Abstract
The risk of thromboembolism in patients with CHA2DS2-VASc score of 0 to 1 was low, and the anticoagulant therapy was not recommended. Although the CHA2DS2-VASc score was low, there were still many patients suffered from thrombotic events and stroke. We aim to investigate the risk factors of thrombotic events in nonvalvular atrial fibrillation (NVAF) patients with low CHA2DS2-VASc score.
We retrospectively enrolled 595 consecutive NVAF patients with low CHA2DS2-VASc score (male: CHA2DS2-VASc = 0, female: CHA2DS2-VASc = 1). The general clinical data, blood biochemical data, and echocardiography results of the 595 patients were collected. Multivariate logistic regression models were used to evaluate risk factors of thrombosis. Receiver operating characteristic curve was used to identify the optimal cut-off value of the independent risk factors. A P value of <.05 (2-sided) was considered to be statistically significant.
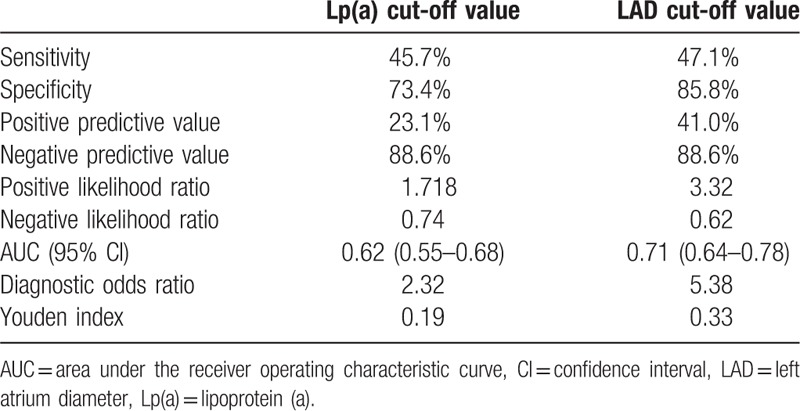
In multivariate analysis, lipoprotein (a) (Lp(a)) plasma level and left atrium diameter (LAD) were positively related to thromboembolism in NVAF patients with CHA2DS2-VASc score of 0 to 1 after adjustment for age, gender, and other variables (odds ratio [OR] = 1.02, 95% confidence interval [CI]: 1.01–1.03; OR = 1.13, 95% CI: 1.06–1.18). Lp(a) exerted a significant predictive value with area under the curve (AUC) of 0.62 (95% CI: 0.55–0.68, P < .01). The optimal cut-off value for Lp(a) predicting thrombotic events was 27.2 mg/dL (sensitivity 45.7%, specificity 73.4%). LAD showed a significant predictive value with AUC of 0.71 (95% CI: 0.64–0.78, P < .01). The optimal cut-off point for LAD predicting thrombotic events was 43.5 mm (sensitivity 47.1%, specificity 85.8%).
High Lp(a) plasma level and left atrial dilatation might be independent risk factors of thrombotic events for NVAF patients with low CHA2DS2-VASc score.
Keywords: CHA2DS2-VASc score, nonvalvular atrial fibrillation, thromboembolism
1. Introduction
Atrial fibrillation (AF), the most common sustained cardiac arrhythmia in clinical practice, can result in a series of adverse outcomes. Thromboembolism is one of the most serious complications to increase mortality and disability in AF patients. Nonvalvular atrial fibrillation (NVAF) increases the risk of ischemic stroke by 5.6 times.[1] Guidelines for the management of patients with AF had recommended that assessment of thrombotic risk in patients with NVAF should be based on the CHA2DS2-VASc scoring system.[2] According to the guidelines, NVAF patients with CHA2DS2-VASc score of 0 to 1, defined as low thrombotic risk, were not necessary to take antithrombotic therapy.
Although low CHA2DS2-VASc score indicated low risk of thrombosis, there were still patients suffering from thromboembolism. A large-scale cohort study in Danish showed that the annual risk of ischemic stroke was 0.64% for NVAF patients with a CHA2DS2-VASc score of 0 in the absence of anticoagulants.[3] Even in some studies, the annual incidence of stroke in Asia's AF population with CHA2DS2-VASc score of 0 was significantly higher than in the West.[4,5] In recent years, some studies found that the CHA2DS2-VASc scoring system was not comprehensive prediction for thrombotic risk in NVAF patients, there were still other clinical factors associated with the risk of thrombotic events.[6,7] In a cohort study of 15,806 patients with AF in Taiwan, the age threshold was reset. It was suggested that age younger than 50 years was a true low risk.[8] Therefore, retrospective analysis of 595 NVAF patients’ clinical data were made to identify the potential risk factors for thrombotic events in NVAF patients with CHA2DS2-VASc score of 0 to 1, and to help guiding clinical practice. The innovation of this study was to limit the NVAF population with CHA2DS2-VASc score of 0 to 1, which further purified the clinical factors.
