Abstract
Background/Aims
To investigate how the serum 25-hydroxyvitamin D (25(OH)D) level change in patients with inflammatory bowel disease (IBD) and investigate the intestinal mucosa vitamin D receptor (VDR) and vitamin D1-α hydroxylase (CYP27B1) expressions.
Materials and Methods
A total of 105 patients with IBD were enrolled in the present study, including 49 cases with ulcerative colitis (UC) and 56 cases with Crohn’s disease (CD), compared with 45 healthy controls (CON) during the same period by testing the permeability of the intestinal mucosa. The expressions of VDR and CYP27B1 in the intestinal mucosa were detected, so as the serum endotoxin, tumor necrosis factor (TNF)-α, and 25(OH)D levels.
Results
The lactulose and mannitol absorption ratio (LMR) and serum endotoxin and TNF-α levels were significantly higher in the IBD group than in the CON group (p<0.05). The levels of LMR, endotoxin, and TNF-α were higher in the UC group than in the CD group, but 25(OH)D was lower (p<0.05). VDR in the IBD and UC groups was down-regulated when compared with the CON group (p<0.05), but there was no significance between them (p>0.05). CYP27B1 in the IBD and CD groups was significantly up-regulated compared with the CON group (p<0.05), with no significant difference between them (p>0.05).
Conclusion
Patients with IBD exhibit vitamin D metabolism imbalance, lower serum 25(OH)D, and lower VDR expression, but higher CYP27B1 expression in the colonic mucosa. However, VDR and CYP27B1 cannot be used to distinguish UC and CD.
Keywords: Inflammatory bowel disease, Vitamin D, vitamin D, vitamin D receptor, vitamin D1-α hydroxylase
INTRODUCTION
Inflammatory bowel disease (IBD) is a group of chronic, recurrent, non-specific intestinal inflammatory diseases (1). According to the onset sites (2), IBD included ulcerative colitis (UC), which mainly violates the large intestinal mucosa and submucosa, and Crohn’s disease (CD), which mainly violates the small intestine. Recently, it has become a worldwide healthcare problem with a continually increasing incidence (3), and patients with IBD have an increased risk of vascular complications (4), as well as non-melanoma skin cancer and small bowel cancer (5), and colon cancer (6).
New epidemiological data suggest that the incidence and prevalence of the diseases are increasing, which was commonly found in North America and Europe (7). Currently, UC has the highest incidence in Europe (approximately 24.3/100,000 person per year), and CD has the highest incidence in North America (approximately 20.2/100,000 person per year). IBD not only impacts individuals at a young age, thus compromising their work productivity (1), but also affects older ones, in whom with dysbiosis and dysregulation of the immune system playing a more significant role (8). In addition to UC in Europe, IBD in the rest of the world is low but appears to increase recent years, which also has become a common disease of the digestive system in China (9, 10). Although genetic studies provide insights into the disease mechanisms, the environmental influence on the microbiota may be an essential factor in disease development (11), among which the relationships of IBD with vitamin D are attracting scholars’ attention. Vitamin D circulates in vivo in the form of 25-hydroxyvitamin D (25(OH)D), and 1α,25-dihydroxy vitamin D3 (125D) is the active form of vitamin D, which plays its biological roles through specifically combining with vitamin D receptors (VDRs) in a variety of tissues (12); vitamin D1-α hydroxylase (CYP27B1) is a key enzyme that converts the circulating form of vitamin D (25(OH)D) into the active form 125D. Serum 25(OH)D, VDR, and CYP27B1 are all related to the metabolism of vitamin D, but the relationships between IBD and vitamin D had been less studied. The present study examined the relationships between the above three indicators and IBD, aiming to provide references for the pathological characteristics and clinical treatment of IBD.
