ABSTRACT
Background
Sarcopenia and low skeletal muscle radiodensity (SMD) have been associated with adverse outcomes in patients with colorectal cancer (CRC); however, factors contributing to these 2 muscle abnormalities are unclear.
Objectives
The aim of this study was to investigate the association of medical and demographic characteristics with muscle abnormalities among patients with nonmetastatic CRC.
Methods
Patients with stage I–III invasive CRC (2006–11) who had diagnostic computed tomography (CT) available from Kaiser Permanente Northern California electronic medical records were included. CT-assessed sarcopenia and low SMD were defined according to optimal stratification. Logistic regressions including age, stage, site, total adipose tissue (TAT), race/ethnicity, neutrophil-lymphocyte ratio, smoking history, alcohol use, and Charlson Comorbidity Score were performed to identify characteristics associated with muscle abnormalities.
Results
The study included 3262 patients (49.9% females) with a mean ± SD age of 62.6 ± 11.4 y. Sarcopenia and low SMD were highly prevalent (42.4% and 29.6%, respectively). Age and sex interactions were noted for muscle mass, but not SMD. Age was associated with higher odds of muscle abnormalities in a dose-response manner. Compared with those aged ≤50 y, patients aged 70–80 y had considerably higher odds (OR: 6.19; 95% CI: 4.72, 8.11) of sarcopenia, and low SMD (OR: 17.81; 95% CI: 11.73, 27.03). High TAT was related to a higher odds of low SMD (OR: 9.62; 95% CI: 7.37, 12.56), but lower odds of sarcopenia (OR: 0.59; 95% CI: 0.48, 0.71). Compared with Caucasians, African Americans had lower odds of sarcopenia and low SMD. Patients with a higher neutrophil-lymphocyte ratio had higher odds of having both muscle abnormalities. Patients who were smokers or had any comorbidity had higher odds of low SMD, but not sarcopenia.
Conclusions
Muscle abnormalities were common in patients with nonmetastatic CRC, with great variability in muscle mass and SMD across age, TAT, and race/ethnicity. Factors associated with muscle abnormalities may be used to facilitate risk stratification and the guidance of targeted strategies to counteract these abnormalities.
Keywords: sarcopenia, muscle radiodensity, nonmetastatic colorectal cancer, computed tomography (CT), adiposity, inflammation, race/ethnicity
Introduction
Skeletal muscle constitutes the largest fraction of the lean soft tissue compartment and is the primary site of body protein storage (1). In addition, ∼80% of glucose disposal in the human body occurs in skeletal muscle (2). Therefore, skeletal muscle is crucial for maintaining glucose homeostasis and represents a patient's physiologic reserve and overall health status. The term "sarcopenia" was originally used to describe age-associated decline in muscle mass (primary sarcopenia) (3). Secondary sarcopenia, however, is the loss of muscle mass observed in multiple pathologic and physiologic disorders, such as illnesses requiring critical care, end-stage renal disease, and malignant disease (3).
Colorectal cancer (CRC) is the third leading cause of cancer-related death in women and second in men in the United States (4). It has been reported that, depending on study cohort characteristics, 12–60% of patients with CRC are affected by sarcopenia (5–8). CRC patients with sarcopenia have poor functional capacity, increased postoperative morbidity, greater chemotherapy toxicity, shorter time to cancer progression, and decreased life expectancy (9). Moreover, sarcopenia has been associated with a higher rate of major complications after CRC resection, longer recovery time, and greater need for rehabilitation care (10).
Computed tomography (CT) allows precise quantification of muscle mass, and hence, sarcopenia. Additionally, this technique allows the assessment of low skeletal muscle radiodensity (SMD), reflective of a higher degree of fat infiltration (i.e., myosteatosis) in muscle (11, 12). Low SMD is an emerging prognostic factor in CRC and in other cancers (13). Little is known about risk factors for sarcopenia and low SMD in cancer. Recognizing these factors may aid in the prediction of overall patient prognosis. Additionally, attempts could be made to improve modifiable risk factors related to these conditions, thus improving short- and long-term prognosis. The aim of this study was to assess the prevalence and major factors associated with sarcopenia and low SMD in a large cohort of 3262 nonmetastatic CRC patients.
Methods
Study population and setting
We included patients aged 18–80 y at Kaiser Permanente Northern California (KPNC) diagnosed with stage I–III invasive CRC between 2006 and 2011. Patients who had abdominal CT scans around diagnosis with sufficient image quality for body composition assessment were included (Supplemental Figure 1). The primary study outcome was the presence of sarcopenia or low SMD (as binary variables) (14). This study was approved by the KPNC Institutional Review Board and the University of Alberta Health Research Ethics Board.
Body mass index and body composition variables
We selected patients’ height and weight closest to cancer diagnosis measured by KPNC medical assistants and computed “at-diagnosis” BMI and classified patients according to WHO guidelines: underweight (<18.5 kg/m2), normal weight (18.5 –< 25 kg/m2), overweight (25 –< 30 kg/m2), Class I obesity (30 –< 35kg/m2), and Class II/III obesity (≥35 kg/m2).
Body composition was measured from diagnostic CT scans taken before any chemotherapy or radiation treatment (83% presurgical). The median time between diagnosis and scan was 0.2 mo (range: −2.0 to 3.8 mo). A single image at the third lumbar vertebra (L3) was selected for body composition quantification, including skeletal muscle mass, intermuscular adipose tissue (IMAT), visceral adipose tissue (VAT), and subcutaneous adipose tissue (SAT) cross-sectional areas (cm2). A single L3 image strongly correlates with whole-body muscle and total adipose tissue (TAT) in healthy individuals and patients with cancer (15, 16). Tissue areas were measured according to the standard Hounsfield unit (HU) range of −29 to 150 for muscle (17), −150 to −50 for VAT (18), and −190 to −30 for IMAT and SAT (16) with the use of SliceOmatic Software version 5.0 (TomoVision). SMD in HU was generated by the software as the mean radiation attenuation value of the measured muscle groups at L3. The interobserver coefficients of variation were 1.2% for skeletal muscle, 0.7% for SMD, and 1% for TAT. The skeletal muscle index (SMI) was calculated from skeletal muscle cross-sectional area divided by height squared (cm2/m2). TAT was calculated as the sum of VAT, IMAT, and SAT.
Definitions of sarcopenia and low SMD
Threshold values of muscle abnormalities (i.e., sarcopenia or low SMD, or a combination) were developed through the use of the optimal stratification approach (13, 19). This method identifies cutpoints that best separate patients’ risk with respect to time to death, which has been increasingly accepted as a clinically relevant approach for patient risk stratification. Accordingly, we adopted cutpoints of sarcopenia and low SMD derived from this study cohort, as previously described (14, 20). We further classified patients into 4 phenotype groups: nonsarcopenic, normal SMD; nonsarcopenic, low SMD; sarcopenic, normal SMD; and sarcopenic, low SMD.
