Abstract
Because there are limited clinical reports on the impact of human T-lymphotropic virus type 1 (HTLV-1) on organ transplantation, its effects on the development of adult T-cell leukemia-lymphoma (ATL), post-transplantation lymphoproliferative disorder (PTLD) and HTLV-1–associated myelopathy (HAM) or atypical HAM after organ transplantation remain unclear.
We retrospectively analyzed the impact of HTLV-1 in 54 allogeneic hematopoietic stem cell transplantation (allo-HSCT) cases and 31 renal transplantation cases between January 2006 and December 2016.
Among the 54 allo-HSCT cases, nine recipients with ATL tested positive for HTLV-1, and one was found to be an HTLV-1 carrier. All donors tested negative for HTLV-1. Only one HTLV-1 carrier did not present with ATL or HAM development after allo-HSCT. Among nine ATL cases after allo-HSCT, four eventually relapsed due to proliferation of recipient-derived ATL cells. However, in one ATL case, atypical HAM developed rapidly at 5 months after allo-HSCT.
Among the 31 renal transplantation cases, all donors tested negative for HTLV-1, and only recipients tested positive. Only one HTLV-1 carrier recipient did not present with ATL or HAM development after renal transplantation. However, one HTLV-1-negative recipient developed PTLD in the brain 10 years after renal transplantation.
In clinical practice, careful follow-up of HTLV-1 infected recipients after organ transplantation is important because atypical HAM can develop in ATL patients after allo-HSCT. Furthermore, to clarify the risk of ATL or HAM development in HTLV-1 infected recipients, we prospectively followed up our cohort.
Keywords: HTLV-1, ATL, atypical HAM, PTLD, organ transplantation
INTRODUCTION
HTLV-1 infection is rare, serious, and life-threatening, and leads to the development of adult T-cell leukemia-lymphoma (ATL) or HTLV-1–associated myelopathy (HAM) in patients undergoing immunosuppressive treatment after bone marrow transplantation (BMT) and organ transplantation because of HTLV-1 reactivation in recipient or donor cells.1
Regarding the ATL development mechanism, the major pathogenesis is proviral genome mutations, such as HTLV-1 tax or HTLV-1 bZIP factor (HBZ), and host gene mutations such as the activation of TCR/NF-κB signaling.2,3
As for the mechanism of HAM development, the major pathogenesis is chronic myelitis because of inflammation caused by CD4+ CXCR3+ CCR4+ cells, and the production of Th1 cytokines such as CXCL10 and neopterin.4,5
ATL development after allogeneic hematopoietic stem cell transplantation (allo-HSCT) and organ transplantation has been reported in three and 13 cases, respectively.6-20 Similarly, the development of HAM-mimicking myelitis after allo-HSCT and HAM after organ transplantation has been reported in one and 13 cases, respectively.21-33 On the other hand, the clinical impact of HTLV-1 infection after allo-HSCT and organ transplantation is unclear and controversial.
Previously, we reported three cases of acute-type ATL development that originated from a recipient in eight HTLV-1 carriers among 164 living-donor liver transplant recipients undergoing immunosuppressive treatment.13 In this study, we retrospectively analyzed the clinical impact of HTLV-1 on organ transplantation in 54 allo-HSCT cases and 31 renal transplantation cases.
PATIENTS AND METHODS
We retrospectively analyzed the impact of HTLV-1 in patients who underwent organ transplantation, including 54 allo-HSCT cases and 31 renal transplantation cases, for an 11-year period from January 2006 to December 2016.
Among the 54 allo-HSCT cases, the HTLV-1 status of the allo-HSCT recipients was ATL in nine and one HTLV-1 carrier. All donors for allo-HSCT tested negative for HTLV-1. We previously reported nine patients with aggressive-type ATL who underwent allo-HSCT at a single institution in Miyazaki between January 2006 and July 2015.31 Of the nine patients with ATL, three received mogamulizumab therapy before allo-HSCT due to the poor control of refractory ATL.34
At our institution, based on the allo-HSCT treatment guidelines by the Fukuoka Bone Marrow Transplantation Group (FBMTG),35 the indications for allo-HSCT were as follows: age, <65 years; Eastern Cooperative Oncology Group PS, 0–2; adequate liver and kidney function; serum bilirubin, <2.0 mg/dl; and serum creatinine, <2.0 mg/dl. Patients <55 years old were preconditioned with a myeloablative conditioning regimen (MAC) consisting of total body irradiation (TBI) of 12 Gray (Gy) and cyclophosphamide administration (CPA; 120 mg/kg), whereas patients aged between 55 and 65 years were preconditioned with a reduced intensity (RI) conditioning regimen, consisting of fludarabine (Flu; 180 mg/m2), busulfan (BU; 6.4 mg/kg), and low-dose TBI (2 Gy), for myeloid malignancy and Flu (125 mg/m2), melphalan (L-PAM; 150 mg/m2), and low-dose TBI (2 Gy) for lymphoid malignancy. For those with poor disease control (PD) before allo-HSCT, high-dose cytarabine (HDAC) or etoposide (VP-16) was additionally administered during the conditioning regimen for lymphoid malignancy. Eligible donors included human leukocyte antigen (HLA)-identical related and HLA-identical unrelated donors from the Japan Marrow Donation Program [unrelated bone marrow (UR-BM)] as well as cord blood (CB) from the Japan Cord Blood Bank Network (JCBBN). Prophylaxis for graft-versus-host disease (GVHD) was performed using short-term methotrexate (MTX) plus cyclosporine (CSP) on CB donors for allo-HSCT, and short-term MTX plus tacrolimus (FK 506) on UR-BM donors for allo-HSCT.
Regarding renal transplantation, the 31 cases comprised 22 living-donor and nine cadaveric-donor renal transplants. The status of HTLV-1 infection among the recipients and donors, and the protocol of immunosuppressive pharmacotherapy to prevent graft rejection after renal transplantation are described below. All donors for renal transplantation tested negative for HTLV-1, and we excluded an HTLV-1 positive donor based on the recommendation of the Japanese Society for Clinical Renal Society and the Ministry of Health, Labour and Welfare in 2014. Thus, two HTLV-1 carrier donors were excluded as renal transplantation donors at the health check-up. Only one recipient undergoing renal transplantation tested positive for HTLV-1. The immunosuppressive pharmacotherapy protocol to prevent graft rejection in patients after renal transplantation was as follows: induction therapy with basiliximab (20 mg daily at transplant and on postoperative day 4), followed by mycophenolate mofetil (MMF; 1000 mg twice daily, tapered to 500 mg twice daily at approximately 3 months post-transplant), and corticosteroids (250-mg bolus injection intraoperatively, 24 mg daily in the first week, tapered to 4 mg daily by 1 month post-transplant). Rituximab was administered 1 week before transplant to patients who received repeat transplants and ABO-incompatible transplants.36
This retrospective study was conducted in compliance with good clinical practice and the ethical principles of the Declaration of Helsinki. The appropriate ethics committees and institutional review boards approved this study.
RESULTS
We retrospectively analyzed the impact of HTLV-1 on organ transplantation in 54 allo-HSCT BMT cases and 31 renal transplantation cases (Table 1).
