ABSTRACT
Background
A higher protein intake is suggested to preserve muscle mass during aging and may therefore reduce the risk of sarcopenia.
Objectives
We explored whether the amount and type (animal or vegetable) of protein intake were associated with 5-y change in mid-thigh muscle cross-sectional area (CSA) in older adults (n = 1561).
Methods
Protein intake was assessed at year 2 by a Block food-frequency questionnaire in participants (aged 70–79 y) of the Health, Aging, and Body Composition (Health ABC) Study, a prospective cohort study. At year 1 and year 6 mid-thigh muscle CSA in square centimeters was measured by computed tomography. Multiple linear regression analysis was used to examine the association between energy-adjusted protein residuals in grams per day (total, animal, and vegetable protein) and muscle CSA at year 6, adjusted for muscle CSA at year 1 and potential confounders including prevalent health conditions, physical activity, and 5-y change in fat mass.
Results
Mean (95% CI) protein intake was 0.90 (0.88, 0.92) g · kg–1 · d–1 and mean (95% CI) 5-y change in muscle CSA was −9.8 (−10.6, −8.9) cm2. No association was observed between energy-adjusted total (β = −0.00; 95% CI: −0.06, 0.06 cm2; P = 0.982), animal (β = −0.00; 95% CI: −0.06, 0.05 cm2; P = 0.923), or plant (β = +0.07; 95% CI: −0.06, 0.21 cm2; P = 0.276) protein intake and muscle CSA at year 6, adjusted for baseline mid-thigh muscle CSA and potential confounders.
Conclusions
This study suggests that a higher total, animal, or vegetable protein intake is not associated with 5-y change in mid-thigh muscle CSA in older adults. This conclusion contradicts some, but not all, previous research. This trial was registered at www.trialregister.nl as NTR6930.
Keywords: dietary protein intake, cross-sectional muscle area, computed tomography, older adults, age-related muscle loss
Introduction
Indicators of low muscle mass and in particular low strength have been associated with functional decline and disability in older adults (1, 2). Previous studies have indicated that dietary protein intake affects protein synthesis and net protein balance in older adults. Therefore, an adequate protein intake may help to slow the process of age-related muscle loss (3, 4).
Muscle loss in older adults is associated with loss of sensitivity of the skeletal muscle to protein ingestion (5), insulin resistance, and a higher extraction of amino acids by splanchnic tissue, which results in a lower availability of amino acids for muscle protein synthesis (6). Thus, a higher protein intake may be necessary to reduce the loss of muscle mass with aging. These findings support recent suggestions (7) that the current Recommended Daily Allowance for protein for older adults of 0.8 g · kg body weight–1 · d–1 (8) potentially underestimates the true requirement.
Until now, only a few studies have investigated the relation between dietary protein intake and longitudinal changes in lean mass in older adults. Houston et al. (9) studied the relation between protein intake and longitudinal changes in lean mass assessed by dual-energy X-ray absorptiometry (DXA) over a 3-y period. They demonstrated that older adults in the highest quintile of protein intake (mean intake 1.2 g · kg–1 · d–1) lost nearly 40% less appendicular lean mass than those in the lowest quintile (mean intake 0.7 g · kg–1 · d–1). This observation (9) is supported by cross-sectional studies in which higher protein intakes are associated with more lean body mass assessed by DXA in older persons (10, 11). However, Chan et al. (12) did not find an association between total protein intake and change in appendicular muscle mass (assessed by DXA) in an older (65 y and older) Chinese population over a 4-y period.
Not only the total amount of protein intake, but protein source and amino acid composition might play a role in the age-related change in muscle mass. The essential amino acids (EAAs) deliver substrates for protein synthesis and are primarily responsible for its regulation. Of the EAAs leucine is recognized to have a specific stimulating role in muscle protein synthesis (13, 14). However, the role of leucine in the age-related change in muscle mass remains unclear because prospective observational studies and supplementation trials have provided conflicting results (15, 16).
To our knowledge, only a few longitudinal observational studies have investigated the association of protein intake, protein source (animal or protein), and leucine intake in older persons with lean mass change (9, 12, 15), with contrasting findings. Therefore, we investigated the association of the amount, type (animal or vegetable), and amino acid composition of protein intake with 5-y change in mid-thigh muscle cross-sectional area (CSA) as measured by computed tomography (CT) in older adults.
Methods
Study sample
Data from the Health, Aging, and Body Composition (Health ABC) study were used. The Health ABC study is a prospective cohort study and investigates the association among body composition, weight-related health conditions, and functional limitations in older adults (for more information, see https://healthabc.nia.nih.gov/). Between April, 1997 and June, 1998, 3075 well-functioning black and white men and women aged 70–79 y were enrolled. Participants were recruited from a random sample of white Medicare-eligible residents and all of the black Medicare-eligible residents in the Pittsburgh, PA, and Memphis, TN, metropolitan areas. Subjects were eligible if they reported no difficulties in walking one-fourth of a mile, climbing up 10 steps, or performing basic activities of daily living; no history of active cancer in the 3 y before the study; that they planned to remain in the geographic area for ≥3 y; and were not enrolled in lifestyle intervention trials. All participants gave written informed consent. All protocols were approved by the Human Investigation and Review Boards at the University of Pittsburgh and the University of Tennessee at Memphis.
Participants were included in the data analyses (registered at www.trialregister.nl as NTR6930) if they had good-quality CT data of the mid-thigh muscle at both the clinical visit at baseline (year 1) and year 6 (see below) (n = 1675) and completed the food-frequency questionnaire (FFQ) which was administered at the 12-mo follow-up clinic visit (year 2) (n = 2713). Participants were excluded if they had an FFQ with serious errors or reported energy intakes <500 kcal/d or >3500 kcal/d (women) or <800 kcal/d or >4000 kcal/d (men) (n = 116) (17). In total, 1561 participants were included in the data analyses (Figure 1).
FIGURE 1.
