ABSTRACT
Background
Patients with cystic fibrosis (CF) have increased risk of vitamin D deficiency owing to fat malabsorption and other factors. Vitamin D deficiency has been associated with increased risk of pulmonary exacerbations of CF.
Objectives
The primary objective of this study was to examine the impact of a single high-dose bolus of vitamin D3 followed by maintenance treatment given to adults with CF during an acute pulmonary exacerbation on future recurrence of pulmonary exacerbations.
Methods
This was a multicenter, double-blind, placebo-controlled, intent-to-treat clinical trial. Subjects with CF were randomly assigned to oral vitamin D3 given as a single dose of 250,000 International Units (IU) or to placebo within 72 h of hospital admission for an acute pulmonary exacerbation, followed by 50,000 IU of vitamin D3 or an identically matched placebo pill taken orally every other week starting at 3 mo after random assignment. The primary outcome was the composite endpoint of the time to next pulmonary exacerbation or death within 1 y. The secondary outcomes included circulating concentrations of the antimicrobial peptide cathelicidin and recovery of lung function as assessed by the percentage of predicted forced expiratory volume in 1 s (FEV1%).
Results
A total of 91 subjects were enrolled in the study. There were no differences between the vitamin D3 and placebo groups in time to next pulmonary exacerbation or death at 1 y. In addition, there were no differences in serial recovery of lung function after pulmonary exacerbation by FEV1% or in serial concentrations of plasma cathelicidin.
Conclusions
Vitamin D3 initially given at the time of pulmonary exacerbation of CF did not alter the time to the next pulmonary exacerbation, 12-mo mortality, serial lung function, or serial plasma cathelicidin concentrations. This trial was registered at clinicaltrials.gov as NCT01426256.
Keywords: vitamin D, cystic fibrosis, pulmonary exacerbation, cathelicidin, clinical trial, nutrition, lung function
Introduction
Patients with cystic fibrosis (CF) have a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene which results in derangements in chloride transport across epithelial surfaces, leading to abnormally thickened mucus on the surfaces of the lung, pancreas, intestines, and other organs (1). Resulting chronic infection and inflammation of the airways lead to progressive destruction of the lung, respiratory failure, and ultimately a shortened life span. Acute pulmonary exacerbations are a common clinical event in patients with CF and are associated with increased morbidity and mortality (2–4). Pulmonary exacerbations are usually triggered by a viral or bacterial infection and are associated with worsened pulmonary function, including increased shortness of breath, cough, sputum production, fatigue, and weight loss (5, 6). Approximately 50% of patients with CF will experience an acute pulmonary exacerbation yearly and will have accelerated loss of lung function compared with those patients with CF who do not experience an acute pulmonary exacerbation (4, 7). Despite aggressive treatment, lung function often does not fully recover after an acute pulmonary exacerbation (8). Antibiotic therapy is recommended for treatment of pulmonary exacerbations but few other therapies, other than routine clinical pulmonary support, exist (7).
Vitamin D is proposed to be an important mediator of immunity and respiratory health (9). Vitamin D deficiency, as determined by circulating 25-hydroxyvitamin D [25(OH)D] concentrations, is associated with higher risk of chronic lung diseases and rates of upper respiratory tract infection (10). Vitamin D upregulates production of an important antimicrobial peptide, cathelicidin (LL-37), in in vitro and in clinical studies (11–13), providing a strong mechanistic basis for its immunomodulatory effects. Patients with CF commonly experience vitamin D depletion and frank deficiency due to fat malabsorption secondary to exocrine pancreatic insufficiency, decreased sunlight exposure, poor nutritional intake, and changes in vitamin D metabolism (14–17). Several studies have documented that vitamin D deficiency increases the risk of pulmonary exacerbation in children and adults with CF (18–20). Recent small pilot studies in individuals with CF have demonstrated that vitamin D administration improved recovery after a pulmonary exacerbation of CF (21–23).
Our single-center pilot study demonstrated that a single oral dose of 250,000 International Units (IU) vitamin D3 given to adults at the time of hospital admission for a pulmonary exacerbation of CF was associated with improved 12-mo survival, recovery of lung function, and decreased inflammatory cytokine concentrations in blood compared with placebo (22, 23).
Informed by these pilot study data, we conducted a multicenter, double-blind, randomized, placebo-controlled clinical trial to investigate the impact of high-dose vitamin D3 administered to adults with CF during and after an acute pulmonary exacerbation. The primary endpoint of this study was the time to next pulmonary exacerbation or death within 12 mo after random assignment. Secondary serial outcomes included number of pulmonary exacerbations, lung function, plasma LL-37 concentrations, and markers of safety of the intervention.
Methods
Study procedures
This was a multicenter study (NCT01426256) conducted by 5 Cystic Fibrosis Foundation Therapeutics Development Network Centers: Emory University and Emory University Hospital (Atlanta, GA), The University of Alabama Hospital at Birmingham (Birmingham, AL), Case Western Reserve University and Rainbow Babies and Children's Hospital (Cleveland, OH), University of Iowa and University of Iowa Hospitals and Clinic (Iowa City, IA), and the University of Cincinnati and University of Cincinnati Medical Center (Cincinnati, OH). All study sites received human studies approval from their local Institutional Review Boards before participation in the trial. Our study methods and procedures were published in detail before completion of this study (24).
An independent data and safety monitoring board (DSMB) was established before the initiation of the study and convened every 6 mo to review the progress of study enrollment, primary outcomes, and safety measurements. All investigators and members of the DSMB remained blinded to the study drug assignment except for 1 biostatistician who prepared the biannual safety reports for the DSMB. Annual in-person audits of all of the study sites were conducted by the Principal Investigator of the coordinating site (VT of Emory University) to ensure compliance with the study protocol and the completeness of data collection.
