Abstract
Anhedonia, induced by nicotine withdrawal, may serve as an important affective sign that reinforces tobacco use and smoking relapse rates in humans. Animal models provide a way to investigate the underlying neurobiological factors involved in the decrease in responding for positive affective stimuli during nicotine withdrawal and may aid in drug development for nicotine dependence. Thus, we explored the use of the sucrose preference test to measure nicotine withdrawal-induced reduction in response for positive affective stimuli in mice. C57BL/6J and knockout (KO) mice were chronically exposed to different doses of nicotine through surgically implanted subcutaneous osmotic minipumps for 14 days and underwent spontaneous nicotine withdrawal on day 15. A sucrose preference time course was performed and the results were compared to another well-established affective sign of nicotine withdrawal, the reduction in time spent in light side, using the Light Dark Box test. Subsequently, our results demonstrated a time-dependent and dose-related reduction in sucrose preference in nicotine withdrawn male C57BL/6J mice, indicative of a decrease in responding for positive affective stimuli. Furthermore, the sucrose preference reduction during nicotine withdrawal was consistent with decrease in time spent in the light side of the Light Dark Box test. We also found the reduction for positive affective stimuli and time spent in the light side was not present in nicotine withdrawn β2 and α6 KO mice, suggesting that these nicotinic subunits are involved in the affective signs of nicotine withdrawal. Thus, this report highlights the potential utility of the sucrose preference test as a useful measure of the decrease in responding for positive affective stimuli during spontaneous nicotine withdrawal.
Keywords: nicotine, withdrawal, Light Dark Box, sucrose preference test
1. Introduction
Nicotine dependence is not only based on the positive reinforcing and hedonic effects of nicotine, but it is also associated with a withdrawal syndrome that results from smoking cessation (George et al., 2007; Kenny and Markou, 2001). Indeed, the nicotine withdrawal syndrome in humans is represented by a variety of signs such as somatic signs that include gastrointestinal disturbances, weight gain, decreased heart rate (American Psychiatric Association 2000), sweating, dizziness (Hughes and Hatsukami 1986), fatigue, nausea, constipation, and diarrhea (Shiffman, 1979). In addition to these physical signs, there are other unpleasant negative mood symptoms that occur during smoking cessation in humans. Anxiety and anhedonia are affective features of nicotine withdrawal that are thought to contribute to continued tobacco use (Dawkins et al., 2007; Cook et al., 2014; American Psychiatric Association, 2000; Hughes, 2007). It has been reported that tobacco smokers may continue smoking to escape the loss of pleasure induced by nicotine withdrawal (Dawkins et al., 2007; Perkins & Karelitz, 2013; Cook et al., 2014). Also, laboratory human studies suggest that nicotine deprivation results in anhedonia (Al-Adawi and Powell, 1997; Dawkins et al., 2006; Powell et al, 2002; Powell et al, 2004). Therefore, assessment of anhedonia induced by the nicotine withdrawal syndrome may have clinical implications in terms of treatment of tobacco addiction.
Similarly, in animal studies, it has also been reported that nicotine withdrawal following chronic nicotine administration leads to reduction of operant responding for rewarding electrical brain stimulation using intracranial self-stimulation (ICSS) in rats and mice, a measure of reduction in responding for positive affective stimuli (Jordan et al., 1998; Hilario et al., 2012; Johnson et al., 2008; Stoker et al., 2015). However, the ICSS procedure is an operant conditioning method that requires surgery, which can be labor-intensive. It also involves a special training schedule and a distinctive apparatus (Carlezon Jr and Charltoff et al., 2007). The two-bottle choice procedure for assessing sucrose preference is another useful test to investigate reduction in positive affective stimuli in laboratory rodents (Thompson and Grant, 1971). Compared to the ICSS procedure, the sucrose preference test is simple, fast, does not require surgery, or long term testing and training sessions.
Accordingly, the current study aimed to establish a mouse model of spontaneous nicotine withdrawal-induced decrease in affect using the sucrose preference test. We assessed the time-course and dose-dependency of sucrose preference during spontaneous nicotine withdrawal in C57BL/6J mice. In addition, we investigated the role of the β2 and α6 nicotinic subunits in this behavior by utilizing β2 and α6 nicotinic knockout (KO) and wild-type (WT) mice. It has been previously reported that the affective signs of nicotine withdrawal including reduction in responding for positive affective stimuli were absent in these KO mice (Jackson et al. 2008; 2009; Stoker et al., 2008). Finally, we compared the results of the sucrose preference test to another well-established affective sign of nicotine withdrawal, the reduction in time spent in light side by using the Light Dark Box (LDB) test.
2. Methods
2. 1. Animals
8-week-old male C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). C57BL/6J provided the background strain for our α6 and β2 KO and WT mice. The β2 KO mice (Institut Pasteur, Paris, France) and their WT littermates were bred in an animal care facility at Virginia Commonwealth University. Healthy, viable mice null for the α6 nicotinic subunit were provided by Dr. Uwe Maskos at Institut Pasteur (Paris, France) (Champtiaux et al., 2002). All mice used in each experiment were backcrossed at least 10 to 12 generations. Mutant and wild types were obtained from crossing heterozygote mice. This breeding scheme controlled for any irregularities that might occur with crossing solely mutant animals. Animals were 8–10 weeks of age at the beginning of the experiments and were group-housed (four animals per group with ad libitum access to food and water under a 12 hr light/dark cycle in a 21 °C). Experiments were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University and followed the National Institutes of Health Guidelines for the Care and Use of laboratory animals.