Table 1. The impact of HTLV-1 on organ transplantation [allogeneic hematopoietic stem cell transplantation (allo-HSCT) or renal transplantation] at our institution.
| Organ transplantation |
Cases (an 11-year period from January 2006 to December 2016) |
HTLV-1 status of organ transplantation |
After organ transplantation | ||
|---|---|---|---|---|---|
| ATL development |
atypical HAM |
PTLD | |||
| Bone Marrow Transplantation |
54 cases | Recipient: ATL (9) HTLV-1 carrier (1) Donor (0) |
ATL relapse (recipient) 4 |
+ 5 months |
- |
| Renal Transplantation |
31cases Living donor:22, cadaveric donor:9 (cardiopulmonary arrest donor 6, brain-dead donor 3) |
Recipient (1) HTLV-1 carrier (1) Donor(0) |
- | - | + 10 years brain |
We retrospectively analyzed the impact of HTLV-1 in 54 allo-HSCT cases and 31 renal transplantation cases.
In the 54 allo-HSCT cases, the HTLV-1 status of the organ transplantation recipients was as follows: nine ATL cases and one HTLV-1 case. All donors tested negative for HTLV-1. After allo-HSCT, ATL development was not observed. However, atypical HAM developed demonstrating the absence of anti-HTLV-1 antibodies and the presence of HTLV-1 proviral DNA, with rapid development and progression of the symptoms at 5 months.
Of the 31 renal transplantation cases, 22 were living-donor and nine were cadaveric-donor transplants. All donors tested negative for HTLV-1. One recipient was an HTLV-1 carrier. We excluded an HTLV-1–positive donor according to the recommendation of the Japanese Society for Clinical Renal Society and the Ministry of Health, Labour and Welfare in 2014. At our institution, one HTLV-1 carrier who underwent renal transplantation did not develop ATL or HAM during careful follow-up. One HTLV-1 non-carrier developed PTLD in the brain 10 years after renal transplantation.
The impact of HTLV-1 after allo-HSCT
Among the 54 allo-HSCT cases, the HTLV-1 status of the allo-HSCT recipients was ATL in nine and one HTLV-1 carrier (Table 2). All donors for allo-HSCT tested negative for HTLV-1. Among the nine ATL cases after allo-HSCT, four eventually relapsed due to proliferation of recipient-derived ATL cells, as confirmed by PCR analysis for donor chimerism. Only one HTLV-1 carrier recipient who underwent allo-HSCT did not present with ATL or HAM development after allo-HSCT during follow-up. However, HAM-mimicking myelitis developed in the absence of anti-HTLV-1 antibody production with rapid development and progression of clinical symptoms at 5 months after allo-HSCT.
Table 2. Nine ATL cases and one HTLV-1 carrier among allo-HSCT cases.
| Case | Age | Sex | Subtype | Chemo-therapy | Disease status |
Allo-HSCT (Source) |
Allo-HSCT (Conditioning) |
GVHD prophylaxis |
Acute GVHD |
Survival |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | F | Lymphoma | CHOP (1) +Mog (8) |
SD | Unrelated HLA full matched BM | TBI/CY | CSP+sMTX | Grade 2 skin | 355 days (dead) |
| 2 | 46 | F | acute | VCAP- VMPV-ECP (1)+Mog (4) |
CR | Unrelated HLA full matched BM | TBI/CY | FK+sMTX | Grade 2 skin | 295 days (alive) |
| 3 | 54 | M | acute | VCAP- VMPV-ECP (1)+Mog (8) |
CR | Unrelated HLA full matched BM | TBI/CY | FK+sMTX | Grade 2 skin | 546 days (alive) |
| 4 | 49 | F | Lymphoma | CHOP-VMMV | CR | CB | TBI/CY | CSP+sMTX | - | 138 days (dead, relapse) |
| 5 | 42 | M | Lymphoma | CHOP-VMMV | CR | CB | TBI/CY | CSP+sMTX | - | 135 days (dead, relapse) |
| 6 | 61 | F | acute | CHOP-VMMV | PR | CB | BU/FLU/TBI | CSP+sMTX | - | 28 days (dead, relapse) |
| 7 | 44 | F | acute | VCAP- VMPV-ECP |
CR | Unrelated HLA full matched BM | TBI/CY | FK+sMTX | - | 1084 days (alive) |
| 8 | 56 | F | acute | VCAP- VMPV-ECP |
CR | Unrelated HLA full matched BM | TBI/CY | FK+sMTX | - | 1156 days (alive) |
| 9 | 41 | F | acute | VCAP- VMPV-ECP |
CR | Unrelated HLA full matched BM | TBI/CY | FK+sMTX | - | 122 days (dead) |
| 10 | 60 | M | HTLV-1 carrier AML | AZA | PR | UR-BMT | FLU/BU/TBI | FK+sMTX | - | 1080 days (alive) |
Abbreviations: F:female, M:male, allo-HSCT: allogeneic hematopoietic stem cell transplantation, CR: complete response, PR: partial response, TBI: total body irradiation, CY: cyclophosphamide, FLU: Fludarabine, BU: busulfan, CB: cord blood, BM: bone marrow
HTLV-1 carrier who underwent allo-HSCT: case 10
A 60-year-old male HTLV-1 carrier was diagnosed with myelodysplastic syndrome (MDS)-derived AML, and was subsequently treated with azacitidine (AZA) for bridging therapy after allo-HSCT with 30 mg/m2 of fludarabine (FLU) for 6 days, 0.8 mg/kg of intravenous busulfan (BU) every 6 hr for a total of eight dose, and 2 Gy of total body irradiation (TBI). Moreover, for GVHD prophylaxis, the patient received FK plus short-term MTX (Table 2). The patient did not develop ATL or HAM after allo-HSCT during follow-up.
A patient with atypical HAM after allo-HSCT: case 2
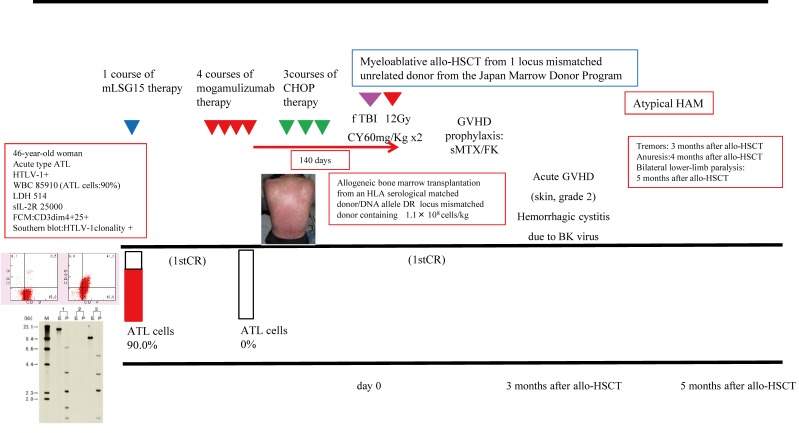
A 46-year-old woman was diagnosed with atypical HAM at 5 months post-allo-HSCT with acute-type ATL (Figures 1A and 1B). Complete remission (CR) of ATL was observed after one cycle of modified LSG15 therapy, four cycles of mogamulizumab therapy, and three cycles of CHOP therapy. Allo-HSCT was performed by MAC consisting of TBI 12 Gy and CPA 120 mg/kg for the patient from an unrelated donor of the Japan Marrow Donor Program with one locus HLA mismatch in a DNA allele. The number of days from the last mogamulizumab administration to allo-HSCT was 140. The patient developed tremors at 3 months, anuresis at 4 months, and bilateral lower-limb paralysis at 5 months after allo-HSCT for ATL.
Fig. 1A.
The outline of chemotherapy and allo-HSCT for acute-type ATL (case 2).