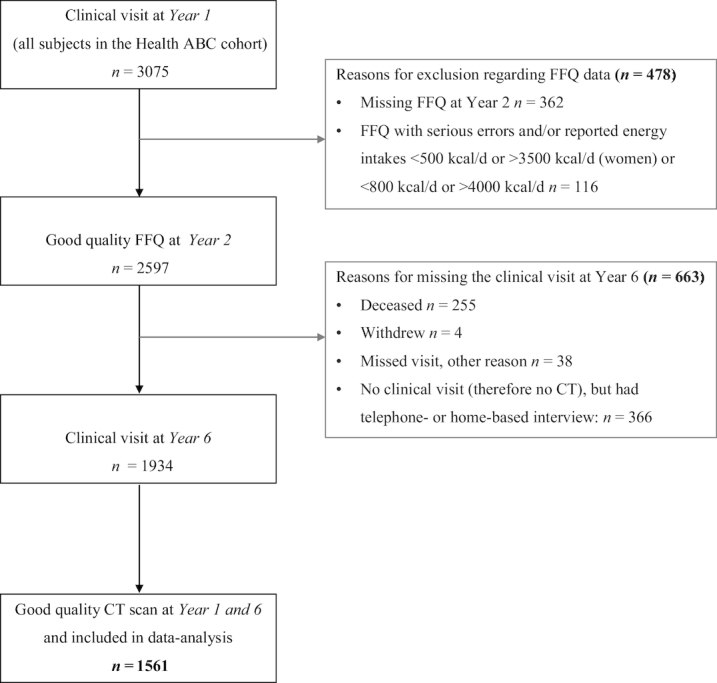
Flow chart for inclusion of participants of the Health ABC study in the data analyses. CT, computed tomography; FFQ, food-frequency questionnaire; Health ABC, Health, Aging, and Body Composition.
CT of mid-thigh muscle
The sum of the CSA (in cm2) of muscle in both thighs was analyzed. Muscle area was measured by CT (Memphis clinic site: Somatom Plus 4, Siemens, or PQ 2000S, Marconi Medical Systems; Pittsburgh clinic site: 9800 Advantage, General Electric). Year 1 and year 6 mid-thigh CSA were measured with the same CT device for each subject. An anterior–posterior scout scan of the entire right femur was used to localize the mid-thigh position. The femoral length was measured in the cranial–caudal dimension, and the midpoint was determined of the distance between the medial edge of the greater trochanter and the intercondyloid fossa. A single 10-mm-thick axial image was then obtained at the femoral midpoint, making sure that the entire circumference of both thighs was included in the field of view. These scans were completed at 120 kVp, 200–250 mA. All CT scans of both sites were transferred to one reading center and were analyzed by a single observer on a SUN Workstation (SPARCstation II, Sun Microsystems). Skeletal muscle and adipose tissue areas of the mid-thigh were calculated from the axial CT images using IDL development software (RSI Systems). Muscle and adipose tissue areas were calculated by multiplying the number of pixels of a given tissue type by the pixel area. Density values were determined by averaging the pixel density values (defined on a Hounsfield Unit scale) of the regions outlined on the images. The external contours of the thigh were determined using a threshold of 224 HU, and the external bone contours were derived at 150 HU. For each participant, the determination of soft tissue type was made using the bimodal image distribution histogram resulting from the distribution numbers in adipose tissue and muscle tissue. Intermuscular and visible intramuscular adipose tissue were separated from subcutaneous adipose tissue by manual drawing of contours among the deep fascial plane surrounding the thigh muscles. The total (left + right) mid-thigh CSA of nonadipose, nonbone tissue within the deep fascial plane was used as a measure of muscle mass. CT scans were rated for quality based on scanning the same leg (right or left) at both points in time and a slice location on the femur within 20 mm of the first location. Data were included when CT scans of the same leg were performed at both points in time and slice location criteria were satisfied. Reproducibility of measuring muscle area at the mid-thigh of both legs was assessed by reanalyzing a 5% convenience sample of the study cohort and showed a CV of 5% (18).
Dietary assessment
Participants completed a 108-item interviewer-administered modified version of the Block FFQ (Block Dietary Data Systems) (19) to estimate usual nutrient intake over the previous year. This FFQ was developed specifically for Health ABC by Block Dietary Data Systems using the NHANES III 24-h recall data for older (>65 y) non-Hispanic white and black adults residing in the northeast or southern United States. Trained and certified interviewers used wood blocks, food models, standard kitchen measures, and flash cards to help participants estimate portion sizes for each food. Interviews were monitored once per month per certified interviewer throughout the study to ensure the quality and consistency of the data collection procedures. The Health ABC FFQ was analyzed for micro- and macronutrient content by Block Dietary Data Systems. Total energy and protein intake were calculated, as well as the source of protein (animal or vegetable) and the total amount of EAAs, branched-chain amino acids (BCAAs), and leucine. Block et al. (19) evaluated the validity of the original FFQ, in which the food items and portion sizes are based on NHANES II instead of NHANES III. Correlations of this FFQ with a dietary food record yielded correlations >0.7 for energy and 17 selected nutrients including protein. Amino acids were not evaluated in this study, but a study of Ishihara et al. (20) demonstrated that correlation coefficients for amino acids were similar to that for protein when comparing an FFQ with a 28-d weighted dietary record.
Potential confounders
Demographic characteristics (age, sex, race, and study site), smoking status, alcohol consumption, and physical activity were established by an interviewer-administered questionnaire at baseline. Body mass index (BMI; in kg/m2) was calculated from measured body weight and body height. Body weight was measured using a standard balance beam scale. Body height was measured with a wall-mounted Harpenden stadiometer. Alcohol drinking was categorized as ≤1 or >1 alcoholic drink per day. Smoking was categorized as never, former, or current. Physical activity was based on the estimated calories per week spent on walking and exercise over the previous 7 d. The prevalence of diabetes, coronary heart disease, congestive heart failure, cerebrovascular disease, chronic obstructive pulmonary disease (COPD), and cancer (excluding skin) was determined at baseline by using algorithms based on self-report, medication use, blood values (fasting glucose and oral-glucose-tolerance test for diabetes), and measurements (pulmonary function testing for COPD). The use of oral steroids was determined from drug data coded by using the Iowa Drug Information System ingredient codes. Change in total body fat mass (in kg) over the 5-y follow-up was assessed by using the DXA whole body scan (4500A, version 8.20a; Hologic) at year 1 and year 6. Interim hospitalizations, defined as an overnight stay, during the 5 y of follow-up were categorized as 0 or ≥1 hospitalizations.