MATERIALS AND METHODS
Subjects
Patients with clinical manifestations, laboratory tests, imaging results, and endoscopic/histological findings that were consistent with the diagnosis and treatment consensus of IBD were included in the study (10). Patients with a history of intestinal tract surgery; taking vitamin D preparations; certain disease that may cause severe vitamin D metabolic disorders, such as primary hyperparathyroidism, severe liver and kidney dysfunction (including dialysis), severe malnutrition, or dyspepsia were excluded from the study. A total of 105 IBD inpatients and outpatients treated in the Department of Gastroenterology affiliated to Huashan Hospital of Fudan University were collected in 2014 (49 cases with active UC and 56 cases with active CD affecting the colon). UC was diagnosed based on clinical, colonoscopic, and histologic findings by a gastroenterologist. Meanwhile, 45 healthy controls (CON) were selected from the Medical Center affiliated to Huashan Hospital of Fudan University during the same period. The present study was conducted in accordance with the Declaration of Helsinki. The ethics committee of Fudan University approved the study. Written informed consent was obtained from all the participants.
Permeability of the intestinal mucosa
A urine sample was collected from each patient. Each patient was restricted diet one night (at 20:00 PM) before the test and emptied urine under fasting status in the next morning (6:30 AM). Each patient was orally administrated 50 mL of lactulose–mannitol test solution (containing 10 g of lactulose and 5 g of mannitol), and urine was collected within 6 h after 0.5 h water and 2 h diet restriction. The total amount of urine was recorded and determined by high-performance liquid chromatography. According to the standard curves, the concentrations of lactose and mannitol were determined and calculated by the lactulose and mannitol absorption ratio (LMR).
Determination of endotoxin and TNF-α
A fasting blood sample was collected from each patient to detect the serum endotoxin and tumor necrosis factor (TNF)-α. The serum endotoxin was determined by the tachypleus amebocyte lysate–azole colorimetric assay. The test is based on the natural antibacterial defense mechanism of horseshoe crab (Limulus polyphemus), in which Gram-negative infection leads to the coagulation of its hemolymph. The coagulation process is initiated by the reaction of protein with an endotoxin molecule, leading to the formation of the active form of factor C enzyme (13). TNF-α was determined by the enzyme-linked immunosorbent assay. This technique uses an enzyme-linked antibody binding to a surface-attached antigen. Thereafter, a substrate is added to produce either a color change or light signal correlating to the amount of the antigen present in the original sample (14). The operation steps were in strict accordance with the manufacturer’s instructions.
Detection of serum 25(OH)D
Serum 25(OH)D in the peripheral blood was detected by competitive radioimmunoassay. The basis is a selective immunoextraction of 25(OH)D from serum or plasma with a specific monoclonal antibody bound to a solid support, and this antibody is directed toward the H-hydroxylated A ring of 25(OH)D (15).
Expressions of VDR and CYP27B1
Sample preparation
Certain specimen wax blocks were borrowed from the Department of Pathology of Huashan Hospital affiliated to Fudan University. For the healthy controls, one piece of the sigmoid colon tissue was sampled 20 cm away from the anus using disposable biopsy forceps under the condition of obtaining an informed consent from the subject. The sample was then preserved at 4°C in 10% formaldehyde solution. The wax block was prepared by Shanghai Biotech Co., Ltd.