Subject eligibility
Potentially eligible patients were screened upon hospital admission by reviewing the electronic medical record for eligibility criteria. As previously published, the inclusion criteria included the following: 1) adult and adolescent patients with a diagnosis of CF ≥16 y of age; 2) admission to the hospital for treatment of an acute pulmonary exacerbation of CF; 3) enrollment into the study within 72 h of hospital admission; 4) ability to tolerate oral medication; and 5) expectation to survive the hospital admission. We previously published a number of exclusion criteria that included 1) inability to provide written informed consent; 2) serum total 25(OH)D concentration <10 or >55 ng/mL within the previous 12 mo; 3) vitamin D intake that was >2000 IU daily exclusive of vitamin D contained in a multivitamin or >10,000 IU vitamin D bolus dose within the past 60 d; 4) plans for pregnancy in the next 12 mo; 5) conditions that could be exacerbated by vitamin D including current hypercalcemia (albumin-corrected calcium >10.8 mg/dL or ionized calcium >5.2 mg/dL) or history of nephrolithiasis in the past 2 y; 6) conditions that affect vitamin D metabolism including chronic kidney disease with an estimated glomerular filtration rate <60 mL/min, oral or intravenous glucocorticoid use in the previous month, hepatic dysfunction, and use of cytotoxic medications or immunosuppressive drugs; 7) conditions that affected survival including history of organ transplantation or plans for organ transplantation, HIV/AIDS, illicit drug abuse, or any other serious medical illness that in the opinion of the Principal Investigator would affect subject safety or 1-y survival; and 8) enrollment in any other invention trial.
Random assignment and masking
Subjects were randomly assigned and stratified by site using blocks of 10 in a 1:1 ratio of assignment to the groups receiving vitamin D and placebo. The randomization sequence was created by an independent biostatistician. The study drug assignment was kept blinded from all of the study investigators, research coordinators, dispensing pharmacists, and subjects. The study medication was identical in size, color, and shape and was dispensed in identical containers to conceal the identity of the study drug.
Intervention
The subjects randomly assigned to vitamin D received an oral dose of 5 capsules of vitamin D3 containing 50,000 IU in each capsule (BioTech Pharmacal, Inc.) to equal a total dose of 250,000 IU. Vitamin D3 was chosen based on the recommendations of the CF Foundation and studies suggesting that vitamin D3 had improved bioavailability in patients with CF as compared to vitamin D2 (16, 25). The subjects randomly assigned to placebo received an oral dose of 5 capsules that were identical in size, shape, and color to the vitamin D3 capsule produced by the same manufacturer. The study medication (vitamin D or placebo) was ingested with 240 mL of a standard liquid meal replacement beverage (Boost; Nestlé) and under direct observation by the study coordinator. The amount of vitamin D contained in the study capsules was verified by an independent laboratory (ARL Bio Pharma). Subjects who were not on any chronic vitamin D replacement were given 800 IU vitamin D as part of standard of care to prevent severe vitamin D deficiency.
At the 3-mo study visit, subjects randomly assigned to vitamin D received a bottle containing 20 capsules of 50,000 IU vitamin D3 and were instructed to take a capsule every 2 wk for the remainder of the study. Subjects randomly assigned to placebo received a bottle containing 20 capsules of placebo and were given the same instructions to take a capsule every 2 wk for the remainder of the study. The coordinating site (Emory) provided reminder phone calls every 2 wk to all of the enrolled study subjects.
Subjects had study assessments at the time of enrollment (within 72 h of hospital admission); on days 1, 2, 3, and 7 during their hospitalization (if they remained hospitalized); and at 1, 3, 6, and 12 mo after random assignment, usually during a scheduled outpatient visit for routine CF care.
Outcomes
The study sought to demonstrate that a single large dose of vitamin D3 given at the time of pulmonary exacerbation of CF would improve 1-y survival and, over the 12-mo period of observation, time to next pulmonary exacerbation and number of pulmonary exacerbations, lung function as assessed by percentage of predicted forced expiratory volume in 1 s (FEV1%), and innate immunity as assessed by plasma concentrations of LL-37. The primary outcome was the composite of time to next pulmonary exacerbation or death (24). Pulmonary exacerbations were identified by study investigators when a subject initiated new antibiotics during the study period. The pulmonary exacerbations were defined using criteria adapted from previously conducted trials in the CF Foundation Therapeutics Development Network and confirmed by the site Principal Investigator (26, 27). The criteria for a pulmonary exacerbation were defined as the presence of 1 major criterion (decrease in FEV1% of >10%, oxygen saturation of <90% on room air or absolute decrease of ≥5%, changes on chest radiograph indicative of a new infiltrate, effusion or atelectasis, and new hemoptysis) or ≥2 minor criteria lasting >3 d in the absence of 1 major criterion (increased respiratory rate or work of breathing, new or worsening breath sounds on exam, >5% loss of body weight, increased cough, decreased exercise activity, increased chest congestion or sputum) (26, 27). Secondary outcomes included recovery of lung function from time of enrollment as assessed by FEV1%, plasma LL-37 concentrations, and total number of pulmonary exacerbations.
Safety measurements (adverse event outcomes) at each study visit included total serum 25(OH)D concentration, serum calcium concentration corrected for serum albumin concentration, serum creatinine concentration, and spot urine calcium:creatinine ratio. At each study visit, subjects were also queried for symptoms of hypercalcemia which included polyuria, polydipsia, and kidney stones.
Laboratory measurements
Serum 25(OH)D concentrations were measured using the Immunodiagnostic Systems Inc. iSYS chemiluminescent automated system in a laboratory participating in the vitamin D quality assessment scheme (DEQAS) and NIH Vitamin D QA Program. Plasma LL-37 concentrations were determined by ELISA (Hycult Biotech). Serum and urine calcium and creatinine concentrations were measured by standard hospital methods, as was serum albumin.
Statistical analysis
The power calculation for the target sample size has been previously published (24). In brief, a sample size of 45 subjects in each study group (90 total) was expected to provide 80% power to detect an HR of 0.5 between the vitamin D arm and the placebo arm, assuming an event rate of 86% in the placebo arm at 1-y follow-up with a 0.05 significance level. A dropout rate of 10% was assumed in the sample size calculation.
All analyses were intention-to-treat analyses and carried out in SAS version 9.3 (SAS Institute Inc.), and plots were generated using R version 3.4.1 (R Foundation). The primary study outcome was the time to first composite event, defined as death or pulmonary exacerbation. The overall treatment effect on time to first composite event was evaluated using a Cox proportional hazards model, and the proportional hazards assumption was checked using the Schoenfeld residuals. The overall number of composite events was modeled using negative binomial regression due to overdispersion.
