Abstract
OBJECTIVES
The goal of this study was to describe short- and long-term outcomes in all patients referred for inappropriate sinus tachycardia ablation, along with the potential complications of the intervention.
BACKGROUND
Sinus node (SN) ablation/modification has been proposed for patients refractory to pharmacological therapy. However, available data derive from limited series.
METHODS
The electronic databases MEDLINE, Embase, CINAHL, Cochrane, and Scopus were systematically searched (January 1, 1995–December 31, 2015). Studies were screened according to predefined inclusion and exclusion criteria.
RESULTS
A total of 153 patients were included. Their mean age was 35.18 ± 10.02 years, and 139 (90.8%) were female. All patients had failed to respond to maximum tolerated doses of pharmacological therapy (3.5 ± 2.4 drugs). Mean baseline heart rates averaged 101.3 ± 16.4 beats/min according to electrocardiography and 104.5 ± 13.5 beats/min according to 24-h Holter monitoring. Two electrophysiological strategies were used, SN ablation and SN modification, with the latter being used more. Procedural acute success (using variably defined pre-determined endpoints) was 88.9%. Consistently, all groups reported high-output pacing from the ablation catheter to confirm absence of phrenic nerve stimulation before radiofrequency delivery. Need of pericardial access varied between 0% and 76.9%. Thirteen patients(8.5%) experienced severe procedural complications, and 15 patients (9.8%) required implantation of a pacemaker. At a mean follow-up interval of 28.1 ± 12.6 months, 86.4% of patients demonstrated successful outcomes. The symptomatic recurrence rate was 19.6%, and 29.8% of patients continued to receive antiarrhythmic drug therapy after procedural intervention.
CONCLUSIONS
Inappropriate sinus tachycardia ablation/modification achieves acute success in the vast majority of patients. Complications are fairly common and diverse. However, symptomatic relief decreases substantially over longer follow-up periods, with a corresponding high recurrence rate.
Keywords: inappropriate sinus tachycardia, sinus node ablation, sinus node modification
Inappropriate sinus tachycardia (IST) is syndrome characterized by unexpectedly fast sinus rates at rest, with minimal physical activity, or both. It is manifest by a spectrum of debilitating symptoms, including palpitations, weakness, fatigue, dizziness, and near-syncope (1,2). Available pharmacological therapy often falls short in providing reliable symptomatic relief, although multiple recent studies have elucidated a potential value of the If blocker ivabradine in the treatment of IST (2). Sinus node (SN) modification via radiofrequency (RF) ablation is considered for drug-refractory cases (3). However, there is no consensus on the optimal procedural approach. Physicians must choose between SN modification and ablation, use open-chested or conventional intravascular access, and conform to one of many available mapping methods. Acute and chronic success rates have varied widely between series reported thus far. Moreover, techniques remain subject to potential complications. These include permanent pacing requirement (3), phrenic nerve (PN) paralysis (4), and transient superior vena cava (SVC) syndrome (5,6), among others. Accordingly, IST ablation is not recommended as a routine intervention by the HRS Expert Consensus Document on IST (Class III) (7). However, the latter document does sanction the use of IST ablation in highly selected circumstances or as part of research protocols.
The goal of the present study was to conduct a systematic review of the different approaches described to date, their short- and long-term results, and the potential complications that can occur during IST ablation/modification.
MATERIALS AND METHODS
A systematic review and best-evidence synthesis was conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (8).
LITERATURE SEARCH.
Electronic databases MEDLINE, Embase, CINAHL, Cochrane, and Scopus were systematically searched from January 1, 1995, to December 31, 2015. The following search terms were used: inappropriate sinus tachycardia ablation, inappropriate sinus tachycardia modification, and sinus node ablation. Each article’s references were screened for additional relevant papers that may have been missed by the initial search.
ELIGIBILITY CRITERIA.
Titles and abstracts were screened for eligibility via the following criteria. Inclusion:
Languages: English, French, Swedish, Arabic, Norwegian, Danish, and Spanish.
Publication type: original manuscripts in peer-reviewed journals.
Study design: systematic reviews, meta-analyses, randomized controlled trials, case–control, and cohort studies. Studies reporting a minimum of 2 IST ablation cases were included.
Study population: human participants of all ages who have undergone SN ablation/modification.
Case definition: IST must be defined in compliance with the definition provided in the Expert Consensus Statement on the Diagnosis and Treatment of Inappropriate Sinus Tachycardia (7). It states: “The syndrome of IST is defined as a sinus heart rate >100 beats/min at rest (with a mean 24-h heart rate >90 beats/min not due to primary causes) and is associated with distressing symptoms of palpitations.” Accordingly, studies including patients with postural orthostatic tachycardia syndrome (POTS) were excluded (9).