We describe the case of a 46-year-old woman who was diagnosed with atypical HAM at 5 months after allo-HSCT for acute-type ATL. She demonstrated CR of ATL after one cycle of modified LSG15 therapy, four cycles of mogamulizumab therapy, and three cycles of CHOP therapy. Allo-HSCT was performed by MAC consisting of TBI 12 Gy and CPA 120 mg/kg for the patient from an unrelated donor of the Japan Marrow Donor Program with one locus HLA mismatch in a DNA allele. The number of days from the last mogamulizumab administration to allo-HSCT was 140. The patient developed tremors at 3 months, anuresis at 4 months, and bilateral lower-limb paralysis at 5 months after allo-HSCT for ATL.
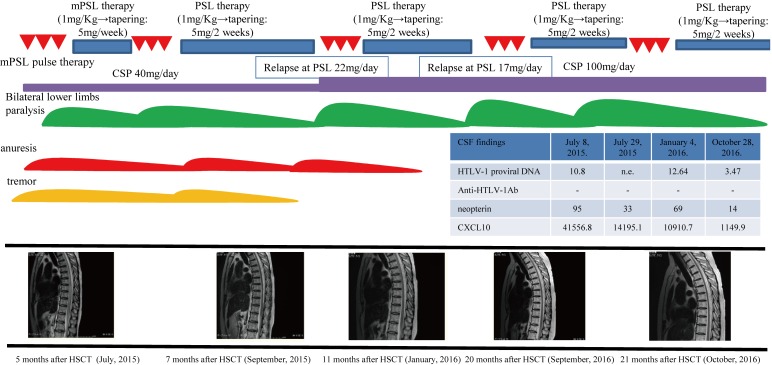
Fig. 1B.
The timing of diagnosis and treatment for atypical HAM (case 2). We made a final diagnosis of acute-onset atypical HAM based on the following findings: the symptoms of tremors, enuresis, and paralysis, the upregulation of inflammatory cells (82/mm3), the total protein level (150 mg/dl), and Th1 cytokine levels [neopterin, 95 pmol/ml; CXCL10 (also known as IP-10), 41556.8 pg/ml] in the CSF, radiological findings (swelling of the spinal cord with enhancement), the absence of anti-HTLV-1 antibodies and ATL cells, and the presence of HTLV-1 DNA in the CSF according to PCR analysis. The criteria of HAM were not fulfilled due to the lack of anti-HTLV-1 antibodies in the CSF after allo-HSCT. Treatment with two cycles of mPSL pulse therapy and maintenance PSL therapy with rehabilitation led to remission of the symptoms, and normalization of laboratory and radiological findings without relapse. However, atypical HAM relapsed during PSL tapering. Thus, we again administered mPSL pulse therapy with cyclosporine for atypical HAM. These treatments resolved the symptoms and returned the laboratory and radiological findings to CR status of acute-type ATL.
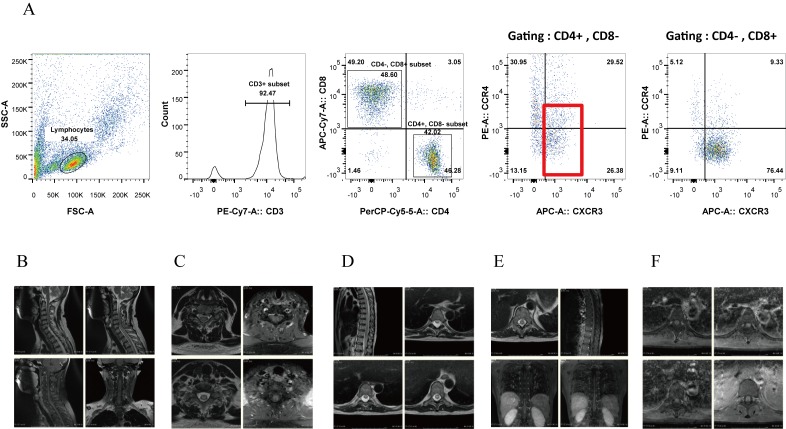
Laboratory testing of cerebrospinal fluid (CSF) demonstrated upregulation of inflammatory cells (82/mm3) and the total protein level (150 mg/dl), an oligoclonal band by immunoelectrophoresis (IEF), elevation of Th1 cytokines (neopterin, 95 pmol/ml; CXCL10, 41556 pg/ml), the absence of anti-HTLV-1 antibodies, and the presence of HTLV-1 DNA by PCR (Table 3A). Transition of an anti-HTLV-1 antibody revealed that serum anti-HTLV-1 antibodies had disappeared before the onset of atypical HAM (Table 3B). On flow cytometry (FCM) analysis, the proliferating inflammatory cells were CD4+ CXCR3+ (mostly CCR4- and partially CCR4+) (Figure 2A). Cytological analysis of CSF revealed the absence of ATL cells. On MRI, cervical (lower than C5/C6), thoracic, and lumbar areas of the spinal cord exhibited diffuse swelling with an enhanced irregular T2 high-intensity signal (Figures 2B–F).
Table 3A. Laboratory testing of cerebrospinal fluid (CSF) at admission (case 2).
| Laboratory data | Results | |
|---|---|---|
| CSF at admission | CSF cells | 82 Multi-nucleated cells 23% Mononucleated cells 77% |
| TP | 150 | |
| Sugar | 67 | |
| Cl | 118 | |
| HSV/CMV/HHV-6/VZV DNA | <100 | |
| MBP | 114 (normal range: <102) |
|
| IEF | Oligoclonal band | |
| IgG (CSF) | 14.9 mg/dl | |
| Aquaporin 4(AQP4) Ab | <1.3 | |
| Provival DNA in CSF | 10.80 copies/100 cells | |
| Anti-HTLV-1 Ab (PA method) |
Negative (over 16:positieve) |
|
| Neopterin | 95 (pmol/mL) (control < 5 pmol/mL) |
|
| CXCL10 | 41556.8 (pg/mL) (control <200.0 pg/mL) |
Table 3B. Laboratory findings regarding anti–HLTV-1 antibody transition in serum (case 2).
| March, 2015 | April, 2015 | May, 2015 | June, 2015 | |
|---|---|---|---|---|
| HTLV-1 antibody in serum chemistry | 2^7 | 2^7 | 2^5 | - |
Fig. 2.
Flow-cytometry analysis of CSF (A) and MRI findings (B–F) at admission (case 2).
Thus, we made a final diagnosis of acute-onset atypical HAM based on the following findings: the symptoms of tremors, anuresis, and paralysis, the upregulation of inflammatory cells (82/mm3), the total protein level (150 mg/dl), and Th1 cytokine levels [neopterin, 95 pmol/ml; CXCL10 (also known as IP-10), 41556.8 pg/ml] in the CSF, radiological findings (swelling of the spinal cord with enhancement), the absence of an anti-HTLV-1 antibody and ATL cells, and the presence of HTLV-1 DNA in the CSF according to PCR analysis. The criteria of HAM were not fulfilled due to the lack of anti-HTLV-1 antibodies in the CSF after allo-HSCT. The bone marrow after allo-HSCT was found to contain donor-derived hematopoietic cells by PCR analysis for donor chimerism, but the inflammatory cells in the CSF in this case were not subjected to PCR analysis for donor chimerism. Treatment with two cycles of mPSL pulse therapy and maintenance PSL therapy with rehabilitation led to remission of the symptoms, and normalization of laboratory and radiological findings without relapse. However, atypical HAM relapsed during PSL tapering. Thus, we again administered mPSL pulse therapy with cyclosporine for atypical HAM. These treatments resolved the clinical symptoms, and returned the laboratory and radiological findings to CR of acute-type ATL.
