Abstract
Background/Aims
Multiple factors have been linked to pathogenesis of pancreatic cancer and cholangiocarcinoma. Until now, few studies have investigated the role of small intestinal bacterial overgrowth (SIBO) and toll-like receptor 4 (TLR-4) signaling in these diseases. This study aimed to examine the relationship between the prevalence of SIBO and the TLR-4 expression in patients with pancreatic carcinoma and cholangiocarcinoma.
Materials and Methods
A total of 90 human subjects suffering from pancreatic carcinoma (n=30), cholangiocarcinoma (n=30), and healthy controls (n=30) were enrolled in the study. A glucose hydrogen breath test (GHBT) was used to evaluate SIBO. The TLR4 protein expression was measured by immunohistochemistry (IHC).
Results
The positive rate of SIBO was 63.3% in the pancreatic cancer group and 46.7% in patients with cholangiocarcinoma, which was significantly greater than 13.3% in the healthy control group (p<0.05). An IHC analysis revealed that the TLR-4 protein expression in the SIBO-positive pancreatic carcinoma patients was significantly higher than that in the SIBO-negative patients (p<0.05), and the same result was in the cholangiocarcinoma subjects. In addition, a correlation analysis identified the positive relationship between the prevalence of SIBO and the TLR-4 protein expression in pancreatic carcinoma (r=0.489), and the same result was in the cholangiocarcinoma subjects.
Conclusion
Our findings indicate a high prevalence of SIBO in pancreatic carcinoma and cholangiocarcinoma, and SIBO displays a positive correlation with the TLR-4 expression, suggesting that SIBO could be a risk factor for the pathogenesis of pancreatic carcinoma and cholangiocarcinoma, in which the TLR4 signaling may be involved.
Keywords: Small intestinal bacterial overgrowth, glucose-hydrogen breath test, pancreatic carcinoma, cholangiocarcinoma, toll-like receptor 4
INTRODUCTION
Pancreatic carcinoma is one of the most common causes of cancer-related deaths worldwide. The prevalence of pancreatic carcinoma has been on the rise in China, with the mortality rates close to the incidence rates, and with the survival rate lower than 5%, representing the lowest survival rate in all forms of cancers. A large proportion of patients with pancreatic carcinoma suffer from severe pain and fatigue, and they have a poor quality of life (1–3). Although a number of factors have been reported to contribute to the development and progression of pancreatic cancer, the underlying pathogenesis remains unclear. Like pancreatic cancer, cholangiocarcinoma, known as bile duct cancer, is also a highly malignant but rare form of cancer with the origin of the bile duct epithelial cells or cholangiocytes lining the intrahepatic and extrahepatic bile duct. It has been reported that two-thirds of cholangiocarcinomas become metastatic at the time of diagnosis due to the challenges in both screening and early detection of cholangiocarcinoma (4). Therefore, identifying the causative factors and understanding the molecular mechanisms underlying the development, invasion, and metastasis are the key to the prevention and the development of novel therapy for cholangiocarcinoma.
Small intestinal bacterial overgrowth (SIBO) is a clinical condition, mainly characterized by excessive Gram-positive aerobic microorganisms in the upper gut, exceeding 105–106 bacterial organisms/ml in the small intestine. Patients with SIBO suffer from symptoms such as bloating, chronic diarrhea, weight loss, malabsorption, nutritional deficiencies, osteoporosis, and anemia (5). It has been reported that a diminished gastric acid secretion, small intestine dysmotility, disturbances in the gut immune function, and anatomical abnormalities of the gastrointestinal tract increase the risk of developing SIBO (6). Therefore, patients with digestive disease, including digestive cancers such as pancreatic carcinoma and cholangiocarcinoma, are prone to SIBO. In addition, lipopolysaccharides (LPS), the outer membrane molecule of Gram-negative bacteria are markedly increased in SIBO, have been well-documented to be active and bind to the toll-like receptor 4 (TLR-4), affecting the tissue homeostasis by regulating the inflammatory and tissue repair responses to injury (7–8). In fact, the activation of TLRs including TLR-4 on the tumor cells can not only promote cell proliferation and suppress apoptosis, but it also contributes to tumor invasion, transference, and immune escape (9).