Study outcomes: acute success, complication rates, maneuvers to avoid PN injury, and long-term follow-up.
Exclusion:
Study design: nonsystematic reviews, cadaveric, biomechanical, and laboratory studies.
Study population: animals.
SCREENING.
Initially, 1 reviewer read all titles retrieved from the database search and removed citations clearly unrelated to IST. An abstract review was subsequently conducted. Full-text articles were obtained for all abstracts except those that clearly did not meet the eligibility criteria. If after analyzing the full text, the eligibility of an article remained uncertain, a second reviewer conducted a full-text analysis of the article to determine eligibility. A third reviewer was consulted in the event of disagreement. Level of agreement on study eligibility was tested by using the kappa statistic and 95% confidence intervals.
CRITICAL APPRAISAL.
Two reviewers appraised eligible papers by using the modified Scottish Inter-collegiate Guidelines Network criteria (10). Reviewers were international scientists and/or had experience in systematic review methodology.
DATA EXTRACTION.
Extracted data included the following: 1) study name, authors, and publication date; 2) publication language; 3) publication type; 4) geographic origin; 5) pre-procedural evaluation; 6) IST definition; 7) population size; 8) study design; 9) participant characteristics; 10) electrophysiological details of the SN ablation/modification procedure;11) prognostic factors/outcomes; 12) complications;13) follow-up periods; and 14) key findings. These endpoints were summarized in 4 tables (Tables 1 to 5).
TABLE 1.
Demographic Characteristic of the Study Population
| First Author (Ref. #), Year | Population, n | Age, yrs | Female | No. of Failed Drugs* | Beta-Blockers | Calcium-Channel Blockers | HR, Baseline ECG, beats/min* | HR, 24-h Holter ECG, beats/min* |
|---|---|---|---|---|---|---|---|---|
| Lee et aL. (3), 1995 | 16 | 35.4 ± 2.1 | 15 (94%) | 4.2 ± 0.3 | 16 (100%) | 13 (81.2%) | 114 ± 19 | NA |
| CaLLans et aL. (5), 1999 | 10 | 28 ± 7 | 9 (90%) | NA | 10 (100%) | 5 (50%) | NA | NA |
| Man et aL. (4), 2000 | 29 | 37 ± 12 | 26 (90%) | 4.7 ± 2.9 | NA | NA | 93 ± 9 | NA |
| Marrouche et aL. (16), 2002 | 39 | 31 ± 9 | 35 (90%) | NA | 33 (84.6%) | NA | 99 ± 14 | 108 ± 5 |
| Bonhomme et aL. (13), 2003 | 2 | 26.5 ± 2.1 | 2 (100%) | 1.5 ± 0.7 | 2 (100%) | 1 (50%) | NA | NA |
| Takemoto et aL. (15), 2011 | 6 | 43 ± 3 | 3 (50%) | 1.5 ± 0.5 | 6 (100%) | 2 (33.3%) | NA | 93 ± 1 |
| FrankeL et aL. (18), 2012 | 33 | 39.9 ± 11.5 | 31 (93.93%) | NA | 25 (%) | 23 (69.7%) | NA | 102.5 ± 19.1 |
| Jacobson et aL. (17), 2014 | 5 | 36.4 ± 4.2 | 5 (100%) | 2.2 ± 1.1 | 4 (80%) | 4 (80%) | 119 ± 20 | NA |
| Ibarra-Cortez et aL. (14), 2015 | 13 | 34.2 ± 7.7 | 13 (100%) | 1.6 | 12 (92.3%) | 5 (38.4%) | 104 ± 18 | NA |
| Total | 153 | 35.18 ± 10.02 | 139 (90.8%) | 3.48 ± 2.35 | 108/124 (87%) | 53/85 (62.3%) | 101.26 ± 16.37 | 104.54 ± 13.50 |
Values are n, mean ± SD, n (%), n/N (%).
ECG = electrocardiography; HR = heart rate; NA = nonapplicable.
TABLE 5.