Demographic and clinical variables
We reviewed all patients’ electronic medical records and the KPNC Cancer Registry for information on demographics, lifestyle, and medical history. Age at diagnosis, sex, disease stage, race/ethnicity, comorbidities (1 y before cancer diagnosis), prediagnostic weight change (i.e., the subtraction of diagnosis weight from the weight taken 18 mo prior to diagnosis), smoking history, and alcohol use (any time prior to and closest to cancer diagnosis) were obtained. Laboratory values of albumin and neutrophil-lymphocyte ratio (NLR) were obtained as part of routine blood tests; all measurements were taken within 1 y (albumin, median −0.26 mo; range: −11.94 to 1.48 mo) or 2 y (NLR, median −0.36 mo; range: −17.99 to 1.55 mo) of CRC diagnosis, and prior to the diagnostic scan, surgery, or other treatment. Standard cutoffs were used to categorize NLR into normal (<3), moderate (3 –< 5), and high (≥5) (21), and albumin into low (<3.5 g/dL) and normal (≥3.5 g/dL) groups (22).
Statistical analysis
Differences in descriptive statistics by muscle abnormalities and differences in body composition variables by patient subgroups defined by age, BMI, and sex were analyzed by 1-way analysis of variance or Pearson's chi-square tests, where appropriate. Mean difference and marginal means of body composition components by race/ethnicity were estimated from generalized linear models. Simple linear regression models were used to examine the impact of age and sex on SMI, SMD, and other body composition components as continuous variables. Logistic regression models were applied to determine clinical and demographic predictors of outcome variables (i.e., sarcopenia and low SMD as binary variables) in univariate and multivariable analysis. All statistical analyses were performed with STATA version 14.2 (StataCorp LP), with statistical significance established with 2-sided tests with P < 0.05.
Results
Patient characteristics
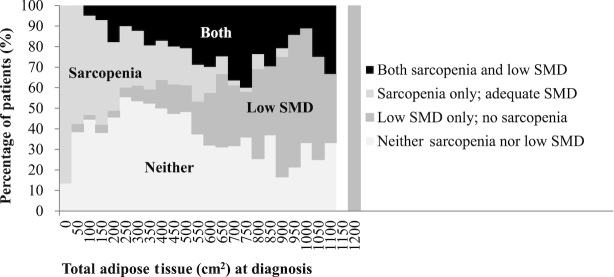
A total of 3262 patients were included. Patient demographic, clinical, and body composition characteristics are given in Table 1. Males presented with higher SMI and SMD than females, although low SMD prevalence did not differ between sexes. The prevalence of sarcopenia and low SMD was 45.3% and 28.4% in males and 39.5% and 30.9% in females, respectively. Although sarcopenia and low SMD were more common in older patients, both conditions occurred across the age spectrum: for example, 114 (6.7%) of patients <65 y at diagnosis had both sarcopenia and low SMD (data not shown). Figure 1 shows the percentage of patients who had sarcopenia, low SMD, or both across the TAT distribution. Most patients who only had sarcopenia were in the lower end of the TAT distribution, whereas patients with only low SMD tended to be at the high end of the distribution (Figure 1).
TABLE 1.
Characteristics of patients with nonmetastatic CRC1
| Overall (n = 3262) | Males (n = 1634) | Females (n = 1628) | P value2 | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 62.6 ± 11.4 | 62.0 ± 11.3 | 63.2 ± 11.5 | 0.002 |
| BMI, kg/m2 | 28.1 ± 6.0 | 28.3 ± 5.2 | 27.9 ± 6.7 | 0.088 |
| BMI categories, n (%) | ||||
| Underweight | 61(1.9) | 14 (0.9) | 47 (2.9) | <0.001 |
| Normal weight | 1007 (30.9) | 416 (25.5) | 591 (36.3) | |
| Overweight | 1164 (35.7) | 687 (42.0) | 477 (29.3) | |
| Class I obesity | 645 (19.8) | 360 (22.0) | 285 (17.5) | |
| Class II/III obesity | 385 (11.8) | 157 (9.6) | 228 (14.0) | |
| Weight change history,3n (%) | ||||
| Stable‚ <5% change | 1150 (35.3) | 545 (33.4) | 605 (37.2) | 0.001 |
| ≥5% loss | 548 (16.8) | 255 (15.6) | 293 (18.0) | |
| ≥5% gain | 137 (4.2) | 62 (3.8) | 75 (4.6) | |
| Race/ethnicity, n (%) | ||||
| Caucasian | 2118 (65.0) | 1063 (65.1) | 1055 (64.9) | 0.442 |
| African American | 234 (7.2) | 105 (6.4) | 129 (7.9) | |
| Hispanic or Latino | 365 (11.2) | 193 (11.8) | 172 (10.6) | |
| Asian/Pacific Islander | 520 (16.0) | 261 (16.0) | 259 (15.9) | |
| Other | 21 (0.6) | 10 (0.6) | 11 (0.7) | |
| Clinical variables | ||||
| Site of cancer, n (%) | ||||
| Proximal | 1436 (44.0) | 644 (39.4) | 792 (48.7) | <0.001 |
| Distal | 879 (27.0) | 438 (26.8) | 441 (27.1) | |
| Rectal | 947 (29.0) | 552 (33.8) | 395 (24.3) | |
| Cancer stage, n (%) | ||||
| I | 979 (30.0) | 501 (30.7) | 478 (29.4) | 0.179 |
| II | 1030 (31.6) | 531 (32.5) | 499 (30.7) | |
| III | 1253 (38.4) | 602 (36.8) | 651 (40.0) | |
| Neutrophil lymphocyte ratio4 | 3.94 ± 4.13 | 4.10 ± 4.51 | 3.79 ± 3.72 | 0.066 |
| Albumin5 | 3.90 ± 0.63 | 3.96 ± 0.61 | 3.85 ± 0.63 | 0.019 |
| Smoking history, n (%) | ||||
| Never | 1516 (46.5) | 634 (38.9) | 882 (54.2) | <0.001 |
| Former | 1347 (41.3) | 771 (47.3) | 576 (35.4) | |
| Current | 396 (12.2) | 226 (13.9) | 170 (10.4) | |
| Alcohol,6n (%) | ||||
| Never | 797 (24.4) | 276 (16.9) | 521 (32.0) | <0.001 |
| Former | 38 (1.2) | 16 (1.0) | 22 (1.4) | |
| Current | 928 (28.5) | 509 (31.2) | 419 (25.7) | |
| Charlson Comorbidity Score, n (%) | ||||
| 0 | 1770 (54.3) | 867 (53.1) | 903 (55.5) | 0.479 |
| 1–2 | 946 (29.0) | 482 (29.5) | 464 (28.5) | |
| ≥3 | 321 (9.8) | 171 (10.5) | 150 (9.2) | |
| Missing | 225 (6.9) | 114 (7.0) | 111 (6.8) | |
| Body composition | ||||
| SMI, cm2/m2 | 48.6 ± 10.1 | 54.2 ± 9.2 | 43.1 ± 7.5 | <0.001 |
| SMD, HU | 39.0 ± 9.9 | 40.6 ± 9.6 | 37.5 ± 10.0 | <0.001 |
| VAT, cm2 | 155.7 ± 109.9 | 201.2 ± 116.2 | 109.9 ± 80.6 | <0.001 |
| SAT, cm2 | 212.5 ± 120.3 | 187.4 ± 105.3 | 237.8 ± 129.0 | <0.001 |
| TAT, cm2 | 381.4 ± 196.0 | 401.9 ± 195.6 | 360.8 ± 194.3 | <0.001 |
| Sarcopenia, n (%) | 1383 (42.4) | 740 (45.3) | 643 (39.5) | 0.001 |
| Low SMD, n (%) | 967 (29.6) | 464 (28.4) | 503 (30.9) | 0.118 |
Values are means ± SDs or n (%). For BMI <30 kg/m2, sarcopenia cutpoints were <52.3 cm2/m2 and <38.6 cm2/m2 for men and women, respectively; for BMI ≥30 kg/m2, sarcopenia cutpoints were <54.3 cm2/m2 and <46.6 cm2/m2 for men and women, respectively (14). Low SMD cutpoints were 35.5 HU for men and 32.5 for women (20). CRC, colorectal cancer; HU, Hounsfield unit; SAT, subcutaneous adipose tissue; SMD, skeletal muscle radiodensity; SMI, skeletal muscle index; VAT, visceral adipose tissue; TAT, total adipose tissue.