The impact of HTLV-1 on renal transplantation
Regarding renal transplantation, the 31 cases comprised 22 living-donor and nine cadaveric-donor renal transplants. All donors for renal transplantation tested negative for HTLV-1. One recipient was an HTLV-1 carrier. We excluded HTLV-1-positive donors according to the recommendation of the Japanese Society for Clinical Renal Society and the Ministry of Health, Labour and Welfare in 2014. Thus, two HTLV-1 carrier donors were excluded as renal transplantation donors at the health check-up. At our institution, only one recipient did not present with ATL or HAM development after renal transplantation during follow-up. Only one HTLV-1-negative recipient developed PTLD in the brain 10 years after renal transplantation.
An HTLV-1 carrier with renal transplantation: case 11
We present the clinical course of one HTLV-1 carrier recipient who underwent renal transplantation (Table 4). A 65-year-old male was diagnosed with end stage renal disease (ESRD, unknown etiology) and subsequently underwent renal transplantation. The patient was found to be an HTLV-1 carrier before renal transplantation. Laboratory tests confirmed the HTLV-1 carrier status, and approximately 1% of the proliferating ATL cells in the peripheral blood expressed CD4 and CD25. He had normal lactate dehydrogenase (LDH) levels, upregulation of soluble IL-2 receptor (sIL-2R) (2257 U/ml), no clonality of the HTLV-1 genome according to Southern blot analysis, and no systemic lymphadenopathy/splenomegaly. Thus far, the patient has not developed ATL or HAM during careful follow-up at 120 days after renal transplantation.
Table 4. An HTLV-1 carrier with renal transplantation (case 11).
| Case | Age | Sex | Subtype | ATL cells in peripheral blood | FCM analysis | sIL-2R | Southern blot analysis | CT (Chest/Abdominal CT) |
Renal transplantation (source) |
ATL development |
HAM development |
Survival |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11 | 65 | M | HTLV -1 carrier | 1% | CD4 +CD 25+ |
2257 | No clona lity |
No systemic lymphadenopathy /no splenomegaly |
Living donor | - | - | 120 days (alive) |
A 65-year-old male (case 11) was diagnosed with ESRD (unknown etiology) and underwent renal transplantation before the health check-up. The patient was found to be an HTLV-1 carrier before renal transplantation examination. Laboratory testing confirmed the HTLV-1 carrier status, and approximately 1% of the proliferating ATL cells in the peripheral blood expressed CD4 and CD25. He had a normal LDH level, upregulation of sIL-2R, no clonality of the HTLV-1 genome according to Southern blot analysis, and no systemic lymphadenopathy/splenomegaly. After renal transplantation, the patient did not develop ATL or HAM during careful follow-up.
Development of PTLD in the brain of a patient 10 years after renal transplantation: case 12
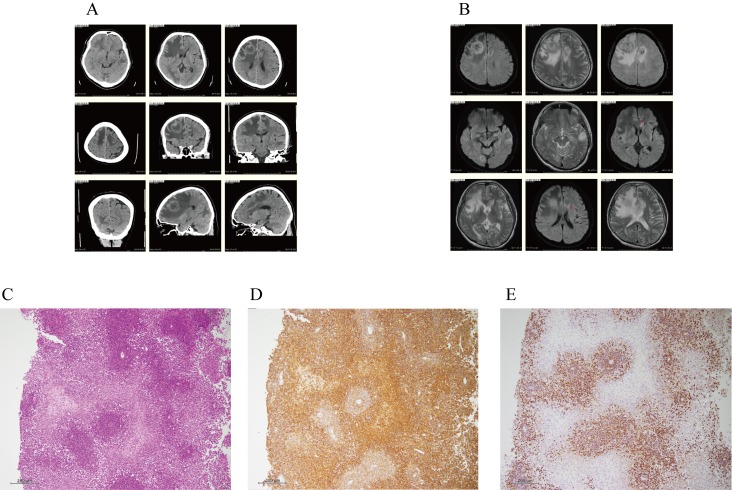
A 60-year-old male HTLV-1 non-carrier was diagnosed with ESRD due to diabetes mellitus (DM) and subsequently underwent renal transplantation on June 23, 2006. During follow-up, the patient developed aggressive lower-limb paralysis on January 6, 2016. CT demonstrated a 3-cm, low-density area with a ring enhancement lesion in the right anterior lobe, and multiple low-density areas in the right occipital, left anterior, and temporary lobes (Figure 3A). Furthermore, MRI revealed a 3-cm, heterogeneous high-intensity area, and multiple T2 high-intensity lesions in the right occipital, left anterior, and temporary lobes (Figure 3B). These observations suggested multiple brain metastases or PTLD in the brain. On subsequent histological analysis of a brain biopsy specimen, diffuse proliferation of abnormal lymphoid cells expressing B-cell markers (CD20 and CD79a) and Epstein-Barr virus (EBV) infection markers were noted [EBV-encoded small RNA (EBER) according to fluorescence in situ hybridization (FISH)] (Figures 3C–E). We diagnosed him with PTLD (EBV+) based on the results of the brain biopsy. Therefore, FK and MMF administration was immediately ceased. PTLD in the brain remained unchanged, and he was treated with rituximab at a dose of 375 mg/m2 and high-dose cytarabine (HDAC) at 3 g/body every 12 hr for a total of four doses during hemodialysis. Even after one cycle of HDAC therapy, PTLD in the brain progressed aggressively. The patient was given rituximab (375 mg/m2) and high-dose methotrexate (HD-MTX) at 3.5 g/body during hemodialysis. However, PTLD in the brain continued progressing aggressively, and he died.
Fig. 3.
A and B. CT for case 11 demonstrated a 3-cm, low-density area with a ring enhancement lesion in the right anterior lobe, and multiple low-density areas in the right occipital, left anterior, and temporary lobes (A). Furthermore, MRI revealed a 3-cm, heterogeneous high-intensity area, and multiple T2 high-intensity lesions in the right occipital, left anterior, and temporary lobes (B). These observations suggested multiple brain metastases or PTLD in the brain.
C, D and E. On histology the brain biopsy specimen of case 11, the abnormal lymphoid cells had larger and hyperchromatic nuclei, and had diffusely proliferated predominantly in the perivascular region with marked necrosis (C). Immunohistochemically, the cells were positive for L26 (CD20), CD79a, MUM1, and Bcl-2, and negative for CD3, UCHL1 (CD45Ro), CD5, CD10, and Bcl-6, indicating non-GC type diffuse large B-cell lymphoma (DLBCL) (D). Moreover, all lymphoid cells expressed EBV markers, as assessed by EBER-FISH (E). Thus, these findings are consistent with monomorphic post-transplant lymphoproliferative disorders [DLBCL with EBV (+)].
DISCUSSION
In our retrospective study on 54 cases of allo-HSCT, one HTLV-1 carrier who underwent allo-HSCT for MDS-derived AML did not develop ATL or HAM during the follow-up period. Among nine ATL cases after allo-HSCT, four eventually relapsed due to proliferation of recipient-derived ATL cells. Of note, only one patient with ATL who underwent allo-HSCT presented with rapid and progressive development of atypical HAM at 5 months post-allo-HSCT.