Requirement of Pericardial Access for Phrenic Nerve Protection or Epicardial Ablation Along With the Complications Encountered
| First Author (Ref. #), Year | Ablation Limited by PN Proximity | Maneuvers to Avoid PN Injury | Pericardial Access | Endocardial RF Lesions* | Epicardial RF Lesions |
|---|---|---|---|---|---|
| Lee et al. (3), 1995 | NA | NA | NA | 3.6 ± 0.8 (SN modification ICE guided); 10.4 ± 2.1 (SN modification non–ICE guided) | NA |
| Callans et al. (5), 1999 | 0 | NA | NA | 16.4 ± 2.9 | NA |
| Man et al. (4), 2000 | NA | NA | NA | 22 ± 15 | NA |
| Marrouche et al. (16), 2002 | 2 (5.13%) | Lesions were delivered at sites showing capture of the PN (in 1 of them ablation induced diaphragm paralysis, which recovered 7 months later) | NA | 29 ± 11 | NA |
| Bonhomme et al. (13), 2003 | 1 (50%) | Open surgical procedure with repositioning of the PN | NA | NA | NA |
| Takemoto et al. (15), 2011 | NA | NA | NA | NA | NA |
| Frankel et al. (18), 2012 | 0 | NA | NA | NA | NA |
| Jacobson et al. (17), 2014 | 3 (60%) | Pericardial access. Two patients required injection of saline and air. In 2 patients, holding ventilation at end-expiration during RF applications | 5 (posterior) | 12.8 ± 7.8 | 22.4 ± 21.8 |
| Ibarra-Cortez et al. (14), 2015 | 12 (92.3%) | 10 patients required pericardial balloon insertion. In 2 patients, PN was avoided by holding ventilation | 4 (posterior), 6 (anterior) | 16 ± 4 | NA |
| Total | 18/102 (17.6%) | 15 (9.8%) | 20.01 ± 13.14 |
QUALITY ASSESSMENT.
A thorough evaluation of bias took place, as described previously (11,12). Appraisal included reporting bias, external validity bias, internal validity bias, internal validity confounding, and power. Each study was assigned a numerical indicator for the degree of each bias type, after which it was designated a title of low, medium, or high risk for that specific bias type. External validity was at low risk for bias in all studies except that of Bonhomme et al. (13). Internal validity confounding and power were at high risk for bias in all studies. All studies were at intermediate risk for reporting and internal validity bias with the exception of Ibarra-Cortez et al. (14), which had low risk for reporting bias. Overall, studies included were designated an intermediate risk for bias.
STATISTICAL ANALYSIS.
Categorical data are presented as numbers and percentages, as well as a range from minimal to maximal reported values, with a corresponding grouped median. Continuous data are expressed as mean ± SD.
For pooled analyses, after evaluating for heterogeneity between studies, a combined mean ± SD was calculated by using the sample size, mean, and SD of the individual studies.
RESULTS
DEMOGRAPHIC CHARACTERISTICS.
A total of 153 patients from 9 different studies were included in the analysis (3–5,13–19) (Table 1). Mean age was 35.18 ± 10.02 years, and 139 (90.8%) of the patients were female. Marrouche et al. (16) reported 100% prevalence of psychiatric disorders in their 39 patients. In the series by Ibarra-Cortez et al. (14), 12 of 13 patients were maintained by using anxiolytic or antidepressant agents. Frankel et al. (18) described a history of syncope in 15 of 33 patients.
All patients had failed to respond to the maximum tolerated doses of pharmacological therapy. Beta-blockers were assigned as first-line therapy in all studies (108 of 124 patients for whom this information was specified [87%]). The number of antiarrhythmic drugs tried before procedural intervention ranged from 4.7 ± 2.1 (4) to 1.5 ± 0.5 (15), averaging 3.5 ± 2.3. Two additional patients were maintained on flecainide (16), 4 on sotalol (14,18), and 2 were receiving ivabradine (14).
The majority of patients were free of structural heart disease. Only 8 patients (5%) exhibited overt cardiomyopathy (3,4,6,17,18), and 7 patients (18) had a history of slow pathway ablation before enrollment (with consequent pacemaker [PCM] implantation in 6 of these patients).
Mean baseline sinus heart rate was 101.26 ± 16.37 beats/min according to electrocardiography and 104.54 ± 13.50 beats/min via 24-h Holter monitoring.
Details regarding the pre-procedural evaluation are given in Table 2.
TABLE 2.