Secondary analyses involved evaluation of the treatment on measures of plasma LL-37, serum calcium, albumin, and creatinine concentrations. Secondary outcomes over time were examined using linear mixed-effects models with treatment as the main effect as well as the following covariates: age, sex, BMI, cystic fibrosis–related diabetes status, FEV1 volume, and FEV1% predicted. All secondary outcomes were assessed for normality. Following our pilot study results by Grossmann et al. (23), return to baseline lung function was evaluated by taking the ratio of the lung function at 3 mo into the study to the best lung function in the year before the study (i.e., FEV1% at 3 mo:Best FEV1%). Serum 25(OH)D concentrations over time were modeled using a mixed-effects analysis with treatment group as the primary exposure.
Results
Study participants
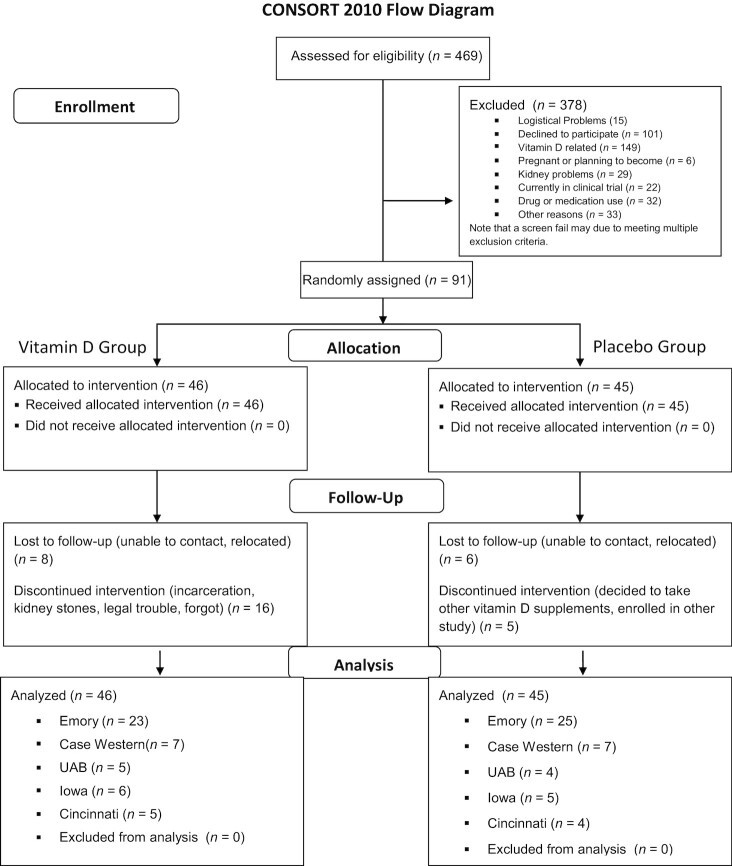
The first subject enrolled on 26 January, 2012 and the last subject enrolled on 22 March, 2016 and completed the study on 24 April, 2017. A total of 91 patients were included in the analysis (46 in the treatment group, 45 in the control group) (Figure 1, CONSORT diagram). The baseline characteristics between the treatment and placebo groups were similar. Approximately 29.7% of the patients were taking various doses of vitamin D supplements at the start of the study (Table 1). Only 2 subjects in the trial were on a CFTR modulator drug (ivacaftor), both in the placebo group.
FIGURE 1.
CONSORT study diagram for the vitamin D for the Immune System (DISC) Study: a multicenter, double-blind, randomized, placebo-controlled trial. CONSORT, Consolidated Standards of Reporting Trials; UAB, The University of Alabama at Birmingham.
TABLE 1.
Patient demographics1
| All(n = 91) | Vitamin D group(n = 46) | Placebo group(n = 45) | |
|---|---|---|---|
| Age at consent, y | 28.8 ± 7.9 | 29.8 ± 8.4 | 27.8 ± 7.3 |
| Gender, male | 50 (55.0) | 24 (52.2) | 26 (57.8) |
| Race, white | 83 (91.2) | 40 (87.0) | 43 (95.6) |
| Genotype, ΔF508 homozygous | 45 (49.5) | 27 (58.7) | 18 (40.0) |
| BMI, kg/m2 | 21.37 ± 3.9 | 21.28 ± 3.8 | 21.46 ± 3.8 |
| FEV1%2 | 48.51 ± 20.7 | 44.76 ± 16.1 | 52.27 ± 20.2 |
| Pancreatic insufficiency | 85 (93.4) | 42 (91.3) | 43 (95.6) |
| CFRD | 30 (33.0) | 11 (23.9) | 19 (42.2) |
| Reported vitamin D supplementation | 27 (29.7) | 13 (28.3) | 14 (31.1) |
| Number of hospital-free days in previous year | 350.9 ± 20.6 | 348.8 ± 21.5 | 353.0 ± 19.7 |
| Season of admission, spring/summer | 47 (51.7) | 23 (50.0) | 24 (53.3) |
| 25(OH)D,3 ng/mL | 27.0 ± 10.9 | 27.1 ± 11.3 | 26.8 ± 10.0 |
| 25(OH)D,3 <30 ng/mL | 54 (62.1) | 25 (58.1) | 29 (65.9) |
| 25(OH)D,3 <20 ng/mL | 22 (25.3) | 12 (27.9) | 10 (22.7) |
Values are means ± SDs or n (%). CFRD, cystic fibrosis–related diabetes.
One missing value in the vitamin D group.
Three missing values in the vitamin D group and 1 in the placebo group.
Primary outcomes
Figure 2 displays the Kaplan–Meier curve for time to first composite event. Cox proportional hazards regression did not reveal a significant treatment effect on time to first composite event (estimated HR of vitamin D group to placebo group: 0.85; 95% CI: 0.54, 1.34; P = 0.48). The Schoenfeld residual plot did not provide any indication that the proportional hazards assumption was violated. Table 2 displays the number of composite events experienced in each group, broken down by the number of subjects experiencing each event count (i.e., 1 event, 2 events, and so on). Table 3 displays the organ systems affected by other adverse events, broken down by treatment group.
FIGURE 2.