2. Methods
2.1. Study population
The study received approval from the Medical Ethics Committee of the Second Affiliated Hospital of Nanchang University. And the informed consent was waived because of the retrospective nature of this study.
We retrospectively screened consecutive NVAF patients who were admitted to the Second Affiliated Hospital of Nanchang University between January 1, 2009 and December 31, 2016. Then we graded them with CHA2DS2-VASc system. Considering the CHA2DS2-VASc score and first-onset thromboembolism, we divided the study population into thrombotic events group and control group. Enrolled patients had no long-term medication history before admission. The control group included the NVAF patients with CHA2DS2-VASc score of 0 to 1 (male: CHA2DS2-VASc = 0, female: CHA2DS2-VASc = 1). While the NVAF patients with previous CHA2DS2-VASc score of 0 to 1 hospitalized for first-onset thromboembolism, were enrolled in thrombotic events group. All selected patients were older than 18. Those who met one of the following conditions were excluded: diagnosed with Structural Heart Disease, thyroid disorder, kidney failure, chronic obstructive pulmonary disease; CHA2DS2-VASc score ≥1 in male, CHA2DS2-VASc score ≥2 in female; and with incomplete data.
2.2. Assessment of thrombotic events
The diagnosis of acute ischemic stroke was based on the diagnostic criteria developed by the American Heart Association and the American Stroke Association: acute onset, focal neurological deficit (one side of the face or limb weakness or numbness, language disorders, and other symptoms), a few were neurological deficits, there were responsible ischemic lesions in head recorded by computed tomography (CT) or magnetic resonance imaging (MRI), vascular causes were excluded, and cerebral hemorrhage was excluded by CT or MRI.[9]
Peripheral arterial embolism includes limb arterial embolism, mesenteric artery embolization, renal artery embolization, and splenic artery embolization. The diagnostic criteria of peripheral arterial embolism are based on “Guidelines for the diagnosis and treatment of peripheral arterial disease”[10] and could be proved by CT angiography or magnetic resonance angiography. Left atrial thrombus was diagnosed by transesophageal echocardiography and/or left atrial CT imaging.
2.3. Data collection
Subjects’ general clinical data including gender, age, history of hyperlipidemia, history of smoking, history of drinking, systolic blood pressure, diastolic blood pressure, heart rate, and urinary protein positive rate on admission. Subjects’ echocardiographic data and laboratory test results on empty stomach at the second day of admission were collected.
2.4. Statistical analysis
Statistical analysis was performed using Empower stats and SPSS version 19.0. The baseline data were described as mean ± standard deviation 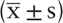 for continuous variables and as percentages for categorical variables. Continuous variables were compared using the Student t test and categorical variables were compared using chi-squared test. Multivariate logistic regression models were used to evaluate risk factors of thrombosis. The receiver operating characteristic (ROC) curve was used to evaluate the ability of related risk factors to predict thromboembolism and to identify the optimal cut-off value, and the area under the curve (AUC) was used to determine the predictive value. A P value of <.05 (2-sided) was considered statistically significant in all analyses.
for continuous variables and as percentages for categorical variables. Continuous variables were compared using the Student t test and categorical variables were compared using chi-squared test. Multivariate logistic regression models were used to evaluate risk factors of thrombosis. The receiver operating characteristic (ROC) curve was used to evaluate the ability of related risk factors to predict thromboembolism and to identify the optimal cut-off value, and the area under the curve (AUC) was used to determine the predictive value. A P value of <.05 (2-sided) was considered statistically significant in all analyses.
3. Results
3.1. Baseline characteristics
A total of 595 participants with an average age of 55.1 years (standard deviation, 8.5 years) were enrolled. There were 68 NVAF patients included in the thrombotic events group, with previous CHA2DS2-VASc score of 0 to 1 hospitalized for first-onset thromboembolism. And there were 527 NVAF patients with CHA2DS2-VASc score of 0 to 1 in the control group. The incidence of thrombotic events was 11.4%, including 51 males and 17 females. Of the participants in nonthrombotic events group, 68.9% were male. There were no patients with congestive heart failure, hypertension, age ≥65 years, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease.