Pre-Procedural Evaluation
| First Author (Ref. #), Year | History, Physical Examination | Complete Blood Cell Counts | Thyroid Function | 24-h Holter | 24-h Urine Collection for Metanephrines | Autonomic Testing | Treadmill Exercise Testing | Exclusion of POTS | Electrophysiology Studies |
|---|---|---|---|---|---|---|---|---|---|
| Lee et al. (3), 1995 | Yes | Yes | ND | Yes | ND | Yes | Yes | ND | Yes |
| Callans et al. (5), 1999 | Yes | ND | ND | Yes | ND | Yes | Yes | ND | ND |
| Man et al. (4), 2000 | Yes | Yes | Yes | ND | ND | ND | ND | Yes | Yes |
| Marrouche et al. (16), 2002 | Yes | ND | ND | Yes | ND | Yes | Yes | ND | Yes |
| Bonhomme et al. (13), 2003 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Takemoto et al. (15), 2011 | Yes | Yes | ND | Yes | ND | ND | Yes | ND | Yes |
| Frankel et al. (18), 2012 | Yes | ND | ND | ND | ND | ND | ND | ND | Yes |
| Jacobson et al. (17), 2014 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Ibarra-Cortez et al. (14), 2015 | Yes | Yes | Yes | Yes | ND | ND | ND | Yes | Yes |
ND = nondescribed; POTS = postural orthostatic tachycardia syndrome.
DEFINITION OF IST.
The definition of IST was fairly consistent throughout the studies we evaluated in accordance with the Expert Consensus Statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope (7).
ENDPOINT DEFINITION.
In terms of ablation versus modification, only Lee et al. (3) made the distinction between SN ablation and SN modification. They defined SN ablation as a slowing of >50% from the baseline rate of tachycardia along with a junctional escape rhythm. SN modification was defined as a minimum 25% reduction in the sinus heart rate under the same conditions of catecholamine infusion, with either retention of the normal P-wave axis in the frontal and horizontal planes or a transient low escape rhythm.
Definitions of procedural success varied slightly between series; however, they all overlapped on the broad premise of reduction of heart rate (to <90 beats/min [4] or >25% reduction [4,5,16,14,18] from baseline) coupled with P-wave inversion. Each study’s definition of success is detailed in Table 3. In multiple studies, isoproterenol infusion was used to document persistence of these features (5) or a $20% reduction in the sinus rate during infusion (4,16).
TABLE 3.
Electrophysiological Characteristics of the Ablation Procedure
| First Author (Ref. #), Year | Nonfluoroscopic System | Isoprenaline Provocation | RF Catheter | Acute Success | Definition of Success | Junctional Rhythm | Abrupt Response | Fluoroscopic Time | Complications |
|---|---|---|---|---|---|---|---|---|---|
| Lee et al. (3), 1995 | No | Yes | Standard 4 or 10 mm | 16 (100%) | SN ablation: a reduction in HR >50% of the tachycardia HR with a junctional escape rhythm SN modification: at least a 25% reduction in the HR under the same conditions of catecholamine infusion with either retention of the normal P-wave axis in frontal and horizontal planes or transient low atrial escape rhythm |
4 (25%) | NA | 3.6 ± 0.8 min (ICE guided); 58.5 ± 8.4 min (non–ICE guided) | 1 case of right diaphragmatic paralysis 1 case of SVC syndrome |
| Callans et al. (5), 1999 | Yes | Yes | Standard 4 or 8 mm | 8 (80%) | 1) an abrupt decrease (30 beats/min) in sinus rate during RF lesion delivery; 2) the sudden appearance of superiorly directed P-wave morphology (negative P-wave in lead III); 3) the persistence of these features despite infusion of isoproterenol for at least 30 min after the delivery of the final RF lesion | NA | 11 (84.6%) | NA | Nonprocedure-related complications |
| Man et al. (4), 2000 | No | Yes | Standard 4 mm | 22 (75.8%) | Reduction of the baseline sinus rate to 90 beats/min, and ≥20% reduction in the sinus rate during infusion of isoproterenol | 8 (27.6%) | 9 (41%) | 59 ± 37 min | 1 case of PN paralysis (persisted for 41 months) |
| Marrouche et al. (16), 2002 | Yes | Yes | Standard 4 mm | NA | Ablation was terminated if the HR dropped to <120 beats/min during isoproterenol infusion at 2 mg/min alone or in combination with aminophylline infusion | NA | NA | 21 ± 6 min | 1 case of SVC syndrome |
| Bonhomme et al. (13), 2003 | Yes | Yes | Standard 4 mm | 2 (100%) | The ablation strategy described in the studies by Lee et al. and Marrouche et al. was used | 0 | NA | 29 min | 2 cases of pericarditis |
| Takemoto et al. (15), 2011 | Yes | Yes | Standard 4 mm | 6 (100%) | Breakout sites observed during HR of >100 beats/min moved completely from the tall P-wave zone to the normal P-wave zone with and without the intravenous administration of isoproterenol in accordance with the abolishment of the tall P-waves on the 12-lead ECG during the RF energy delivery | NA | NA | NA | Nonprocedure-related complications |
| Frankel et al. (18), 2012 | Yes (75.8%); no (24.2%) |
Yes | Open-irrigated 3.5 mm and irrigated ablation 4 mm tip | NA | Decrease of >25% in resting HR, with a shift of P-wave morphology from positive to flat or negative in leads III and aVF | NA | NA | NA | 1 procedure was complicated by AV fistula. 1 retroperitoneal bleed |
| Jacobson et al. (17), 2014 | Yes | Yes | Open-irrigated 3.5 mm | 5 (100%) | 25% reduction in sinus HR and inversion of P-wave axis | 4 (80%) | NA | 3 cases of pericarditis. 1 case of RV puncture during pericardial access |
|
| Ibarra-Cortez et al. (14), 2015 | Yes | Yes | Open-irrigated 3.5 mm | 13 (100%) | A decrease in HR ≥20% from baseline off isoproterenol and an associated change in the P-wave morphology in lead III and aVF from a positive to a flat or negative deflection | 11 (84.6%) | NA | 22.56 ± 15.6 min | 1 case of cardiac tamponade |
| Total | — | — | — | 72/81 (88.9%) | — | 27/65 (41.6%) | NA | 38.76 ± 28.41 min | 13 (8.5%) |
AV = arteriovenous; ICE = intracardiac echocardiography; PN = phrenic nerve; RF = radiofrequency; RV = right ventricular; SN = sinus node; SVC = superior vena cava; other abbreviations as in Table 1.