Horseradish peroxidase labeling: IHC staining
The wax sample was sliced using a continuous slicing method (paraffin slicing machine; Leica Instruments Co., Ltd., Germany). There were three slices for each specimen: one slice used phosphate buffered saline (PBS) instead of the primary antibody for negative control, one slice performed VDR immunohistochemistry (IHC) staining, and the last performed CYP27B1 IHC staining (automated immunohistochemical staining instrument; Dako, Denmark). The steps were as follows: (1) the slice was placed in an incubator at 60 °C for 1 h, followed by immersion in xylene for 20 min, immersion in anhydrous ethanol, 95% ethanol, 80% ethanol, and 70% ethanol for 2 min, respectively, and rinsed with distilled water twice. (2) A 3% H2O2 was then added on the slice and allowed to stand at room temperature for 3 min, followed by rinsing with distilled water twice. (3) Normal 5% calf serum was then added on the slice and allowed to stand at room temperature for 20 min. (4) The antibody diluent–diluted primary antibodies (VDR=1:500, mouse anti-human VDR polyclonal antibody, EMD Millipore, USA and CYP27B1=1:500, rabbit anti-human CYP27B1 monoclonal antibody, EMD Millipore, USA) were then added on the slice (the negative control was dropwisely added PBS), allowed to stand at room temperature for 1 h, and rinsed with PBS three times. (5) The slice was allowed to stand at room temperature for 30 min and rinsed with PBS three times. (6) 3,3′-Diaminobenzidine tetrahydrochloride (DAB) coloration was then added for 5 min and then fully rinsed with tap water to stop DAB coloration (Wuhan Boster Biological Technology Co., Ltd., China). (7) The slice was then restained with hematoxylin for 30 s, followed by rinsing with tap water, hot water differentiation, and tap water re-rinsing. (8) The slice was then dehydrated by 70% ethanol, 80% ethanol, 95% ethanol, and absolute ethanol, respectively. (9) The slice was applied phenol/xylene, xylene I, and xylene II, respectively, for 1 min. (10) Neutral gum was then used to seal the slice.
The results were determined under a microscope (optical microscope; Leica Instruments Co., Ltd., Germany), and the percentage of positive cells in 10 randomly selected high-power fields, combined with staining intensity, for each slice was calculated. Positive cells were identified as yellow or brown particles in the cytoplasm and (or) nuclei. According to staining depth, the results can be divided into four grades (0, 1+, 2+, and 3+): grade 0: the cytoplasm (nuclei) exhibited almost the same staining as the surrounding stroma, grade 1+: the staining of the cytoplasm (nuclei) can be distinguished from the surrounding interstitial substance, grade 3+: the cytoplasm (nuclei) was stained tan and can be significantly distinguished from the surrounding interstitial substance, and grade 2+: between the above two situations. The ratio of positive cells >10% and the staining depth 2+/3+ were defined as high expression, and the rest was defined as low expression (microscope imaging system; Leica Instruments Co., Ltd., Germany).
Statistical analysis
Statistical package for social sciences (SPSS) version 18.0 (IBM Corp.; Armonk, NY, USA) was used for statistical analysis, together with Microsoft Office Excel 2013 (Microsoft, Redmond, USA). Data were expressed as mean±standard deviation. A comparison of continuous mean values between the two groups used the independent-samples t-test. A p value <0.05 was considered as statistically significant.
RESULTS
Demographic data and clinical features
The IBD group included 105 patients, including 49 cases with UC and 56 cases with CD. There was no significant difference in age, sex, and body mass index between the IBD and CON groups (p>0.05) (Table 1).
Table 1.
Comparison of basic information between the IBD and CON groups
| Group | n | M/F | Age (years) | BMI (kg/m2) | Disease duration (months) |
|---|---|---|---|---|---|
| CON | 45 | 32/13 | 45.7±11.8 | 20.6±2.3 | 0±0 |
| IBD | 105 | 69/36 | 41.0±15.5 | 21.3±1.6 | 34±44 |
| UC | 49 | 30/19 | 43.4±14.0 | 21.2±1.8 | 38±43 |
| CD | 56 | 39/17 | 38.9±15.3 | 21.4±1.7 | 31±47 |
| F | 1.319 | 2.112 | 1.993 | 9.038 | |
| p | 0.715 | 0.099 | 0.115 | 0.000 |
CON: control group, IBD: inflammatory bowel disease, UC: ulcerative colitis, CD: Crohn’s disease
Comparison of LMR, endotoxin, TNF-α, and 25(OH)D
Lactulose and mannitol absorption ratio in the IBD group was 57.7±12.6% (63.2±13.9% in the UC group and 52.9±11.3% in the CD group). Endotoxin in the IBD group was 106.9±26.4 EU/μL (118.9±19.7 EU/μL in the UC group and 96.4±10.6 EU/μL in the CD group). TNF-α in the IBD group was 95.4±17.7 pg/mL (109.6±18.4 pg/mL in the UC group and 83.0±16.1 pg/mL in the CD group). 25(OH)D in the IBD group was 41.8±10.6 nmol/L (36.6±9.7 nmol/L in the UC group and 46.4±9.4 nmol/L in the CD group). Compared with the CON group, LMR, endotoxin, and TNF-α in the IBD group were significantly increased (p<0.05), whereas 25(OH)D was significantly reduced (p<0.05). The levels of LMR, endotoxin, and TNF-α in the UC group were significantly higher than those in the CD group (p<0.05) (Table 2).