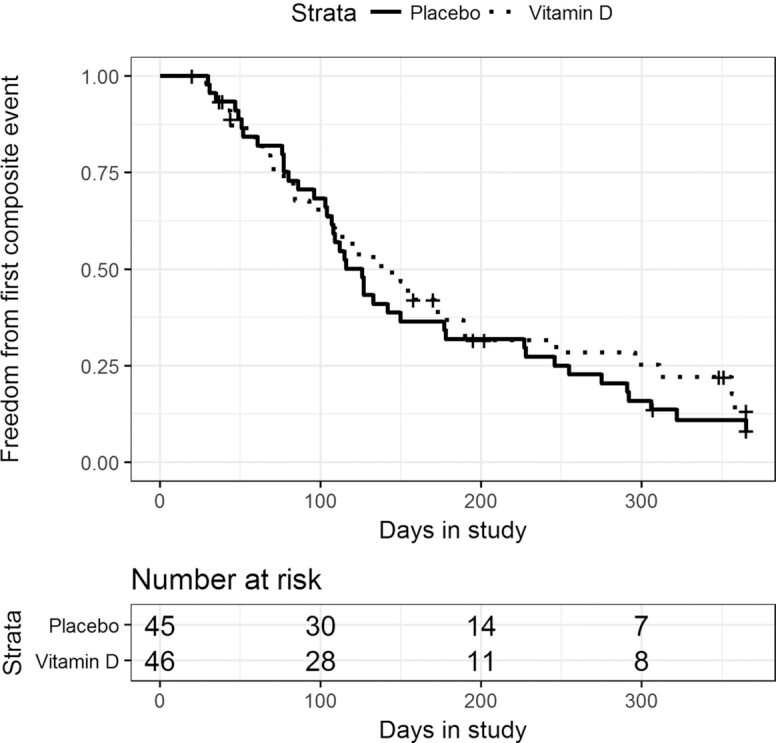
Time to first composite event (death or pulmonary exacerbation) by treatment group in the vitamin D for the Immune System (DISC) Study. The Kaplan–Meier curve for the time to first composite event by vitamin D (dotted line) and placebo (solid line) group is displayed. There were no differences in time to first composite event in the vitamin D and placebo groups in adults with cystic fibrosis followed for 1 y as assessed by Cox proportional hazards regression (95% CI: 0.54, 1.34, P = 0.48).
TABLE 2.
Total number of composite events after randomization for ≤12 mo1
| Placebo group | Vitamin D group | |||
|---|---|---|---|---|
| Composite event count | Number of subjects | Percentage of group | Number of subjects | Percentage of group |
| 0 | 5 | 11.11 | 12 | 26.07 |
| 1 | 16 | 35.56 | 13 | 28.26 |
| 2 | 8 | 17.78 | 4 | 8.70 |
| 3 | 8 | 17.78 | 7 | 15.22 |
| 4 | 5 | 11.11 | 5 | 10.87 |
| 6 | 2 | 4.44 | 4 | 8.70 |
| 7 | 1 | 2.22 | 1 | 2.17 |
| Total | 45 | 100.00 | 46 | 100.00 |
1The number of composite events by total count of events (pulmonary exacerbation of cystic fibrosis) is divided by randomization group: vitamin D or placebo. All of the composite events represented pulmonary exacerbations except for 3 deaths in the vitamin D group.
TABLE 3.
Number of subjects in each group experiencing an adverse event by organ system1
| Organ system | Vitamin D | Placebo |
|---|---|---|
| Total gastrointestinal (GI) | 0 | 2 |
| GI—diarrhea | 0 | 1 |
| GI—nausea | 0 | 1 |
| Total neurologic | 4 | 5 |
| Neurologic—fatigue | 1 | 3 |
| Neurologic—headaches | 0 | 1 |
| Neurologic—increased confusion | 3 | 1 |
| Total pulmonary | 11 | 14 |
| Pulmonary—cough | 1 | 4 |
| Pulmonary—chest pain | 2 | 0 |
| Pulmonary—decreased lung function | 2 | 2 |
| Pulmonary—dyspnea | 1 | 2 |
| Pulmonary—hemoptysis | 1 | 2 |
| Pulmonary—increased sputum | 2 | 2 |
| Pulmonary—upper respiratory tract infection | 2 | 2 |
| Total renal | 11 | 3 |
| Renal—elevated creatinine | 2 | 0 |
| Renal—nephrolithiasis | 1 | 0 |
| Renal—polydipsia | 3 | 2 |
| Renal—polyuria | 5 | 1 |
| Other—not otherwise classified | 6 | 9 |
The total number of reported adverse events occurring during the 12-mo trial is divided by the vitamin D or the placebo group.
Secondary outcomes
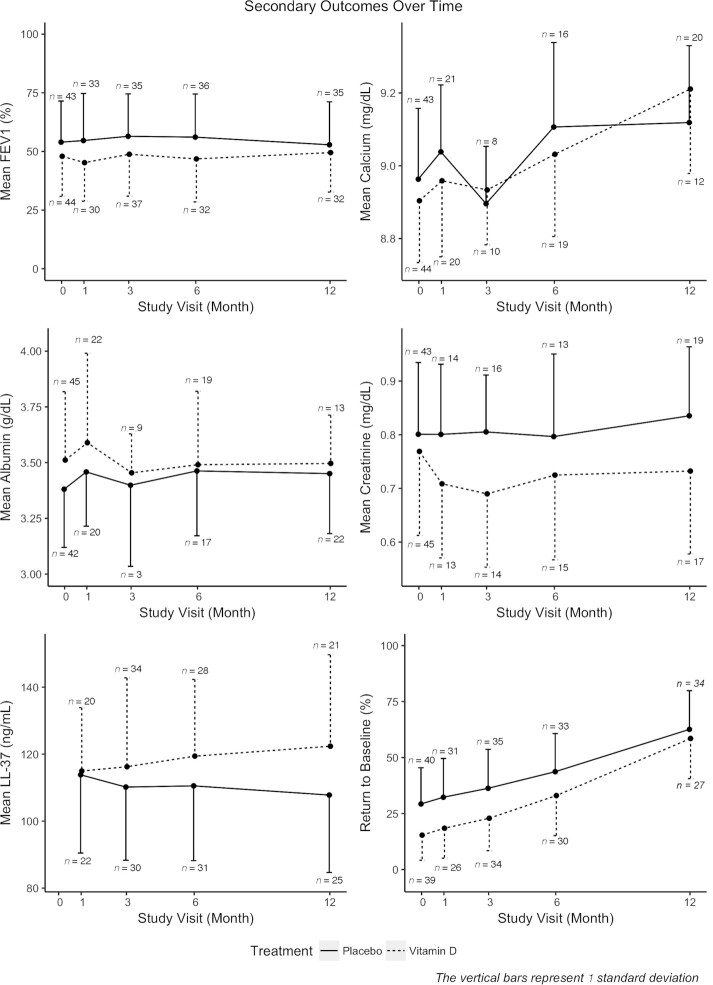
Figure 3 displays the means of the secondary outcomes tabulated by group and study visit where the length of the error bars corresponds to the SD. Two subjects were removed from the LL-37 analysis (1 from each treatment group) for having medically implausible LL-37 values. Mixed-effects analyses did not reveal significant treatment effects on any of the measures.