2.2. Drugs
(−)-Nicotine hydrogen tartrate [(−)-1-methyl-2-(3-pyridyl) pyrrolidine (þ)-bitartrate], sucrose and saccharine were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). Nicotine was dissolved in sterile physiological saline (0.9% sodium chloride). Nicotine (12 and 24 mg/kg/day) was infused through 14-day subcutaneous osmotic minipumps (model 2002, Alzet, Palo Alto, CA, USA). The doses were expressed as the free base of the drug. The doses were used according to the previous literature (Damaj et al., 2003; Salas et al., 2004). Sucrose (2%) and saccharine (0.4%) were dissolved in water and given orally in the two-bottle choice procedure.
2.3. Induction of Nicotine Withdrawal
Mice were anesthetized by inhaling isoflurane/oxygen vapor mixture (1–3%). Alzet osmotic minipumps were then implanted subcutaneously within the mice for 14 days. The Alzet minipumps were filled with either nicotine or saline solutions and inserted by making an incision parallel to the spine at shoulder level of the mice. The wound was closed using wound clips and the mice were placed in a surgery room for recovery before using them in experiments. Post-operative care was done for 14 days by observing the mice daily. For all of the procedures, the doses of nicotine were 12 and 24 mg/kg/day, calculated according to body weight. On day 15 spontaneous nicotine withdrawal was induced by removing the minipumps under isoflurane anesthesia in aseptic surgical conditions. The experiment was adapted as previously described (Damaj et al., 2003; Jackson et al., 2008). On day 16, mice were tested for sucrose preference, saccharin preference and LDB for several days. Summary of time and duration of each behavioral test and the mice that used in each experiment was shown in Supplementary Table 1.
2.4. Sucrose Preference Test
Sucrose preference test was used to investigate the reduction in responding for positive affective stimuli in rodents after the induction of nicotine withdrawal (Thompson and Grant, 1971). In this experiment, mice were individually housed and acclimated to cages with food and water. Mice had free access to two 30 ml sipper tubes containing tap water for 3 days as a baseline. Animals then were exposed to two 30 ml sipper tubes, one with tap water and the other with 2% sucrose solution. Measurements of consumed water and sucrose solution were taken every 24 hrs. To prevent any bias, tube placement was switched daily. 24 hrs following saline and nicotine minipump removal, the same cohort of C57BL/6J mice were tested for sucrose preference for 9 consecutive days. For the experiments using KO mice, the measurements of sucrose preference were taken at day 2 after removal of osmotic minipumps. Sucrose preference was determined as the percentage of 2% sucrose volume consumed over the total fluid intake volume. Sucrose preference (percentage) was calculated as follows: preference = [sucrose solution intake (ml)/total fluid intake (ml)] × 100. The experiment was adapted as previously described (Toma et al., 2017; Pothion et al., 2004). Same cohorts of C57BL/6J male mice (n =11 per group) were tested for 11 consecutive days during chronic nicotine exposure and 9 consecutive days after nicotine minipumps removal. For KO mice, we tested (n=8 per group) at day 2 (48hrs) after removal of minipumps.
2.5. Saccharine Preference Test
Male C57BL/6J mice were individually housed and acclimated to cages with free access to food and water for 3 days. Same cohort of mice were tested for 24 hrs with access to two 30 ml sipper tubes. One tube was filled with tap water and the other with 0.4% saccharin solution. The measurements were taken at 24, 48 and 96 hrs after removal of nicotine or saline minipumps. Saccharin preference (percentage) was calculated as follows: preference = [saccharine solution intake (ml)/total fluid intake (ml)] × 100. The experiment was conducted as previously described (Jastrzębska et al., 2016). We used same cohorts of C57BL/6J male mice (n=8) for 3 days after removal of minipumps.
2.6. Light-Dark Box (LDB) Test
The LDB procedure depends on the innate aversive behavior of rodents to bright areas as well as their stress induced-natural exploratory response (Crawley and Goodwin, 1980). The test was slightly modified as previously reported (Wilkerson et al., 2016). The LDB apparatus is composed of a small, enclosed dark or black compartment (36 × 10 × 34 cm) with a passageway (6 × 6 cm) extending to a larger, light or white compartment (36 × 21 × 34 cm). The mice were habituated to the experiment room for 30 minutes before testing. First, mice were placed in the light chamber and allowed to freely explore the apparatus for 5 min. Then the number of entries into the light compartment, the number of transitions and the total time spent in the light compartment were recorded for 5 min by a video monitoring technique and ANY-MAZE software (Stoelting Co., Wood Dale, IL). Same cohorts of C57BL/6J male mice (n =10 per group) were tested at days 1, 2 and 5 after nicotine minipumps removal. For KO mice, we tested same cohort of male mice (n=8 per group) at day 2 (48hrs) after removal of minipumps.
