Abstract
Lymphomas are solid tumors characterized by the malignant proliferation of lymphoid cells. Neurologic signs encountered in patients with Hodgkin’s lymphoma can be due to the direct spread of tumor to the nervous system, secondary to chemotherapy or radiation, secondary to tumor mass compression, infectious causes and paraneoplastic syndromes. Paraneoplastic neurologic syndromes are rarely encountered in patients with Hodgkin’s lymphoma and non-Hodgkin’s lymphoma. Except for paraneoplastic cerebellar degeneration in Hodgkin’s lymphoma and dermato/polymyositis in both Hodgkin’s lymphoma and non-Hodgkin’s lymphoma, other paraneoplastic syndromes are uncommon and have only been reported as isolated case reports or short series. Here, we present a patient with Hodgkin’s lymphoma with symptoms of bilateral lower extremity weakness and loss of sensation before the start of therapy, who was eventually diagnosed as having motor and sensory axonal neuropathy.
Keywords: Guillain-Barré syndrome, Hodgkin’s lymphoma, paraneoplastic syndrome
Introduction
Lymphomas are tumors that occur as a result of abnormal growth of lymphoid cells. Hodgkin lymphoma (HL) constitutes about 9% of pediatric cancers (1). Neurologic findings related to HL are rare and generally observed in advancing disease. In HL, neurologic findings may occur as invasion to the nervous system, secondary to chemotherapy and radiotherapy, as a result of mass compression, in relation to infection or in the form of paraneoplastic syndrome (PNS) (2, 3). Although sensory and motor neuropathy are observed more frequently in non-Hodgkin lymphoma (NHL), the most commonly observed PNSs in HL include cerebellar syndrome and limbic encephalitis (3, 4).
Guillain-Barré syndrome (GBS) is an inflammatory disease that leads to acute, ascending neuropathy by causing myelination loss and axonal injury in the peripheral nervous system. It is the most common cause of acute flaccid paralysis. The diagnosis is made with evaluation of the associated clinical, laboratory, and neurophysiologic findings. The clinical picture ranges from mild findings to life-threatening paralysis. In 1995, it was classified in 4 subgroups according to the histopathologic and neurophysiological findings (5).
Acute motor and sensory axonal neuropathy (AMSAN) is one of the subtypes of GBS. It has an acute onset and is characterized by weakness in distal muscles, loss of deep tendon reflexes, and sensory findings. In electrophysiologic studies, decreased sensory and motor nerve axon potentials are observed in association with reduced nerve conduction rate (5). In this article, we present a patient with association of HL and AMSAN, which is observed rarely.
Case
A thirteen-year-old female patient presented with symptoms of weight loss (12 kg in the last two months), night sweats, and malaise. The physical examination was normal except for mild paleness and firm lymphadenopathy (LAP) with a size of 2×2 cm in the right supraclavicular area. The complete blood count was as follows: hemoglobin: 9.5 g/dL, hematocrit (Hct): 30%, white blood cells (WBC): 20 600/mm3, platelets: 491 000/mm3; C-reactive protein (CRP): 239 mg/L (normal: <5 mg/L), erythrocyte sedimentation rate: 40 mm/h, lactate dehydrogenase (LDH): 537 U/L, and ferritin 1748 ng/mL. Other biochemical tests were found to be normal. A peripheral blood smear revealed normochromic normocytic erythrocytes and neutrophil predominance; no atypical cells were found. Viral markers (Epstein-Barr virus, cytomegalovirus, parvovirus, hepatitis B and C viruses) showed no active infection. Ultrasonographic examination revealed multiple hypoechoic peripheral lymphadenopathies (LAPs), the largest one having a size of 31 mm. Marked mediastinal widening was observed on antero-posterior lung radiography. Lung computed tomography (CT) revealed multiple, conglomerated LAPs reaching a size of 5 cm in bilateral jugular chains, the anteroposterior cervical chain, anterior mediastinum, paraaortic area, paratracheal area, and in bilateral para-hilar areas (Picture 1). Abdominal CT was found to be normal. Disease involvement was not found in bilateral bone aspiration and biopsy. Fever and pleural effusion occurred during hospitalization. In the follow-up, respiratory distress and hoarse voice, and loss of motor strength and superficial sensory loss in the lower extremities emerged. The lymph node in the right supraclavicular region was removed surgically. The pathology result was CD 30(+), CD 20(−) classic type (subtype mixed cellular) HL. Positron emission tomography (PET) revealed conglomerated hypermetabolic LAPs in the bilateral subpectoral area, right axillary area, and in the anterior mediastinum [maximum standardized uptake value (SUV max): 12.4]. The patient was considered as stage IIB HL and an adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) chemotherapy protocol was initiated. In the follow-up, weakness and sensory loss in the lower extremities increased. On physical examination, muscle strength was found as 1/5 in the proximal and distal muscles in bilateral upper and lower extremities; deep tendon reflexes were absent in the lower extremities. In addition, the patient had superficial and deep sensory loss in bilateral lower extremities. The patient’s consciousness and cranial nerve examination were found to be normal and she had no autonomic dysfunction. Considering paraneoplastic polyneuropathy, cerebrospinal fluid (CSF) was obtained; CSF cytology revealed no cells and CSF protein and glucose levels were found to be normal. Brain and whole spinal magnetic resonance imaging (MRI) was evaluated to be normal. Reduced amplitude was found on nerve conduction studies in the upper and lower extremities on electromyography (EMG) and sensory and motor responses could not be obtained. H reflex was absent and F response was reduced in the upper and lower extremities. The findings supported AMSAN, which is a variant of Guillain-Barré syndrome. High-dose (a total of 2 g/kg) intravenous immunoglobulin (IVIG) treatment was administered. In the second week of treatment, partial recovery was observed, especially in sensory findings. It was planned to continue IVIG treatment with a dosage of 0.5 g/kg/month and to initiate physical therapy exercises. Prednisolone at a dosage of 40 mg/m2/day for 14 days in each course of treatment was added to treatment and vinblastine was removed from treatment with a committee decision considering that it might worsen the neurologic findings. On positron emission tomography (PET) evaluation after two courses of ABVD chemotherapy, it was observed that the disease regressed partially. In the follow-up, sensory and motor functions together with the hoarse voice began to improve from the 3rd course of treatment. At the end of the fourth course, a neurologic examination revealed that her speech was understandable and her muscle strength was 3/5 in the lower extremities bilaterally; the patient could walk with support. Chemotherapy is still continuing, monthly IVIG is being administered and physical therapy exercises are continuing. Verbal consent was obtained from the patient’s parents.
Discussion
Paraneoplastic syndrome is a neurologic picture that occurs in patients with cancer, which does not occur with the direct and regional effects of the underlying tumor and cannot be explained by metastasis, opportunistic infections, and adverse effects of cancer treatment. It is accepted that a great majority of cases occur with autoimmune mechanisms (6). It is thought that an autoimmune response developing as a result of similar antigenic properties between the underlying tumor and the nervous system is involved in the pathogenesis. The detection of neuronal autoantibodies helps to confirm the diagnosis; however, their absence does not exclude PNS (4, 6). The association of PNSs with lymphoma is rare. Other paraneoplastic neurologic findings excluding cerebellar syndrome and dermato-polymyositis are only at the level of case reports (4). The type and frequency of PNS show differences in HL and NHL. Although limbic encephalitis and cerebellar degeneration occur predominantly in Hodgkin lymphoma, sensory and motor neuropathy are observed frequently in NHL (4, 7). AMSAN is a rare subtype of GBS and there are case reports showing its association mostly with NHL (8). No case of association of HL and AMSAN has been reported in the literature to date. GBS was considered in our patient, because symmetrical and ascending neuropathy findings were present and deep tendon reflexes were negative. Paraneoplastic upper motor neuron diseases were excluded, because upper motor neuron findings were absent and cranial MRI was found to be normal. Although albumino-cytologic incompatibility was not found in the cerebrospinal fluid examination, the EMG findings and clinical course were found to be sufficient for making a diagnosis of AMSAN. Possible infectious causes and malignant involvement were excluded with the examination of the CSF. As far as we know, our patient is the first patient with an association of HL and AMSAN to be reported in the literature.