Until now, few studies have assessed the role of SIBO and LPS/TLR-4 signaling in both pancreatic cancer and cholangiocarcinoma. In the present study, we aimed to examine the relationship between the prevalence of SIBO and the expression of TLR-4 in patients with pancreatic carcinoma and cholangiocarcinoma. To the best of our knowledge, the results gained from this study may provide the first evidence for the potential role SIBO and the LPS/TLR-4 signaling in the pathogenesis of pancreatic cancer and cholangiocarcinoma.
MATERIALS AND METHODS
Study Subjects
A total of 30 patients with pancreatic carcinoma, 30 patients with cholangiocarcinoma, and 30 healthy control individuals were included in this prospective study between September 2014 and October 2015 in the Department of Gastroenterology and the Department of Hepatobiliary Surgery of Qingdao Municipal Hospital affiliated with Qingdao University Medical College. The following were the inclusion criteria: adult patients diagnosed with pancreatic carcinoma or cholangiocarcinoma by the computed tomography scanning, ultrasonic imaging, and biochemical tests. Patients with the following conditions were excluded from this study: diabetes; thyroid disease; intestinal pseudo-obstruction; irritable bowel syndrome; lactose intolerance; the use of antibiotics and acid-suppressive drugs; probiotics application a month prior to enrollment; a history of using hormones, antidepressants, and opiate drugs; a long-term smoking history; colonoscopy and enema a month prior to enrollment, medical history of renal insufficiency, and infectious diseases. Controls were those individuals who had no specific gastrointestinal complaints and diseases as diagnosed at the outpatient’s clinic, and who were willing to participate in this prospective study. Pancreatic carcinoma, cholangiocarcinoma, and paired para-carcinoma tissues were resected from patients who underwent a surgical procedure at the Qingdao Municipal Hospital affiliated with Qingdao University Medical College (Qingdao, China). Thirty specimens each of resected human pancreatic carcinoma, paired para-carcinoma normal pancreatic tissues, cholangiocarcinoma, and para-carcinoma normal tissues of biliary tract were pathologically validated, and no tumor cells were pathologically identified in all para-tumor tissues.
All patients signed the written informed consent. This prospective study was reviewed and approved by the Institutional Research Ethics Board of Qingdao Municipal Hospital.
Glucose-hydrogen breath test
All enrolled patients and control individuals were instructed to undergo the glucose-hydrogen breath test (GHBT). A day before the GHBT, they were asked to comply with the instructions as follows: (1) Fast for 12 h with only water to drink and with no food. (2) Avoid foods that digest slowly, including beans, certain vegetables, and dairy products. (3) No smoking and vigorous excise. In addition, diet was not allowed during the test.
The GHBT was conducted on a gas analyzer (Shenzhen Zhonghe Headway BIO-SCI&TECH CO., LTD). Prior to the ingestion of glucose, the baseline breath hydrogen levels were measured, following which 50 g of glucose (Fuzhou Neptunus Fuyao Pharmaceuticals CO., LTD) dissolved in 250 mL of water were given serial alveolar samples were collected every 20 minutes for over 120 minutes. The H2 excretion levels were measured using the gas analyzer. SIBO was defined as an increase in the hydrogen excretion over the basal peak of >12 parts per million (ppm), the cutoff value for the positive result of SIBO. A value for the fasting breath hydrogen >20 ppm, despite adequate preparation, was also recorded as potentially indicative for SIBO as reported previously (10).
