 2012;2012:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Patsatsi A, Vyzantiadis TA, Devliotou-Panagiotidou D, et al. Detection of anti-BP180NC16a and anti-BP230 autoantibodies in blister fluid of patients with bullous pemphigoid: the first survey in Greece. Clin Exp Dermatol 2008;33:183–5. [DOI] [PubMed] [Google Scholar]
- [14].Schmidt E, Obe K, Bröcker EB, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol 2000;136:174–8. [DOI] [PubMed] [Google Scholar]
- [15].Euroimmun Italia. Reagenti per la diagnostica medica di laboratorio [Internet]. 2018. Available from: http://www.euroimmun.it Accessed June 29, 2018. [Google Scholar]
- [16].Sitaru C, Schmidt E, Petermann S, et al. Autoantibodies to bullous pemphigoid antigen 180 induce dermal-epidermal separation in cryosections of human skin. J Invest Dermatol 2002;118:664–71. [DOI] [PubMed] [Google Scholar]
- [17].Herrero-González JE, Brauns O, Egner R, et al. Immunoadsorption against two distinct epitopes on human type XVII collagen abolishes dermal-epidermal separation induced in vitro by autoantibodies from pemphigoid gestationis patients. Eur J Immunol 2006;36:1039–48. [DOI] [PubMed] [Google Scholar]
- [18].Liu Z, Diaz LA, Swartz SJ, et al. Molecular mapping of a pathogenically relevant BP180 epitope associated with experimentally induced murine bullous pemphigoid. J Immunol 1995;155:5449–54. [PubMed] [Google Scholar]
- [19].Tampoia M, Giavarina D, Di Giorgio C, et al. Diagnostic accuracy of enzyme-linked immunosorbent assays (ELISA) to detect anti-skin autoantibodies in autoimmune blistering skin diseases: a systematic review and meta-analysis. Autoimmun Rev 2012;12:121–6. [DOI] [PubMed] [Google Scholar]
- [20].MBL Medical & Biological Laboratories Co., Ltd. [Internet]. 2018. Available from: http://www.mbl.co.jp/e/ Accessed June 29, 2018. [Google Scholar]
- [21].Sitaru C, Dähnrich C, Probst C, et al. Enzyme-linked immunosorbent assay using multimers of the 16th non-collagenous domain of the BP180 antigen for sensitive and specific detection of pemphigoid autoantibodies. Exp Dermatol 2007;19:770–7. [DOI] [PubMed] [Google Scholar]
- [22].Van Beek N, Rentzsch K, Probst C, et al. Serological diagnosis of autoimmune bullous skin diseases: prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis 2012;7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fairley JA, Bream M, Fullenkamp C, et al. Missing the target: characterization of bullous pemphigoid patients who are negative using the BP180 enzyme-linked immunosorbant assay. J Am Acad Dermatol 2013;68:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]