FIGURE 3.
Markers of lung function, calcium status, and antimicrobial peptide concentrations in adult subjects randomly assigned to vitamin D or placebo by study visit up to 1 y. Secondary endpoints in subjects randomly assigned to vitamin D (dotted line) and placebo (solid line) were measured at baseline and 1, 3, 6, and 12 mo after random assignment. Mixed-effects analyses did not reveal any differences between the 2 groups in the 6 measures: FEV1% predicted (marker of lung function), total serum calcium, serum albumin, serum creatinine, LL-37, or return to baseline lung function (%). Each point on the plot is the estimated group mean at that time point, and the vertical bars correspond to 1 SD. The sample size at each time point is reported near the error bar. FEV1%, percentage of predicted forced expiratory volume in 1 s; LL-37, cathelicidin; 25(OH)D, 25-hydroxyvitamin D.
Return to baseline lung function was assessed in study month 3. Patients whose FEV1% predicted returned to within 95% of baseline were said to have returned to baseline lung function. At study month 3, 25.3% of the placebo group and 35.3% of the vitamin D group had returned to baseline lung function. A chi-squared test of the resulting table (Returned/Did not return) by treatment group did not reveal any significant difference between groups at month 3 (P = 0.55).
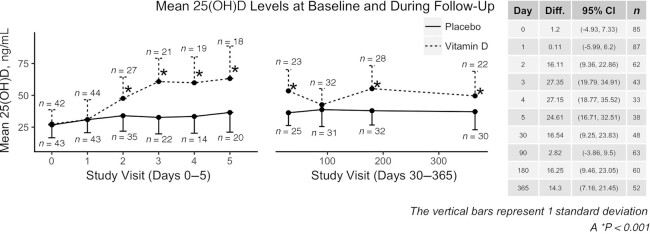
Serum 25(OH)D concentrations
Serum 25(OH)D concentrations were significantly higher in the vitamin D3 treatment group at days 2–5 and months 1, 6, and 12. The difference in serum 25(OH)D at 3 mo was not statistically significant compared with placebo (P = 0.47). Figure 4 displays the 25(OH)D concentrations over time in each group.
FIGURE 4.
Mean 25-hydroxyvitamin D concentrations in the vitamin D and placebo groups. Serum 25(OH)D concentrations in the group randomly assigned to vitamin D (dotted line) were significantly higher than in the placebo group (solid line) at days 1–7 and at 1, 6, and 12 mo in a mixed-effects regression analysis. Each point on the plot is the estimated group mean at that time point, and the vertical bars correspond to 1 SD. The sample size at each time point is reported near the error bar. 25(OH)D, 25-hydroxyvitamin D.
Adverse events
Negative binomial regression did not reveal a significant treatment effect (estimated treatment effect on the difference in the log expected counts: 0.05; 95% CI: −0.33, 0.42; P = 0.82) on the overall number of adverse events (Table 3).
Discussion
This multicenter study demonstrated that high-dose vitamin D3 administration to adults with CF initiated at the time of a pulmonary exacerbation did not improve time to next pulmonary exacerbation or 1-y survival. There were also no differences in the planned secondary outcomes of recovery of lung function or plasma concentrations of the antimicrobial peptide LL-37. Vitamin D has been reported to have pleiotropic effects on the immune and respiratory system by enhancing the innate immune response through upregulation of antimicrobial peptides and by improving lung function (11, 21). Our study does not support a role of vitamin D as an adjunctive treatment during acute pulmonary exacerbation of CF.
Pulmonary exacerbations of CF remain a common clinical entity which is associated with increased mortality and decline of lung function (28). Factors that are associated with a pulmonary exacerbation of CF include previous intravenous (IV) antibiotics in the past year, duration of previous IV antibiotics, previous hospitalization, inhaled aminoglycoside use, leukotriene modifier use, and high-dose ibuprofen use (29). Nutritional factors, such as deficiency in vitamins A, E, and D, have also been associated with increased risk of pulmonary exacerbation in adults and children with CF (18, 19, 20, 30). Early studies suggested a potential benefit of vitamin D on lung function and markers of inflammation and innate immunity (21, 22, 23, 31). The current study design was based on our previous pilot, double-blind, randomized, placebo-controlled study of 30 adults with CF who were hospitalized for an acute pulmonary exacerbation of CF (22, 23). In our pilot study, oral administration of 250,000 IU vitamin D3 improved 1-y survival and decreased blood concentrations of the proinflammatory cytokines TNFα and IL-6, with trends toward improvement of hospital-free days and lung function (FEV1%) compared with placebo (22, 23). As in our pilot study, we found robust increases in serum 25(OH)D concentrations after the bolus dose of vitamin D3 starting at day 1 after administration, reaching a peak serum 25(OH)D of >60 ng/mL by day 7 and remaining higher than placebo by the completion of the study. The subjects in our multicenter study were similar in age, gender, BMI, baseline vitamin D status and vitamin D intake, and prevalence of cystic fibrosis–related diabetes as compared with our initial pilot study. However, in contrast to our pilot study, here we were not able to demonstrate a benefit of oral high-dose vitamin D3 as an adjunctive therapy in acute pulmonary exacerbation of CF.