Statistical analysis
Baseline characteristics of participants were compared between quintiles of energy-adjusted protein intake by calculating a P value for trend. For the continuous variables this was done by using the median value in each quintile as a continuous variable in the linear regression model and for the dichotomous or categorical variables by using chi-square tests.
Multiple linear regression was used to examine the association between protein intake and mid-thigh muscle area at year 6 adjusted for mid-thigh muscle area at year 1 using IBM SPSS version 22 (SPSS Inc.). Energy-adjusted protein residuals (continuous variable) were used as the independent variable for protein intake and were calculated by regressing absolute protein intake on total energy intake. One unit protein residual higher is to be interpreted as a 1-g higher protein intake than expected based on energy intake. An advantage of this method is that it provides a measure of protein intake that is independent of total energy intake (21). The outcome variable was total mid-thigh muscle area (in cm2) at year 6 and all models adjusted for baseline total mid-thigh muscle area (year 1). Different models are presented adjusting for demographic characteristics and study site (model 1), making additional adjustment for health behavior (smoking and alcohol consumption), prevalent health conditions (coronary heart disease, congestive heart failure, COPD, cerebrovascular disease, diabetes, cancer, and use of oral steroids), height, and energy intake (model 2), and in addition for physical activity, hospitalizations (yes or no), and 5-y change in body fat mass (model 3). To investigate whether sex, age, race, study site, or baseline thigh muscle area modified the association under study, the interaction terms sex × protein intake, age × protein intake, race × protein intake, study site × protein intake, and baseline thigh muscle area × protein intake were tested but were nonsignificant (P > 0.20); therefore, all analyses are presented in the total sample. For animal protein, vegetable protein, and EAA intake similar statistical procedures were followed. Energy-adjusted animal or vegetable protein intake residuals were used. Models for animal protein were also adjusted for vegetable protein intake and vice versa. Total EAA, BCAA, and leucine intake were expressed as percentages of total protein intake.
The association between protein intake and change in thigh muscle area was also evaluated categorically by using sex-specific quintiles of energy-adjusted protein intake. Sex-specific quintiles were used to avoid the distribution of sex over the quintiles being skewed. Tests for linear trends across quintiles of protein intake were conducted by using the median value in each protein category as a continuous variable in the linear regression models. The outcome variable was 5-y change in thigh muscle area; adjustments were made for baseline thigh muscle area, age, sex, race, and study site (model 1) and in addition for health behaviors, prevalent health conditions, height, energy intake, physical activity, hospitalizations, and 5-y change in body fat mass (model 3).
Finally we analyzed whether a higher protein intake (≥0.8 g · kg–1 · d–1 vs. <0.8 g · kg–1 · d–1 and ≥1.2 g · kg–1 · d–1vs. <1.2 g · kg–1 · d–1) was associated with a higher thigh muscle area at year 6 adjusted for baseline values and all potential confounders (model 3 as already described) as compared with a lower protein intake and we performed a sensitivity analysis including nondiabetic subjects only because diabetes influences the decline in muscle mass (22).
Results
Of all 3075 participants included in the Health ABC study, 1561 were included for the data analyses (Figure 1). Participants excluded from the analysis (n = 1514) were slightly older, less physically active, had a higher protein intake in grams and grams per kilogram of body weight, were more likely to be black, and were more likely to smoke (P < 0.05). No significant differences were observed for thigh muscle area at baseline, BMI, gender distribution, and alcohol consumption (data not shown).
The mean (95% CI) age of the study sample was 73.4 (73.2, 73.5) y, with 52% being female and 33% being black. The mean (95% CI) protein intake was 66.0 (64.7, 67.2) g/d or 0.90 (0.88, 0.92) g · kg body weight–1 · d–1. Mean (95% CI) 5-y decline in thigh muscle area was −9.8 (−10.6, −8.9) cm2 or −4.0% (−4.4%, −3.6%). Table 1 describes the characteristics of the study sample (n = 1561) by quintile of energy-adjusted total protein intake. Participants in the lower quintiles of protein intake were more likely to be black, less physically active, and less likely to have diabetes. No differences in baseline thigh muscle area were observed.
TABLE 1.