One-way ANOVA or Pearson's chi-square tests.
Missing values for each variable respectively: 1427, 896, 2557, 1499.
FIGURE 1.
Percentage of patients with sarcopenia or low SMD across the spectrum of total adiposity (N = 3262). SMD, skeletal muscle radiodensity.
Body composition features, medical and demographic characteristics, and muscle abnormalities
Variations in body composition components by age and sex are shown in Table 2. Significant age and sex interactions were noted for all components except SMD. Mean SMI and SMD were lower in the higher age categories for both sexes. In contrast, VAT was higher with older age except in those 70–80 y of age for both sexes. In multivariable logistic regression analysis, older age was associated with higher odds of sarcopenia and low SMD in a dose-response manner. Compared with patients aged <50 y, the ORs for sarcopenia were higher in older age groups (Table 3 ); patients aged 70–80 y had the highest odds of sarcopenia (OR: 6.19; 95% CI: 4.72, 8.11). The dose-response effect of age on SMD was more pronounced (Table 4 ). It is noteworthy that patients who were >70 y had a substantially higher odds of having low SMD compared with those who were <50 y (OR: 17.81; 95% CI: 11.73, 27.03). In simple linear regression analyses, SMI decreased 0.27 cm2 and SMD decreased 0.44 HU for every 1-y increase in age; on average, females had 11.05 cm2 lower SMI and 3.05 HU lower SMD than that of their male counterparts (Tables 3 and 4).
TABLE 2.
Variation of body composition by age and sex in patients with nonmetastatic CRC1
| SMM, cm2 | SMI, cm2/m2 | SMD, HU | VAT, cm2 | SAT, cm2 | TAT, cm2 | |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| Males | ||||||
| <50 (n = 273) | 185.7 ± 30.6 | 59.2 ± 8.9 | 48.6 ± 8.0 | 153.9 ± 100.5 | 207.2 ± 130.3 | 369.3 ± 212.3 |
| 50–60 (n = 422) | 176.1 ± 28.4 | 56.6 ± 8.6 | 43.0 ± 8.1 | 188.2 ± 104.8 | 199.4 ± 117.3 | 399.0 ± 198.8 |
| 60–70 (n = 498) | 167.8 ± 28.6 | 53.5 ± 8.7 | 39.0 ± 8.5 | 224.1 ± 123.0 | 188.0 ± 96.2 | 426.4 ± 195.3 |
| 70–80 (n = 441) | 151.8 ± 26.2 | 49.4 ± 8.3 | 35.0 ± 9.0 | 217.2 ± 117.8 | 163.0 ± 77.6 | 397.2 ± 178.3 |
| P value2 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.04 |
| Females | ||||||
| <50 (n = 250) | 121.1 ± 20.7 | 45.6 ± 7.5 | 46.2 ± 8.6 | 72.9 ± 63.2 | 244.4 ± 136.6 | 324.5 ± 187.6 |
| 50–60 (n = 388) | 119.4 ± 20.1 | 45.4 ± 7.9 | 40.7 ± 8.6 | 111.1 ± 78.1 | 267.2 ± 140.2 | 388.9 ± 206.3 |
| 60–70 (n = 451) | 111.4 ± 19.8 | 42.5 ± 6.9 | 36.2 ± 8.8 | 121.9 ± 83.9 | 244.7 ± 127.0 | 380.9 ± 198.0 |
| 70–80 (n = 539) | 105.0 ± 17.5 | 40.8 ± 6.7 | 32.3 ± 8.9 | 116.2 ± 82.0 | 207.7 ± 111.5 | 340.6 ± 180.4 |
| P value2 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.66 |
| Age and sex interaction, P value3 | <0.001 | <0.001 | 0.27 | <0.001 | <0.001 | <0.001 |
Values are means ± SDs. CRC, colorectal cancer; HU, Hounsfield unit; SAT, subcutaneous adipose tissue; SMD, skeletal muscle radiodensity; SMI, skeletal muscle index; SMM, skeletal muscle mass; VAT, visceral adipose tissue; TAT, total adipose tissue.
Simple linear regression tests with age group as the predictor.
Likelihood ratio tests and multivariate linear regression models for interaction terms.
TABLE 3.