There is strong evidence from in vitro and clinical studies that vitamin D may play an important role in the innate immune system. Liu et al. (13) first described that vitamin D upregulated the antimicrobial response to toll-like receptor activation by bacteria in cultured human macrophages. Subsequent studies conducted in humans demonstrated that vitamin D administration in humans upregulated the local mRNA expression of cathelicidin in peripheral blood monocytes but not circulating concentrations of LL-37 (cleaved product of cathelicidin) (32). In vitro, cultured bronchial epithelial cells with the ΔF508 mutation in the CFTR have increased mRNA expression of cathelicidin in response to treatment with vitamin D (33). Furthermore, Schögler et al. (34) demonstrated that vitamin D increased mRNA expression of cathelicidin from primary bronchial epithelial cells collected from CF patients. In our study of patients with CF during acute pulmonary exacerbation, we did not show changes in circulating protein concentrations of LL-37 in response to vitamin D. One possibility for the lack of changes seen in response to vitamin D in circulation could be that the antibiotic therapies used during pulmonary exacerbation may interfere with the vitamin D induction of LL-37 (35). Another possibility: LL-37 is complexed with anionic bacterial molecules such as endotoxin (LPS) and capsular polysaccharides, as well as with DNA in neutrophil extracellular traps and host-derived glycosaminoglycan (36–39). The abundance of bacterial-derived anionic molecules in the CF host, especially during pulmonary exacerbations, inhibits LL-37 activity and may reduce the concentrations of plasma LL-37. Also, the decision by clinicians to start IV antibiotics during acute pulmonary exacerbation is not well standardized and may have affected our findings (40). In addition, 25–50% of patients with CF fail to return to their baseline lung function despite IV antibiotics (41, 42). Another reason for the lack of observed changes could be that potential local changes in lung LL-37 concentrations were not determined. Examining the local mRNA expression of cathelicidin from peripheral blood monocytes or monocytes and epithelial cells collected from bronchial alveolar lavage may provide better insight as to whether vitamin D enhances these local cellular responses.
Although we did not demonstrate an improvement in time to next pulmonary exacerbation and return to baseline lung function, these endpoints may be insensitive markers for improved health in patients with CF. Using a randomized controlled trial design, Pincikova et al. (21) demonstrated that vitamin D therapy in children was associated with lower IL-8 concentrations in blood, a marker of inflammation. Other surrogate markers of inflammation, which may be more sensitive markers of immune health, would be of interest in trials examining the role of vitamin D as an adjunctive therapy during pulmonary exacerbation in CF. Plasma metabolomic profiling may also provide further insights in the pathways activated by vitamin D. We recently demonstrated that high-dose vitamin D activated anticatabolic pathways in patients with CF during an acute pulmonary exacerbation (43). Other pathways important for immune and lung function should be studied as well.
One of the positive findings from the study was the demonstration of the safety of a single bolus dose in rapidly correcting vitamin D status. As previously demonstrated in our pilot study (23), we confirmed that our regimen of 250,000 IU vitamin D3 given as a single bolus dose rapidly increased serum 25(OH)D within 1 wk to the accepted healthy range in patients with CF and that these concentrations could be maintained by ingesting 50,000 IU vitamin D3 every 2 wk (23).
Despite being one of the largest studies to examine the role of supplemental vitamin D administration in CF during pulmonary exacerbation, this study has some limitations. Not all subjects in the trial were vitamin D insufficient [25(OH)D <30 ng/mL] nor did we withhold vitamin D therapy from some subjects owing to the high risk of potential adverse outcomes stemming from untreated vitamin D deficiency. The study investigators did not believe it would be ethical to have a true placebo arm in patients who were vitamin D deficient as this might have had long-term consequences on the bone health of these individuals. The allowance of supplemental vitamin D in both groups may have diminished any group differences; however, we do demonstrate a significant difference in serum 25(OH)D concentrations between both groups. Another potential limitation is that the use of IV antibiotics for treatment of pulmonary exacerbation of CF may have limited any potential differences seen between the vitamin D and placebo groups. As pulmonary exacerbation of CF is a life-threatening condition, it would be unethical to withhold such therapy. Another potential limitation is that we had an unbalanced number of subjects randomly assigned to the vitamin D group who discontinued maintenance study medication or did not complete the full 12-mo trial (n = 22) as compared with the placebo group (n = 11), which may have affected our ability to detect group differences in our primary and secondary endpoints. However, we can confirm all subjects received the bolus study medication as this was performed under direct observation, and we recorded all outpatient and inpatient pulmonary exacerbations during the study period because these were available in our respective electronic medical records. Another limitation is the amount of missing secondary outcome data due to some of the follow-up appointments being optional. Given the associations of vitamin D deficiency with risk of pulmonary exacerbation, preventative intervention with high-dose vitamin D3 before the onset of a pulmonary exacerbation may be an approach deserving further study. Finally, circulating 25(OH)D concentrations may be a marker of overall poor health in CF which predicts a pulmonary exacerbation and may not be causal in the disease pathway of pulmonary exacerbations.
We conclude that a high-dose vitamin D3 bolus, combined with maintenance therapy given to adults with CF during acute pulmonary exacerbation of CF did not improve 1-y survival, recovery of lung function, or circulating LL-37 concentrations. Given the strong association between vitamin D deficiency and risk of pulmonary exacerbation of CF, future investigations should evaluate whether long-term maintenance of adequate vitamin D status may prevent future pulmonary exacerbations of CF rather than acting as a treatment of pulmonary exacerbation of CF.