2.7. Statistical Analysis
The data were analyzed with GraphPad Prism software, version 6 (GraphPad Software, Inc., La Jolla, CA), and expressed as mean ± SEM. Studies using mutant mice were analyzed with an ordinary two-way analysis of variance (ANOVA) test with genotype (KO versus WT) and treatment (saline versus nicotine) as between-subject factors in conjunction with a Tukey post-hoc test. Experiments using C57BL/6J mice were analyzed with two-way repeated measure (RM) ANOVA with treatment (nicotine versus saline) and multiple time points as a between subject design followed by the Sidak post hoc test. Prior to the ANOVA test, results were first assessed for the normality of the residuals and homogeneity of variance. Variances were similar between groups and were assessed using either the F‐test or Levene’s test. All data passed these tests. Differences were considered to be significant at p < 0.05.
3. Results
3.1. Decrease in responding for positive affective stimuli in sucrose preference test during nicotine withdrawal
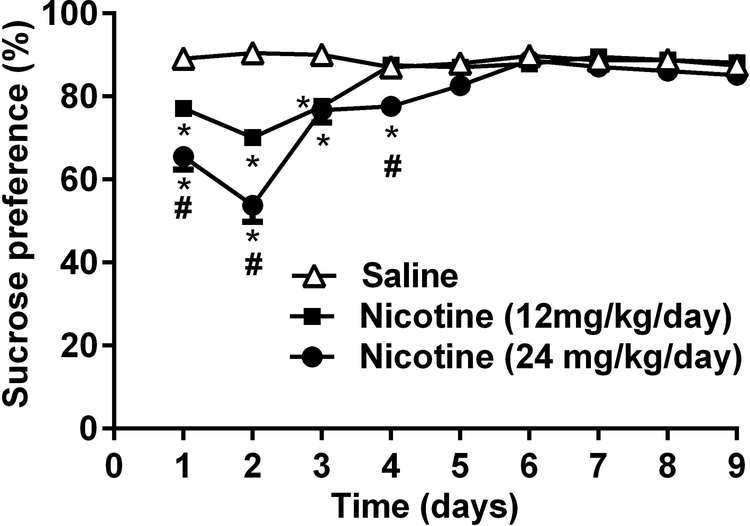
To assess the attenuation in responding for positive affective stimuli as a measurable sign for nicotine withdrawal, we assessed sucrose preference in nicotine withdrawn C57BL/6J mice for 9 days after nicotine minipump removal (see Supplementary Table 1). A reduction in sucrose preference is thought to be an indication of reduction in positive affective stimuli (Thompson and Grant, 1971). In Fig. 1, a two-way RM ANOVA revealed a significant reduction of sucrose preference in nicotine-infused groups in a dose and time dependent manner [Ftreatment (2, 20) = 71.17, p<0.001, Ftime (8, 80) = 24.61 and Finteraction (16,160) = 10.8, p<0.001; n=11]. At days 1, 2 and 3, mice treated with 12 mg/kg/day of nicotine demonstrated significant attenuation in sucrose preference when compared to the saline group (p<0.001). Similarly, at days 1, 2, 3 and 4, the group infused with 24 mg/kg/day of nicotine had significantly less sucrose preference in comparison to the saline group (p<0.001). Furthermore, at days 1, 2, and 4 after removal of minipumps, mice implanted with 24 mg/kg/day nicotine minipumps showed significant decreases in sucrose preference when compared to the group that received 12 mg/kg/day of nicotine (p<0.001). In addition, two way RM ANOVA revealed no significance difference in sucrose preference between chronically exposed nicotine (24 mg/kg/day) and saline treated mice [Ftreatment (1,10)= 1.343, p= 0.2734, Ftime (10,100)= 1.355, p= 0.2123 and Finteraction (10,100)= 0.8335, p= 0.5975; n=11; Supplementary Fig. 1]. Furthermore, in order to rule out a change in sweet taste after nicotine withdrawal, we conducted in a separate cohort of mice, the saccharine preference test (0.4%) in C57BL/6J male mice on days 1, 2 and 4 after removal of nicotine (24 mg/kg/day for 14 days) and saline minipumps. As shown in Supplementary Fig. 2, no reduction of saccharine preference was observed after nicotine withdrawal. There were no significant differences between nicotine and control groups on saccharine preference [Ftreatment (1, 7) =1.188, p= 0.3117; Ftime (2, 14) = 8.719, p= 0.0035; Finteraction (2, 14) = 0.905, p= 0.4268; n=8].
Figure 1. Sucrose preference test during spontaneous nicotine withdrawal in C57BL/6J mice.
C57BL/6J male mice were exposed to two sipper tubes, one containing normal drinking water and the other containing a 2% sucrose solution, for 24 hrs per day. Sucrose preference was determined as the percentage of 2% sucrose volume consumed over the total fluid intake volume. Mice were chronically exposed to nicotine (12 and 24 mg/kg/day) via osmotic minipumps then the sucrose preference was measured for 9 consecutive days after removal of minipumps. Sucrose preference deficit was observed in nicotine withdrawn groups when compared to saline group. Also, there was a dose dependent effect in nicotine treated groups at days 1, 2 and 4 during nicotine withdrawal. Each point represents the mean ± S.E.M. of 11 mice per group. *Denotes P < 0.05 from saline group; # Denotes P < 0.05 from nicotine 12 mg/kg/day treated-group.