Neurologic syndromes with unknown cause occurring in presence of tumor should not always be evaluated as paraneoplastic syndrome. Two definitions as ‘definite’ and ‘possible’ are used for the diagnosis of PNS (4, 6). When using these two definitions, the type of neurologic syndrome, the presence of onconeuronal antibodies, and the presence of cancer are considered criteria. When these syndromes show association with tumor or when the presence of onconeuronal antibodies is detected, a diagnosis of definite PNS is made. In non-classic syndromes including sensory and motor neuropathy, the presence of onconeuronal antibodies should be demonstrated or neuropathy findings should improve with treatment of the underlying tumor in order to make a diagnosis of definite PNS (4). On the other hand, making a diagnosis of definite PNS in lymphomas is not possible most of the time because the presence of onconeuronal antibodies cannot be detected in most patients with lymphoma who develop PNS (4, 6). In most cases, paraneoplastic neuropathy emerges before the diagnosis of cancer or when cancer is in the early stage and can be treated (6), but there are also studies showing that it occurs after the diagnosis is made or in the advanced stage (3, 4). In our patient, the finding of paraneoplastic neuropathy emerged at the stage of diagnosis.
In many patients with paraneoplastic neuropathy, improvement in neurologic status may not occur even if the underlying tumor is removed. This has been associated with the action of autoimmune mechanisms (3). Treatment outcomes are variable in paraneoplastic peripheral neuropathies associated with HL. In some studies, it was reported that neurologic findings did not regress with treatment of the underlying malignancy, and in contrast, they increased secondary to chemotherapy (9). However, IVIG and steroid treatment were given in association, in addition to chemotherapy, because PNS occurred at an advanced level in our patient. Marked improvement was found in the PNS when the underlying disease was controlled. It is not possible to evaluate what caused the regression in the neurological findings because the immunotherapy and treatment of the underlying malignancy were administered simultaneously in our patient. Although plasmapheresis can be used in the treatment of Guillain-Barré syndrome, it could not be performed in our patient because the diagnosis could not be made in the acute stage.
Vinca alkaloids are chemotherapeutic agents, which are known to cause neurotoxicity. When the cumulative dose is 5 mg, sensory neurologic symptoms may occur, but motor neuropathy findings are observed only when the cumulative dose reaches 30–35 mg (10). Vinblastine toxicity was excluded in our patient because the neuropathy findings occurred before chemotherapy was initiated, but the drug was removed from treatment in order to avoid neurotoxic action. Besides, critical patient polyneuropathy included in the differential diagnosis is the most common neuromuscular change in patients hospitalized in intensive care units. It is an axonal neuropathy and should be considered in the differential diagnosis in patients who cannot be separated from a ventilator even if respiratory and cardiac problems are absent (11). Critical patient neuropathy was not considered in our patient because there was no history of intensive care hospitalization and the neurologic findings occurred immediately after the disease was diagnosed.
We presented this case because we found an association of HL and AMSAN, which is a subtype of Guillain-Barré syndrome, occurring as PNS in this patient. It should be considered in the differential diagnosis when neuropathy develops in patients with lymphoma.
Figure 1.