ELECTROPHYSIOLOGICAL STRATEGY.
A wide variation in ablation protocol was observed. A 3-dimensional nonfluoroscopic mapping system was used in 7 of the 9 studies we evaluated (5,13–18). Furthermore, a noncontact mapping system (EnSite Array multi-electrode catheter; Endocardial Solutions, St. Paul, Minnesota) was used in 3 of the studies (13,17,18).
The first study to report the feasibility of IST ablation/modification (3) localized the SN at the region of earliest atrial activation in sinus rhythm and further defined it by using intracardiac echo-cardiography (ICE) in 9 patients.
Callans et al. (5) relied primarily on anatomic localization, but mapping of the earliest atrial activation during episodes of IST further confirmed the appropriateness of ablation site targets.
Man et al. (4) performed point-by-point activation mapping in each patient. RF energy was delivered at the site of the earliest bipolar electrogram relative to the surface P wave.
Marrouche et al. (16) and Ibarra-Cortez et al. (14) systematically created 3-dimensional electro-anatomical maps during sinus rhythm (after administration of esmolol, during infusion of isoproterenol and/or aminophylline). Ablation was initiated at the earliest site of activation, and any decrease in heart rate prompted the acquisition of a new right atrial (RA) 3-dimensional map. If SN acceleration could not be achieved, the catheter was moved cranially first, and then caudally, in search of sites where ablation would result in SN acceleration.
Bonhomme et al. (13) and Takemoto et al. (15) identified the site of earliest activation at peak heart rate with the maximum dose of isoproterenol. RF was delivered when the atrial signal on the ablation catheter was confirmed to be earlier than the surface P-wave. With each change in heart rate or in the P-wave morphology, the site of earliest activation was reassessed, and the new site was targeted.
Frankel et al. (18) used 2 different strategies: 1) an anatomic strategy (24.2%) targeting the supero-lateral crista terminalis at the junction of the SVC and RA appendage; and 2) the electrophysiological mapping approach (75.8%) as previously described.
Jacobson et al. (17) adopted the aforementioned mapping protocols. However, because their patients had previously failed to respond to endocardial ablation, they further performed contact mapping of the epicardium in efforts to optimize efficacy.
Consistently, all groups performed pacing from the ablation catheter at a maximum output of 10 mA with a pulse width of 2 ms to confirm the absence of PN stimulation before each RF lesion delivery.
PROCEDURAL OUTCOMES.
Acute results.
Acute success rates were consistently high in all studies(88.9%) (Table 3). Man et al. (4) reported that 13 of 22 patients exhibited a cranial to caudal migration of their activation sites with an eventual abrupt change in P-wave morphology. Using 3-dimensional mapping, Marrouche et al. (16) quantified the caudal shift at an average of 23 ± 11 mm consequent to ablation.
Junctional rhythm was achieved in 27 (41.6%) of 65 patients. All except 1 patient recovered a higher RA rhythm within 24 h of ablation (3,14). Only 2 studies described whether there was an abrupt response to ablation: 84.6% in the study by Callans et al. (5) and 41% described by Man et al. (4) (Table 3).
Long-term results.