ACKNOWLEDGEMENTS
The study investigators acknowledge the support from the Cystic Fibrosis Foundation Therapeutics Drug Network and the Data Safety and Monitoring Board (DSMB) members (Chair: Laurie LeClair; Members: Octavian Ioachimescu, Eric Felner, Traci Leong) for their oversight and safety review of the study. Research coordinators: Elizabeth Ivie (Emory), Phong Tran (Emory), Mary Teresi (University of Iowa), Traci Major (University of Cincinnati), Colette Bucur (Case Western Reserve University), Tyler Farber (University of Iowa), Thomas Santacroce (University of Iowa), Tiffany Grimes (The University of Alabama), and Emily Elliott (The University of Alabama); laboratory technicians, Milton Brown (Emory), Li Hao (Emory), and Sachinkumar Singh (University of Iowa). The authors’ contributions were as follows—VT and JAA: designed the study and obtained funding; VT, JAA, JFC, REG, AG, PMJ, ESM, and TRZ: performed and supervised the conduct of the trial; VT, JAA, JL, YC, JNGB, JFC, REG, AG, PMJ, ESM, and TRZ: verified the data, analyzed and interpreted the data, and drafted and revised the manuscript; MJL, SC, and WAH: recruited study participants, collected data, and revised the manuscript; JL, YC, and JNGB: performed the statistical analyses; SEJ: performed the pretrial statistical analyses and power calculations and provided the randomization code; SEJ, RT, and SMZ: collected data, analyzed and interpreted the data, and revised the manuscript. All of the authors read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Notes
Supported by Cystic Fibrosis Foundation grants TANGPR11A0 (to VT) and JOSEPH15YO (to PMJ) and NIH grants UL1 TR000454 (Emory CTSA), UL1 TR000165 (UAB CTSA), T32 DK007298 (to JAA), T32 DK007734 (to ESM), K24 DK096574 (to TRZ), and K01 DK102851 (to JAA).
The CF Foundation and the NIH had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Abbreviations used: CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; DSMB, data and safety monitoring board; FEV1%, percentage of predicted forced expiratory volume in 1 s; IU, International Unit; IV, intravenous; LL-37, cathelicidin; 25(OH)D, 25-hydroxyvitamin D.
References
- 1. Elborn JS. Cystic fibrosis. Lancet. 2016;388(10059):2519–31. [DOI] [PubMed] [Google Scholar]
- 2. Ferkol T, Rosenfeld M, Milla CE. Cystic fibrosis pulmonary exacerbations. J Pediatr. 2006;148(2):259–64. [DOI] [PubMed] [Google Scholar]
- 3. Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: epidemiology and pathogenesis. Thorax. 2007;62(4):360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Waters V, Stanojevic S, Atenafu EG, Lu A, Yau Y, Tullis E, Ratjen F. Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur Respir J. 2012;40(1):61–6. [DOI] [PubMed] [Google Scholar]
- 5. Flume PA, Mogayzel PJ Jr, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, Marshall BC; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180(9):802–8. [DOI] [PubMed] [Google Scholar]
- 6. Cogen JD, Oron AP, Gibson RL, Hoffman LR, Kronman MP, Ong T, Rosenfeld M. Characterization of inpatient cystic fibrosis pulmonary exacerbations. Pediatrics. 2017;139(2):e20162642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Boer K, Vandemheen KL, Tullis E, Doucette S, Fergusson D, Freitag A, Paterson N, Jackson M, Lougheed MD, Kumar Vet al.. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax. 2011;66(8):680–5. [DOI] [PubMed] [Google Scholar]
- 8. Flume PA, Wainwright CE, Elizabeth Tullis D, Rodriguez S, Niknian M, Higgins M, Davies JC, Wagener JS. Recovery of lung function following a pulmonary exacerbation in patients with cystic fibrosis and the G551D-CFTR mutation treated with ivacaftor. J Cystic Fibrosis. 2018;17:83–8. [DOI] [PubMed] [Google Scholar]
- 9. Finklea JD, Grossmann RE, Tangpricha V. Vitamin D and chronic lung disease: a review of molecular mechanisms and clinical studies. Adv Nutr. 2011;2(3):244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AAet al.. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quraishi SA, De Pascale G, Needleman JS, Nakazawa H, Kaneki M, Bajwa EK, Camargo CA Jr, Bhan I. Effect of cholecalciferol supplementation on vitamin D status and cathelicidin levels in sepsis: a randomized, placebo-controlled trial. Crit Care Med. 2015;43(9):1928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramos-Martínez E, López-Vancell MR, Fernández de Córdova-Aguirre JC, Rojas-Serrano J, Chavarría A, Velasco-Medina A, Velázquez-Sámano G. Reduction of respiratory infections in asthma patients supplemented with vitamin D is related to increased serum IL-10 and IFNγ levels and cathelicidin expression. Cytokine. 2018;108:239–46. [DOI] [PubMed] [Google Scholar]
- 13. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken Cet al.. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–3. [DOI] [PubMed] [Google Scholar]
- 14. Chesdachai S, Tangpricha V. Treatment of vitamin D deficiency in cystic fibrosis. J Steroid Biochem Mol Biol. 2016;164:36–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Siwamogsatham O, Alvarez JA, Tangpricha V. Diagnosis and treatment of endocrine comorbidities in patients with cystic fibrosis. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tangpricha V, Kelly A, Stephenson A, Maguiness K, Enders J, Robinson KA, Marshall BC, Borowitz D; Cystic Fibrosis Foundation Vitamin D Evidence-Based Review Committee. An update on the screening, diagnosis, management, and treatment of vitamin D deficiency in individuals with cystic fibrosis: evidence-based recommendations from the Cystic Fibrosis Foundation. J Clin Endocrinol Metab. 2012;97(4):1082–93. [DOI] [PubMed] [Google Scholar]
- 17. Wolfenden LL, Judd SE, Shah R, Sanyal R, Ziegler TR, Tangpricha V. Vitamin D and bone health in adults with cystic fibrosis. Clin Endocrinol (Oxf). 2008;69(3):374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vanstone MB, Egan ME, Zhang JH, Carpenter TO. Association between serum 25-hydroxyvitamin D level and pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol. 2015;50(5):441–6. [DOI] [PubMed] [Google Scholar]
- 19. Sexauer WP, Hadeh A, Ohman-Strickland PA, Zanni RL, Varlotta L, Holsclaw D, Fiel S, Graff GR, Atlas A, Bisberg Det al.. Vitamin D deficiency is associated with pulmonary dysfunction in cystic fibrosis. J Cyst Fibros. 2015;14(4):497–506. [DOI] [PubMed] [Google Scholar]