Table 2.
Comparison of serum 25(OH)D among different groups
| Group | n | LMR (%) | Endotoxin (EU/mL) | TNF-α (pg/mL) | 25(OH)D (nmol/L) |
|---|---|---|---|---|---|
| CON | 45 | 3.3±1.2 | 34.2±5.6 | 0.6±0.3 | 49.6±10.9 |
| IBD | 105 | 57.7±12.6* | 106.9±26.4* | 95.4±17.7* | 41.8±10.6* |
| UC | 49 | 63.2±13.9* | 118.9±19.7* | 109.6±18.4* | 36.6±9.7* |
| CD | 56 | 52.9±11.3# | 96.4±10.6# | 83.0±16.1# | 46.4±9.4# |
| F | 286.232 | 177.299 | 466.230 | 15.139 | |
| p | 0.000 | 0.000 | 0.000 | 0.005 |
CON: control group, IBD: inflammatory bowel disease, UC: ulcerative colitis, CD: Crohn’s disease
p<0.05 compared with the CON group
p<0.05 compared with the UC group
Comparison of expressions of VDR and CYP27B1
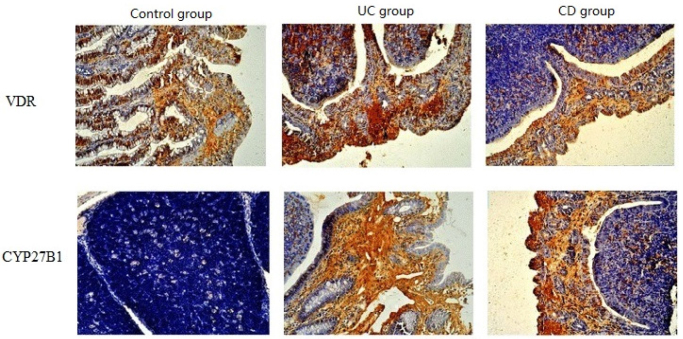
Vitamin D receptor and CYP27B1 are both expressed in the mucosal epithelium, mainly in the glandular columnar epithelium while not in the lamina propria and mucosal muscular layer. The stained area is the cytoplasm, and the nuclei will not be stained. Some patients with IBD exhibited the infiltration of a large number of inflammatory cells on the epithelium, which also have the expressions of VDR and CYP27B1. The staining levels of VDR and CYP27B1 IHC range from negative (blue) to strong positive (tan). VDR is mostly expressed in normal tissues while less expressed in the tissue of patients with IBD. In the present study, the CON group had 36 (80.0%) cases with high VDR expression and 9 (20.0%) cases with low VDR expression. The IBD group had 49 (46.7%) cases with high VDR expression and 56 (53.3%) cases with low VDR expression, among which the UC group had 18 (36.7%) cases with high VDR expression and 31 (63.3%) cases with low VDR expression, whereas the CD group had 31 (55.4%) cases with high VDR expression and 25 (44.6%) cases with low VDR expression. The expression of VDR was significantly higher in the CON group than the IBD and UC groups (p<0.05), but the difference between the CON and CD groups was not significant (p>0.05). There was no significant difference between the UC and CD groups (p>0.05). Most cases in the CON group exhibited negative expression of CYP27B1, but the IBD group exhibited high expression of CYP27B1. The CON group had 10 (22.2%) cases with high CYP27B1 expression and 35 (77.8%) cases with low CYP27B1 expression. The IBD group had 56 (53.3%) cases with high CYP27B1 expression and 49 (46.7%) cases with low CYP27B1 expression, among which the UC group had 13 (38.8%) cases with high CYP27B1 expression and 36 (61.2%) cases with low CYP27B1 expression, whereas the CD group had 43 (53.6%) cases with high CYP27B1 expression and 13 (64.3%) cases with low CYP27B1 expression. The expression of CYP27B1 was significantly lower in the CON group than in the IBD and CD groups (p<0.05), but the difference between the CON and UC groups was not significant (p>0.05). There was no significant difference between the UC and CD groups (p>0.05) (Table 3, Figure 1).