In the thrombotic events group, there were 49 cases of acute cerebral ischemic stroke, lower limb artery embolization in 3 cases, renal artery embolization in 3 cases, 3 cases of splenic artery embolism (including renal artery combined splenic artery embolization in 1 case), mesenteric artery embolism in 1 case, and 10 cases of left atrial thrombus.
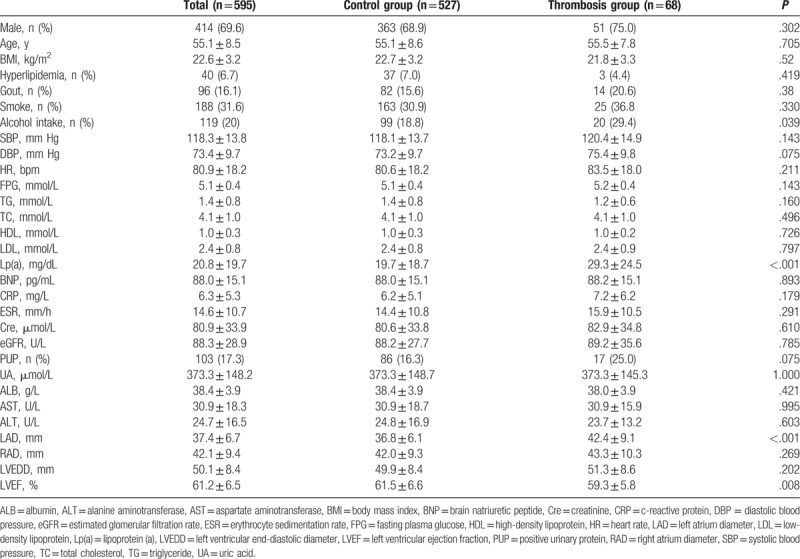
The comparisons of the baseline characteristics in the thrombosis group with control group are shown in Table 1. Compared with control group, the alcohol use rate (20, 29.4% vs. 99, 18.8%, P = .039), lipoprotein (a) (Lp(a)) plasma level (29.3 ± 24.5 mg/dL vs. 19.7 ± 18.7 mg/dL, P < .001), and left atrium diameter (LAD) (42.4 ± 9.1 mm vs. 36.8 ± 6.1 mm, P < .001) were significantly higher in the thrombotic event group. However, the LVEF (59.3 ± 5.8% vs. 61.5 ± 6.6%, P = .008) were obviously lower than in control group. Meanwhile, there was no significant difference in systolic blood pressure, heart rate, history of hyperlipidemia, history of smoking, FPG, plasma TG level, TC level, Cre level, left ventricular end-diastolic diameter, and other parameters in the 2 groups (Table 1).
Table 1.
Comparison of baseline characteristics in control group with thrombosis group.
3.2. Risk factors of thrombotic events in NVAF patients
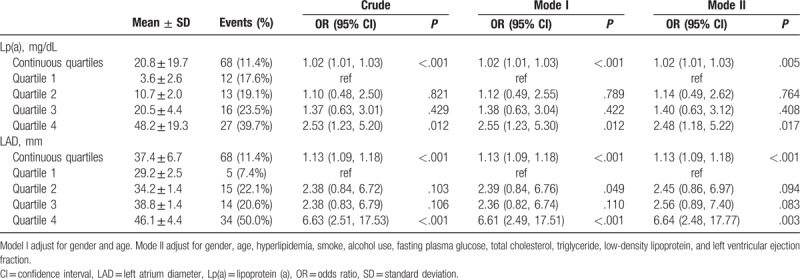
To investigate the risk factors of thrombotic events in NVAF patients with CHA2DS2-VASc score of 0 to 1, multivariate regression analysis was used to evaluate the effects of the related factors on thrombosis. The results showed that the odds of Lp(a) plasma level and LAD were positively related to thrombosis, and these relationships remained statistically significant after adjustment for age, gender, and various other baseline parameters (Table 2). They were independent predictors of thrombotic events after adjusting for other covariables. The parameters not significantly associated with thrombotic events were not listed.
Table 2.
Multivariate logistic analysis of thrombotic events with plasma Lp(a) level and LAD.