Medical and demographic characteristics associated with sarcopenia among nonmetastatic CRC patients1
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Characteristics | OR (95% CI) | P value | OR (95% CI) | P value |
| Age, per 5 y | 1.06 (1.05,1.06) | <0.001 | 1.06 (1.05,1.07) | <0.001 |
| Age, y | ||||
| ≤50 | Reference | Reference | ||
| 50–60 | 1.72 (1.33,2.22) | <0.001 | 1.90 (1.45,2.48) | <0.001 |
| 60–70 | 3.21 (2.51,4.10) | <0.001 | 3.71 (2.85,4.81) | <0.001 |
| 70–80 | 5.29 (4.14,6.76) | <0.001 | 6.19 (4.72,8.11) | <0.001 |
| Sex | ||||
| Males | Reference | Reference | ||
| Females | 0.79 (0.69,0.91) | 0.001 | 0.71 (0.61,0.83) | <0.001 |
| TAT tertiles | ||||
| Tertile 1 | Reference | Reference | ||
| Tertile 2 | 0.56 (0.47,0.67) | <0.001 | 0.52 (0.43,0.62) | <0.001 |
| Tertile 3 | 0.59 (0.50,0.70) | <0.001 | 0.59 (0.48,0.71) | <0.001 |
| Race/ethnicity | ||||
| Caucasian | Reference | Reference | ||
| African American | 0.47 (0.35,0.64) | <0.001 | 0.53 (0.38,0.72) | <0.001 |
| Hispanic or Latino | 0.53 (0.42,0.68) | <0.001 | 0.67 (0.52,0.86) | 0.002 |
| Asian | 1.03 (0.85,1.24) | 0.79 | 1.09 (0.87,1.35) | 0.45 |
| Weight change history2 | ||||
| Stable‚ <5% change | Reference | Reference | ||
| ≥5% loss | 1.20 (0.98,1.47) | 0.08 | 1.04 (0.83,1.29) | 0.76 |
| ≥5% gain | 1.07 (0.75,1.53) | 0.70 | 1.10 (0.75,1.61) | 0.64 |
| Stage | ||||
| Stage I | Reference | Reference | ||
| Stage II | 1.42 (1.19,1.69) | <0.001 | 1.30 (1.07,1.57) | 0.008 |
| Stage III | 1.14 (0.96,1.35) | 0.13 | 1.21 (1.01,1.46) | 0.04 |
| Site | ||||
| Colon | Reference | Reference | ||
| Rectal | 0.74 (0.63,0.87) | <0.001 | 0.89 (0.75,1.06) | 0.20 |
| Charlson Comorbidity Score | ||||
| 0 | Reference | Reference | ||
| 1 or 2 | 1.08 (0.92,1.27) | 0.33 | 0.86 (0.72,1.03) | 0.10 |
| ≥3 | 1.12 (0.88,1.43) | 0.35 | 0.65 (0.49,0.86) | 0.003 |
| Neutrophil-lymphocyte ratio3 | ||||
| <3 | Reference | Reference | ||
| 3–5 | 1.38 (1.13,1.68) | 0.002 | 1.26 (1.02,1.57) | 0.03 |
| ≥5 | 1.82 (1.48,2.26) | <0.001 | 1.65 (1.31,2.08) | <0.001 |
| Albumin4 | ||||
| ≥3.5 g/dL | Reference | Reference | ||
| <3.5 g/dL | 1.35 (0.93,1.96) | 0.11 | 0.96 (0.64,1.44) | 0.85 |
| Smoking history | ||||
| Never smoker | Reference | Reference | ||
| Former smoker | 1.25 (1.08,1.45) | 0.003 | 1.02 (0.86,1.20) | 0.84 |
| Current smoker | 1.18 (0.94,1.47) | 0.15 | 1.14 (0.90,1.46) | 0.28 |
| Alcohol5 | ||||
| Never | Reference | Reference | ||
| Former | 1.15 (0.60,2.20) | 0.68 | 0.98 (0.48,1.99) | 0.96 |
| Current | 1.03 (0.85,1.25) | 0.73 | 1.04 (0.84,1.29) | 0.72 |
| Simple linear regression | ||||
| Coefficient | Standard error | P value | 95% CI | |
| Age, per year | −0.27 | 0.01 | <0.001 | (−0.29,−0.24) |
| Sex (male as reference) | −11.05 | 0.29 | <0.001 | (−11.63,−10.48) |
Multivariable models were adjusted for age at diagnosis (either categoric or continuous), sex, race/ethnicity, cancer stage, cancer site, prediagnostic weight change history, Charlson Comorbidity Score, smoking history, alcohol use, neutrophil lymphocyte ratio, albumin, and TAT tertiles at diagnosis. For BMI <30 kg/m2, sarcopenia cutpoints were <52.3 and <38.6 cm2/m2 for men and women, respectively; for BMI ≥30 kg/m2, sarcopenia cutpoints were <54.3 and <46.6 cm2/m2 for men and women, respectively (14). Low SMD cutpoints were 35.5 HU for men and 32.5 HU for women (20). CRC, colorectal cancer; SMD, skeletal muscle radiodensity; TAT, total adipose tissue.
2,3,4,5Missing values for each variable respectively:1427, 896, 2557, 1499.
TABLE 4.
Medical and demographic characteristics associated with low SMD among nonmetastatic CRC patients1
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Characteristics | OR (95% CI) | P value | OR (95% CI) | P value |
| Age, per 5 y | 1.09 (1.08,1.10) | <0.001 | 1.10 (1.09,1.11) | <0.001 |
| Age, y | ||||
| ≤50 | Reference | Reference | ||
| 50–60 | 2.83 (1.90,4.19) | <0.001 | 2.51 (1.64,3.83) | <0.001 |
| 60–70 | 6.20 (4.27,9.02) | <0.001 | 5.26 (3.50,7.90) | <0.001 |
| 70–80 | 15.86 (10.96,22.96) | <0.001 | 17.81 (11.73,27.03) | <0.001 |
| Sex | ||||
| Males | Reference | Reference | ||
| Females | 1.13 (0.97,1.31) | 0.12 | 1.16 (0.96,1.41) | 0.12 |
| TAT tertiles | ||||
| Tertile 1 | Reference | Reference | ||
| Tertile 2 | 2.58 (2.06,3.23) | <0.001 | 2.75 (2.13,3.56) | <0.001 |