Table 3.
Expressions of VDR and CYP27B1 in different groups (n, %)
| Group | n | VDR | CYP27B1 | ||
|---|---|---|---|---|---|
|
|
|
||||
| High expression | Low expression | High expression | Low expression | ||
| CON | 45 | 36 (80.0) | 9 (20.0) | 10 (22.2) | 35 (77.8) |
| IBD | 105 | 49 (46.7)* | 56 (53.3)* | 56 (53.3)* | 49 (46.7)* |
| UC | 49 | 18 (36.7)* | 31 (63.3)* | 13 (38.8) | 36 (61.2) |
| CD | 56 | 31 (55.4) | 25 (44.6) | 43 (53.6)* | 13 (64.3)* |
| c2 | 20.148 | 42.544 | |||
| P | 0.000 | 0.000 | |||
Note. CON: control group; IBD: inflammatory bowel disease; UC: ulcerative colitis; CD: Crohn’s disease
p<0.05 compared with the CON group
Figure 1.
Expressions of VDR and CYP27B1 in different groups (40)
DISCUSSION
Relationships between serum 25(OH)D and IBD
Recent studies have shown that vitamin D deficiency can increase the risk of immune-related diseases, but it still needs further evidence to verify whether it can be used to speculate that vitamin D deficiency or insufficiency is related to the occurrence of IBD (16). Domestic and foreign studies have shown that the serum 25(OH)D level in patients with IBD is different from normal controls. For example, Silvennoinen reported that the serum 25(OH)D level in patients with IBD is significantly lower than that in the CON group (28.4±12.0 nmol/L vs 36.1±16.7 nmol/L), but there was no significant difference between the UC and CD groups (17). El-Matary investigated 60 newly diagnosed pedia patients with IBD (including 21 UC cases and 39 CD cases), and their serum 25(OH)D level was significantly lower than the CON group, and there was also no significant difference between the UC and CD groups (18). Increasing preclinical and clinical evidence suggests the role of vitamin D deficiency in the development and severity of IBD (19). Tajika studied 33 patients with CD, and the results revealed that the serum 25(OH)D level in the CD group has no significant difference than that in the CON group (20). The results of the present study are similar to most of the other studies. The serum 25(OH)D level is significantly lower in the IBD group than in the CON group (41.8±10.6 nmol/L vs 49.6±10.9 nmol/L). Considering the decrease of 25(OH)D as the cause of IBD may include, from the viewpoint of epidemiology, with the industrialization and urbanization progress and air pollution aggravation, the prevalence of IBD increases, so they are independent risk factors of IBD (21,22). At the same time, the above-mentioned environmental degradation is also an important reason for the decline of vitamin D in humans. The effect of illumination on vitamin D is greater, and the illumination amount of minimal erythemal dose allows the skin to produce 20,000 IU of vitamin D, which is equivalent to 200 times of vitamin D contained in 8 ounces of milk (12). Air contamination leads to lighting reduction, as well as the process of industrialization reduces the time of human outdoor work, so the vitamin D level in human bodies also declines (23,24). Patients with IBD have less outdoor activities due to physical weakness, so their lighting time is less than healthy people; the absorption site of vitamin D is the small intestine, so CD involving the small intestine can affect the absorption of vitamin D due to mucosal inflammation (25). CYP27B1 is up-regulated in inflammatory intestinal tissues, and as mentioned before, it is the hydroxylase of vitamin D1-α and can transfer 25(OH)D into 125D, thus consuming 25(OH)D in the circulation (26). Whether the 25(OH)D level is causally related to IBD, it can be considered that the 25(OH)D level is closely related to the occurrence and development of IBD.