Descriptive characteristics of Health ABC study participants at baseline by sex-specific quintiles (Q) of energy-adjusted total protein intake residuals1
| Q1 (n = 312) | Q2 (n = 312) | Q3 (n = 313) | Q4 (n = 312) | Q5 (n = 312) | P 2 | |
|---|---|---|---|---|---|---|
| Age, y | 73.4 (73.0, 73.7) | 73.4 (73.1, 73.7) | 73.4 (73.0, 73.7) | 73.5 (73.2, 73.8) | 73.2 (72.9, 73.6) | 0.631 |
| Female | 51.9 | 51.9 | 52.1 | 51.9 | 51.9 | 1.000 |
| Black | 42.3 | 33.0 | 29.7 | 29.8 | 31.1 | 0.002 |
| Current smoker | 7.4 | 7.4 | 6.4 | 7.7 | 4.8 | 0.291 |
| Alcohol consumption, >1 unit/d | 11.5 | 6.1 | 6.1 | 7.7 | 8.0 | 0.295 |
| Walking and exercise, kcal/wk | 892 (711, 1074) | 1066 (868, 1264) | 1176 (989, 1362) | 1350 (1066, 1634) | 1446 (1188, 1704) | <0.001 |
| Prevalent health conditions | ||||||
| Diabetes | 16.5 | 17.3 | 23.5 | 21.5 | 24.7 | 0.005 |
| Coronary heart disease | 20.0 | 18.2 | 17.6 | 18.5 | 20.8 | 0.788 |
| Congestive heart failure | 1.3 | 0.6 | 2.3 | 2.9 | 1.9 | 0.140 |
| Cerebrovascular disease | 6.8 | 6.8 | 4.5 | 6.1 | 6.5 | 0.792 |
| COPD | 13.3 | 20.1 | 17.1 | 17.1 | 15.2 | 0.932 |
| Cancer | 14.1 | 15.4 | 18.9 | 20.0 | 17.7 | 0.083 |
| Oral steroid use | 2.6 | 1.3 | 2.6 | 2.6 | 1.9 | 1.000 |
| BMI, kg/m2 | 27.2 (26.7, 27.8) | 27.2 (26.7, 27.7) | 27.1 (26.6, 27.6) | 26.8 (26.3, 27.3) | 27.9 (27.4, 28.5) | 0.108 |
| Obese (BMI ≥ 30 kg/m2) | 24.4 | 21.5 | 19.8 | 26.0 | 28.8 | 0.701 |
| Body composition | ||||||
| FFM DXA, kg | 49.0 (47.8, 50.0) | 48.4 (47.3, 49.6) | 48.6 (47.4, 49.8) | 48.3 (47.0, 49.5) | 49.5 (48.4, 50.7) | 0.504 |
| FM DXA, kg | 27.0 (26.0, 27.9) | 26.8 (25.9, 27.8) | 26.4 (25.6, 27.4) | 26.1 (25.2, 27.0) | 27.5 (26.5, 28.5) | 0.600 |
| Thigh muscle area, cm2 | 225 (219, 231) | 222 (216, 228) | 222 (216, 228) | 219 (213, 225) | 229 (223, 236) | 0.391 |
| Dietary intake | ||||||
| Total energy, kcal | 2052 (1983, 2120) | 1700 (1634, 1766) | 1690 (1629, 1751) | 1714 (1649, 1780) | 2014 (1942, 2085) | 0.652 |
| Fat, % of energy | 34.8 (34.0, 35.6) | 33.2 (32.4, 34.0) | 33.6 (32.8, 34.4) | 32.0 (31.2, 32.8) | 32.0 (31.1, 32.9) | <0.001 |
| Carbohydrate, % of energy | 54.8 (53.9, 55.7) | 55.1 (54.2, 56.1) | 53.1 (52.2, 54.0) | 53.1 (52.3, 54.0) | 50.4 (49.4, 51.3) | <0.001 |
| Protein, % of energy | 10.9 (10.8, 11.0) | 12.8 (12.8, 12.9) | 14.3 (14.2, 14.3) | 16.0 (15.8, 16.1) | 18.7 (18.4, 19.0) | <0.001 |
| Protein, g/d | 56.2 (54.2, 58.2) | 54.5 (52.4, 56.7) | 59.7 (57.7, 61.8) | 67.2 (65.0, 69.3) | 92.2 (89.2, 95.3) | <0.001 |
| Protein, g · kg–1 · d–1 | 0.77 (0.74, 0.80) | 0.75 (0.72, 0.78) | 0.82 (0.79, 0.85) | 0.94 (0.91, 0.98) | 1.23 (1.19, 1.27) | <0.001 |
| Protein, g · kg FFM–1 · d–1 | 1.19 (1.14, 1.24) | 1.17 (1.12, 1.21) | 1.27 (1.22, 1.32) | 1.45 (1.40, 1.50) | 1.91 (1.85, 1.97) | <0.001 |
| Animal protein, g/d | 26.6 (25.4, 27.8) | 28.1 (26.8, 29.4) | 33.8 (32.5, 35.0) | 40.2 (38.8, 41.6) | 61.6 (59.3, 63.9) | <0.001 |
| Vegetable protein, g/d | 29.5 (28.3, 30.7) | 26.5 (25.3, 27.7) | 26.0 (24.9, 27.0) | 27.0 (25.8, 28.1) | 30.6 (29.2, 32.0) | 0.045 |
Values are means (95% CIs) or percentages. COPD, chronic obstructive pulmonary disease; DXA, dual-energy X-ray absorptiometry; FFM, fat-free mass; FM, fat mass; Health ABC, Health, Aging, and Body Composition; Q, quintile.
P for trend across quintiles. For continuous variables: by using the median value in each quintile as a continuous variable in the linear regression model; for the dichotomous variables: by using the linear-by-linear association within chi-square tests.
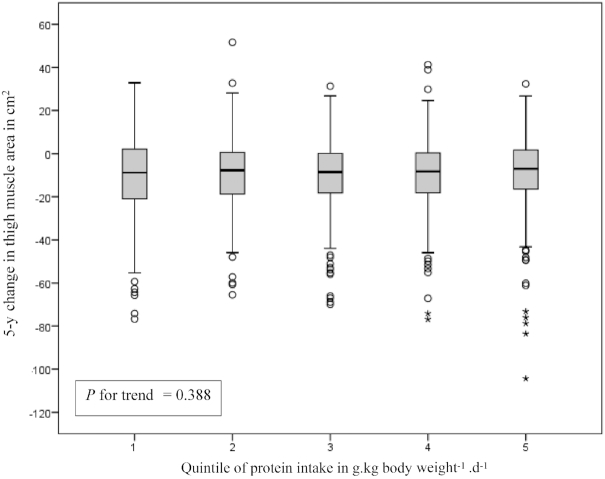
Figure 2 displays the crude 5-y change in mid-thigh muscle area by quintiles of protein intake (in g · kg–1 · d–1), showing no trend across the quintiles. The association between quintiles of sex-specific energy-adjusted total protein intake and thigh muscle area at year 6 adjusted for baseline thigh muscle area and potential confounders shows similar results (Figure 3). Table 2 shows no associations of total, animal, and plant protein intake with thigh muscle area at year 6, adjusted for baseline thigh muscle area and potential confounders. In line with total protein intake, total EAA, BCAA, and leucine intake were not significantly associated with change in thigh muscle area.