| Tertile 3 | 6.87 (5.54,8.52) | <0.001 | 9.62 (7.37,12.56) | <0.001 |
| Race/ethnicity | ||||
| Caucasian | Reference | Reference | ||
| African American | 0.38 (0.27,0.54) | <0.001 | 0.37 (0.24,0.55) | <0.001 |
| Hispanic or Latino | 0.88 (0.70,1.12) | 0.30 | 1.19 (0.89,1.59) | 0.24 |
| Asian | 0.24 (0.18,0.31) | <0.001 | 0.38 (0.27,0.53) | <0.001 |
| Weight change history2 | ||||
| Stable‚ <5% change | Reference | Reference | ||
| ≥5% loss | 1.02 (0.82,1.27) | 0.84 | 1.00 (0.77,1.30) | 1.00 |
| ≥5% gain | 1.33 (0.92,1.92) | 0.13 | 1.24 (0.79,1.93) | 0.35 |
| Stage | ||||
| Stage I | Reference | Reference | ||
| Stage II | 1.16 (0.96,1.41) | 0.12 | 1.10 (0.87,1.39) | 0.42 |
| Stage III | 1.02 (0.85,1.23) | 0.84 | 1.17 (0.93,1.47) | 0.18 |
| Site | ||||
| Colon | Reference | Reference | ||
| Rectal | 0.52 (0.44,0.63) | <0.001 | 0.77 (0.62,0.96) | 0.02 |
| Charlson Comorbidity Score | ||||
| 0 | Reference | Reference | ||
| 1 or 2 | 2.18 (1.83,2.59) | <0.001 | 1.40 (1.14,1.73) | 0.002 |
| ≥3 | 4.83 (3.77,6.20) | <0.001 | 2.00 (1.46,2.74) | <0.001 |
| Neutrophil-lymphocyte ratio3 | ||||
| <3 | Reference | Reference | ||
| 3 to 5 | 1.56 (1.26,1.92) | <0.001 | 1.48 (1.15,1.92) | 0.003 |
| ≥5 | 1.91 (1.53,2.38) | <0.001 | 2.07 (1.57,2.74) | <0.001 |
| Albumin4 | ||||
| ≥3.5 g/dL | Reference | Reference | ||
| <3.5 g/dL | 2.09 (1.44,3.04) | <0.001 | 1.80 (1.14,2.84) | 0.01 |
| Smoking history | ||||
| Never smoker | Reference | Reference | ||
| Former smoker | 1.98 (1.68,2.33) | <0.001 | 1.45 (1.19,1.78) | <0.001 |
| Current smoker | 1.63 (1.28,2.07) | <0.001 | 2.07 (1.54,2.79) | <0.001 |
| Alcohol5 | ||||
| Never | Reference | Reference | ||
| Former | 1.43 (0.73,2.78) | 0.30 | 1.06 (0.47,2.39) | 0.88 |
| Current | 0.91 (0.74,1.12) | 0.38 | 1.11 (0.85,1.45) | 0.43 |
| Simple linear regression | ||||
| Coefficient | Standard error | P value | 95% CI | |
| Age, per year | −0.44 | 0.01 | <0.001 | (−0.46,−0.41) |
| Sex (male as reference) | −3.05 | 0.34 | <0.001 | (−3.72,−2.38) |
Multivariable models were adjusted for age at diagnosis (either categoric or continuous), sex, race/ethnicity, cancer stage, cancer site, prediagnostic weight change history, Charlson Comorbidity Score, smoking history, alcohol use, neutrophil lymphocyte ratio, albumin, and TAT tertiles at diagnosis. For BMI <30 kg/m2, sarcopenia cutpoints were <52.3 and <38.6 cm2/m2 for men and women, respectively; for BMI ≥30 kg/m2, sarcopenia cutpoints were <54.3 and <46.6 cm2/m2 for men and women, respectively (14). Low SMD cutpoints were 35.5 HU for men and 32.5 HU for women (20). CRC, colorectal cancer; SMD, skeletal muscle radiodensity; TAT, total adipose tissue.
2,3,4,5Missing values for each variable respectively: 1427, 896, 2557, 1499.
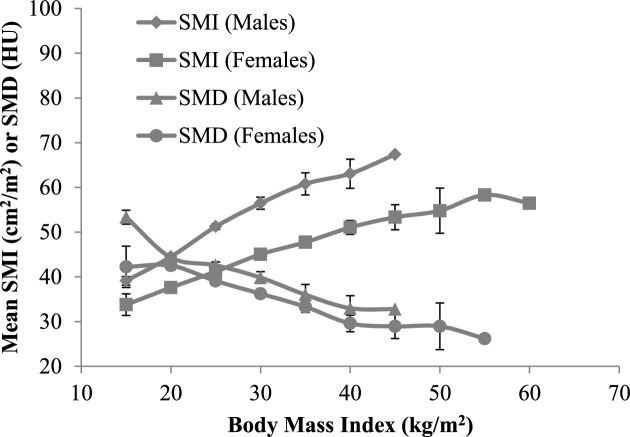
Within each age group, there was a direct, dose-response relationship between BMI and SMI in both sexes (Supplemental Tables 1 and 2). In contrast, SMD was lower in those in the higher BMI categories, consistent with the overall linear trend shown in Figure 2. Patients with higher BMI had more of each type of adipose tissue (Supplemental Tables 1 and 2). Multivariable regression analysis showed that associations varied in direction between the degree of adiposity and sarcopenia or low SMD. Similar to the distribution shown in Figure 1, those in the highest TAT tertile had a lower risk of sarcopenia but higher risk of low SMD, after adjusting for confounding factors (Tables 3 and 4).
FIGURE 2.
Mean SMI and SMD by BMI and sex at diagnosis among patients with nonmetastatic CRC (males = 1634; females = 1628). CRC, colorectal cancer; HU, Hounsfield unit; SMI, skeletal muscle index; SMD, skeletal muscle radiodensity.
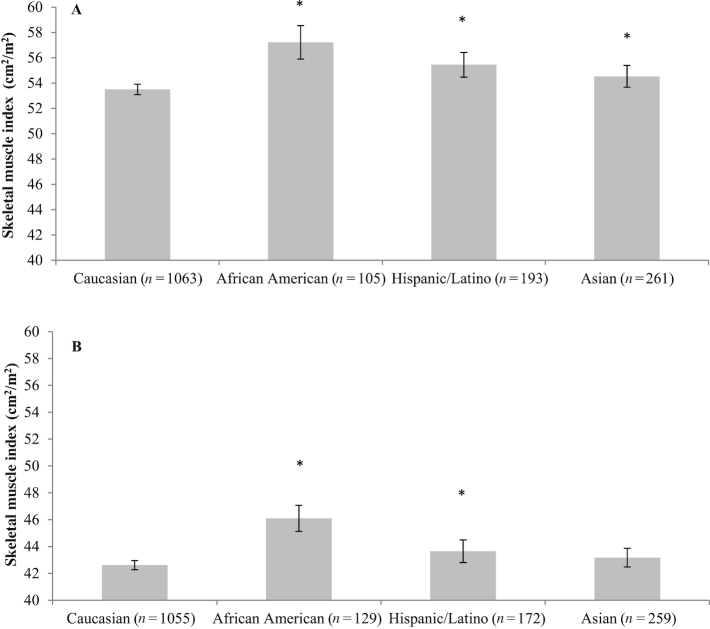
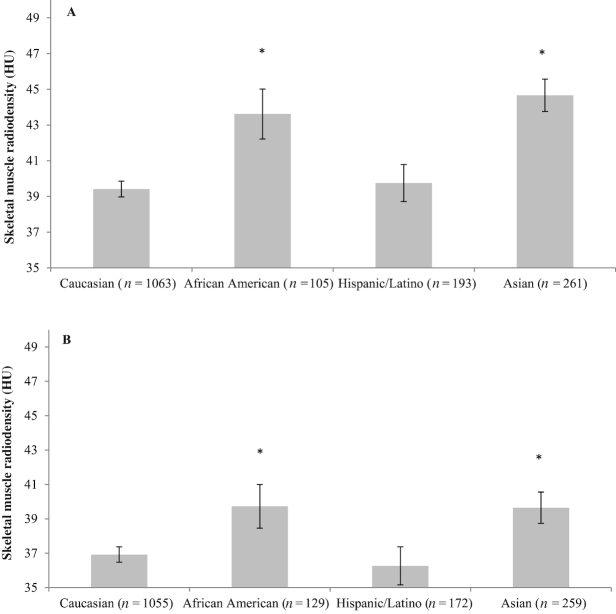
Race/ethnicity difference in muscle abnormalities (Figures 3 and 4) and adipose tissue components (Supplemental Figures 2 and 3) were observed. In males, Caucasians had the lowest SMI among all race/ethnicity, whereas in females, Caucasians had lower SMI than African Americans and Hispanics or Latinos. In both sexes, African Americans and Asians had higher SMD compared with Caucasians. Additionally, VAT was lower in African Americans than that of Caucasians. TAT was lower in African Americans and Asians compared to Caucasians.