Immunohistochemistry
The TLR4 protein expression was measured by immunohistochemistry using the rabbit anti-TLR4 primary antibody (Beijing Boisynthesis Biotechnology, Beijing, China) and goat anti-rabbit IgG (Beijing Zhong Shan-Golden Bridge Biological Technology, Beijing, China) according to the manufacturers’ instructions. The staining intensity was rated as follows: 0 point, no intensity; 1 point, weak intensity; 2 points, moderate intensity; and 3 points, strong intensity. The percentage of TLR-4-positive cells was defined as follows: 0 point, <5% TLR-4-positive tumor cells; 1 point, 5%–25% TLR-4-positive cells; 2 points, 26%–50% TLR-4-positive cells; 3 points, 51%–75% TLR-4-positive cells; and 4 points, >75% TLR-4-positive cells. Points for expression and percentage of positive cells were added, and the specimens were attributed to four groups according to their overall score: negative (−), 0–1 point; weak expression (+), 2–3 points; moderate expression (++), 4–6 points; and strong expression (+++), 7 points. Negative (−) and weak expression (+) classify as negative, and moderate expression (++) and strong expression (+++) classify as positive (11).
Statistical analysis
A statistical analysis was performed using the statistic software SPSS version 19.0 (IBM Corp.; Armonk, NY, USA). The group comparison was conducted with the Kolmogorov-Smirnov test and chi-squared test. A correlation coefficient analysis was carried out to analyze the association between SIBO and the TLR-4 expression. A p-value <0.05 was considered to be statistically significant.
RESULTS
The prevalence of SIBO in patients with pancreatic carcinoma and cholangiocarcinoma of the 90 subjects, the prevalence of SIBO was 63.3% among the patients with pancreatic carcinoma, 46.7% among the patients with cholangiocarcinoma, which was significantly higher than the positive rate of 13.3% in the healthy controls (χ2=15.864 and p<0.05 in the pancreatic carcinoma group versus the control group; χ2=7.937 and p<0.05 in the cholangiocarcinoma group versus the control group). The results are displayed in Table 1. The expiratory hydrogen concentrations were contrasted between the groups of patients with pancreatic carcinoma, the patients with cholangiocarcinoma, and the control group. Both the basic value and post-prandial expiratory hydrogen concentrations were higher in patients with pancreatic carcinoma and cholangiocarcinoma, and these differences were statistically significant (p<0.05). The results are displayed in Figure 1.
Table 1.
Demographic characteristics of the patients with and without Helicobacter pylori infection
| SIBO | Total | Positive Rate | χ2 | p | ||
|---|---|---|---|---|---|---|
|
| ||||||
| +(Cases) | −(Cases) | |||||
| Pancreatic carcinoma group | 19 | 11 | 30 | 63.3% | 15.864 | <0.05 |
| Cholangiocarcinoma group | 14 | 16 | 30 | 46.7% | 7.937 | <0.05 |
| Control group | 4 | 26 | 30 | 13.3% | ||
SIBO: small intestinal bacterial overgrowth
Figure 1.
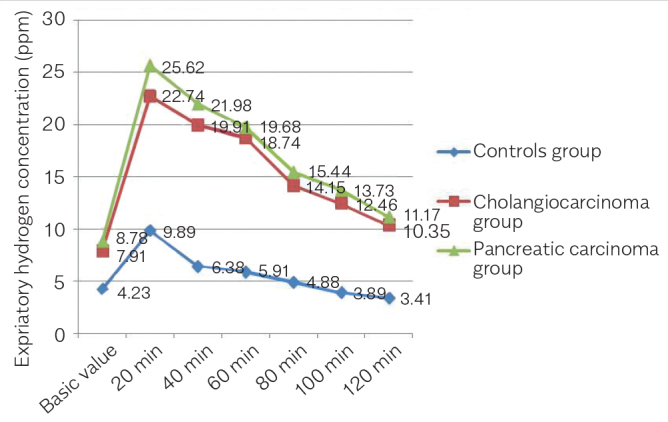
The expiratory hydrogen concentrations were contrasted between the groups of patients with pancreatic carcinoma, the patients with cholangiocarcinoma, and the control group. Both the basic value and post-prandial expiratory hydrogen concentrations were higher in patients with pancreatic carcinoma and cholangiocarcinoma, and these differences were statistically significant (p<0.05)