FIGURE 2.
Five-year crude thigh muscle area change by quintile of protein intake in grams per kilogram body weight per day in 1561 older participants of the Health ABC study. Median intakes of protein in grams per kilogram per day were 0.50 for Q1, 0.68 for Q2, 0.85 for Q3, 1.03 for Q4, and 1.39 for Q5. Gray boxes represent the IQR (P25–P75), the black horizontal line within the gray box represents the median value (P50), whiskers display the lowest or highest value that is not an outlier or extreme, open dots represent outliers (>1.5 and ≤3 times the IQR), and asterisks represent extremes (>3 times the IQR). Tests for a linear trend across the quintiles were conducted by using the median value in each quintile as a continuous variable in the linear regression model. Health ABC, Health, Aging, and Body Composition; P, percentile; Q, quintile.
FIGURE 3.
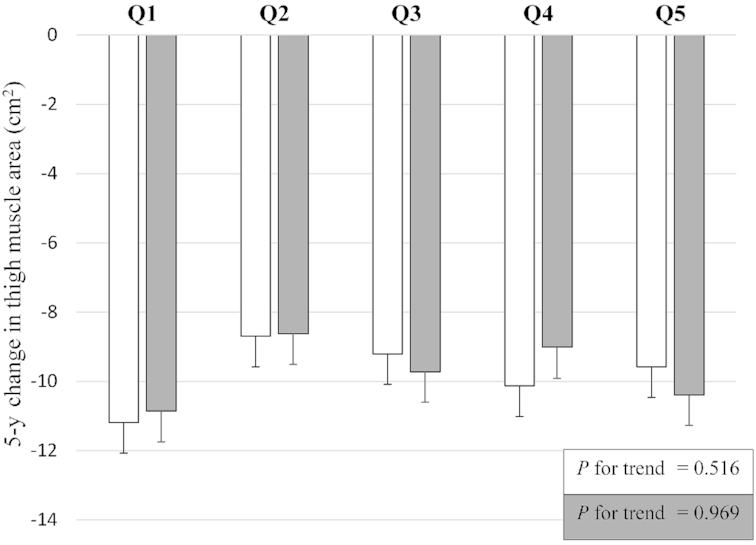
Five-year thigh muscle area loss by baseline sex-specific quintiles (Q) of energy-adjusted total protein intake residuals and adjusted for baseline thigh muscle area and potential confounders in 2 models (white and grey bars) in 1561 older participants of the Health ABC study. White bars represent estimated marginal means with SEs as calculated with general linear models of changes in thigh muscle area with adjustments for baseline thigh muscle, age, sex, race, and study site. Gray bars represent estimated marginal means with SEs as calculated with general linear models of changes in thigh muscle area with additional adjustments for smoking, alcohol consumption, prevalent health conditions (coronary heart disease, congestive heart failure, chronic obstructive pulmonary disease, cerebrovascular disease, diabetes, cancer, use of oral steroids), height, energy intake, physical activity, interim hospitalization, and change in fat mass. Tests for a linear trend across the quintiles were conducted by using the median value in each quintile as a continuous variable in the linear regression model. Health ABC, Health, Aging, and Body Composition; Q, quintile.
TABLE 2.
Association between protein intake and thigh muscle area at year 6, adjusted for baseline thigh muscle area and potential confounders in 1561 older participants of the Health ABC study1
| Thigh muscle area (cm2) | ||
|---|---|---|
| β (95% CI) | P | |
| Energy-adjusted total protein intake residuals (g)2 | ||
| Model 13 | 0.011 (−0.045, 0.067) | 0.704 |
| Model 24 | 0.015 (−0.047, 0.077) | 0.636 |
| Model 35 | −0.001 (−0.056, 0.055) | 0.982 |
| Energy-adjusted animal protein intake residuals (g) 6 | ||
| Model 13 | 0.007 (−0.050, 0.063) | 0.813 |
| Model 24 | 0.012 (−0.050, 0.074) | 0.702 |
| Model 35 | −0.003 (−0.058, 0.053) | 0.923 |
| Energy-adjusted vegetable protein intake residuals (g)6 | ||
| Model 13 | 0.132 (−0.002, 0.266) | 0.054 |
| Model 24 | 0.107 (−0.043, 0.256) | 0.162 |
| Model 35 | 0.075 (−0.060, 0.210) | 0.276 |
Health ABC, Health, Aging, and Body Composition.
Energy-adjusted total protein intake residuals in grams of protein (1 unit higher is to be interpreted as 1-g higher protein intake than expected based on energy intake).
Model 1 is adjusted for baseline thigh muscle area, age, sex, race, and study site.
4Model 2 is adjusted for determinants in model 1 and smoking, alcohol consumption, prevalent health conditions (coronary heart disease, congestive heart failure, chronic obstructive pulmonary disease, cerebrovascular disease, diabetes, cancer, use of oral steroids), height, and energy intake.
Model 3 is corrected for determinants in model 2 and physical activity, interim hospitalization, and change in fat mass.
All models for energy-adjusted animal protein residuals were also adjusted for energy-adjusted vegetable protein residuals and vice versa.
The mean difference (95% CI) in CT slice location between years 1 and 6 was 3.9 (3.5, 4.2) mm. When performing the analysis only on the leg on which the CT scout scan was performed, results were in line with the results in Table 2. The βs (with 95% CIs) and P values of the fully adjusted model for energy-adjusted total protein intake residuals, energy-adjusted animal protein intake residuals, and energy-adjusted vegetable protein intake residuals were 0.004 (−0.025, 0.033), P = 0.782; 0.003 (−0.026, 0.032), P = 0.821; and 0.031 (−0.040, 0.101) cm2, P = 0.395, respectively.