Relationships of VDR and IBD
VDR is the target of active vitamin D, which is located in the center of the vitamin D pathway. The activation of VDR can regulate the expressions of multiple genes. A study about the IBD genome-wide association (the ample size was >75,000) has shown that the VDR gene is a susceptible gene for UC and CD (27). Animal experimental studies have also confirmed the protective roles of VDR in the intestinal tract. Froicu et al. (28,29) established the VDR knockout mouse model and compared those with wild gene type, and the results show that colitis is more serious, proinflammatory factors are up-regulated, and mortality is significantly increased. As for the studies about human tissues, Liu et al. (30) found that VDR is highly expressed in normal colorectal epithelium, and the expression of VDR is lower in patients with UC and CD than in normal populations. The expression of VDR in active patients with CD is significantly lower than that in the remission phase, and the VDR expression in the same patient is significantly lower in the inflammatory site than in the normal mucosa. The present study also confirms that patients with IBD exhibit significantly lower VDR expression in the colonic inflammatory site than normal populations, and it may be associated with the decrease in the expression of mucosal VDR in patients with IBD due to vitamin D deficiency.
Relationships between CYP27B1 and IBD
CYP27B1 is also expressed in such peripheral tissues as the intestinal tract and the immune cells, such as the macrophages and dendritic cells. Liu et al. (31) found that in the mouse model with dextrane sulfate sodium (DSS)-induced acute colitis, the expression of CYP27B1 in the inflammatory region is significantly decreased, but the expression of CYP27B1 in the peri-inflammatory tissue is significantly increased; meanwhile, CYP27B1 knockout mice are much more sensitive to DSS, appearing more serious colitis and higher mortality, suggesting that CYP27B1 has protective effects on the colonic mucosa. Fritsche et al. (32) demonstrated that the lipopolysaccharide can stimulate the expression of CYP27B1 in human monocytes and dendritic cells. It can be seen that when stimulated by inflammatory factors, the body can mobilize the vitamin D system to participate in anti-inflammatory responses. In studies of human tissues, Abreu et al. (26) reported that CYP27B1 is up-regulated in the colonic tissue of patients with CD than normal populations. The expression of CYP27B1 in the colonic tissue of patients with UC is also significantly increased, similar to the results obtained in the present study.
The present study shows that when IBD occurs, VDR is decreased, but CYP27B1 is increased, indicating that 25(OH)D in the peripheral circulation is consumed, so it is difficult for the body to control inflammation, thus leading to the continuous development of IBD. Supplementing vitamin D may be beneficial for preventing or treating IBD. Cantorna et al. (33) demonstrated that oral administration of vitamin D3 can alleviate the symptoms of enteritis in VDR/IL-10 dual-gene knockout mice. Zhu et al. (34) had shown that oral administration of Ca preparations and 125D can reduce the symptoms of experimental colitis in mice and reduce the expression of proinflammatory cytokine TNF-α. Therefore, supplementing vitamin D may be used as a method of clinical prevention or treatment of IBD.
In addition, the present study shows a result different from previous studies. LMR, endotoxin, TNF-α, and 25(OH)D have differences between patients with UC or CD. The contents of LMR, endotoxin, and TNF-α are higher in the UC group than in the CD group, but the content of 25(OH)D is lower in the UC group than in the CD group, and no literature has similar reports that whether the onset age of CD is generally earlier than that of UC (in the present study, the mean age in the UC group was 43.4±14.0 years, and that in the CD group was 38.9±15.3 years). However, VDR and CYP27B1, which are closely related to 25(OH)D, show no significant difference between the patients with UC or CD, suggesting that it may be difficult to consider VDR and CYP27B1 as differential markers for the diagnosis of UC and CD, and it is also possible to confirm the misdiagnosis of UC and CD due to their similar clinical manifestations.