The expression of the TLR-4 protein in pancreatic carcinoma and cholangiocarcinoma
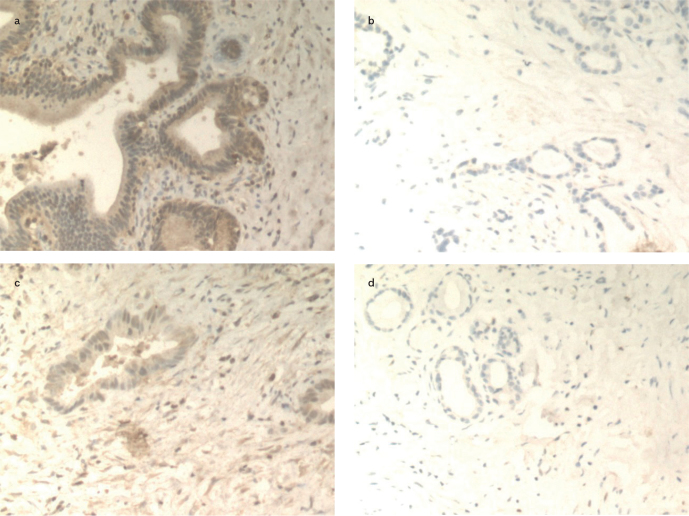
We next examined the protein levels of TLR-4 in pancreatic carcinoma, cholangiocarcinoma, and the para-carcinoma normal tissues. As shown in Figure 2 and Table 2, the percentage of TLR-4-positive cells in pancreatic carcinoma was 66.7%, which was significantly higher than the percentage in para-carcinoma normal tissues (30.0%), and the difference between the percentages in the two groups was statistically significant (χ2=8.076, p<0.05). Similar results were observed for the TLR-4 protein expression and TLR-4-postitive cells in cholangiocarcinoma versus adjacent normal tissues (Figure 2 and Table 3). The level of the TLR-4 protein in cholangiocarcinoma tissues was 56.7%, which was significantly greater than that in para-carcinoma normal tissues (26.7%) (χ2=5.554, P<0.05).
Figure 2. a–d.
An immunohistochemical analysis of the TLR-4 protein expression in pancreatic carcinoma, cholangiocarcinoma, and their adjacent normal tissues: Expression of the TLR-4 protein in pancreatic carcinoma tissues (×100) (a); expression of the TLR-4 protein in paracarcinoma normal tissues (×100) (B); expression of the TLR-4 protein in cholangiocarcinoma tissues (×100) (c); expression of the TLR-4 protein in para-carcinoma normal tissues (×100) (d)
TLR-4: toll-like receptor
Table 2.
The expression of the TLR-4 protein in pancreatic carcinoma and para-carcinoma normal tissues
| TLR-4 | Total | Positive Rate | χ2 | p | ||
|---|---|---|---|---|---|---|
|
| ||||||
| +(Cases) | −(Cases) | |||||
| Pancreatic carcinoma group | 20 | 10 | 30 | 66.7% | 8.076 | <0.05 |
| Para-carcinoma group | 9 | 21 | 30 | 30.0% | ||
TLR-4: toll-like receptor 4
Table 3.
The expression of the TLR-4 protein in cholangiocarcinoma and para-carcinoma normal tissues
| TLR-4 | Total | Positive Rate | χ2 | p | ||
|---|---|---|---|---|---|---|
|
| ||||||
| +(Cases) | −(Cases) | |||||
| Cholangiocarcinoma group | 17 | 13 | 30 | 56.7% | 5.554 | <0.05 |
| Para-carcinoma group | 8 | 22 | 30 | 26.7% | ||
TLR-4: toll-like receptor 4
Correlation between SIBO and the TLR-4 protein expression in pancreatic carcinoma and cholangiocarcinoma
The mean percentage of TLR-4-postive cells in pancreatic carcinoma tissues of the patients who were detected for SIBO was 70.6%, which was significantly higher than 61.5% in pancreatic carcinoma tissues that were negative for SIBO; the difference was statistically significant (χ2=7.177, p<0.05) (Table 4). In addition, the positive correlation between the prevalence of SIBO and protein expression of TLR-4 in pancreatic carcinoma was identified (r=0.489) (Table 4). In parallel, we observed that the mean percentage of TLR-4-positive cells in cholangiocarcinoma tissues of the patients who were SIBO-positive was 78.6%, which was markedly higher than 37.5% in the cholangiocarcinoma tissues of the patients who were SIBO-negative; the difference between the two groups was statistically significant (χ2=5.129, p<0.05). Furthermore, the prevalence of SIBO and protein expression of TLR-4 in cholangiocarcinoma was positively correlated (r=0.413) (Table 5).