FIGURE 3.
Mean SMI by race/ethnicity and sex for patients with nonmetastatic CRC: (A) male; (B) female. The estimated marginal means and conference intervals are from generalized linear models adjusted for age and BMI (*P value of mean difference <0.05 using Caucasians as the reference group). The interaction term between sex and race/ethnicity was tested using the likelihood ratio test and multivariate linear regression model (P < 0.001). CRC, colorectal cancer; SMI, skeletal muscle index.
FIGURE 4.
Mean SMD by race/ethnicity and sex for patients with nonmetastatic CRC: (A) male; (B) female. The estimated marginal means and conference intervals are from generalized linear models adjusted for age and BMI (*P value of mean difference <0.05 based on Caucasians as the reference group). The interaction term between sex and race/ethnicity was tested with the likelihood ratio test and multivariate linear regression model (P < 0.001). CRC, colorectal cancer; HU, Hounsfield unit; SMD, skeletal muscle radiodensity.
In multivariable logistic regression analysis, race/ethnicity predicted muscle abnormalities. Compared with Caucasians as a reference group, African Americans had 47% lower odds of sarcopenia and 63% lower odds of low SMD. Hispanics or Latinos were 33% less likely to have sarcopenia, whereas Asians were 62% less likely to have low SMD (Tables 3 and 4).
As shown in Tables 3 and 4, female patients were less likely to be sarcopenic than their male counterparts. Patients with stage II and III CRC had a higher likelihood of sarcopenia than those who had stage I cancer. In addition, compared to patients with colon cancer, those with rectal cancer were less likely to have low SMD. A higher NLR was associated with higher odds of both sarcopenia and low SMD in a dose-response manner. Patients with a low albumin concentration had a higher risk of having a low SMD than patients with a normal albumin concentration. Results were not different for NLR or albumin measurements restricted to values within 1 mo of CRC diagnosis.
Regarding lifestyle and clinical factors, alcohol use was not associated with either muscle abnormality. Current and former smokers were more likely to have low SMD, but not sarcopenia, compared with nonsmokers. Likewise, patients with a Charlson Comorbidity Score ≥1 were more likely to have a low SMD, and patients with a comorbidity score of ≥3 had a lower risk of having sarcopenia than patients without any comorbidity.
Results did not differ in sensitivity analyses adjusting for chemotherapy/radiation treatment; therefore, treatment was excluded from the models. Additionally, restricting the analyses to patients with presurgical CT scans (n = 2701) did not affect the ORs, except that cancer site was not associated with low SMD.
Discussion
To our knowledge, this is the largest and first study to examine medical and demographic characteristics associated with sarcopenia and low SMD in patients with CRC. Sarcopenia was found in 42% and low SMD in 30% of patients, despite the wide BMI range. Older age was strongly correlated with both muscle abnormalities with a more pronounced effect on low SMD. Higher TAT was associated with a lower risk of sarcopenia but a higher risk of low SMD. Compared with Caucasians, African American and Hispanic or Latino patients had lower risks of sarcopenia, whereas African American and Asian patients had lower risk of low SMD.
Aging is characterized by an accelerated muscle loss (23, 24) and higher adipose tissue accumulation within or between skeletal muscle (11, 25). The mean age in our cohort was 62.6 y; as expected, the likelihood of each muscle abnormality increased with age. The associations between muscle abnormalities and advanced age have been reported in other cancer cohorts (26–28), nonmalignant diseases (29), and normal aging (30). With the rapidly aging population, and the surging number of older adults with cancer, understanding the interactions between age and lifestyle factors and the connections of these with body composition abnormalities is essential for targeted preventive/interventional strategies. The lower likelihood of sarcopenia in females is consistent with previous studies (8, 13). Sexual dimorphism in skeletal muscle mass, such as fiber type, fiber size, and response to tumors, could possibly explain the greater susceptibility to sarcopenia observed in males (31).
We found that BMI and TAT were positively related to SMI, which is consistent with previous studies across cancer types (8, 13). The prevalence of overweight/obesity was 67.3% in the current cohort, and muscle mass may increase concurrent with the increase in adipose tissue for most patients (32), which may partially explain the lower risk of sarcopenia in patients with higher TAT. Despite this lower risk, the prevalence of sarcopenia was 31.6% in patients who were overweight or obese, and sarcopenic obesity (concurrence of sarcopenia and obesity) was prevalent at 10.7%. Furthermore, our previous report demonstrated that sarcopenia was predictive of a higher risk of mortality, independent of the amount of adipose tissue (14). Because BMI cannot distinguish muscle from fat, patients with sarcopenic obesity or other body composition phenotypes that increase risk of poor oncologic outcomes often go undetected. The negative relationship between high TAT/BMI and low SMD has been previously shown in patients with metastatic lung or gastrointestinal cancer and patients with diabetes or obesity (28, 33). The precise mechanism leading to SMD decline in cancer has not been determined. Nevertheless, it is reasonable to speculate that the ectopic fat infiltrates into surrounding organs with advanced age, in this case skeletal muscle, resulting in the radiologic manifestation of low SMD (11). Additionally, high circulating free fatty acid concentrations or disuse of muscle have also been suggested to impair mitochondria oxidation and lipid metabolism within muscle, both leading to fat accumulation into muscle (11, 34). Future studies are warranted to investigate the precise relationship between these metabolic disturbances and SMD decline in cancer patients.
We also observed race/ethnicity differences in body composition. African Americans presented the highest mean SMI (age and BMI adjusted) among all race/ethnicities for both sexes, which is consistent with previous multiethnic studies in individuals with or without cancer (35–37). Race difference in SMD is less understood. We found African Americans and Asians had higher mean SMD and were less likely to have low SMD compared with Caucasians. Nevertheless, earlier studies in noncancer individuals suggested greater amounts of intramuscular fat among individuals with African heritage compared with Caucasians (38–40). More research is needed to elucidate differences in muscle fat infiltration across race/ethnicities and the determining factors/underlying mechanism of this variability (41). As for lifestyle risk factors, those who smoke or had smoking history also had higher amounts of TAT (P < 0.001, data not shown); it is possible that smoking affected the risk of SMD through high TAT. Other studies have suggested that smoking induces insulin resistance and oxidative stress in skeletal muscle (42, 43). These alterations could lead to impaired lipid metabolism and therefore the accumulation of intramuscular adipose tissue. Likewise, the Charlson Comorbidity Score was associated with low SMD, echoing our previous report in this cohort that 6 (out of 11) Charlson comorbidities were associated with a higher likelihood of low SMD at diagnosis, whereas most of them were not associated with sarcopenia (44).