In our retrospective study on 31 cases of renal transplantation, one HTLV-1 carrier recipient who underwent renal transplantation did not develop ATL or HAM during follow-up, one HTLV-1 non-carrier recipient developed PTLD in the brain 10 years after renal transplantation, and two HTLV-1 carrier donors were excluded based on the recommendation of the Japanese Society for Clinical Renal Society and the Ministry of Health, Labour and Welfare in 2014.
Our clinical study demonstrated the following:
(i) No development of ATL or HAM in 2 HTLV-1 carriers after organ transplantation (allo-HSCT and renal transplantation).
(ii) The development of atypical HAM after allo-HSCT in one ATL case during the follow-up period.
(iii) No new HTLV-1 infection via organ transplantation from HTLV-1 carrier donors to HTLV-1 negative recipients (allo-HSCT and renal transplantation).
We focused on HTLV-1 infection and HTLV-1-associated diseases, including ATL and HAM or atypical HAM, after organ transplantation.
We observed no development of ATL or HAM in two HTLV-1 carriers after organ transplantation (allo-HSCT or renal transplantation) during the follow-up period.
Regarding HAM development, one case after allo-HSCT and 13 cases after organ transplantation, including 10 cases after renal transplantation, two cases after liver transplantation, and one case after heart transplantation have been reported (Table 5).21-33 The majority of the cases were due to donor-derived HAM.22,23,28 Thus, the Japanese Society for Clinical Renal Society and the Ministry of Health, Labour and Welfare in 2014 recommended the exclusion of HTLV-1 positive carriers from renal transplantation donors based on the high incidence of HAM after renal transplantation. The presence of anti-HTLV-1 antibodies, and rapid development and progression during several months to several years were characteristic of HAM after organ transplantation, differing from the slowly progressing HAM generally observed in patients aged 40 to 60 years.21-33 In our study, one HTLV-1 carrier who underwent allo-HSCT and one HTLV-1 carrier who underwent renal transplantation did not develop HAM during careful follow-up.
Table 5. Previous reports of HAM-mimicking myelitis after allo-HSCT and HAM after organ transplantation.
| Year | HAM | Recipient after OTP |
Development of HAM | Age, sex | Transplantation | Recipient HTLV-1 |
Donor HTLV-1 |
Recipient HTLV-1 after organ transplantation |
HTLV-1 DNA |
Neopterin | Immune suppression |
Donor cells | Therapy | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kawamata et al. | 2014 | HAM- mimicking myelitis |
- | 20 months | 63, F | BMT | + | - | - | - | 8 | CSP | CD4+ CXCR3+ CCR4+ |
mPSL | Imp |
| Present case | 2017 | Atypical HAM |
- | 5 months | 46, F | BMT | + | - | - | 10.8 copies/ 1000 cells) |
95 | FK+sMTx →CSP |
CD4+ CXCR3+ partially CCR4+ |
mPSL | Imp |
| Nagamine et al. | 2014 | HAM | + | 2 months | 38, F | RTP | - | + | + | 15/1000(PB) | 51 | FK/MMF | CD4+:54% | IFNα | Imp |
| Ramanan et al. | 2014 | HAM | + | 5 months | 56, M | RTP | - | + | + | n.d. | n.d. | FK/MMF PSL |
n.d. | PSL AZT |
Imp |
| Inose et al. | 2010 | HAM | + | 10 months | 51, M | RTP | - | n.e. | + | 7.4% | n.d. | CY/PSL | n.d. | IFNα | Imp |
| Kuroki et al. | 2007 | HAM | n.e. | 17 months | 40, M | RTP | n.e. | n.e. | n.e. | 0.83% | n.d. | CY/MMF/PSL | n.d. | PSL | Imp |
| Toro C et
al. Zarranz Imirizaidu JJ et al. |
2003 | HAM | + | 24 months | 54, F | RTP | - | + | + | 12.44% (164.4/1000) |
n.d. | CY | n.d. | PSL | PD |
| Toro C et
al. Zarranz Imirizaidu JJ et al. |
2003 | HAM | + | 20 months | 57, M | RTP | - | + | + | 16.44% (124.4/1000) |
n.d. | CY | n.d. | PSL | PD |
| Nakamura N et al. | 2001 | HAM | + | < 1 year | 48, M | RTP | n.e. | n.e. | + | n.d. | 121 | n.d. | n.d. | PE | Imp |
| Shintani et al. | 2001 | HAM | + | 7 years | 56, M | RTP | n.e. | n.e. | + | n.d. | n.d. | n.d. | n.d. | PSL | n.d. |
| Nakatsuji Y et al. | 2010 | HAM | + | 4 years | 50, M | RTP | - | n.e. | + | n.d. | n.d. | CY/MMF/PSL | n.d. | n.d. | n.d. |
| Kuroda Y et al. | 1992 | HAM | + | 11 months | 32, M | RTP | n.e. | + | + | n.d. | n.d. | CY/MZR PSL.AZA |
n.d. | PSL | Imp |
| Soyama A | 2008 | HAM | + | 20 months | 58, M | Liver TRP | + | + | + | 180/1000 | n.d. | TAC/PSL | n.d. | IFNα ribavium |
unchange |
| Toro C et
al. Zarranz Imirizaidu JJ et al. |
2003 | HAM | + | 18 months | 44, F | Liver TRP | - | + | + | + | n.d. | TAC | n.d. | PSL IFNα |
SD |
| Ozen S et al. | 2001 | HAM | + | 5 months | 41, M | Heart TRP | - | - | + | n.d. | n.d. | n.d. | n.d. | PSL | Imp |
In the present and previous reports of HAM-mimicking myelitis or atypical HAM after allo-HSCT and of HAM after organ transplantation (renal, liver, or heart transplantation), two cases were after allo-HSCT, and 13 cases were after organ transplantation, including 10 cases after renal transplantation, two cases after liver transplantation, and one case after heart transplantation. The majority of cases were donor-derived HAM. The presence of anti-HTLV-1 antibodies, and rapid development and progression of HAM were characteristic of HAM after organ transplantation. In one case after allo-HSCT, HAM-mimicking myelitis developed in the absence of anti–HTLV-1 antibodies and the presence of HTLV-1 proviral DNA, with rapid development and progression of the symptoms. In our case after allo-HSCT, atypical HAM developed in the absence of anti–HTLV-1 antibodies and presence of HTLV-1 proviral DNA, with rapid development and progression of the symptoms.
Regarding ATL development after allo-HSCT and organ transplantation (renal or liver transplantation), two cases were reported after allo-HSCT and 11 cases after organ transplantation, including six cases after renal transplantation and five cases after liver transplantation (Table 6).6-20 The majority of the cases were recipient-derived ATL,7,8,13 except for two cases of ATL that developed after allo-HSCT.17,18 The rapid development and progression of ATL during several months to several years is characteristic of ATL after allo-HSCT and organ transplantation, different from the slowly progressing ATL generally observed in patients aged 40 to 60 years.6-20 We previously reported three cases of acute-type ATL development that originated from a recipient in eight HTLV-1 carriers among 164 living-donor liver transplant recipients undergoing immunosuppressive treatment.13 At our institution, among nine ATL cases after allo-HSCT, four eventually relapsed because of recipient-derived ATL cells confirmed to have recipient chimerism by PCR analysis. This is different from the study of donor-derived ATL development by Kitamura et al., in which the development of HTLV-1-associated donor-derived PTLD occurred after allo-HSCT by virus transmission from recipient to donor cells.19 Moreover, one HTLV-1 carrier who underwent allo-HSCT and one HTLV-1 carrier who received renal transplantation did not develop ATL or HAM during careful follow-up. Further follow-up is needed for early detection and immediate treatment of ATL or HAM after allo-HSCT or renal transplantation. One HTLV-1 non-carrier in our study developed PTLD (EBV+) in the brain after renal transplantation instead of ATL. Immediate diagnosis and subsequent treatment, including rituximab plus HDAC or HD-MTX, did not improve PTLD in the brain according to a review by Nagel et al.,37 a previous report of PTLD in the brain,15,38 and previous reports on PTLD treatments.39-46 Thus, careful follow-up may also be necessary to detect the development of PTLD after renal transplantation.