3.2.1. Multivariate logistic analysis of thrombotic events with plasma Lp(a)
Table 2 shows the effect of plasma Lp(a) level on thrombosis in the multivariate regression analysis. Continuous plasma Lp(a) level was positively associated with thrombosis (odds ratio [OR] = 1.02, 95% confidence interval [CI]: 1.01–1.03). The incidence of thrombotic events was significantly higher in highest Lp(a) quartile than the lowest quartile. Compared with the lowest quartile (3.6 ± 2.6 mg/dL), subjects in highest Lp(a) quartile (48.2 ± 19.3 mg/dL) had a higher risk of thrombotic events (OR = 2.48, 95% CI: 1.18–5.22) after adjusting for other covariables. There was no significant difference in thrombotic risk among the other 3 quartiles.
3.2.2. Multivariate logistic analysis of thrombotic events with LAD
The effect of LAD on thrombosis in the multivariate regression analysis is shown in Table 2. Both continuous and categorical LAD were significantly associated with thrombotic events. Continuous LAD was positively associated with thrombotic events (OR = 1.13, 95% CI: 1.09–1.18). Thrombotic risk was significantly higher in highest LAD quartile (46.1 ± 4.4 mm) compared with the lowest quartile (29.2 ± 2.5 mm) after adjusting for age, gender, and various other parameters (OR = 6.64, 95% CI: 2.48–17.77). Subjects in the second LAD quartile and third LAD quartile did not have significant different incidences compared with the lowest quartile (Table 2).
3.3. Lp(a) and LAD in predicting thrombotic events
Multivariable logistic regression analysis showed that hyper-Lp(a) and left atrial dilatation were thrombotic risk factors for NVAF patients with CHA2DS2-VASc score of 0 to 1. Compared with the lowest quartile, subjects in highest Lp(a) and LAD quartile had a higher risk of thrombotic events after adjusting for other covariables. To identify the optimal cut-off value, we used ROC curve to evaluate the ability of Lp(a) and LAD to predict thromboembolism.
On the basis of ROC curve analysis in Fig. 1, Lp(a) exerted a significant predictive value with AUC of 0.62 (95% CI: 0.55–0.68, P < .01). And the optimal cut-off value for Lp(a) predicting thrombotic events was 27.2 mg/dL, with a sensitivity of 45.7% and a specificity of 73.4%. The incidence of thrombosis in patients with Lp(a) ≥ 27.2 mg/dL was 18.50%, which was significantly higher than the 8.7% in Lp(a) < 27.2 mg/dL patients. The diagnostic efficacy of Lp(a) cut-off value was shown in Table 3, including sensitivity, specificity, positive and negative predictive value, positive and negative likelihood ratio, area under the ROC curve, diagnostic OR, and Youden index.
Figure 1.
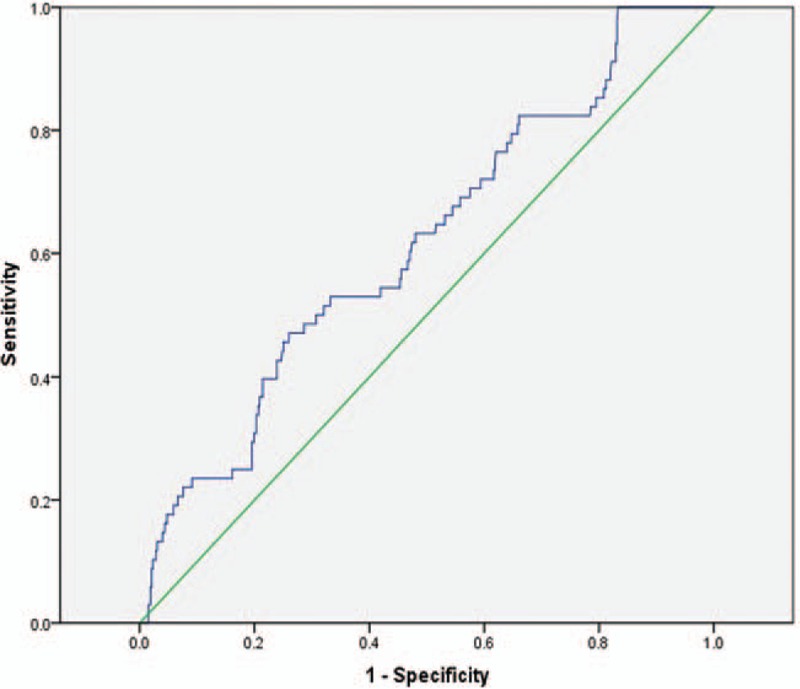
Receiver operating characteristic working curve of lipoprotein (a).
Table 3.
The diagnostic efficacy of Lp(a) cut-off value and LAD cut-off value.