Consistently, all series reported recurrence of IST within their designated study period; the overall frequency of recurrence was 19.6% (Table 4). The majority of cases were treated with repeat ablation (4,5,16), while some received pharmacological therapy (3,14) (including amiodarone), 1 patient underwent His ablation plus implantation of a PCM (3) and 1 patient was referred for combined minimally invasive surgical/catheter SN cryoablation(16).
TABLE 4.
Outcomes in the Long-Term Follow-Up of Patients Referred for SN Ablation/Modification
| First Author (Ref. #), Year | Mean HR at Follow-Up | Symptoms Relief | Drugs in the Follow-Up | Requirement of Recurrence Months of Follow-Up PCM Implantation | ||
|---|---|---|---|---|---|---|
| Lee et aL. (3), 1995 | 54.1 ± 5.3 beats/min (24-h HoLter) | 14 (87.5%) | 4 (25%) | 2 (1 patient treated with amiodarone and 1 patient treated with AVN abLation plus PCM) | 20.5 ± 0.3 | 3 (2 of those referred for SN abLation, who presented pre-syncope; and 1 in the SN modification due to recurrence, which was treated with AVN abLation pLus PCM. Three patients had a PCM previousLy impLanted) |
| CaLLans et aL. (5), 1999 | NA | NA | NA | 3 (30%) | NA | 1 patient required PCM implantation after complete SN abLation |
| Man et aL. (4), 2000 | 80 ± 8 beats/min (ECG) | 19 (66%) | 5 (17.2%) | 6 (27%) (redo procedure) | 32 ± 12 | 6 (1 due to sinus pauses, 5 due to persistence of the symptoms reason why they were referred for AVN abLation pLus PCM impLantation) |
| Marrouche et aL. (16), 2002 | 85 ± 5 (24-h HoLter) | 39 (100%) | NA | 8 (21%) (redo procedure) | 32 ± 9 | 0 (2 patients had previous PCM) |
| Bonhomme et aL. (13), 2003 | 70–80 beats/min (first patient); 60 beats/min (second patient) (24-h HoLter) | 2 (100%) | 0 | 0 | 1 | 0 |
| Takemoto et aL. (15), 2011 | 79 ± 1 beats/min (24-h HoLter) | 6 (100%) | 2 (33%) | 1 (16.6%) (redo procedure) | 12 | 0 |
| FrankeL et aL. (18), 2012 | 83.5 ± 15.7 beats/min | NA | 18 (54.54%) | 6 (18.2%) | 24 ± 18 | 4 (12.1%) |
| Jacobson et aL. (17), 2014 | NA | 5 (100%) | 0 | 1 (invasive surgical ablation) | 30.4 ± 18.4 | 0 (3 patients had previous implanted ICD or PCM) |
| Ibarra-Cortez et aL. (14), 2015 | 71.9 ± 10.8 beats/min | 10 (77%) | 2 (15.3%) | 3 (23%) | 27 ± 1.4 | 1 (1 patients had previous PCM) |
| TotaL | 78.26 ± 13.63 beats/min | 95/110 (86.36%) | 31/104 (29.8%) | 30 (19.6%) | 28.14 ± 12.64 | 15 (9.8%) (9 had previous PCM) |
Interestingly, recurrence occurred within a wide range of post-ablation intervals, anywhere from a few weeks (3,16,18) to several months (4,18) after the procedure. As such, repeat procedures were performed between 1 and 8 months after each initial ablation. Frankel et al. (18) established predictors of time-to-tachyarrhythmia recurrence through multivariate analysis. Reliable predictors included higher resting heart rates post-ablation and smaller cranial to caudal shifts (p = 0.03 and p = 0.05, respectively). Similarly, Marrouche et al. (16) found that patients with a longer history of IST and those reporting near syncope/syncope had a higher probability of recurrence.
At a mean follow up interval of 28.14 ± 12.64 months, 95 (86.36%) of 110 patients for whom this endpoint was clearly reported exhibited successful outcomes, whereas 15 (15.3%) experienced a lack of symptom relief. One-third of patients continued to receive antiarrhythmic drug therapy at the end of the follow-up period (n = 31 [29.8%]).
Takemoto et al. (15) documented a significant drop in B-type natriuretic peptide levels 6 to 12 months’ post-ablation (from 34 pg/dl to 10 pg/dl; p < 0.05). In the study by Marrouche et al. (16), 81% of patients who had been diagnosed with panic disorder terminated their follow-up in the psychiatric clinic. Similarly, 61% of patients diagnosed with somatoform disorder no longer required psychiatric care. Moreover, direct access to allied health professionals reduced the need for emergency department visits and unscheduled clinic visits after the ablation procedure. Furthermore, all 67% of patients from the study by Takemoto et al. who were previously diagnosed with depression achieved resolution of the condition after ablation.