In an additional analysis we analyzed whether a higher protein intake (≥0.8 g · kg–1 · d–1 or ≥1.2 g · kg–1 · d–1) was associated with a greater thigh muscle area at year 6 adjusted for baseline thigh muscle area and confounders. When categorizing protein intake of our study population into ≥0.8 g · kg–1 · d–1 compared with <0.8 g · kg–1 · d–1, analyses revealed no difference between the groups with regard to adjusted thigh muscle area at year 6 (β: −1.08 cm2; 95% CI: −3.09, 0.93 cm2, P = 0.291). Categorizing protein intake into ≥1.2 g · kg–1 · d–1 compared with <1.2 gave similar results (β: −1.17 cm2; 95% CI: −3.65, 1.32 cm2, P = 0.358). Protein intake in grams per kilogram per day as a continuous variable tended to be negatively associated with change in thigh muscle area (fully adjusted model β: −3.58 cm2; 95% CI: −7.21, 0.06 cm2, P = 0.054). Furthermore, a sensitivity analysis restricted to nondiabetic subjects (n = 1205) showed no significant association between energy-adjusted protein residuals and change in thigh muscle area (fully adjusted model β: −0.01 cm2; 95% CI: −0.07, 0.05 cm2, P = 0.815).
Discussion
This study is the first longitudinal study, to our knowledge, investigating the association between protein intake and thigh muscle area assessed by CT scan in a large study population of older adults. This study shows no association between total, animal, or vegetable protein intake and EAA intake and change in thigh muscle area over a 5-y period. These results are contrary to our initial hypotheses. Because a higher protein intake has been shown to stimulate protein synthesis and a more positive protein balance, we expected that a higher protein intake and more specifically a higher animal protein and leucine intake would be associated with maintenance of muscle CSA (7, 23).
Like Houston et al. (9) we used data from the Health ABC study. In contrast to our findings, they did find an association between protein intake and change in lean mass. There are 2 potential explanations for this difference in results. First, their sample size was different from our study (n = 2066 compared with 1561) because of the difference in outcome measure (DXA compared with CT), a difference in follow-up period (3 compared with 5 y), and a difference in the time points of the used data (year 2 and year 5 as opposed to year 1 and year 6). The shorter follow-up period in their study resulted in less dropout due to mortality (188 compared with 255). Our study population therefore might have been somewhat healthier at baseline. A difference in health conditions might affect the association under study because, for example, the presence and severity of insulin resistance have been shown to affect the level of inhibition of the protein synthesis stimulating pathway mTOR (24, 25). The potential impact of health status should be explored in future studies. Second, we used a different outcome measure. Whereas Houston et al. (9) used (appendicular) lean mass assessed by DXA, we used thigh muscle area using the CT scan. One of the major advantages of using CT is the ability to measure fat infiltration into skeletal muscle, and therefore to measure actual muscle tissue area (26, 27). Furthermore, DXA probably also measures nonskeletal muscle tissues as lean mass (e.g., the fat-free mass in adipose tissue) (28) and is therefore probably more confounded by body size than is CT. Another advantage of CT is the ability to detect smaller changes in thigh muscle compared to DXA owing to smaller measurement errors (29). Thus, mid-thigh muscle area by CT is a more reliable method to assess actual muscle tissue and more sensitive to change over time. However, a potential drawback of using the single-slice CT muscle area is that it only assesses the muscle area at 1 location in the body and does not necessarily reflect whole-body muscle mass (28).
Chan et al. (12) also studied the association between total protein intake and longitudinal change in appendicular lean mass (by DXA) over a 4-y period in 2726 Chinese elderly. In line with our results, they did not find an association. The intake of total protein, however, was relatively high in their study compared to ours with <0.9 g · kg–1 · d–1 in the lowest quartile and ≥1.6 g · kg–1 · d–1 in the highest quartile (compared with 0.77 g · kg–1 · d–1 in the lowest quintile and 1.23 g · kg–1 · d–1 in the highest quintile in our study). The authors argue that this high protein intake is one of the main reasons for finding no effect. They also studied the association between type of protein and muscle mass loss. In contrast to their expectations, higher vegetable, but not higher animal protein intake was associated with reduced muscle loss. Animal-based products generally contain more leucine. Because leucine has muscle protein synthesis–stimulating properties, one might expect that a higher animal protein intake would have a positive effect on muscle protein synthesis (13, 14). McDonald et al. (15) did demonstrate that a higher intake of leucine in the diet (∼7 g leucine/d) in conjunction with a sufficient amount of protein among 79 older adults was associated with retention of lean mass assessed by bioelectrical impedance analysis after 6 y. The protein intake in the lowest quartile of leucine intake was 0.61 g · kg–1 · d–1 compared with 1.26 g · kg–1 · d–1 in the highest quartile, with a slightly wider range than ours (0.77 g · kg–1 · d–1 and 1.23 g · kg–1 · d–1 in the lowest and highest quintiles, respectively). They analyzed whether a higher leucine intake in conjunction with a higher protein intake was beneficial, therefore the effect of leucine per se could not be determined in their study. In contrast to their findings, a meta-analysis of 8 supplementation trials showed that protein or amino acid supplementation did not increase muscle mass in older people (30). Two recent supplementation trials, that were not included in this meta-analysis, used a whey protein, leucine, and vitamin D–enriched supplement and showed an increase in appendicular lean mass (31, 32) and fractional synthesis rate (32) in older adults. These findings are in line with a recent 10-wk trial in which a diet providing 1.6 g · kg–1 · d–1 protein compared with 0.8 g · kg–1 · d–1, on which older subjects lost appendicular lean mass, had a beneficial effect on lean body mass in older men (33). In summary, the relation between protein intake and amino acid composition of the diet and change in muscle mass over time in older adults remains unclear.
Several factors that potentially influence muscle mass change over time and are related to protein intake could not be taken into account in our analysis. First, older adults might require a higher threshold of protein per meal to raise muscle protein synthesis levels. Previous studies showed that a minimal amount of 25–30 g of high-quality protein per meal is needed to stimulate protein synthesis above baseline levels (4, 34) in the short term. Whether the distribution of protein intake affects muscle mass over the longer term remains to be elucidated (35, 36). Second, the effect of protein supplementation or a high-protein diet on lean mass may be more pronounced in combination with resistance exercise (37–41). For future research, longitudinal studies are warranted that take into account the amount of protein ingested per meal, the distribution of protein intake over the day, and the potential interaction of protein intake with resistance exercise.