Table 6. Previous reports of ATL development after allo-HSCT and organ transplantation.
| Year | ATL | Development of ATL | Age, sex | Transplantation | Recipient HTLV-1 |
Donor HTLV-1 |
Immune suppression | Therapy for ATL | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|
| Tamaki et al. | 2006 | ATL | 2 months | 63, F | BMT | + | + | CSP/PSL | Irradiation of 3060 cGy and rapid reduction of immunosuppressive agents |
32months alive |
| Nakamizo et al. | 2011 | ATL | 6 years | 46, F | BMT | + | + | CSP | Whole brain irradiation and localised irradiation |
27 months alive |
| Kitamura et al. | 2017 | ATL | 16 months | 52, M | BMT | + | - | FK/PSL | PSL | Several months, PD |
| Kawano and Yoshizumi et al. |
2006 | ATL | 6 months | 39, F | Liver trp | + | + | FK/PSL | Discontinuation of immune suppression agents |
15 days dead |
| Kawano and Yoshizumi et al. |
2006 | ATL | 2 years | 45, M | Liver trp | + | + | FK/PSL | Chemotherapy LSG 15 ③ |
3 months dead |
| Kawano and Yoshizumi et al. |
2006 | ATL | 9 months | 67, M | Liver trp | + | + | FK/PSL | Chemotherapy CHOP① and CHOV-VMMV③ |
5 months dead |
| Yoshizumi et al. | 2012 | ATL | 4 years | 48, M | Liver trp | - | + | CSP/PSL | Chemotherapy | 15 months dead |
| Iiona et al. | 2013 | ATL | 2 years | 59, M | Liver trp | - | + | FK/PSL | Chemotherapy | 27 months dead |
| Zanke et al. | 1989 | ATL | 2 years | 43, M | Renal trp | n.d. | n.d. | CSP/PSL | Chemotherapy CVP④ |
20 months alive |
| Tsurumi et al. | 1992 | ATL | 4 years | 32, M | Renal trp | + | - | CSP/ mizoribine |
Chemotherapy CHOP⑥, KM2210 |
13 months alive |
| Willizms et al. | 1994 | ATL | 13 years | 42, M | Renal trp | n.d. | n.d. | Azathioprine/PSL | No treatment | 1 day dead |
| Jenks et al. | 1995 | ATL | 9 months | 61, M | Renal trp | + | - | CSP/azathioprine/PSL | Chemotherapy CHOP① |
5 days dead |
| Mouri et al. | 2000 | ATL | 3 years | 49, F | Renal trp | n.d. | n.d. | FK/ azathioprine/PSL |
Chemotherapy CHOP① |
1month dead |
| Ichikawa et al. | 2000 | ATL | 10 years | 42, M | Renal trp | - | n.d. | CSP/azathioprine/PSL | Chemotherapy CHOP+radiation |
3months dead |
| Glowacka et al. | 2013 | ATL | 2 years | 59, M | Liver trp | - | + | FK | PSL | alive |
| Glowacka et al. | 2013 | ATL | 3 years | 28, M | Renal trp | - | n.d. | FK/MMF | PSL | alive |
In the present and previous reports of ATL development after allo-HSCT and organ transplantation (renal or liver transplantation), three cases were after allo-HSCT and 13 cases were after organ transplantation, including six cases after renal transplantation and five cases after liver transplantation. The majority of the cases were recipient-derived ATL, except for ATL development after allo-HSCT. Rapid development and progression were characteristic of ATL development after allo-HSCT and organ transplantation. At our institution, two HTLV-1 carriers who underwent allo-HSCT and renal transplantation did not develop ATL or HAM during careful follow-up.
In the present study, negative clinical data was considered to indicate the absence of ATL or HAM development in 2 HTLV-1 carrier cases after organ transplantation. Although our study population was small and the cohort study period was too short, the clinical data of two HTLV-1 carriers who did not develop ATL or HAM after organ transplantation may help clarify the impact of HTLV-1 infection on organ transplantation. In the future, further accumulation of cases or systemic review of HTLV-1 carriers after organ transplantation is needed.
Second, in one ATL case after allo-HSCT, atypical HAM rapidly developed at five months after allo-HSCT. Kawamata et al. first reported HAM-mimicking myelitis after allo-HSCT for ATL.21 In this report, the patient developed crural paresis 14 months after allo-HSCT. Initially, she was diagnosed with CNS relapse of ATL, and was treated by intrathecal injection and whole-brain and spine irradiation. However, the neurological symptoms recurred 5 months later. She was diagnosed with HAM-mimicking myelitis based on her symptoms, MRI findings, CSF analysis demonstrating increased CD4+ CXCR3+ CCR4+ cell numbers and Th1 cytokine levels [neopterin and CXCL10 (also known as IP-10)], without anti-HTLV-1 antibodies or HTLV-1 proviral DNA. Based on the diagnosis of HAM-mimicking myelitis, the reported patient was successfully treated with PSL.
Although we noted similar clinical findings, MRI results, and CSF findings as those reported by Kawamata et al., the HTLV-1 proviral genome in CSF was detected in our case. Thus, it may have been a neurogenerative disease caused by HTLV-1 infection. As anti-HTLV-1 antibodies were not detected in the CSF, we made a diagnosis of atypical HAM without anti-HTLV-1 antibodies in the CSF instead of HAM-mimicking myelitis. Previous reports regarding ATL development after organ transplantation have demonstrated that the transmission of HTLV-1 between donor cells and recipient cells can occur in organ transplantation patients.19,20 In particular, Kitamura et al. reported HTLV-1-associated PTLD after allo-HSCT by virus transmission from recipient to donor cells.19 Similar with the report by Kitamura et al., the patient in our study may have developed atypical HAM after HTLV-1 transmission from recipient to donor cells based on the following findings: an HTLV-1-positive recipient and HTLV-1-negative donor before allo-HSCT, and the presence of HTLV-1 proviral DNA in the CSF and donor-derived hematopoietic cells after allo-HSCT.
Moreover, Yamano et al. proposed the following pathogenesis for HAM: HTLV-1 induces formation of CD4+CXCR3+ CCR4+ cells, designated as HAM/tropical spastic paraparesis (TSP) cells, which produce IFN-γ and subsequently activate astrocytes. These cells then produce Th1 cytokines, which consequently damage neuronal cells.5 In our case, atypical HAM donor cells with CD4+CXCR3+ (mostly CCR4- and partially CCR4+) may have played a role in the pathogenesis of atypical HAM by inducing astrocytes to produce Th1 cytokines. Markedly high Th1 activity may be related to the development of atypical HAM. Thus, immediate diagnosis and immediate PSL treatment are necessary to improve the symptoms and reverse the radiological findings of atypical HAM after allo-HSCT. At present, caution is needed regarding the implications of this case for organ transplantation because only a few cases of HAM-mimicking myelitis or atypical HAM after allo-HSCT have been reported. Thus, further accumulation of similar cases after organ transplantation are required in the future.