Table 4.
Comparison of the TLR-4 protein expression in pancreatic carcinoma tissues of the SIBO-positive versus SIBO-negative patients
| TLR-4 | Total | Positive Rate | χ2 | p | ||
|---|---|---|---|---|---|---|
|
| ||||||
| +(Cases) | −(Cases) | |||||
| Pancreatic carcinoma who have the SIBO-positive group | 16 | 3 | 19 | 70.6% | 7.117 | <0.05 |
| Pancreatic carcinoma who have the SIBO-negative group | 4 | 7 | 11 | 61.5% | ||
SIBO: small intestinal bacterial overgrowth; TLR-4: toll-like receptor 4
Table 5.
Comparison of the TLR-4 protein expression in the cholangiocarcinoma tissues of the SIBO-positive versus SIBO-negative patients
| TLR-4 | Total | Positive Rate | χ2 | p | ||
|---|---|---|---|---|---|---|
|
| ||||||
| +(Cases) | −(Cases) | |||||
| Cholangiocarcinoma who have the SIBO-positive group | 11 | 3 | 14 | 78.6% | 5.129 | <0.05 |
| Cholangiocarcinoma who have the SIBO-negative group | 6 | 10 | 16 | 37.5% | ||
SIBO: small intestinal bacterial overgrowth; TLR-4: toll-like receptor 4
DISCUSSION
The mobility and mortality of pancreatic carcinoma and cholangiocarcinoma continue to rise each year, mainly due to difficulties in early diagnosis. A number of risk factors and their roles in the pathogenesis of the diseases have been investigated. However, to date, SIBO and the SIBO-mediated activation of the LPS/TLR-4 signaling in both pancreatic cancer and cholangiocarcinoma have not been explored. The major novel findings of the present prospective study were summarized as follows: (1) SIBO is significantly prevalent in patients with pancreatic carcinoma or cholangiocarcinoma compared with healthy controls (Table 1). (2) The TLR-4 protein is significantly up-regulated in the SIBO-positive pancreatic carcinoma or patients with cholangiocarcinoma compared with SIBO-negative patients (Table 4, 5). (3) The over-expression of the TLR-4 protein is positively associated with the prevalence of SIBO in pancreatic carcinoma or cholangiocarcinoma (Figure 2, Table 4, 5).
Recent studies demonstrated that a large proportion of irritable bowel syndrome (IBS) patients (up to 80%) were SIBO-positive using the hydrogen breath test, the IBS-related symptoms were significantly improved after the eradication of SIBO by antibiotics in IBS patients with SIBO, and the recurrence of SIBO was related with the return of the IBS symptoms (12–13). Sánchez et al. found that an increase in the population of intestinal aerobic bacteria in experimental cirrhosis was related to bacterial translocation (14). In a case study of a 62-year-old Caucasian male with a history of advanced pancreatic cancer by Ivan Bustillo (15), diarrhea was greatly improved following a SIBO therapy. It has been also found that the SIBO-positive rate in the patients with esophageal cancer, gastric cancer, and liver cancer were 47.1% (16/34), 49.4% (41/83), and 76.5% (39/51), respectively (16), suggesting that patients with digestive disease are prone to SIBO. In our study, a high prevalence of SIBO, 63.3% in pancreatic carcinoma and 46.7% in cholangiocarcinoma, was observed.