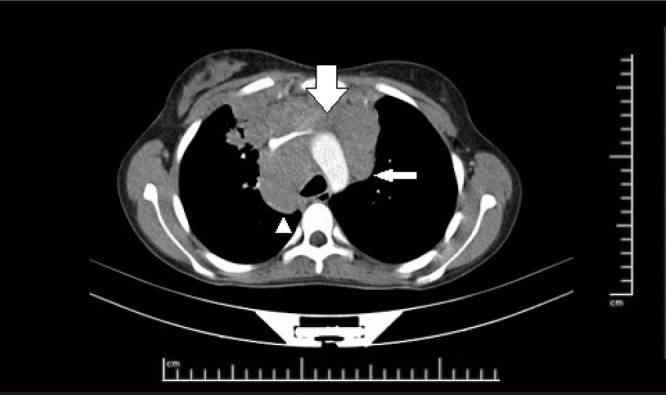
Enlarged lymph nodes in the anterior mediastinum (thick arrow), aorticopulmonary window (thin arrow) and paratracheal area (arrow tip) on computed tomography
Footnotes
Informed Consent: Verbal informed consent were obtained from patients’ parents.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - G.N.Ö., A.A.; Design - E.U.P.; Supervision - C.B.; Data Collection and/or Processing - I.O.A., B.K., C.B., E.U.P., E.P.Y, A.A., M.Ç., G.N.Ö.; Analysis and/or Interpretation - G.N.Ö., C.B.; Literature Review - I.O.A.; Writing - I.O.A.; Critical Review - G.N.Ö., A.A.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Friedman DL. Hodgkin lymphoma. In: Lanzkowsky P, Lipton JM, Fish JD, editors. Lanzkowsky’s manual of pedıatrıc hematology and oncology. 6th ed. Elsevier; 2016. pp. 429–41. [DOI] [Google Scholar]
- 2.Correale J, Monteverde DA, Bueri JA, Reich EG. Peripheral nervous system and spinal cord involvement in lymphoma. Acta Neurol Scand. 1991;83:45–51. doi: 10.1111/j.1600-0404.1991.tb03957.x. [DOI] [PubMed] [Google Scholar]
- 3.Flanagan EP, Sandroni P, Pittock SJ, Inwards DJ, Jones LK. Paraneoplastic lower motor neuropathy associated with Hodgkin lymphoma. Muscle Nerve. 2012;46:823–7. doi: 10.1002/mus.23464. [DOI] [PubMed] [Google Scholar]
- 4.Graus F, Ariño H, Dalmau J. Paraneoplastic neurological syndromes in Hodgkin and non-Hodgkin lymphomas. Blood. 2014;123:3230–8. doi: 10.1182/blood-2014-03-537506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi AG, Maisuria BA, Patel RG, Arora B. General consideration of Guilliain Barre Syndrome. IJPSR. 2012;3:4135–41. [Google Scholar]
- 6.Tüzün E. Nörolojik tutulumla seyreden paraneoplastik sendromlar. Klinik gelişim dergisi. 2010;23:71–7. [Google Scholar]
- 7.Polo-Romero FJ, Sánchez-Beteta P, Perona-Buendía P, Pérez-García AM. Guillain-Barré syndrome as first presentation of non-Hodgkin lymphoma. Neurologia. 2012;27:511–3. doi: 10.1016/j.nrl.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Wanschitz J, Dichtl W, Budka H, Löscher WN, Boesch S. Acute motor and sensory axonal neuropathy in Burkitt-like lymphoma. Muscle Nerve. 2006;34:494–8. doi: 10.1002/mus.20569. [DOI] [PubMed] [Google Scholar]
- 9.Forsyth PA, Dalmau J, Graus F, Cwik V, Rosenblum MK, Posner JB. Motor neuron syndromes in cancer patients. Ann Neurol. 1997;41:722–30. doi: 10.1002/ana.410410608. [DOI] [PubMed] [Google Scholar]
- 10.Casey EB, Jellife AM, Le Quesne PM, Millett YL. Vincristine neuropathy. Clinical and electrophysiological observations. Brain. 1973;96:69–86. doi: 10.1093/brain/96.1.69. [DOI] [PubMed] [Google Scholar]
- 11.Latronico N, Bertolini G, Guarneri B, et al. Simplified electrophysiological evaluation of peripheral nerves in critically ill patients: the Italian multi-centre CRIMYNE study. Critical Care. 2007;11:1–11. doi: 10.1186/cc5671. [DOI] [PMC free article] [PubMed] [Google Scholar]