In endemic areas of HTLV-1 infection, such as our institution in Miyazaki prefecture, we did not perform organ transplantation from HTLV-1 carrier donors to HTLV-1-negative recipients in order to prevent newly HTLV-1 infection. In our study on 54 patients who underwent allo-HSCT, including nine ATL patients and one HTLV-1 carrier, and 31 patients who underwent renal transplantation, all donors tested negative for HTLV-1. In 2014, the Japanese Society for Clinical Renal Society and the Ministry of Health, Labour and Welfare recommended the exclusion of HTLV-1-positive donors for renal transplantation. Thus, to prevent new HTLV-1 infection, organ transplantation from HTLV-1 carrier donors to HTLV-1-negative recipients should be avoided in clinical practice.
In conclusion, in our study, only one of 54 patients who underwent allo-HSCT developed atypical HAM. Furthermore, of the 31 patients who underwent renal transplantation, one HTLV-1 non-carrier developed PTLD in the brain. One HTLV-1 carrier who received renal transplantation and one HTLV-1 carrier who underwent allo-HSCT did not develop ATL or HAM during follow-up. Although our study population was small and the cohort study period was too short, careful follow-up of HTLV-1 infected recipients after organ transplantation is important because atypical HAM can develop in ATL patients after allo-HSCT. In addition, to determine the risk for ATL or HAM development in HTLV-1 infected recipients, we prospectively followed up our cohort. In the future, further accumulation of cases or systemic review of HTLV-1 carriers after organ transplantation may clarify the clinical impact of HTLV-1 on organ transplantation.
Footnotes
CONFLICT OF INTEREST: None of the authors has any conflicts of interest.
REFERENCES
- 1.Taylor GP. Human T-lymphotropic virus type 1 infection and solid organ transplantation. Rev Med Virol. 2018; 28: e1970. 10.1002/rmv.1970 [DOI] [PubMed] [Google Scholar]
- 2.Miyoshi H, Ohshima K. Epidemiology of malignant lymphoma and recent progress in research on adult T-cell leukemia/lymphoma in Japan. Int J Hematol. 2018; 107: 420-427. 10.1007/s12185-018-2430-6 [DOI] [PubMed] [Google Scholar]
- 3.Kogure Y, Kataoka K. Genetic alterations in adult T-cell leukemia/lymphoma. Cancer Sci. 2017; 108: 1719-1725. 10.1111/cas.13303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osame M, Usuku K, Izumo S, et al. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986; 327: 1031-1032. 10.1016/S0140-6736(86)91298-5 [DOI] [PubMed] [Google Scholar]
- 5.Yamano Y, Coler-Reilly A. HTLV-1 induces a Th1-like state in CD4+CCR4+ T cells that produces an inflammatory positive feedback loop via astrocytes in HAM/TSP. J Neuroimmunol. 2017; 304: 51-55. 10.1016/j.jneuroim.2016.08.012 [DOI] [PubMed] [Google Scholar]
- 6.Zanke BW, Rush DN, Jeffery JR, et al. HTLV-1 T cell lymphoma in a cyclosporine-treated renal transplant patient. Transplantation. 1989; 48: 695-697. [PubMed] [Google Scholar]
- 7.Tsurumi H, Tani K, Tsuruta T, et al. Adult T-cell leukemia developing during immunosuppressive treatment in a renal transplant recipient. Am J Hematol. 1992; 41: 292-294. 10.1002/ajh.2830410414 [DOI] [PubMed] [Google Scholar]
- 8.Jenks PJ, Barrett WY, Raftery MJ, et al. Development of human T-cell lymphotropic virus type I-associated adult T-cell leukemia/lymphoma during immunosuppressive treatment following renal transplantation. Clin Infect Dis. 1995; 21: 992-993. 10.1093/clinids/21.4.992 [DOI] [PubMed] [Google Scholar]
- 9.Williams NP, Buchner LM, Shah DJ, et al. Adult T-cell leukemia/lymphoma in a renal transplant recipient: an opportunistic occurrence. Am J Nephrol. 1994; 14: 226-229. 10.1159/000168722 [DOI] [PubMed] [Google Scholar]
- 10.Mori J, Kamiryo Y, Yano S, et al. Adult T-cell leukemia following ABO incompatible renal transplant patients in Japan. Renal Transplantation Vascular Surgery. 2000; 12: 137-141. [Google Scholar]
- 11.Hoshida Y, Li T, Dong Z, et al. Lymphoproliferative disorders in renal transplant patients in Japan. Int J Cancer. 2001; 91: 869-875. [DOI] [PubMed] [Google Scholar]
- 12.Ichikawa Y, Iida M, Ebisui C, et al. A case study of adult t-cell lymphoma in a kidney transplant patient. Transplant Proc. 2000; 32: 1982-1983. 10.1016/S0041-1345(00)01521-9 [DOI] [PubMed] [Google Scholar]
- 13.Kawano N, Shimoda K, Ishikawa F, et al. Adult T-cell leukemia development from a human T-cell leukemia virus type I carrier after a living-donor liver transplantation. Transplantation. 2006; 82: 840-843. 10.1097/01.tp.0000235186.30113.c7 [DOI] [PubMed] [Google Scholar]
- 14.Yoshizumi T, Shirabe K, Ikegami T, et al. Impact of human T cell leukemia virus type 1 in living donor liver transplantation. Am J Transplant. 2012; 12: 1479-1485. 10.1111/j.1600-6143.2012.04037.x [DOI] [PubMed] [Google Scholar]
- 15.Yoshizumi T, Takada Y, Shirabe K, et al. Impact of human T-cell leukemia virus type 1 on living donor liver transplantation: a multi-center study in Japan. J Hepatobiliary Pancreat Sci. 2016; 23: 333-341. 10.1002/jhbp.345 [DOI] [PubMed] [Google Scholar]
- 16.Nakamura N, Tamaru S, Ohshima K, et al. Prognosis of HTLV-I-positive renal transplant recipients. Transplant Proc. 2005; 37: 1779-1782. 10.1016/j.transproceed.2005.03.132 [DOI] [PubMed] [Google Scholar]
- 17.Tamaki H, Matsuoka M. Donor-derived T-cell leukemia after bone marrow transplantation. N Engl J Med. 2006; 354: 1758-1759. 10.1056/NEJMc053295 [DOI] [PubMed] [Google Scholar]
- 18.Nakamizo A, Akagi Y, Amano T, et al. Donor-derived adult T-cell leukaemia. Lancet. 2011; 377: 1124. 10.1016/S0140-6736(11)60315-2 [DOI] [PubMed] [Google Scholar]
- 19.Kitamura N, Nakanishi T, Yoshida Y, et al. HTLV-I–associated posttransplant lymphoproliferative disorder following virus transmission from recipient to donor cells. Blood. 2017; 130: 84-86. 10.1182/blood-2016-11-749820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glowacka I, Korn K, Potthoff SA, et al. Delayed seroconversion and rapid onset of lymphoproliferative disease after transmission of human T-cell lymphotropic virus type 1 from a multiorgan donor. Clin Infect Dis. 2013; 57: 1417-1424. 10.1093/cid/cit545 [DOI] [PubMed] [Google Scholar]