Limitations
The sample size of the study was small. Patients included have varied ages, as well as medical histories, and the medicine the patients have taken was not included to analyze the effect on vitamin D metabolism. Meanwhile, for patients with IBD, the nutrition support was of great importance, and vitamin D should be one of the nutrients, of which the total intake and release were not considered as one of the factors for further discussion.
In summary, patients with IBD have vitamin D metabolic imbalance, lower serum 25(OH)D level, and lower VDR expression in the colonic mucosa, but higher CYP27B1 expression. The abnormal expressions of the above three indicators are very closely related to the occurrence and development of IBD, but VDR and CYP27B1 cannot be used as indicators to identify UC and CD.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Fudan University.
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - J.H.; Design - Y.L.; Supervision - J.H.; Materials - W.Y.; Data Collection and/or Processing - L.L.; Analysis and/or Interpretation - X.L.; Literature Search - T.C.; Writing Manuscript - J.H.; Critical Review - Y.L.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Büsch K, Sonnenberg A, Bansback N. Impact of inflammatory bowel disease on disability. Curr Gastroenterol Rep. 2014;16:414. doi: 10.1007/s11894-014-0414-0. [DOI] [PubMed] [Google Scholar]
- 2.Malik TA. Inflammatory Bowel Disease: Historical Perspective, Epidemiology, and Risk Factors. Surg Clin North Am. 2015;95:1105–22. doi: 10.1016/j.suc.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Fiocchi C. Inflammatory bowel disease pathogenesis: where are we? J Gastroenterol Hepatol. 2015;30(Suppl 1):12–8. doi: 10.1111/jgh.12751. [DOI] [PubMed] [Google Scholar]
- 4.Zezos P, Kouklakis G, Saibil F. Inflammatory bowel disease and thromboembolism. World J Gastroenterol. 2014;20:13863–78. doi: 10.3748/wjg.v20.i38.13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Algaba A, Guerra I, Marín-Jiménez I, et al. Incidence, management, and course of cancer in patients with inflammatory bowel disease. J Crohns Colitis. 2015;9:326–33. doi: 10.1093/ecco-jcc/jjv032. [DOI] [PubMed] [Google Scholar]
- 6.Gallinger ZR, Weizman AV. Colorectal cancer in inflammatory bowel disease: a shift in risk? Expert Rev Anticancer Ther. 2014;14:847–56. doi: 10.1586/14737140.2014.895936. [DOI] [PubMed] [Google Scholar]
- 7.Burisch J, Jess T, Martinato M, Lakatos PL. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7:322–37. doi: 10.1016/j.crohns.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Taleban S, Colombel JF, Mohler MJ, Fain MJ. Inflammatory bowel disease and the elderly: a review. J Crohns Colitis. 2015;9:507–15. doi: 10.1093/ecco-jcc/jjv059. [DOI] [PubMed] [Google Scholar]
- 9.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 10.Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91–9. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheehan D, Moran C, Shanahan F. The microbiota in inflammatory bowel disease. J Gastroenterol. 2015;50:495–507. doi: 10.1007/s00535-015-1064-1. [DOI] [PubMed] [Google Scholar]
- 12.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 13.Chałupniak A, Waszczuk K, Hałubek-Głuchowska K, Piasecki T, Gotszalk T, Rybka J. Application of quartz tuning forks for detection of endotoxins and Gram-negative bacterial cells by monitoring of Limulus Amebocyte Lysate coagulation. Biosens Bioelectron. 2014;58:132–7. doi: 10.1016/j.bios.2014.02.048. [DOI] [PubMed] [Google Scholar]
- 14.Lin AV. Indirect ELISA. Methods Mol Biol. 2015;1318:51–9. doi: 10.1007/978-1-4939-2742-5_5. [DOI] [PubMed] [Google Scholar]