According to the ROC curve analysis in Fig. 2, LAD showed a significant predictive value with AUC of 0.71 (95% CI: 0.64–0.78, P < .01). And the optimal cut-off value for LAD predicting thrombotic events was 43.5 mm, with a sensitivity of 47.1% and a specificity of 85.8%. The incidence of thrombosis in patients with LAD ≥ 43.5 mm was 29.9%, which was significantly higher than 7.4% in patients with LAD < 43.5 mm. The diagnostic efficacy of LAD cut-off value was shown in Table 3.
Figure 2.
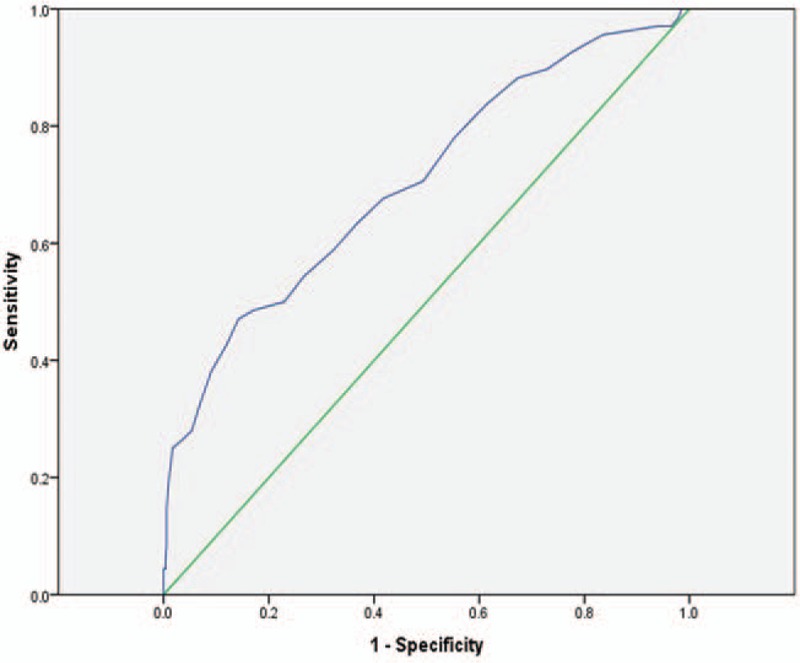
Receiver operating characteristic working curve of left atrial diameter.
4. Discussion
AF is the most frequent cardiac arrhythmia in clinical practice. It occurs when a diffuse and chaotic pattern of electrical activity in the atria replaces the normal sinus rhythm.[11,12] In a broad clinical context, NVAF is associated with consequent diastolic dysfunction and heart failure secondary to hypertension, coronary artery disease, myocarditis, aging, obesity, and diabetes mellitus.[13–16] As the incidence of NVAF is increasing year by year,[13,17] embolism complications is becoming a growing burden for healthcare systems. It was suggested that NVAF patient with a CHA2DS2-VASc score of 0 to 1 has a “real low risk” of thromboembolism. It was not necessary to take anticoagulant therapy for NVAF patient with a score of 0 to 1.[2] However, the present study found that the incidence of thrombotic events was 11.4% in NVAF patients with CHA2DS2-VASc score of 0 to 1. It indicated that there was still a high incidence of thrombotic events in NVAF patients with CHA2DS2-VASc score of 0 to 1, who were considered as low risk of thrombosis in clinical. Therefore, the further research on the risk factors of NVAF has a great clinical significance. We found that Lp(a) plasma level and LAD were positively related to thrombosis in NVAF patients with CHA2DS2-VASc score of 0 to 1 after adjustment for age, gender, and other parameters.
4.1. Association between Lp(a) level and thrombotic events
Lp(a) is a special form of low-density lipoprotein (LDL), composed of apoB in LDL-like particle and apolipoprotein (a) (apo[a]), connected by a single disulfide bond. Lp(a) possesses structural homology with plasminogen.[18] Epidemiological surveys showed that Lp(a) was closely related to cardiovascular disease. Lp(a) was also considered to be an independent risk factor for coronary heart disease.[19] As early as 1987, Jurgens and Koltringer[20] found that the Lp(a) was significantly different between patients with ischemic cerebrovascular disease and the healthy control group. Then, many studies found that Lp(a) was a risk factor for ischemic stroke.[21,22] In 2017, Lange et al[23] found that ischemic stroke patients with elevated Lp(a) had a higher risk of recurrence of vascular events in adult population. However, some studies still suggested that Lp(a) was meaningless in predicting ischemic stroke risk.[24–26] Even some large prospective studies had shown that elevated Lp(a) forecast stroke risk in men only.[27,28] Lp(a) is still controversial in predicting stroke, and the conclusions of each study are dissimilar.