3.2. Sucrose preference during nicotine withdrawal in β2 KO and α6 KO mice
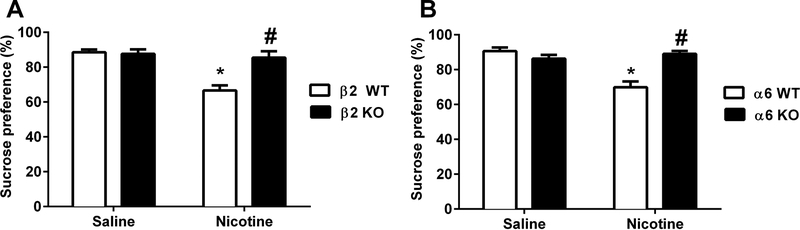
We used the spontaneous nicotine withdrawal model to test the role of β2 and α6 nicotinic subunits in nicotine withdrawal-induced decrease in sucrose preference by using β2 and α6 mutant mice. Based on the time course in Fig. 1, we measured sucrose preference in WT and KO mice at 48 hrs after minipump removal (see Supplementary Table 1). Interestingly, nicotine (24mg/kg/day) treated β2 WT mice showed significantly lower sucrose preference than their saline counterparts [Ftreatment (1, 28) = 18.69; p= 0.0002; n=8], but the effect of nicotine was abolished in β2 KO mice. Indeed, there was a significant effect of genotype when we compared nicotine treated β2 WT and KO groups [Fgenotype (1, 28) = 10.27; p=0.0034]. In addition, the interaction between nicotine treatment and genotype was significant between the same subjects [Finteraction (1, 28) = 12.58; p= 0.0014; n=8; Fig. 2A]. Similarly, nicotine infused α6 WT mice demonstrated an attenuation of sucrose preference when compared to their controls [Ftreatment (1, 28) = 14.14; p= 0.008)]. This effect was absent in α6 KO mice [Fgenotype (1, 28) = 9.516, p= 0.0045]. Two-way ANOVA revealed a significant effect of interaction between genotype and treatment [Finteraction (1, 28) = 24.19; p< 0.0001; n=8; Fig. 2B].
Figure 2. Measurement of sucrose preference after induction of nicotine withdrawal in nicotinic KO mice.
β2 and α6 KO and WT mice were chronically infused with nicotine (24 mg/kg/day) for 14 days. Minipumps were removed from mice on day 15, and sucrose preference (2%) was performed 48 hrs later. A) Sucrose preference test of β2 WT and KO mice at day 2 after removal of minipumps. B) Sucrose preference results of α6 WT and KO mice. Each point represents the mean ± S.E.M. of 8 mice per group. * Denotes p < 0.05 vs. Saline groups, # Denotes p < 0.05 vs. nicotine withdrawn WT mice.
3.3. Decrease in time spent in the light side in LDB during nicotine withdrawal
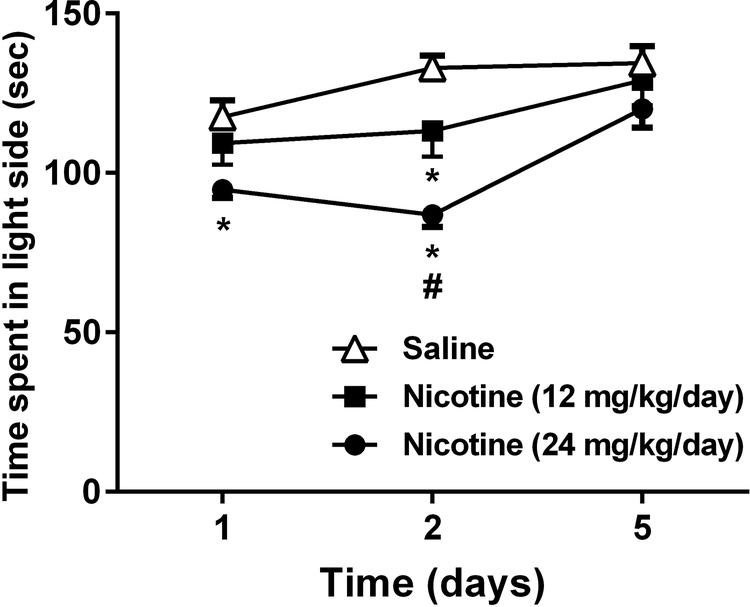
We measured the time spent in the light chamber of nicotine-dependent C57BL/6J mice in the LDB assay. Using the same cohorts of mice that underwent the spontaneous nicotine withdrawal in the sucrose preference test. It has been show that the reduction in time spent in the light side of LDB occur during nicotine withdrawal (Jonkman et al., 2005). There was a significant difference between nicotine withdrawn mice and saline treated mice observed by changes in time spent in the light compartment [2-way RM ANOVA, [Ftreatment (2, 18) = 12.26, p= 0.0004], [Ftime (2, 18) = 10.93, p= 0.0008] and [Finteraction (4, 36) = 2.114, p= 0.0993; n=10; Fig. 3] (see Supplementary Table 1). As seen in Fig. 3, mice undergoing spontaneous nicotine withdrawal spent less time in the light compartment of the LDB apparatus compared to saline controls (p= 0.0004). This experiment continued for 5 days after induction of spontaneous nicotine withdrawal. On day 2 after removal of minipumps, mice that were infused with 12 mg/kg/day of nicotine significantly spent less time in the light side when compared to saline group (p= 0.0004). In addition, at day 1 and 2, the 24 mg/kg/day nicotine exposed group had a significant reduction in the time spent in the light side when compared to their saline counterparts (p= 0.0004). Furthermore, at day 2 there was a significant difference between the two nicotine groups (p= 0.0004). Simultaneously, transition numbers were recorded and serve as an index of activity and exploration. In that regard, no significant difference was found in number of transitions between nicotine and saline treated C57BL/6J male mice [2-way RM ANOVA, Ftreatment (2, 18) =0.162, p= 0.8515, [Ftime (2, 18) = 0.146, p= 0.8645] and [Finteraction (4, 36) = 0.918, p= 0.4637; n=10]; (Supplementary Fig. 3A).