Frankel et al. (18) demonstrated that non-IST tachyarrhythmias (27%) were a more common cause of symptom recurrence than persistent, drug-refractory IST (18%). In fact, 9 of their patients developed non-IST tachyarrhythmias post-ablation. Atrial tachycardia was the most common non-IST tachyarrhythmia after SN modification (71.4% of all non-IST tachyarrhythmias). Three atrial tachycardias were mapped to the SVC and superior crista terminalis. In the series by Ibarra-Cortez et al. (14), a single patient who had undergone 2 previous attempts of SN modification presented a concomitant clockwise RA flutter at the time of the third procedure.
Complications.
Thirteen patients (8.5%) experienced severe procedural complications (Table 3). Three patients had clinical pericarditis that was addressed with medical treatment. Two patients presented with right diaphragmatic paralysis (3,4). One case resolved spontaneously within 1 month while the second persisted after 41 months of follow-up (4). A single procedure was complicated by arteriovenous fistula and another by retroperitoneal bleed (18). Of those who underwent pericardial puncture (n = 14 [12.1%]), 1 patient experienced cardiac tamponade (14) and 1 right ventricular puncture(17) with no subsequent sequelae.
A complication of particular concern was SVC syndrome, documented in 2 patients (both with previous PCM implantation) (3,16). One case resolved spontaneously within 1 h of ablation and the second persisted 4 months’ post-ablation, eventually requiring balloon dilatation. In this regard, Callans et al. (5) explored the potential for ICE assessment of tissue swelling that may lead to venous occlusion during RF ablation. With RF delivery, local and circumferential swelling was observed, causing progressive reduction in the diameter of the SVC-RA junction from 16.4 ± 2.9 mm to 12.6 ± 3.3 mm (24% reduction; p < 0.0001). A reduction in SVC-RA orifice diameter of 30% compared with baseline was observed in 5 patients. However, SVC occlusion and clinical signs of SVC syndrome were not observed. After using ICE to evaluate this endpoint, Ibarra-Cortez et al. (14) observed no incidence of acute SVC syndrome after ablation.
Fifteen patients (9.8%) required PCM implantation. Indications included symptomatic intermittent junctional tachycardia/sinus dysfunction (n = 16) and persistence of severe IST symptoms (n = 6). The latter underwent RF ablation of the atrioventricular junction and implantation of a PCM.
Maneuvers performed to avoid PN injury.
PN injury is a severe and dreaded complication of IST ablation. Proximity of the desirable ablation target to the PN poses a major limitation to liberal RF delivery. Various maneuvers have been described to overcome this limitation. Jacobson et al. (17) established access to the SN via the transverse pericardial sinus by using a posterior epicardial access approach in 6 patients, and the epicardial balloon was used for PN protection in 3 patients. Two patients required injection of saline and air to further protect the PN. Similarly, Ibarra-Cortez et al. (14) performed pericardial balloon insertion whenever PN capture was noted at the desired ablation sites (77% of cases). Notably, pericardial access to optimal balloon positioning time was significantly lower in anterior versus posterior pericardial access (16.3 ± 6 min vs. 58 ± 21.3 min; p < 0.05), as was fluoroscopy time (7.6 ± 1 min vs. 35.9 ± 1.8 min; p < 0.01) (20). Moreover, transient PN capture was seen during end-inspiration in both studies, and holding ventilation solely at the time of ablation proved to be a useful tool in avoiding PN injury in 4 patients (14,16). Requirement of pericar-dial access for PN protection is detailed in Table 5.
DISCUSSION
IST is a phenomenologically defined clinical syndrome whose treatment remains limited by incomplete mechanistic understanding. Interventional treatment has shown promise in drug-refractory cases in multiple reports. Unfortunately, only small population studies have been individually assessed, and no conclusive inference could be drawn owing to this limitation. The present study therefore sought to provide a holistic evaluation of interventional treatment for IST by: 1) summarizing the available literature to draw conclusions from a sufficient population size; 2) comparing available procedures, including SN modification and ablation; 3) assessing demographic and clinical indications for referral to intervention; 4) studying procedural protocols and outcomes; and 5) evaluating the hazards and complications of intervention and their respective preventative maneuvers/measures.