The sample size of our study (n = 1561) was sufficient, as ∼500 subjects were needed to detect an expected difference (9) of 40% less decline in mid-thigh muscle area between the highest and lowest quintiles of energy-adjusted protein intake with a statistical power of 80% (9, 42). A limitation of this study is the use of a single FFQ at year 2 to estimate the usual intake of nutrients over the previous year. For the analysis presented here we assume that this intake did not change during the follow-up period. Eating habits, however, might change over time, including among other possibilities a decline in energy and protein intake because of the onset of chronic conditions or functional limitations. Another limitation of the FFQ in general is that it provides an imprecise means of estimating absolute amounts of nutrient intake including amino acids, but it can be used to rank nutrient intake (20, 43); also, underreporting is more present in subjects with a higher BMI (44). Furthermore, the time point of the CT measurement was in year 1, whereas the FFQ was filled in at year 2 which is methodologically less desirable. These limitations regarding the use of a single FFQ may have reduced the ability to detect an association between dietary protein intake and changes in thigh muscle area. A second limitation is that the scanning location of the mid-thigh muscle area by CT was based on a scout scan of a single leg. The same position on the same leg was used at follow-up. It is therefore unknown whether the scan location on the opposite leg is the same between year 1 and year 6. However, when we repeated the analysis only using data of the leg on which the scout scan was performed, similar nonsignificant associations were observed. When selecting a subgroup of subjects with a follow-up slice location difference within 10 mm of the baseline location (n = 1314), still no significant associations were observed between protein intake and thigh muscle area change. A third limitation is that no cross-calibration of the CT scan between the 2 sites was performed to assess possible differences in relation to detecting changes in mid-thigh muscle area over time, which potentially introduced measurement bias. However, when analyzing the sites separately, still no significant associations were observed between protein intake and thigh muscle area change. Finally, the level of exercise was also based on self-report, which gives an imprecise estimation of the level of exercise, and residual bias may be present.
In conclusion, this study suggests that a higher total, animal, vegetable protein or EAA intake is not associated with 5-y change in mid-thigh muscle CSA in older adults. This conclusion contradicts some, but not all, previous research. More research is required to determine the optimal protein intake for community-dwelling older adults.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—AMV, MFE, PJMW, and MV: analysis and interpretation of the data and drafting of the manuscript; DKH and TBH: study design and critical revision of the manuscript; IAB, PMC, ABN, and FAT: critical revision of the manuscript; MV: had primary responsibility for the final content; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Notes
Supported by National Institute on Aging (NIA) contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050; and National Institute of Nursing Research grant R01-NR012459. This research was funded by the Intramural Research Program of the NIH, National Institute on Aging and by the European Union Horizon 2020 PROMISS Project PRevention Of Malnutrition In Senior Subjects in the EU, grant agreement number: 678732.
Abbreviations used: BCAA, branched-chain amino acid; COPD, chronic obstructive pulmonary disease; CSA, cross-sectional area; CT, computed tomography; DXA, dual-energy X-ray absorptiometry; EAA, essential amino acid; FFQ, food-frequency questionnaire; HU, Hounsfield unit.
References
- 1. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM et al.. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev. 2013;35:51–65. [DOI] [PubMed] [Google Scholar]
- 3. Landi F, Calvani R, Tosato M, Martone AM, Ortolani E, Savera G, D'Angelo E, Sisto A, Marzetti E. Protein intake and muscle health in old age: from biological plausibility to clinical evidence. Nutrients. 2016;8:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Witard OC, Wardle SL, Macnaughton LS, Hodgson AB, Tipton KD. Protein considerations for optimising skeletal muscle mass in healthy young and older adults. Nutrients. 2016;8:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wall BT, Gorissen SH, Pennings B, Koopman R, Groen BB, Verdijk LB, van Loon LJ. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PloS One. 2015;10:e0140903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Courtney-Martin G, Ball RO, Pencharz PB, Elango R. Protein requirements during aging. Nutrients. 2016;8:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, Cederholm T, Cruz-Jentoft A, Krznaric Z, Nair KS et al.. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joint WHO/FAO/UNU Expert Consultation. Protein and amino acid requirements in human nutrition. World Health Organ Tech Rep Ser. 2007;(935):265, back cover. [PubMed] [Google Scholar]
- 9. Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB et al.. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) study. Am J Clin Nutr. 2008;87:150–5. [DOI] [PubMed] [Google Scholar]
- 10. Geirsdottir OG, Arnarson A, Ramel A, Jonsson PV, Thorsdottir I. Dietary protein intake is associated with lean body mass in community-dwelling older adults. Nutr Res. 2013;33:608–12. [DOI] [PubMed] [Google Scholar]
- 11. Isanejad M, Mursu J, Sirola J, Kroger H, Rikkonen T, Tuppurainen M, Erkkila AT. Association of protein intake with the change of lean mass among elderly women: the Osteoporosis Risk Factor and Prevention – Fracture Prevention Study (OSTPRE-FPS). J Nutr Sci. 2015;4:e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chan R, Leung J, Woo J, Kwok T. Associations of dietary protein intake on subsequent decline in muscle mass and physical functions over four years in ambulant older Chinese people. J Nutr Health Aging. 2014;18:171–7. [DOI] [PubMed] [Google Scholar]