Figure 3. Time course of time spent in the light side of nicotine-dependent mice in the LDB test.
Mice were chronically infused with saline or nicotine (12 or 24 mg/kg/day) for 14 days. On day 15 mice underwent spontaneous nicotine withdrawal and the LDB test was performed at days 1, 2 and 5 after removal of minipumps. Each point represents the mean ± S.E.M. of 10 mice per group. *Denotes P < 0.05 from vehicle control, # Denotes from 12 mg/kg/day nicotine withdrawn group.
3.4. Attenuation of time spent in the light side in the LDB test in β2 KO and α6 KO mice undergoing nicotine withdrawal
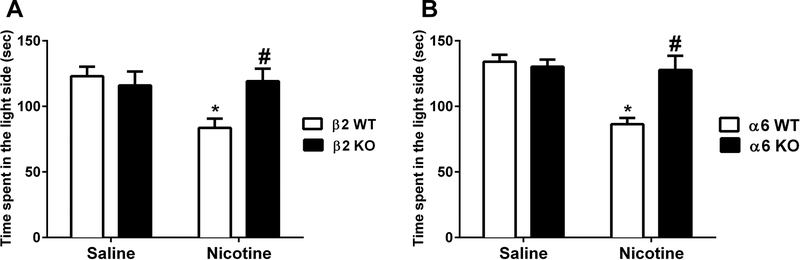
We utilized same β2 and α6 mutant mice cohorts to evaluate the nicotine withdrawal effect on time spent in the light side of the LDB test. Based on the time course in Fig. 2, we measured the time recorded in the light side in the LDB in WT and KO mice 48 hrs after minipump removal. Nicotine (24 mg/kg/day) infused β2 WT mice significantly spent lower time in the light side when compared to their saline controls [2-way ANOVA, [Ftreatment (1, 28) = 4.255, p= 0.0485], [Fgenotype (1, 28) = 2.667, p= 0.013] and [Finteraction (1, 28) = 5.91, p= 0.0217; n=8; Fig. 4A]. However, nicotine withdrawal-induced reduction in time spent in light side was not present in β2 KO mice (p>0.05). Furthermore, nicotine treated β2 WT exhibited lower time spent in the light chamber in comparison to the nicotine β2 KO group (p= 0.013). Similarly, as seen in Fig. 4B, chronic nicotine exposed α6 WT mice demonstrated lower time spent in light side of the apparatus in comparison to the saline treated α6 WT mice [Ftreatment (1,28) = 12.77; p= 0.0013]. However, nicotine dependent α6 KO mice did not show a significant difference when compared to saline treated α6 KO mice (p>0.05). In addition, two-way ANOVA revealed a significant effect of genotype [Fgenotype (1, 28) = 7.212; p= 0.012] and [Finteraction (1, 28) = 10.4; p= 0.032; n=8; Fig. 4B] (see Supplementary Table 1). Nicotine withdrawn α6 WT mice spent lower time in the light compartment when compared to nicotine treated α6 KO mice (p= 0.012). Furthermore, two-way ANOVA did not demonstrate significant difference in number of transitions between saline and nicotine groups in genetically modified β2 [Ftreatment (1,28)=0.002, p= 0.9639], [Fgenotype (1,28)= 0.752, p= 0.3931] and [Finteraction (1,28)= 0.168, p= 0.6843; n=8] (Supplementary Fig. 3B) and α6 mice [Ftreatment (1,28)= 0.633,p= 0.4328],[Fgenotype (1,28)= 0.085,p= 0.7715] and [Finteraction(1,28)= 0.2123, p= 0.6485; n=8] (Supplementary Fig. 3C).
Figure 4. Time spent in the light side of LDB in nicotinic KO mice during nicotine withdrawal.