Under normal circumstances in healthy persons, the microbes in the small intestine are well-balanced. A number of clinical conditions including the immunodeficiency, intestinal obstruction, intestinal bacterial translocation, exocrine pancreatic insufficiency, and the absence of bile have been found to result in an imbalance of the small intestine microorganisms and SIBO. In immunodeficiency, the immune defense system has been damaged, and the barrier of intestinal mucosa can be destroyed, for which an intestinal flora imbalance is frequently seen in patients with immunodeficiency. In the exocrine pancreatic insufficiency, pancreatic enzymes might play an important role in the bacterial flora imbalance of the small intestine and in a high rate of SIBO (17). Bile acid is a metabolite of cholesterol, absorbed in the enterohepatic circulation through the intestinal mucous membrane due to the intestinal bacterial flora. In fact, bile plays an important role in the proper function of the intestinal barrier to prevent the invasion of intestinal bacteria into the underlying tissues, indicating that the intestinal administration of bile to patients with obstructive jaundice could be helpful to reduce infectious complications by inhibiting bacterial translocation from the intestine to other organs (18). Therefore, the deficiency of bile acid can result in bacterial overgrowth, the small bowel function disorders, and endotoxemia.
Immunodeficiency is frequently found in patients with pancreatic carcinoma and cholangiocarcinoma. The damage of immune defense system and the barrier of the intestinal mucosa may lead to an imbalance in the small intestinal bacterial flora. The exocrine pancreatic and pancreatic enzymes insufficiency in pancreatic carcinoma and the bile acid deficiency in cholangiocarcinoma can also lead to intestinal bacterial overgrowth. It was not surprising in our study that the positive rate of SIBO in patients with pancreatic carcinoma and cholangiocarcinoma were both significantly higher than healthy controls. Thus, patients with pancreatic carcinoma and cholangiocarcinoma are more prone to SIBO than healthy individuals. SIBO can aggravate the symptoms of digestive system through multiple factors that affect the small intestine. Researches have shown that the bacteria overgrowth can compete Vitamin B12, interfere with the metabolism of bile salt, and influence the absorption of amino acids, through which it may cause a Vitamin B12 deficiency, diarrhea, and low-protein blood syndrome. In addition, SIBO can also increase the bacterial adhesion to the intestinal wall and produce a number of metabolites and toxins, which can subsequently damage the intestinal mucosa structure and adversely affect the motility of intestinal tract (19).
TLR4 was the first member found in the TLRs family (Medzhitov et al., 1997), and was later identified as a receptor of LPS (20–21) in the LPS/TLR-4 signaling pathway. The translocation of bacterial components termed pathogen-associated molecular patterns triggers inflammatory responses through TLRs in both the early and late disease stages (22). In fact, there are two major TLR pathways with one mediated by the myeloid differentiation factor 88 (MYD88) adaptor proteins, and the other independent of MYD88. TLR4 acts with delayed activation of the NF-κB signaling via MYD88-independent pathways (23). TLR4 was highly expressed in tumor cells of patients with gastric carcinoma and its precursor lesions, intestinal metaplasia, and dysplasia (24). Tumor tissues displayed higher levels of TLRs expression in breast cancer (25). The TLR-4 expression is found to be associated with prostate cancer with recurrence (26), indicating that the abnormal expression of TLRs may be closely related to the occurrence and development of malignant tumor. In the study of ovarian cancer, the functional activity for TLRs was demonstrated by the stimulation of cell lines with specific ligands and subsequent activation and translocation of NFkappaB and release of the proinflammatory cytokines interleukin-6 and CCL-2 (27). In the study of melanoma, the heat shock protein 60 released by tumor cells caused a persistent activation of TLR2 and was critical in the constitutive activation of the transcription factor Stat3, leading to the release of immunosuppressive cytokines and chemokines (28). Therefore, there is a possibility that there may be some endogenous ligands in pancreatic carcinoma and cholangiocarcinoma microenvironment. In the present study, the percentage of the TLR-4-positive cells in pancreatic carcinoma was 66.7%, and 56.7% in cholangiocarcinoma, which were significantly higher than that in para-carcinoma normal tissues. Furthermore, the relationship between the prevalence of SIBO and protein expression of TLR-4 was found to be positively correlated in pancreatic carcinoma (r=0.489) and cholangiocarcinoma (r=0.413). The limitation of our study is that we only demonstrate the association between SIBO and TLR-4 in patients with pancreatic carcinoma and cholangiocarcinoma, but we do not to investigate whether the SIBO could mediate the activation of the LPS/TLR-4 signaling through which it may contribute to the development of pancreatic carcinoma and cholangiocarcinoma. We will further conduct an in-depth study to investigate it.