- 20. McCauley LA, Thomas W, Laguna TA, Regelmann WE, Moran A, Polgreen LE. Vitamin D deficiency is associated with pulmonary exacerbations in children with cystic fibrosis. Ann Am Thorac Soc. 2014;11(2):198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pincikova T, Paquin-Proulx D, Sandberg JK, Flodström-Tullberg M, Hjelte L. Clinical impact of vitamin D treatment in cystic fibrosis: a pilot randomized, controlled trial. Eur J Clin Nutr. 2017;71(2):203–5. [DOI] [PubMed] [Google Scholar]
- 22. Grossmann RE, Zughaier SM, Liu S, Lyles RH, Tangpricha V. Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation. Eur J Clin Nutr. 2012;66(9):1072–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grossmann RE, Zughaier SM, Kumari M, Seydafkan S, Lyles RH, Liu S, Sueblinvong V, Schechter MS, Stecenko AA, Ziegler TRet al.. Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation: a randomized, controlled trial. Dermatoendocrinol. 2012;4(2):191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tangpricha V, Smith EM, Binongo J, Judd SE, Ziegler TR, Walker S, Tirouvanziam R, Zughaier SM, Lee MJ, Chesdachai Set al.. The Vitamin D for Enhancing the Immune System in Cystic Fibrosis (DISC) trial: rationale and design of a multi-center, double-blind, placebo-controlled trial of high dose bolus administration of vitamin D3 during acute pulmonary exacerbation of cystic fibrosis. Contemp Clin Trials Commun. 2017;6:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khazai NB, Judd SE, Jeng L, Wolfenden LL, Stecenko A, Ziegler TR, Tangpricha V. Treatment and prevention of vitamin D insufficiency in cystic fibrosis patients: comparative efficacy of ergocalciferol, cholecalciferol, and UV light. J Clin Endocrinol Metab. 2009;94(6):2037–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saiman L, Anstead M, Mayer-Hamblett N, Lands LC, Kloster M, Hocevar-Trnka J, Goss CH, Rose LM, Burns JL, Marshall BCet al.. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2010;303(17):1707–15. [DOI] [PubMed] [Google Scholar]
- 27. Treggiari MM, Rosenfeld M, Mayer-Hamblett N, Retsch-Bogart G, Gibson RL, Williams J, Emerson J, Kronmal RA, Ramsey BW; EPIC Study Group. Early anti-pseudomonal acquisition in young patients with cystic fibrosis: rationale and design of the EPIC clinical trial and observational study. Contemp Clin Trials. 2009;30(3):256–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Justicia JL, Solé A, Quintana-Gallego E, Gartner S, de Gracia J, Prados C, Máiz L. Management of pulmonary exacerbations in cystic fibrosis: still an unmet medical need in clinical practice. Expert Rev Respir Med. 2015;9(2):183–94. [DOI] [PubMed] [Google Scholar]
- 29. VanDevanter DR, Pasta DJ, Konstan MW. Treatment and demographic factors affecting time to next pulmonary exacerbation in cystic fibrosis. J Cyst Fibros. 2015;14(6):763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hakim F, Kerem E, Rivlin J, Bentur L, Stankiewicz H, Bdolach-Abram T, Wilschanski M. Vitamins A and E and pulmonary exacerbations in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2007;45(3):347–53. [DOI] [PubMed] [Google Scholar]
- 31. Pincikova T, Paquin-Proulx D, Sandberg JK, Flodström-Tullberg M, Hjelte L. Vitamin D treatment modulates immune activation in cystic fibrosis. Clin Exp Immunol. 2017;189(3):359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M. Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182(7):4289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D3. J Cyst Fibros. 2007;6(6):403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schögler A, Muster RJ, Kieninger E, Casaulta C, Tapparel C, Jung A, Moeller A, Geiser T, Regamey N, Alves MP. Vitamin D represses rhinovirus replication in cystic fibrosis cells by inducing LL-37. Eur Respir J. 2016;47(2):520–30. [DOI] [PubMed] [Google Scholar]
- 35. Chesdachai S, Zughaier SM, Hao L, Kempker RR, Blumberg HM, Ziegler TR, Tangpricha V. The effects of first-line anti-tuberculosis drugs on the actions of vitamin D in human macrophages. J Clin Transl Endocrinol. 2016;6:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nagaoka I, Hirota S, Niyonsaba F, Hirata M, Adachi Y, Tamura H, Tanaka S, Heumann D. Augmentation of the lipopolysaccharide-neutralizing activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by replacement with hydrophobic and cationic amino acid residues. Clin Diagn Lab Immunol. 2002;9(5):972–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zughaier SM, Svoboda P, Pohl J, Stephens DS, Shafer WM. The human host defense peptide LL-37 interacts with Neisseria meningitidis capsular polysaccharides and inhibits inflammatory mediators release. PLoS One. 2010;5(10):e13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Neumann A, Völlger L, Berends ET, Molhoek EM, Stapels DA, Midon M, Friães A, Pingoud A, Rooijakkers SH, Gallo RLet al.. Novel role of the antimicrobial peptide LL-37 in the protection of neutrophil extracellular traps against degradation by bacterial nucleases. J Innate Immun. 2014;6(6):860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bergsson G, Reeves EP, McNally P, Chotirmall SH, Greene CM, Greally P, Murphy P, O'Neill SJ, McElvaney NG. LL-37 complexation with glycosaminoglycans in cystic fibrosis lungs inhibits antimicrobial activity, which can be restored by hypertonic saline. J Immunol. 2009;183(1):543–51. [DOI] [PubMed] [Google Scholar]
- 40. Sanders DB, Solomon GM, Beckett VV, West NE, Daines CL, Heltshe SL, VanDevanter DR, Spahr JE, Gibson RL, Nick JAet al.. Standardized Treatment of Pulmonary Exacerbations (STOP) study: observations at the initiation of intravenous antibiotics for cystic fibrosis pulmonary exacerbations. J Cyst Fibros. 2017;16(5):592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med. 2010;182(5):627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Waters VJ, Stanojevic S, Sonneveld N, Klingel M, Grasemann H, Yau YC, Tullis E, Wilcox P, Freitag A, Chilvers Met al.. Factors associated with response to treatment of pulmonary exacerbations in cystic fibrosis patients. J Cyst Fibros. 2015;14:755–62. [DOI] [PubMed] [Google Scholar]
- 43. Alvarez JA, Chong EY, Walker DI, Chandler JD, Michalski ES, Grossmann RE, Uppal K, Li S, Frediani JK, Tirouvanziam Ret al.. Plasma metabolomics in adults with cystic fibrosis during a pulmonary exacerbation: a pilot randomized study of high-dose vitamin D3 administration. Metabolism. 2017;70:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]