β2 and α6 KO/WT mice were chronically infused with nicotine (24 mg/kg/day) for 14 days. Minipumps were removed from mice on day 15, and the LDB test was performed 48 h later. A) Nicotine withdrawn β2 WT mice exhibited an attenuation of time spent in the light side on day 2 after removal of minipumps. But this effect was not seen in β2 KO mice. B) Similarly, α6 WT mice displayed a reduction in the time spent in the light side on day 2 that was not observed in α6 KO mice. Each point represents the mean ± S.E.M. of 8 mice per group. * Denotes p < 0.05 vs. Saline groups, # Denotes p < 0.05 vs. nicotine withdrawn WT mice.
4. Discussion
This is the first report demonstrating the usefulness of the sucrose preference test as a measure of nicotine withdrawal-induced decrease in responding for positive affective stimuli in mice. In our sucrose preference experiments, we found that spontaneous nicotine withdrawal significantly produced a sucrose preference reduction in C57BL/6J mice in a time- and dose-related manner (12 and 24 mg/kg/day of nicotine). According to the time course results, the attenuation of the sucrose preference occurred at day 1 after removal of minipumps and continued for 4 days (Fig. 1). However, chronic nicotine exposure did not alter the sucrose preference compared with their vehicle-treated counterparts (Supplementary Fig. 1) suggesting that the sucrose preference reduction is a result of nicotine withdrawal. The decrease in sucrose preference observed in our studies was not due to nicotine-induced taste aversion since nicotine-dependent mice did not differ from their saline counterparts in saccharine preference (Supplementary Fig. 2). Saccharine and sucrose both are sweet substances, but saccharine is deprived from a caloric confound that is in the sucrose, which is naturally occurring carbohydrate. We performed the saccharine preference as a control experiment to control for taste aversion during nicotine withdrawal. In addition, the decrease in responding for positive affective stimuli observed in the sucrose preference test is probably not due to food consumption-related effects during nicotine withdrawal, because nicotine did not affect sucrose preference during chronic nicotine exposure (Supplementary Fig. 1). Furthermore, no significant increase in body weight in nicotine-infused mice were observed after minipump removal (Data not shown).
Our results in the sucrose preference test are in agreement with two previous studies that used ICSS to evaluate the reduction in rewarding effects and decrease in responding for positive affective stimuli during nicotine withdrawal in C57BL/6J mice (Stoker et al., 2008; Johnson et al., 2008). In Johnson et al., (2008), the results were very similar to our sucrose preference results in terms of dose of nicotine and time course of withdrawal. However, in Stoker et al., (2008), the change in ICSS thresholds or latencies occurred at a higher dose of nicotine (40mg/kg/day) than used in the current study and continued for a shorter period of time.
The sucrose preference of β2 and α6 WT mice was significantly attenuated 48 hrs after removal of nicotine minipumps, while nicotine withdrawn KO mice did not exhibit a reduction in sucrose preference (Fig. 2). The results of β2 KO mice were consistent with Stoker et al. (2015), which used ICSS to measure the reduction in responding for positive affective stimuli in the same nicotine withdrawn mutant mice. While our study is the first to measure decrease in responding for positive affective stimuli using the sucrose preference test in α6 KO mice, the results were consistent with the role of α6 nicotinic subunits in other affective signs (Jackson et al., 2009). In addition, our sucrose preference test results were consistent with other well-established affective symptoms. A previous report demonstrated that other affective behaviors of nicotine withdrawal (i.e., conditioned placed aversion and reduction in time spent in the open arm in the Elevated Plus Maze) were also absent in β2 KO mice (Jackson et al., 2008).
Importantly, attenuation of sucrose preference as an indication of decrease in responding for positive affective stimuli during nicotine withdrawal is consistent with our findings of other emotional behaviors, such as an attenuation in the time spent in the light side of the LDB. Anxiety is another affective symptom of nicotine withdrawal in humans. (American Psychiatric Association, 2000; Hughes, 2007). The time course of time spent in the light side in C57BL/6J mice undergoing nicotine withdrawal was similar to the sucrose preference results. A significant reduction in time spent in light side in the LDB occurred at 48 hrs and dissipated on day 5 after minipump removal (Fig. 3). Consistent with the sucrose preference in KO mice, the time spent in the light side was unaffected in β2 and α6 KO mice at day 2 (Fig. 4). Our findings in LDB were consistent with previous work with the same apparatus (Jonkman et al., 2005) and another test that measures the reduction in time spent in open arm, the Elevated Plus-Maze Test (Jackson et al., 2008; Jackson et al., 2009; Zhao-Shea et al., 2015).
One of the limitations of our study is the use of passive (non-contingent) nicotine exposure via subcutaneous osmotic minipumps. However, our results were consistent with findings of previous studies that used active (contingent) design to provide nicotine in ICSS (Stolker et al., 2008; Johnson et al., 2008). In addition, this study did not test for sex differences, which is an important aspect of nicotine withdrawal.
Collectively, our results show that the sucrose preference test can be used to evaluate nicotine withdrawal affective signs in mice. The findings of the sucrose preference test were consistent with the results of ICSS and demonstrated similar phenotypes and nicotinic receptor mechanisms. These new findings highlight the usefulness of the sucrose preference test for the study of the neurobiological and pharmacological mechanisms of reduction of responding for positive affective stimuli that occur during nicotine withdrawal. This test could be a useful tool for investigating pharmacological and behavioral interventions for nicotine dependence.