Due to the structural homology, Lp(a) could compete with plasminogen on the fibrin binding sites, inhibit plasminogen activity, and reduce the vitality of the fibrinolysis system after thrombosis, which eventually weakened the fibrinolysis. Besides, Lp(a) could promote the secretion of plasminogen activator inhibitor-1 in vascular endothelial cells and hepatocytes, which caused plasma fibrinolysis and coagulation system imbalance. These could make the coagulation function prevail and promote thrombosis.[29,30] Furthermore, Lp(a) could bind heparin and heparan sulfate, which closed the substrate, causing its inhibition of thrombosis inactivation.[31] It suggested that elevated Lp(a) could facilitate coagulation-fibrinolytic system during thrombosis.
To define the relationship between Lp(a) and thrombotic events in the NVAF patients, we first screened the NVAF patients with low CHA2DS2-VASc score, and targeted the risk factors outside the CHA2DS2-VASc score range. The results showed that elevated Lp(a) was an independent risk factor for thrombotic events in NVAF patients with CHA2DS2-VASc score of 0 to 1. Patients in the fourth quartile of the Lp(a) distribution had an increased risk for thromboembolism compared with patients in the first 3 quartiles of the Hcy distribution.
4.2. Association between LAD and thrombotic events
Researches had proved that LAD enlargement was a risk factor for AF occurrence, recurrence, and even death events.[32] However, it was controversial whether LAD increasing thrombotic risk in AF patients. Some studies suggested that LAD enlargement had no significant association with thromboembolism in AF patients.[33,34] In the subgroup of the AFFIRM study,[19] researchers did not think LAD was a risk factor for embolism in AF patients. It could be explained by the reason that the study population excluded patients who had increased LAD and part of patients had oral anticoagulants. Recently, Hamatani et al[35] found that AF patients with the LAD > 45 mm had a higher stroke rate in the AF study. The experiment suggested that the enlargement of LAD was a risk factor for the embolism in AF patients. But the LAD expansion group was significantly higher than the control group in the age, course of AF, the proportion of nonparoxysmal AF, CHA2DS2-VASc score, and the use of oral anticoagulants, which were the defects of AF study. Therefore, its conclusions are restricted. It still needs to be confirmed that whether stroke risk is achieved by LAD expansion.
The present study screened the NVAF patients with CHA2DS2-VASc score of 0 to 1, which controlled the risk factors in CHA2DS2-VASc scoring system. Importantly, our study confirmed that LAD enlargement was an independent risk factor for thrombotic events in NVAF patients with low CHA2DS2-VASc score. Thrombotic risk was significantly higher in highest LAD quartile (46.1 ± 4.4 mm) compared with the lowest quartile (29.2 ± 2.4 mm) after adjusting for age, gender, and various other parameters. It implied that left atrium dilatation could increase thrombotic risk in NVAF patients, which might be associated with hemodynamic disorders, decreased blood flow velocity in left atrial appendage, decline of left ventricular compliance, dysfunction of vascular endothelial and excessive activation of coagulation system.[36,37] On the other hand, left atrium dilatation increased the eddy currents caused by left atrium's irregular contraction in AF patients, which would damage the atrial intima and then cause wall thrombosis.[38]
4.3. Limitations of the study
The study was a retrospective single center analysis. There was controversy over the fact that whether above variables were causes or consequences. Most of the selected cases were the serious patients in clinical disease spectrum, so there was a certain choice bias. Moreover, there were still some shortages such as racial specificity of Lp(a) plasma concentration and the small sample size.
4.4. Future directions
In the future, we need prospective multicenter cohort studies for comprehensive analysis to identify the potential risk factors for thrombotic events in NVAF patients, and to help guiding clinical practice.
5. Conclusion
Our results showed that Lp(a) plasma level and LAD were positively related to thromboembolism in NVAF patients with CHA2DS2-VASc score of 0 to 1 after adjustment for age, gender, and other variables. High Lp(a) plasma level and left atrial dilatation might be independent risk factors of thrombotic events for NVAF patients with low CHA2DS2-VASc score.
Author contributions
Conceptualization: Sujuan Yan, Juxiang Li, Xiaoshu Cheng.
Data curation: Shuangbing Yan, Yichun Wei.
Formal analysis: Shuangbing Yan.
Investigation: Zhen Xia, Juxiang Li.
Methodology: Qing Li, Juxiang Li.