- 13. Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:381. [DOI] [PubMed] [Google Scholar]
- 14. Paddon-Jones D, Campbell WW, Jacques PF, Kritchevsky SB, Moore LL, Rodriguez NR, van Loon LJ. Protein and healthy aging. Am J Clin Nutr. 2015;101(6):1339S–45S. [DOI] [PubMed] [Google Scholar]
- 15. McDonald CK, Ankarfeldt MZ, Capra S, Bauer J, Raymond K, Heitmann BL. Lean body mass change over 6 years is associated with dietary leucine intake in an older Danish population. Br J Nutr. 2016;115:1556–62. [DOI] [PubMed] [Google Scholar]
- 16. Verhoeven S, Vanschoonbeek K, Verdijk LB, Koopman R, Wodzig WK, Dendale P, van Loon LJ. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr. 2009;89:1468–75. [DOI] [PubMed] [Google Scholar]
- 17. Willett W. Nutritional Epidemiology. 3rd ed Oxford, United Kingdom: Oxford University Press; 2012. [Google Scholar]
- 18. Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB. Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:326. [DOI] [PubMed] [Google Scholar]
- 19. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–69. [DOI] [PubMed] [Google Scholar]
- 20. Ishihara J, Todoriki H, Inoue M, Tsugane S; JPHC FFQ Validation Study Group. Validity of a self-administered food-frequency questionnaire in the estimation of amino acid intake. Br J Nutr. 2009;101:1393–9. [DOI] [PubMed] [Google Scholar]
- 21. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–31S. [DOI] [PubMed] [Google Scholar]
- 22. Leenders M, Verdijk LB, van der Hoeven L, Adam JJ, van Kranenburg J, Nilwik R, van Loon LJ. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Dir Assoc. 2013;14:585–92. [DOI] [PubMed] [Google Scholar]
- 23. Nowson C, O'Connell S. Protein requirements and recommendations for older people: a review. Nutrients. 2015;7:6874–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gomes MJ, Martinez PF, Pagan LU, Damatto RL, Cezar MDM, Lima ARR, Okoshi K, Okoshi MP. Skeletal muscle aging: influence of oxidative stress and physical exercise. Oncotarget. 2017;8:20428–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cleasby ME, Jamieson PM, Atherton PJ. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol. 2016;229:67. [DOI] [PubMed] [Google Scholar]
- 26. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985). 1998;85:115–22. [DOI] [PubMed] [Google Scholar]
- 27. Lustgarten MS, Fielding RA. Assessment of analytical methods used to measure changes in body composition in the elderly and recommendations for their use in phase II clinical trials. J Nutr Health Aging. 2011;15:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levine JA, Abboud L, Barry M, Reed JE, Sheedy PF, Jensen MD. Measuring leg muscle and fat mass in humans: comparison of CT and dual-energy X-ray absorptiometry. J Appl Physiol (1985). 2000;88:452–6. [DOI] [PubMed] [Google Scholar]
- 29. Delmonico MJ, Kostek MC, Johns J, Hurley BF, Conway JM. Can dual energy X-ray absorptiometry provide a valid assessment of changes in thigh muscle mass with strength training in older adults?. Eur J Clin Nutr. 2008;62:1372–8. [DOI] [PubMed] [Google Scholar]
- 30. Tieland M, Franssen R, Dullemeijer C, van Dronkelaar C, Kyung Kim H, Ispoglou T, Zhu K, Prince RL, van Loon LJC, de Groot LCPGM. The impact of dietary protein or amino acid supplementation on muscle mass and strength in elderly people: individual participant data and meta-analysis of RCT's. J Nutr Health Aging. 2017;21:994–1001. [DOI] [PubMed] [Google Scholar]
- 31. Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, McMurdo ME, Mets T, Seal C, Wijers SL et al.. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2015;16:740–7. [DOI] [PubMed] [Google Scholar]
- 32. Chanet A, Verlaan S, Salles J, Giraudet C, Patrac V, Pidou V, Pouyet C, Hafnaoui N, Blot A, Cano N et al.. Supplementing breakfast with a vitamin D and leucine-enriched whey protein medical nutrition drink enhances postprandial muscle protein synthesis and muscle mass in healthy older men. J Nutr. 2017;147:2262–71. [DOI] [PubMed] [Google Scholar]
- 33. Mitchell CJ, Milan AM, Mitchell SM, Zeng N, Ramzan F, Sharma P, Knowles SO, Roy NC, Sjodin A, Wagner KH et al.. The effects of dietary protein intake on appendicular lean mass and muscle function in elderly men: a 10-wk randomized controlled trial. Am J Clin Nutr. 2017;106:1375–83. [DOI] [PubMed] [Google Scholar]
- 34. Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. 2015;70:57–62. [DOI] [PubMed] [Google Scholar]
- 35. Loenneke JP, Loprinzi PD, Murphy CH, Phillips SM. Per meal dose and frequency of protein consumption is associated with lean mass and muscle performance. Clin Nutr. 2016;35:1506–11. [DOI] [PubMed] [Google Scholar]
- 36. Kim IY, Schutzler S, Schrader AM, Spencer HJ, Azhar G, Wolfe RR, Ferrando AA. Protein intake distribution pattern does not affect anabolic response, lean body mass, muscle strength or function over 8 weeks in older adults: a randomized-controlled trial. Clin Nutr. 2018;37(2):488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Finger D, Goltz FR, Umpierre D, Meyer E, Rosa LH, Schneider CD. Effects of protein supplementation in older adults undergoing resistance training: a systematic review and meta-analysis. Sports Med. 2015;45:245–55. [DOI] [PubMed] [Google Scholar]
- 38. Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012;96:1454–64. [DOI] [PubMed] [Google Scholar]
- 39. Liao CD, Tsauo JY, Wu YT, Cheng CP, Chen HC, Huang YC, Chen HC, Liou TH. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and meta-analysis. Am J Clin Nutr. 2017;106(4):1078–91. [DOI] [PubMed] [Google Scholar]
- 40. Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, Helms E, Aragon AA, Devries MC, Banfield L, Krieger JW et al.. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52(6):376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thomas DK, Quinn MA, Saunders DH, Greig CA. Protein supplementation does not significantly augment the effects of resistance exercise training in older adults: a systematic review. J Am Med Dir Assoc. 2016;17:959.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Whitley E, Ball J. Statistics review 4: sample size calculations. Crit Care. 2002;6:335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 44. Lissner L. Measuring food intake in studies of obesity. Public Health Nutr. 2002;5:889–92. [DOI] [PubMed] [Google Scholar]