Supplementary Material
Mice were chronically exposed to saline or nicotine (24mg/kg/day) by osmotic subcutaneous minipumps for 14 days. Sucrose preference test (2%) was conducted 24 hrs after implantation of minipumps and continued for 11 consecutive days. There was no significant difference of sucrose preference between nicotine and saline treated groups. Each point represents the mean ± S.E.M. of 11 mice per group.
Two days after removal of minipumps, mice undergoing nicotine withdrawal were exposed to two tubes: one containing drinking water and the other 0.4% of saccharine. After 24, 48 and 96 hrs, measurements of water and saccharine were recorded. Saccharine preference was determined as the percentage of 0.4% saccharine volume consumed over the total fluid intake volume. There was no alteration in saccharine preference in nicotine withdrawn mice compared to their controls. Each point represents the mean ± S.E.M. of 8 mice per group.
Mice were chronically infused with saline or nicotine (12 or 24 mg/kg/day) for 14 days. Spontaneous nicotine withdrawal was induced on day 15 and the LDB test was performed. Along with the time spent in the light side, number of transitions between the dark and light side was recorded. A) Number of transitions on days 1, 2 and 5 after removal of minipumps in C57BL/6J mice (n=10/per group). B) Number of transitions on day 2 of β2 KO mice (n=8/per group) C) Number of transitions on day 2 of α6 KO mice (n=8). Each point represents the mean ± S.E.M. of 8–10 mice per group.
Funding Sources:
This study was supported by NIH grant [DA 005274 and DA032246] to MID.
Abbreviations:
- KO
knockout
- WT
wild-type
- ICSS
intracranial self-stimulation
- LDB
light dark box
Footnotes
Conflicts of interest: None declared.
References
- Al-Adawi S, Powell J, 1997. The influence of smoking on reward responsiveness and cognitive functions: A natural experiment. Addiction 92, 1773–1782. doi: 10.1111/j.1360-0443.1997.tb02897. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. 2000. Diagnostic and statistical manual of mental disorders, (4th ed., text revision). Washington, DC: American Psychiatric Association. [Google Scholar]
- Calcagno C, Lobatto ME, Robson PM, Millon A, 2016. Anhedonia as a Component of the Tobacco Withdrawal Syndrome. J. Abnorm. Psychol 28, 1304–1314. doi: 10.1002/nbm.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Chartoff EH, 2007. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat. Protoc 2, 2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, 1999. Nicotine self-administration in animals as a dependence model. Nicotine Tob. Res 1, 11–20. [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK, 1980. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol. Biochem. Behav 13, 167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, West R, Powell J, Pickering A, 2007. A double-blind placebo-controlled experimental study of nicotine: II - Effects on response inhibition and executive functioning. Psychopharmacology (Berl). 190, 457–467. doi: 10.1007/s00213-006-0634-6. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, West R, Powell J, Pickering A, 2006. A double-blind placebo controlled experimental study of nicotine: I - Effects on incentive motivation. Psychopharmacology (Berl). 189, 355–367. doi: 10.1007/s00213-006-0588-8. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A, 1998. Dramatic decreases in brain reward function during nicotine withdrawal. Nature 393, 76–9. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O’Dell LE, Richardson HN, Koob GF, 2007. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc. Natl. Acad. Sci. U. S. A 104, 17198–203. doi: 10.1073/pnas.0707585104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SM, 2004. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology (Berl). 175, 134–142. doi: 10.1007/s00213-004-1818-6. [DOI] [PubMed] [Google Scholar]
- Hilario MRF, Turner JR, Blendy JA, 2012. Reward Sensitization: Effects of Repeated Nicotine Exposure and Withdrawal in Mice. Neuropsychopharmacology 37, 2661–2670. doi: 10.1038/npp.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J, 2007. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine Tob. Res 9, 315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D, 1986. Signs and symptoms of tobacco withdrawal. Arch. Gen. Psychiatry 43, 289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI, 2008. Differential Role of Nicotinic Acetylcholine Receptor Subunits in Physical and Affective Nicotine Withdrawal Signs. J. Pharmacol. Exp. Ther 325, 302–312. doi: 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Mcintosh JM, Brunzell DH, Sanjakdar SS, Damaj MI, 2009. The Role of a6-Containing Nicotinic Acetylcholine Receptors in Nicotine Reward and Withdrawal. J. Pharmacol. Exp. Ther 331, 547–554. doi: 10.1124/jpet.109.155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastrzębska K, Walczak M, Cieślak PE, Szumiec L, Turbasa M, Engblom D, Błasiak T, Parkitna JR, 2016. Loss of NMDA receptors in dopamine neurons leads to the development of affective disorder-like symptoms in mice. Sci. Rep 6, 37171. doi: 10.1038/srep37171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S, Henry B, Semenova S, Markou A, 2005. Mild anxiogenic effects of nicotine withdrawal in mice. Eur. J. Pharmacol 516, 40–45. doi: 10.1016/j.ejphar.