Systematic inflammation stimulates a number of mediators that directly accelerate muscle catabolism and is a hallmark of cancer cachexia (45). Our dose-response findings between a higher NLR and higher risks of both muscle abnormalities in a large cohort of nonmetastatic CRC patients are novel. We previously reported, based on the use of various inflammatory biomarkers, that prediagnostic systemic inflammation was independently associated with the presence of sarcopenia (46). The findings from our cohort are supported by a recent study of 763 patients with stages I–IV CRC where high NLR (>3) was an independent predictor of sarcopenia (OR: 1.78; 95% CI: 1.29, 2.45) and low SMD (OR: 1.60; 95% CI: 1.03, 2.49) (47). Systemic inflammation has also been reported as higher among patients with pancreatic cancer who had low SMD (48). Despite these and our findings, we cannot conclude whether sarcopenia and low SMD were consequences of an inflammatory milieu occurring at an earlier time point or concurrent with the onset of systemic inflammation due to our retrospective design. Similarly, it is unknown whether smoking and comorbidities lead to muscle abnormalities or vice versa. Future prospective studies are needed to explore the relationships between cancer metabolism, lifestyle factors, and muscle abnormalities in patients with early-stage cancer.
Similar to the aging literature, characteristics associated with muscle abnormalities in patients with cancer included age, sex, adiposity, albumin concentrations, inflammation, and smoking (49, 50). It is possible that other factors reported in healthy aging, such as physical activity, dietary intake, and socioeconomic status (49), may be relevant to these patients. Further studies are also needed to evaluate whether a single CT image represents SMD at the whole-body level, as well as longitudinal changes in muscle mass and SMD during cancer trajectory. In addition, whether the rate and magnitude of muscle mass and SMD decline in CRC are the same as those occurring in the normal aging process remains to be investigated. Although anthropometric and clinical risk factors (i.e., age, race/ethnicity, stage, cancer site) associated with muscle abnormalities cannot be changed, other risk factors (i.e., smoking, TAT, and comorbidities) are modifiable. These factors should be explored in pretreatment rehabilitation programs (51). Resistance exercise or its prescription with nutritional supplementation has shown efficacy for preserving or increasing muscle mass in adults (52, 53); nevertheless, its effects in patients with early-stage CRC have not yet been established.
This is the largest study to demonstrate medical and demographic characteristics associated with sarcopenia and low SMD among nonmetastatic CRC patients. The association of these two abnormalities with different characteristics suggests diverse pathophysiologic mechanisms between muscle depletion and fat infiltration, which may explain why sarcopenia and low SMD uniquely affect short- and long-term prognosis (54). Future studies are needed to explore the overlapping and distinct mechanistic pathways through which identified factors in this study lead to sarcopenia and low SMD. Additionally, the feasibility and efficacy of modifying muscle abnormalities in patients with cancer warrant investigation.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows: JX: contributed to project conception, development of overall research plan, performed the statistical analysis, interpreted the results, wrote the paper, and had primary responsibility for final content; BJC and EMCF: contributed to project conception, development of overall research plan, data analysis, interpretation, editing, and critical review; CHK: contributed to study design, interpretation, and editing; JAM and VEB: contributed to study design, interpretation, editing, and critical review; EW: contributed to analysis and editing; MLK and SEA: contributed to interpretation and editing; ALC: contributed to editing; CMP: contributed to project conception, development of the overall research plan, data analysis, interpretation, editing, and critical review; and all authors: read and approved the final version submitted. None of the authors has declared a conflict of interest.
Notes
Supported by National Cancer Institute grant R01 CA175011-01. CMP is supported by a Canadian Institutes of Health Research (CIHR) New Investigator Salary Award and the Campus Alberta Research Chair Program.
Supplemental Tables 1 and 2 and Supplemental Figures 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CT, computed tomography; CRC, colorectal cancer; HU, Hounsfield unit; IMAT, intermuscular adipose tissue; KPNC, Kaiser Permanente Northern California; NLR, neutrophil-lymphocyte ratio; SMI, skeletal muscle index; SMD, skeletal muscle radiodensity; SAT, subcutaneous adipose tissue; TAT, total adipose tissue; VAT, visceral adipose tissue.
References
- 1. Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. J Parenter Enteral Nutr. 2014;38:940–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kelley DE, Mokan M, Simoneau JA, Mandarino LJ. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest. 1993;92:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM et al.. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–93. [DOI] [PubMed] [Google Scholar]
- 5. Malietzis G, Currie AC, Athanasiou T, Johns N, Anyamene N, Glynne-Jones R, Kennedy RH, Fearon KC, Jenkins JT. Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg. 2016;103:572–80. [DOI] [PubMed] [Google Scholar]
- 6. Huang DD, Wang SL, Zhuang CL, Zheng BS, Lu JX, Chen FF, Zhou CJ, Shen X, Yu Z. Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Colorectal Dis. 2015;17:O256–264. [DOI] [PubMed] [Google Scholar]
- 7. Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107:931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peng PD, van Vledder MG, Tsai S, de Jong MC, Makary M, Ng J, Edil BH, Wolfgang CL, Schulick RD, Choti MA et al.. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford). 2011;13:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prado CM, Cushen SJ, Orsso CE, Ryan AM. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc. 2016;75:188–98. [DOI] [PubMed] [Google Scholar]
- 10. Jones K, Gordon-Weeks A, Coleman C, Silva M. Radiologically determined sarcopenia predicts morbidity and mortality following abdominal surgery: a systematic review and meta-analysis. World J Surg. 2017;41:2266–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miljkovic I, Zmuda JM. Epidemiology of myosteatosis. Curr Opin Clin Nutr Metab Care. 2010;13:260–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, Mazurak VC. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf). 2014;210:489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–47. [DOI] [PubMed] [Google Scholar]
- 14. Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, Xiao J, Weltzien E, Feliciano EC, Castillo AL, Quesenberry CP, Kwan ML et al.. Explaining the obesity paradox: the association between body composition and colorectal cancer survival (C-SCANS Study). Cancer Epidemiol Biomarkers Prev. 2017;26(7):1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, Heymsfield SB, Heshka S. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985). 2004;97:2333–8. [DOI] [PubMed] [Google Scholar]
- 16. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 17. Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, Pierson RN Jr.. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. 1990;52:214–8. [DOI] [PubMed] [Google Scholar]