In conclusion, our findings demonstrate a very high prevalence of SIBO in pancreatic carcinoma and cholangiocarcinoma, and SIBO exhibits a positive correlation with the TLR-4 protein expression. Our results also suggest that SIBO could be a risk factor to contribute to the development and progression of pancreatic carcinoma and cholangiocarcinoma, in which the LPS/TLR4 signaling could play an important role.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Qingdao University.
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - L.X., X.M., H.W., P.Z.; Design - L.X., X.M., H.W., P.Z.; Supervision - P.Z., Z.T.; Resources - L.X., X.M., P.Z.; Materials - X.M., H.W., Z.T.; Data Collection and/or Processing - X.M., H.W., P.Z.; Analysis and/or Interpretation - L.X., X.M., Z.T.; Literature Search - P.Z., Z.T.; Writing Manuscript - X.M., H.W.; Critical Reviews - P.Z., Z.T., X.M.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received financial support by Qingdao Outstanding Health Professional Development Fund.
REFERENCES
- 1.Lin Quan-Jun, Yang Feng, Jin Chen, Fu De-Liang. Current status and progress of pancreatic cancer in China. World J Gastroenterol. 2015;21:7988–8003. doi: 10.3748/wjg.v21.i26.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rui L, Zhang Haiyang, Wang Xia, et al. The miR-24-Bim pathway promotes tumor growth and angiogenesis in pancreatic carcinoma. Oncotarget. 2015;6:43831–42. doi: 10.18632/oncotarget.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bustillo Ivan, Larson Heidi, Saif Muhammad Wasif. Small Intestine Bacterial Overgrowth: An Underdiagnosed Cause of Diarrhea in Patients with Pancreatic Cancer. JOP. 2009;10:576–8. [PubMed] [Google Scholar]
- 4.ZHAO RUI, CHANG YUAN, LIU ZHAO, et al. Effect of vascular endothelial growth factor-C expression on lymph node metastasis in human cholangiocarcinoma. Oncol Lett. 2015;10:1011–5. doi: 10.3892/ol.2015.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dukowicz AC, Lacy BE, Levine GM. Small Intestinal Bacterial Overgrowth: A Comprehensive Review. Gastroenterol Hepatol(N Y) 2007;3(2):112–22. [PMC free article] [PubMed] [Google Scholar]
- 6.Corrazza GR, Menozzi MG, Strocchi A, et al. The diagnosis of small bowel bacterial overgrowth: reliability of jejunal culture and inadequacy of breath hydrogen testing. Gastroenterology. 1990;98:302–9. doi: 10.1016/0016-5085(90)90818-L. [DOI] [PubMed] [Google Scholar]
- 7.Medvedev Andrei E. Toll-Like Receptor Polymorphisms, Inammatory and Infectious Diseases, Allergies, and Cancer. J Interferon Cytokine Res. 2013;33:467–84. doi: 10.1089/jir.2012.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thada S, Valluri VL, Gaddam SL. Influence of Toll-like receptor gene polymorphisms to tuberculosis susceptibility in humans. Scand J Immunol. 2013;78:221–9. doi: 10.1111/sji.12066. [DOI] [PubMed] [Google Scholar]
- 9.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 10.Signoretti M, Stigliano S, Valente R, Piciucchi M, Delle Fave G, Capurso G. Small Intestinal Bacterial Overgrowth in Patients With Chronic Pancreatitis. J Clin Gastroenterol. 2014;48(Suppl 1):S52–5. doi: 10.1097/MCG.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 11.Birner Peter, Schindl Monika, Obermair Andreas, Plank Christina, Breitenecker Gerhard, Oberhuber Georg. Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000;60:4693–6. [PubMed] [Google Scholar]
- 12.Basseri RJ, Weitsman S, Barlow GM, Pimentel M. Antibiotics for the Treatment of Irritable Bowel Syndrome. Gastroenterol Hepatol(N Y) 2011;7:455–93. [PMC free article] [PubMed] [Google Scholar]