- 15.Van Schoor NM, Heymans MW, Lips P. Vitamin D status in relation to physical performance, falls and fractures in the Longitudinal Aging Study Amsterdam: A reanalysis of previous findings using standardized serum 25-hydroxyvitamin D values. J Steroid Biochem Mol Biol. 2017;177:255–60. doi: 10.1016/j.jsbmb.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Palmer MT, Weaver CT. Linking vitamin d deficiency to inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2245–56. doi: 10.1097/MIB.0b013e31828a3b6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silvennoinen J. Relationships between vitamin D, parathyroid hormone and bone mineral density in inflammatory bowel disease. J Intern Med. 1996;239:131–7. doi: 10.1046/j.1365-2796.1996.420765000.x. [DOI] [PubMed] [Google Scholar]
- 18.El-Matary W, Sikora S, Spady D. Bone mineral density, vitamin D, and disease activity in children newly diagnosed with inflammatory bowel disease. Dig Dis Sci. 2011;56:825–9. doi: 10.1007/s10620-010-1380-5. [DOI] [PubMed] [Google Scholar]
- 19.Hlavaty T, Krajcovicova A, Payer J. Vitamin D therapy in inflammatory bowel diseases: who, in what form, and how much? J Crohns Colitis. 2015;9:198–209. doi: 10.1093/ecco-jcc/jju004. [DOI] [PubMed] [Google Scholar]
- 20.Tajika M, Matsuura A, Nakamura T, et al. Risk factors for vitamin D deficiency in patients with Crohn’s disease. J Gastroenterol. 2004;39:527–33. doi: 10.1007/s00535-003-1338-x. [DOI] [PubMed] [Google Scholar]
- 21.Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environment alinfluences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 22.Soon IS, Molodecky NA, Rabi DM, Ghali WA, Barkema HW, Kaplan GG. The relationship between urban environment and the inflammatory bowel diseases: a systematic review and meta-analysis. BMC Gastroenterol. 2012;12:51. doi: 10.1186/1471-230X-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hochberg Z. Rickets-past and present. Introduction. Endocr Dev. 2003;6:1–13. doi: 10.1159/000072763. [DOI] [PubMed] [Google Scholar]
- 24.Khalili H, Huang ES, Ananthakrishnan AN, et al. Geographical variation and incidence of inflammatory bowel disease among US women. Gut. 2012;61:1686–92. doi: 10.1136/gutjnl-2011-301574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farraye FA, Nimitphong H, Stucchi A, Dendrinos K, Boulanger AB, Vijjeswarapu A, et al. Use of a novel vitamin D bioavailability test demonstrates that vitamin D absorption is decreased in patients with quiescent Crohn’s disease. Inflamm Bowel Dis. 2011;17:2116–21. doi: 10.1002/ibd.21595. [DOI] [PubMed] [Google Scholar]
- 26.Abreu MT, Kantorovich V, Vasiliauskas EA, Gruntmanis U, Matuk R, Daigle K, et al. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn’s disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut. 2004;53:1129–36. doi: 10.1136/gut.2003.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–24. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. doi: 10.1186/1471-2172-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17:2386–92. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 30.Liu W, Chen Y, Golan MA, et al. Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J Clin Invest. 2013;123:3983–96. doi: 10.1172/JCI65842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu N, Nguyen L, Chun RF, et al. Altered endocrine and autocrine metabolism of vitamin D in a mouse model of gastrointestinal inflammation. Endocrinology. 2008;149:4799–808. doi: 10.1210/en.2008-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M. Regulation of 25-hydroxyvitamin D3–1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003;102:3314–6. doi: 10.1182/blood-2002-11-3521. [DOI] [PubMed] [Google Scholar]
- 33.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648–52. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 34.Zhu Y, Mahon BD, Froicu M, Cantorna MT. Calcium and 1 alpha, 25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol. 2005;35:217–24. doi: 10.1002/eji.200425491. [DOI] [PubMed] [Google Scholar]