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Hollander JA, Kenny PJ, 2009. Decreased brain reward function during nicotine withdrawal in C57BL6 mice: Evidence from intracranial self-stimulation (ICSS) studies. Pharmacol. Biochem. Behav 90, 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A, 2001. Neurobiology of the nicotine withdrawal syndrome. Pharmacol. Biochem. Behav 70, 531–549. doi: 10.1016/S0091-3057(01)00651-7 [DOI] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR, 2003. Characterization of Spontaneous and Precipitated Nicotine Withdrawal in the Mouse. J. Pharmacol. Exp. Ther 307, 526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, 2013. Influence of reinforcer magnitude and nicotine amount on smoking’s acute reinforcement enhancing effects. Drug Alcohol Depend. 133, 167–171. doi: 10.1016/j.drugalcdep.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothion S, Bizot JC, Trovero F, Belzung C, 2004. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav. Brain Res 155, 135–146. doi: 10.1016/j.bbr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Powell J, Dawkins L, Davis RE, 2002. Smoking, reward responsiveness, and response inhibition: Tests of an incentive motivational model. Biol. Psychiatry 51, 151–163. doi: 10.1016/S0006-3223(01)01208-2. [DOI] [PubMed] [Google Scholar]
- Powell JH, Pickering AD, Dawkins L, West R, Powell JF, 2004. Cognitive and psychological correlates of smoking abstinence, and predictors of successful cessation. Addict. Behav 29, 1407–1426. doi: 10.1016/j.addbeh.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M, 2004. Decreased Signs of Nicotine Withdrawal in Mice Null for the 4 Nicotinic Acetylcholine Receptor Subunit. J. Neurosci 24, 10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman SM 1979. The tobacco withdrawal syndrome In: Krasnegor NA (ed.) Cigarette smoking as a dependence process. NIDA Research Monograph 23. DHEW Publication No, (ADM) 79–800; pp. 158–1X4. [Google Scholar]
- Stoker AK, Semenova S, Markou A, 2008. Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Neuropharmacology 54, 1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AK, Marks MJ, Markou A, 2015. Null mutation of the β2 nicotinic acetylcholine receptor subunit attenuates nicotine withdrawal-induced anhedonia in mice. Eur. J. Pharmacol 1848, 3047–3054. doi: 10.1016/j.bbamem.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RD, Grant CV, 1971. Automated Preference Testing Apparatus for Rating Palatability of Foods. J. Exp. Anal. Behav 15, 215–220. doi: 10.1901/jeab.1971.15-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma W, Kyte SL, Bagdas D, Alkhlaif Y, Alsharari SD, Lichtman AH, Chen ZJ, Del Fabbro E, Bigbee JW, Gewirtz DA, Damaj MI, 2017. Effects of paclitaxel on the development of neuropathy and affective behaviors in the mouse. Neuropharmacology 117, 305–315. doi: 10.1016/j.neuropharm.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JL, Ghosh S, Bagdas D, Mason BL, Crowe MS, Hsu KL, Wise LE, Kinsey SG, Damaj MI, Cravatt BF, Lichtman AH, 2016. Diacylglycerol lipase β inhibition reverses nociceptive behaviour in mouse models of inflammatory and neuropathic pain. Br. J. Pharmacol 173, 1678–1692. doi: 10.1111/bph.13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao-Shea R, DeGroot SR, Liu L, Vallaster M, Pang X, Su Q, Gao G, Rando O,J, Martin GE, George O, Gardner PD, Tapper AR 2016. Increased CRF signaling in a ventral tegmental area- interpeduncular nucleus-medial habenula circuit induces anxiety during nicotine withdrawal. Nat. Commun 28, 1304–1314. doi: 10.1002/nbm.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mice were chronically exposed to saline or nicotine (24mg/kg/day) by osmotic subcutaneous minipumps for 14 days. Sucrose preference test (2%) was conducted 24 hrs after implantation of minipumps and continued for 11 consecutive days. There was no significant difference of sucrose preference between nicotine and saline treated groups. Each point represents the mean ± S.E.M. of 11 mice per group.
Two days after removal of minipumps, mice undergoing nicotine withdrawal were exposed to two tubes: one containing drinking water and the other 0.4% of saccharine. After 24, 48 and 96 hrs, measurements of water and saccharine were recorded. Saccharine preference was determined as the percentage of 0.4% saccharine volume consumed over the total fluid intake volume. There was no alteration in saccharine preference in nicotine withdrawn mice compared to their controls. Each point represents the mean ± S.E.M. of 8 mice per group.
Mice were chronically infused with saline or nicotine (12 or 24 mg/kg/day) for 14 days. Spontaneous nicotine withdrawal was induced on day 15 and the LDB test was performed. Along with the time spent in the light side, number of transitions between the dark and light side was recorded. A) Number of transitions on days 1, 2 and 5 after removal of minipumps in C57BL/6J mice (n=10/per group). B) Number of transitions on day 2 of β2 KO mice (n=8/per group) C) Number of transitions on day 2 of α6 KO mice (n=8). Each point represents the mean ± S.E.M. of 8–10 mice per group.