- 13.Reddymasu Savio C, Sostarich Sandra, McCallum Richard W. Small intestinal bacterial overgrowth in irritable bowel syndrome: are there any predictors? BMC Gastroentero. 2010;10:23. doi: 10.1186/1471-230X-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sánchez E, Casafont F, Guerra A, de Benito I, Pons-Romero F. Role of intestinal bacterial overgrowth and intestinal motility in bacterial translocation in experimental cirrhosis. Rev Esp Enferm Dig. 2005;11:805–14. doi: 10.4321/S1130-01082005001100005. [DOI] [PubMed] [Google Scholar]
- 15.Bustillo I, Larson H, Saif MW. Small intestine bacterial overgrowth: an underdiagnosed cause of diarrhea in patients with pancreatic cancer. JOP. 2009;10:576–8. [PubMed] [Google Scholar]
- 16.Wang W, Liu F, Xu AL, et al. A study on small intestinal bacterial overgrowth in patients with three gastrointestinal malignancies. J Med Rev. 2011;17:946–7. [Google Scholar]
- 17.Pezzilli Raffaele. Chronic pancreatitis:Maldigestion, intestinal ecology and intestinal inflammation. World J Gastroenterol. 2009;15:1673–6. doi: 10.3748/wjg.15.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogata Y, Nishi M, Nakayama H, Kuwahara T, Ohnishi Y, Tashiro S. Role of bile in intestinal barrier function and its inhibitory effect on bacterial translocation in obstructive jaundice in rats. J Surg Res. 115:18–23. doi: 10.1016/S0022-4804(03)00308-1. [DOI] [PubMed] [Google Scholar]
- 19.Liu Yang, Li Yuan, Zhang Dong-Sheng, Zhang Ai-Jun, Xu Lin. Significance of small intestinal bacterial overgrowth in small intestinal tumors. WCJD. 2013;21:3435–9. [Google Scholar]
- 20.O’Neill LA, Bryant CE, Doyle SL. Therapeutic targeting of Toll-like receptors for infectious and inflammatory diseases and cancer. Pharmacol Rev. 2009;61:177–97. doi: 10.1124/pr.109.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song PI, Abraham TA, Park Y, et al. The expression of functional LPS receptor proteins CD14 and toll-like receptor 4 in human corneal cells. Invest Ophthalmol Vis Sci. 2001;42:2867–77. [PubMed] [Google Scholar]
- 22.Dapito DH, Mencin A, Gwak GY, et al. Promotion of Hepatocellular Carcinoma by the Intestinal Microbiota and TLR4. Cancer Cell. 2012;21:504–16. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li TT, Ogino S, Qian ZR. Toll-like receptor signaling in colorectal cancer: carcinogenesis to cancer therapy. World J Gastroenterol. 2014;20:17699–708. doi: 10.3748/wjg.v20.i47.17699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmausser B, Andrulis M, Endrich S, Müller-Hermelink HK, Eck M. Toll-like receptors TLR4, TLR5 and TLR9 on gastric carcinoma cells: an implication for interaction with Helicobacter pylori. Int J Med Microbiol. 2005;295:179–85. doi: 10.1016/j.ijmm.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 25.González-Reyes S, Marín L, González L, et al. Study of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasis. BMC Cancer. 2010;10:665. doi: 10.1186/1471-2407-10-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.González-Reyes S, Fernández JM, González LO, et al. Study of TLR3, TLR4, and TLR9 in prostate carcinomas and their association with biochemical recurrence. Cancer Immunol Immunother. 2011;60:217–26. doi: 10.1007/s00262-010-0931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou M, McFarland-Mancini MM, Funk HM, Husseinzadeh N, Mounajjed T, Drew AF. Toll-like receptor expression in normal ovary and ovarian tumors. Cancer Immunol Immunother. 2009;58:1375–85. doi: 10.1007/s00262-008-0650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang HZ, Cui B, Liu HZ, et al. Blocking TLR2 activity attenuates pulmonary metastases of tumor. PLoS One. 2009;4:e6520. doi: 10.1371/journal.pone.0006520. [DOI] [PMC free article] [PubMed] [Google Scholar]