- 18. Miller KD, Jones E, Yanovski JA, Shankar R, Feuerstein I, Falloon J. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet. 1998;351:871–5. [DOI] [PubMed] [Google Scholar]
- 19. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–35. [DOI] [PubMed] [Google Scholar]
- 20. Kroenke CH, Prado CM, Meyerhardt JA, Weltzien EK, Xiao J, Cespedes Feliciano EM, Caan BJ. Muscle radiodensity and mortality in patients with colorectal cancer. Cancer. 2018;124:3008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B et al.. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. [DOI] [PubMed] [Google Scholar]
- 22. Burtis CA, Ashwood ER. Tietz Textbook of Clinical Chemistry. 3rd ed Philadelphia, PA: WB Saunders; 1998. p. 481–85. [Google Scholar]
- 23. Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports. 1995;5:129–42. [DOI] [PubMed] [Google Scholar]
- 24. Keller K, Engelhardt M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligaments Tendons J. 2013;3:346–50. [PMC free article] [PubMed] [Google Scholar]
- 25. Borkan GA, Hults DE, Gerzof SG, Robbins AH, Silbert CK. Age changes in body composition revealed by computed tomography. J Gerontol. 1983;38:673–7. [DOI] [PubMed] [Google Scholar]
- 26. Carrara G, Pecorelli N, De Cobelli F, Cristel G, Damascelli A, Beretta L, Braga M. Preoperative sarcopenia determinants in pancreatic cancer patients. Clin Nutr. 2017;36:1649–53. [DOI] [PubMed] [Google Scholar]
- 27. Weinberg MS, Shachar SS, Muss HB, Deal AM, Popuri K, Yu H, Nyrop KA, Alston SM, Williams GR. Beyond sarcopenia: characterization and integration of skeletal muscle quantity and radiodensity in a curable breast cancer population. Breast J. 2018;24:278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Esfandiari N, Ghosh S, Prado CM, Martin L, Mazurak V, Baracos VE. Age, obesity, sarcopenia, and proximity to death explain reduced mean muscle attenuation in patients with advanced cancer. J Frailty Aging. 2014;3:3–8. [DOI] [PubMed] [Google Scholar]
- 29. Stangl MK, Bocker W, Chubanov V, Ferrari U, Fischereder M, Gudermann T, Hesse E, Meinke P, Reincke M, Reisch N et al.. Sarcopenia - endocrinological and neurological aspects. Exp Clin Endocrinol Diabetes. 2018 (ahead of print). [DOI] [PubMed] [Google Scholar]
- 30. Ali S, Garcia JM. Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options - a mini-review. Gerontology. 2014;60:294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haizlip KM, Harrison BC, Leinwand LA. Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology (Bethesda). 2015;30:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bosy-Westphal A, Muller MJ. Identification of skeletal muscle mass depletion across age and BMI groups in health and disease—there is need for a unified definition. Int J Obes (Lond). 2015;39:379–86. [DOI] [PubMed] [Google Scholar]
- 33. Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985). 2000;89:104–10. [DOI] [PubMed] [Google Scholar]
- 34. Stein TP, Wade CE. Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135:1824s–8s. [DOI] [PubMed] [Google Scholar]
- 35. Shah AD, Kandula NR, Lin F, Allison MA, Carr J, Herrington D, Liu K, Kanaya AM. Less favorable body composition and adipokines in South Asians compared with other US ethnic groups: results from the MASALA and MESA studies. Int J Obes (Lond). 2016;40:639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taaffe DR, Cauley JA, Danielson M, Nevitt MC, Lang TF, Bauer DC, Harris TB. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2001;16:1343–52. [DOI] [PubMed] [Google Scholar]
- 37. Parsons HA, Baracos VE, Dhillon N, Hong DS, Kurzrock R. Body composition, symptoms, and survival in advanced cancer patients referred to a phase I service. PLoS One. 2012;7:e29330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ryan AS, Nicklas BJ, Berman DM. Racial differences in insulin resistance and mid-thigh fat deposition in postmenopausal women. Obes Res. 2002;10:336–44. [DOI] [PubMed] [Google Scholar]
- 39. Munoz J, Gower BA. Relationship between serum leptin concentration and low-density muscle in postmenopausal women. J Clin Endocrinol Metab. 2003;88:1157–61. [DOI] [PubMed] [Google Scholar]
- 40. Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, Visser M, Harris TB. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81:903–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miljkovic-Gacic I, Wang X, Kammerer CM, Gordon CL, Bunker CH, Kuller LH, Patrick AL, Wheele VW, Evans RW, Zmuda JM. Fat infiltration in muscle: new evidence for familial clustering and associations with diabetes. Obesity (Silver Spring). 2008;16:1854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barreiro E, del Puerto-Nevado L, Puig-Vilanova E, Perez-Rial S, Sanchez F, Martinez-Galan L, Rivera S, Gea J, González-Mangado N, Peces-Barba G. Cigarette smoke-induced oxidative stress in skeletal muscles of mice. Respir Physiol Neurobiol. 2012;182:9–17. [DOI] [PubMed] [Google Scholar]
- 43. Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87:801–9. [DOI] [PubMed] [Google Scholar]
- 44. Xiao J, Caan BJ, Weltzien E, Cespedes Feliciano EM, Kroenke CH, Meyerhardt JA, Baracos VE, Kwan ML, Castillo AL, Prado CM. Associations of pre-existing co-morbidities with skeletal muscle mass and radiodensity in patients with non-metastatic colorectal cancer. J Cachexia Sarcopenia Muscle. 2018;9:654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shinko D, Diakos CI, Clarke SJ, Charles KA. Cancer-related systemic inflammation: the challenges and therapeutic opportunities for personalized medicine. Clin Pharmacol Ther. 2017;102:599–610. [DOI] [PubMed] [Google Scholar]
- 46. Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, Xiao J, Alexeeff S, Corley D, Weltzien E et al.. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS Study. JAMA Oncol. 2017;3:e172319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Malietzis G, Johns N, Al-Hassi HO, Knight SC, Kennedy RH, Fearon KC, Aziz O, Jenkins JT. Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer. Ann Surg. 2016;263:320–5. [DOI] [PubMed] [Google Scholar]
- 48. Rollins KE, Tewari N, Ackner A, Awwad A, Madhusudan S, Macdonald IA, Fearon KC, Lobo DN. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr. 2016;35:1103–9. [DOI] [PubMed] [Google Scholar]
- 49. Kim H, Hirano H, Edahiro A, Ohara Y, Watanabe Y, Kojima N, Kim M, Hosoi E, Yoshida Y, Yoshida H et al.. Sarcopenia: prevalence and associated factors based on different suggested definitions in community-dwelling older adults. Geriatr Gerontol Int. 2016;16 Suppl 1:110–22. [DOI] [PubMed] [Google Scholar]
- 50. Baumgartner RN, Koehler KM, Romero L, Garry PJ. Serum albumin is associated with skeletal muscle in elderly men and women. Am J Clin Nutr. 1996;64:552–8. [DOI] [PubMed] [Google Scholar]
- 51. Glance LG, Osler TM, Neuman MD. Redesigning surgical decision making for high-risk patients. N Engl J Med. 2014;370:1379–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012;96:1454–64. [DOI] [PubMed] [Google Scholar]
- 53. Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc. 2011;43:249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sjoblom B, Gronberg BH, Wentzel-Larsen T, Baracos VE, Hjermstad MJ, Aass N, Bremnes RM, Fløtten Ø, Bye A, Jordhøy M. Skeletal muscle radiodensity is prognostic for survival in patients with advanced non-small cell lung cancer. Clin Nutr. 2016;35:1386–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.