- 21.Kawamata T, Ohno N, Sato K, et al. A case of post-transplant adult T-cell leukemia/lymphoma presenting myelopathy similar to but distinct from human T-cell leukemia virus type I (HTLV- I)-associated myelopathy. Springerplus. 2014; 3: 581. 10.1186/2193-1801-3-581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagamine Y, Hayashi T, Kato Y, et al. Human T lymphotropic virus type-1-associated myelopathy manifesting shortly after living-donor renal transplantation. Intern Med. 2015; 54: 75-78. 10.2169/internalmedicine.54.2950 [DOI] [PubMed] [Google Scholar]
- 23.Ramanan P, Deziel PJ, Norby SM, et al. Donor-transmitted HTLV-1-associated myelopathy in a kidney transplant recipient--case report and literature review. Am J Transplant. 2014; 14: 2417-2421. 10.1111/ajt.12849 [DOI] [PubMed] [Google Scholar]
- 24.Nakatsuji Y, Sugai F, Watanabe S, et al. HTLV-I-associated myelopathy manifested after renal transplantation. J Neurol Sci. 2000; 177: 154-156. 10.1016/S0022-510X(00)00332-4 [DOI] [PubMed] [Google Scholar]
- 25.Kuroda Y, Takashima H, Yukitake M, et al. Development of HTLV-I-associated myelopathy after blood transfusion in a patient with aplastic anemia and a recipient of a renal transplant. J Neurol Sci. 1992; 109: 196-199. 10.1016/0022-510X(92)90168-K [DOI] [PubMed] [Google Scholar]
- 26.Inose Y, Akiyama S, Mochizuki A, et al. A case report of HTLV-1 associated myelopathy (HAM) manifested after renal transplantation. Rinsho Shinkeigaku. 2010; 50: 241-245. 10.5692/clinicalneurol.50.241 [DOI] [PubMed] [Google Scholar]
- 27.Kuroki N, Kin M, Tateishi T. Rinsho Shinkeigaku. 2007; 48: 222 [in Japanese]. [Google Scholar]
- 28.Toro C, Rodés B, Poveda E, et al. Rapid development of subacute myelopathy in three organ transplant recipients after transmission of human T-cell lymphotropic virus type I from a single donor. Transplantation. 2003; 75: 102-104. 10.1097/00007890-200301150-00019 [DOI] [PubMed] [Google Scholar]
- 29.Imirizaldu JJZ, Gomez Esteban JC, Rouco Axpe I, et al. Post-transplantation HTLV-1 myelopathy in three recipients from a single donor. J Neurol Neurosurg Psychiatry. 2003; 74: 1080-1084. 10.1136/jnnp.74.8.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shintani Y, Napou Y, Fujii R, et al. One case of HAM after cadaveric renal transplantation. Ishoku. 2001; 36: 286. [Google Scholar]
- 31.Soyama A, Eguchi S, Takatsuki M, et al. Human T-cell leukemia virus type I–associated myelopathy following living-donor liver transplantation. Liver Transpl. 2008; 14: 647-650. 10.1002/lt.21414 [DOI] [PubMed] [Google Scholar]
- 32.Ozden S, Seilhean D, Gessain A, et al. Severe demyelinating myelopathy with low human T cell lymphotropic virus type 1 expression after transfusion in an immunosuppressed patient. Clin Infect Dis. 2002; 34: 855-860. 10.1086/338868 [DOI] [PubMed] [Google Scholar]
- 33.Narukawa N, Shiizaki K, Kitabata Y, et al. Plasma exchange for the treatment of human T-cell lymphotropic virus type 1 associated myelopathy. Ther Apher Dial. 2001; 5: 491-493. 10.1046/j.1526-0968.2001.00398.x [DOI] [PubMed] [Google Scholar]
- 34.Kawano N, Kuriyama T, Yoshida S, et al. The impact of a humanized CCR4 antibody (Mogamulizumab) on patients with aggressive-type adult T-cell leukemia-lymphoma treated with allogeneic hematopoietic stem cell transplantation. J Clin Exp Hematop. 2017; 56: 135-144. 10.3960/jslrt.56.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagafuji K, Miyamoto T, Eto T, et al. Monitoring of minimal residual disease (MRD) is useful to predict prognosis of adult patients with Ph-negative ALL: results of a prospective study (ALL MRD2002 Study). J Hematol Oncol. 2013; 6: 14. 10.1186/1756-8722-6-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terasaka S, Kitada H, Okabe Y, et al. Living-donor kidney transplant in T-cell and B-cell flow cytometry crossmatch-positive patients. Exp Clin Transplant. 2014; 12: 227-232. [PubMed] [Google Scholar]
- 37.Nagle SJ, Reshef R, Tsai DE. Posttransplant lymphoproliferative disorder in solid organ and hematopoietic stem cell transplantation. Clin Chest Med. 2017; 38: 771-783. 10.1016/j.ccm.2017.08.001 [DOI] [PubMed] [Google Scholar]
- 38.Morris J, Smith C, Streicher A, et al. A rare presentation of isolated CNS posttransplantation lymphoproliferative disorder. Case Rep Oncol Med. 2017; 2017: 1-5. 10.1155/2017/7269147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dotti G, Fiocchi R, Motta T, et al. Lymphomas occurring late after solid-organ transplantation. Transplantation. 2002; 74: 1095-1102. 10.1097/00007890-200210270-00007 [DOI] [PubMed] [Google Scholar]
- 40.Caillard S, Porcher R, Provot F, et al. Post-transplantation lymphoproliferative disorder after kidney transplantation: report of a nationwide French registry and the development of a new prognostic score. J Clin Oncol. 2013; 31: 1302-1309. 10.1200/JCO.2012.43.2344 [DOI] [PubMed] [Google Scholar]
- 41.Choquet S, Trappe R, Leblond V, et al. CHOP-21 for the treatment of post-transplant lymphoproliferative disorders following solid organ transplantation. Haematologica. 2007; 92: 273-274. 10.3324/haematol.10595 [DOI] [PubMed] [Google Scholar]
- 42.Choquet S, Oertel S, LeBlond V, et al. Rituximab in the management of post-transplantation lymphoproliferative disorder after solid organ transplantation: proceed with caution. Ann Hematol. 2007; 86: 599-607. 10.1007/s00277-007-0298-2 [DOI] [PubMed] [Google Scholar]
- 43.Trappe RU, Choquet S, Reinke P, et al. Salvage therapy for relapsed posttransplant lymphoproliferative disorders (PTLD) with a second progression of PTLD after Upfront chemotherapy: the role of single-agent rituximab. Transplantation. 2007; 84: 1708-1712. 10.1097/01.tp.0000295987.12996.19 [DOI] [PubMed] [Google Scholar]
- 44.Trappe R, Oertel S, Leblond V, et al. German PTLD Study Group. European PTLD Network . Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 2012; 13: 196-206. 10.1016/S1470-2045(11)70300-X [DOI] [PubMed] [Google Scholar]
- 45.Gross TG, Bucuvalas JC, Park JR, et al. Low-dose chemotherapy for Epstein-Barr virus-positive post-transplantation lymphoproliferative disease in children after solid organ transplantation. J Clin Oncol. 2005; 23: 6481-6488. 10.1200/JCO.2005.08.074 [DOI] [PubMed] [Google Scholar]
- 46.Dierickx D, Tousseyn T, Gheysens O. How I treat posttransplant lymphoproliferative disorders. Blood. 2015; 126: 2274-2283. 10.1182/blood-2015-05-615872 [DOI] [PubMed] [Google Scholar]