Project administration: Sujuan Yan.
Resources: Yanqing Wu.
Software: Shuangbing Yan.
Supervision: Kui Hong, Juxiang Li.
Validation: Qing Li, Juxiang Li, Xiaoshu Cheng.
Writing – original draft: Shuangbing Yan.
Writing – review & editing: Qing Li.
Qing Li orcid: 0000-0002-7795-481X.
Footnotes
Abbreviations: AF = atrial fibrillation, AUC = area under the curve, CI = confidence interval, CT = computed tomography, LAD = left atrium diameter, LDL = low-density lipoprotein, Lp(a) = lipoprotein (a), MRI = magnetic resonance imaging, NVAF = nonvalvular atrial fibrillation, OR = odds ratio, ROC = receiver operating characteristic.
SY and QL have contributed equally to this work.
This study was supported by funds from the National Natural Science Foundation of China (No. 81200132) and the Major Project of Jiangxi Provincial Natural Science Foundation (No. 20152ACB20025).
The authors have no conflicts of interest to disclose.
References
- [1].Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991;22:983–8. [DOI] [PubMed] [Google Scholar]
- [2].Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg 2016;50:e1–88. [DOI] [PubMed] [Google Scholar]
- [3].Taillandier S, Olesen JB, Clementy N, et al. Prognosis in patients with atrial fibrillation and CHA2DS2-VASc score = 0 in a community-based cohort study. J Cardiovasc Electrophysiol 2012;23:708–13. [DOI] [PubMed] [Google Scholar]
- [4].Chao TF, Liu CJ, Wang KL, et al. Using the CHA2DS2-VASc score for refining stroke risk stratification in “low-risk” Asian patients with atrial fibrillation. J Am Coll Cardiol 2014;64:1658–65. [DOI] [PubMed] [Google Scholar]
- [5].Siu CW, Lip GY, Lam KF, et al. Risk of stroke and intracranial hemorrhage in 9727 Chinese with atrial fibrillation in Hong Kong. Heart Rhythm 2014;11:1401–8. [DOI] [PubMed] [Google Scholar]
- [6].Hijazi Z, Lindback J, Alexander JH, et al. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker-based risk score for predicting stroke in atrial fibrillation. Eur Heart J 2016;37:1582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Garcia-Fernandez A, Roldan V, Rivera-Caravaca JM, et al. Does von Willebrand factor improve the predictive ability of current risk stratification scores in patients with atrial fibrillation? Sci Rep 2017;7:41565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chao TF, Wang KL, Liu CJ, et al. Age threshold for increased stroke risk among patients with atrial fibrillation: a nationwide cohort study from Taiwan. J Am Coll Cardiol 2015;66:1339–47. [DOI] [PubMed] [Google Scholar]
- [9].Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tendera M, Aboyans V, Bartelink ML, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2851–906. [DOI] [PubMed] [Google Scholar]
- [11].Zimetbaum P. Atrial fibrillation. Ann Intern Med 2017;166:33–48. [DOI] [PubMed] [Google Scholar]
- [12].Zhang H, Ye H, Huang W. A meshfree method for simulating myocardial electrical activity. Comput Math Methods Med 2012;2012:936243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Abhayaratna WP, Seward JB, Appleton CP, et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol 2006;47:2357–63. [DOI] [PubMed] [Google Scholar]
- [14].Zhu W, Qiu J, Ma L, et al. A new scoring system for evaluating coronary artery disease by using blood pressure variability. Australas Phys Eng Sci Med 2017;40:751–8. [DOI] [PubMed] [Google Scholar]
- [15].Xu L, Cai Z, Xiong M, et al. Efficacy of an early home-based cardiac rehabilitation program for patients after acute myocardial infarction: a three-dimensional speckle tracking echocardiography randomized trial. Medicine (Baltimore) 2016;95:e5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xu L, Zhao H, Qiu J, et al. The different effects of BMI and WC on organ damage in patients from a cardiac rehabilitation program after acute coronary syndrome. Biomed Res Int 2015;2015:942695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zoni-Berisso M, Lercari F, Carazza T, et al. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol 2014;6:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cai A, Li L, Zhang Y, et al. Lipoprotein(a): a promising marker for residual cardiovascular risk assessment. Dis Markers 2013;35:551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sharrett AR, Ballantyne CM, Coady SA, et al. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2001;104:1108–13. [DOI] [PubMed] [Google Scholar]
- [20].Jurgens G, Koltringer P. Lipoprotein(a) in ischemic cerebrovascular disease: a new approach to the assessment of risk for stroke. Neurology 1987;37:513–5. [DOI] [PubMed] [Google Scholar]
- [21].Li SY, Gao Y, Ma WN, et al. The relationship between serum lipoprotein (a) levels and ischemic stroke risk: a cohort study in the Chinese population. Inflammation 2014;37:686–93. [DOI] [PubMed] [Google Scholar]
- [22].Boden-Albala B, Kargman DE, Lin IF, et al. Increased stroke risk and lipoprotein(a) in a multiethnic community: the Northern Manhattan Stroke Study. Cerebrovasc Dis 2010;30:237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lange KS, Nave AH, Liman TG, et al. Lipoprotein(a) levels and recurrent vascular events after first ischemic stroke. Stroke 2017;48:36–42. [DOI] [PubMed] [Google Scholar]
- [24].Vuckovic BA, Djeric MJ, Ilic TA, et al. Fibrinolytic parameters, lipid status and lipoprotein(a) in ischemic stroke patients. Srp Arh Celok Lek 2010;138suppl 1:12–7. [DOI] [PubMed] [Google Scholar]
- [25].Unal E, Mungan S, Bilen S, et al. The effects of lipoprotein(a) and homocysteine on prognosis and risk factors in acute ischemic stroke. Int J Neurosci 2013;123:532–6. [DOI] [PubMed] [Google Scholar]
- [26].Nagaraj SK, Pai P, Bhat G, et al. Lipoprotein (a) and other lipid profile in patients with thrombotic stroke: is it a reliable marker? J Lab Physicians 2011;3:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wiberg B, Sundstrom J, Arnlov J, et al. Metabolic risk factors for stroke and transient ischemic attacks in middle-aged men: a community-based study with long-term follow-up. Stroke 2006;37:2898–903. [DOI] [PubMed] [Google Scholar]
- [28].Ariyo AA, Thach C, Tracy R. Lp(a) lipoprotein, vascular disease, and mortality in the elderly. N Engl J Med 2003;349:2108–15. [DOI] [PubMed] [Google Scholar]
- [29].Sirikci O, Aytekin V, Demiroglu IC, et al. Association of lipoprotein(a) concentration and apo(a) isoform size with restenosis after percutaneous transluminal coronary angioplasty. Int J Clin Lab Res 2000;30:93–9. [DOI] [PubMed] [Google Scholar]
- [30].Etingin OR, Hajjar DP, Hajjar KA, et al. Lipoprotein (a) regulates plasminogen activator inhibitor-1 expression in endothelial cells. A potential mechanism in thrombogenesis. J Biol Chem 1991;266:2459–65. [PubMed] [Google Scholar]
- [31].Angles-Cano E. Structural basis for the pathophysiology of lipoprotein(a) in the athero-thrombotic process. Braz J Med Biol Res 1997;30:1271–80. [DOI] [PubMed] [Google Scholar]
- [32].Proietti M, Raparelli V, Basili S, et al. Relation of female sex to left atrial diameter and cardiovascular death in atrial fibrillation: the AFFIRM trial. Int J Cardiol 2016;207:258–63. [DOI] [PubMed] [Google Scholar]
- [33].Olshansky B, Heller EN, Mitchell LB, et al. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. J Am Coll Cardiol 2005;45:2026–33. [DOI] [PubMed] [Google Scholar]
- [34].Broughton ST, O’Neal WT, Salahuddin T, et al. The influence of left atrial enlargement on the relationship between atrial fibrillation and stroke. J Stroke Cerebrovasc Dis 2016;25:1396–402. [DOI] [PubMed] [Google Scholar]
- [35].Hamatani Y, Ogawa H, Takabayashi K, et al. Left atrial enlargement is an independent predictor of stroke and systemic embolism in patients with non-valvular atrial fibrillation. Sci Rep 2016;6:31042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kim TW, Jung SW, Song IU, et al. Left atrial dilatation is associated with severe ischemic stroke in men with non-valvular atrial fibrillation. J Neurol Sci 2015;354:97–102. [DOI] [PubMed] [Google Scholar]
- [37].Mondillo S, Sabatini L, Agricola E, et al. Correlation between left atrial size, prothrombotic state and markers of endothelial dysfunction in patients with lone chronic nonrheumatic atrial fibrillation. Int J Cardiol 2000;75:227–32. [DOI] [PubMed] [Google Scholar]
- [38].Sanfilippo AJ, Abascal VM, Sheehan M, et al. Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation 1990;82:792–7. [DOI] [PubMed] [Google Scholar]