Investigators define IST fairly constantly, and all evaluated patients fall within the same constellation of symptoms. Those referred for procedural intervention are predominantly female, and they experience debilitating symptoms despite maximum tolerated pharmacological doses (primarily beta-blockers as the first choice in patients free of structural heart disease [87%]). Two electrophysiological strategies have been widely used; SN modification was evidently preferable to SN ablation. The vast majority of studies were performed by using 3-dimensional nonfluoroscopic mapping systems and noncontact mapping systems, which targeted the earliest site of activation. IST ablation achieved acute success in most cases (88.9%). However, long-term follow-up demonstrated an overall remittance rate of 86.36%. Few studies have addressed predictors of success, but evidence points to less IST recurrence when more aggressive targeting of the SN is implemented. Finally, a 9.1% incidence of severe complications was observed and 14.4% required PCM implantation during the follow-up period.
It is our conviction that the overall results presented here support the current class III recommendation indicated in the Expert Consensus Statement on the Diagnosis and Treatment of Inappropriate Sinus Tachycardia (7). The document states that given the young age of patients with IST and the highly invasive nature of ablative therapy, SN modification/ablation is not recommended as part of routine care. However, the document further specifies that it may be offered in highly select circumstances.
To date, there is no evidence to support long-term symptomatic improvement consequent to ablation. In the present study, however, with a mean follow-up of 28.1 ± 12.6 months, we can confirm that benefits persist in the medium term. As such, the intervention may provide a useful tool when proper patient selection and safe procedural strategies are used. Eventually, long-term follow-up of reported patients will be crucial in documenting long-term efficacy. This approach in turn could augment the usefulness of ablation and ultimately alter recommendations.
TEMPORAL TRENDS.
Studies included within this analysis span from 1995 (3) through 2015 (14). During this time, a shift in available technology and insight into IST treatment were matched by an evolution of the preferred procedural protocol to include non-fluoroscopic 3-dimensional mapping (contact or noncontact) or pericardial access to avoid PN injury. Quantitative data must thus be contextualized to the available technologies before any clinically relevant conclusions can be drawn regarding variations of success over time.
PROCEDURAL SUCCESS.
SN ablation was initially coined the technique of choice, but recent studies have advocated SN modification, although the differences seem arbitrary. It is clear that SN modification is apt in achieving acute reductions in post-procedural heart rate. However, success rates are suboptimal in terms of symptomatic control (86.4%), with a significant recurrence rate of 19.6%. Strikingly, 3 of the studies included here involved patients for whom symptomatic relief could not be achieved despite effective decreases in heart rate (3,14,16). This illustrates our poor understanding of this disease and its manifestations and suggests that IST may be an epiphenomenon of a more complex underlying disease process.
It is also noteworthy that only 4 of 9 studies specify lack of postural orthostatic tachycardia within their inclusion criteria (4,14,16,17). Accordingly, Shen et al.(9) showed that clinical symptoms do not significantly improve after SN modification in this specific population. Their presence within other series may have offset reported success rates. Although both syndromes share some similarities, the available information seems to point toward a stronger autonomic influence in the pathophysiology of IST than POTS (20). Hence, medical and interventional treatment could differ between both entities, and the result of the studies herein collected should not be generalized to both syndromes. It is also likely, however, that the population of patients with POTS recognized in the literature is a significant subset of patients with IST reported in the cardiology and electrophysiology literature, or vice versa.
Furthermore, non-IST tachyarrhythmias can pose a common confounder. These arrhythmias are evidently a more common cause of symptom recurrence than persistent, drug-refractory IST (27% vs. 18%) (18). The investigators hypothesized that the sympathovagal imbalance prominent in patients with IST could predispose to other tachyarrhythmias. Alternatively, they alluded to common myocardial pathology such as inflammation or fibrosis, which may be promoting arrhythmias both in the region of the SN as well as in remote locations.
It remains difficult to determine which cases would benefit from a nonendocardial approach, such as epicardial, hybrid, or surgical. None of the studies included describe markers that could help identify suitable circumstances. Moreover, data concerning high-risk anatomy, electroanatomical mapping outcomes, and consequent probability of failure is also lacking. A case report by Killu et al. (20) affirms that the arcuate ridge should be considered for specific echocardiographically guided mapping and ablation in patients with a diagnosis of refractory IST. Specifically, when multiple early sites of activation are seen in the vicinity of the SVC-RA junction, targeted mapping of the arcuate ridge may reveal the true early site of origin for targeted energy delivery.
ADJUNCTIVE EFFECTS.
IST ablation had pleiotropic effects beyond control of heart rate. Psychiatric outcomes could prove relevant in terms of improvements in quality of life. Although only 2 studies focused on associated psychiatric disorders, it is clear that SN ablation affected the severity of psychiatric disease. As mentioned, Marrouche et al. (17) reported significant improvement in panic disorder, depression, and somatoform disorder after ablation (16), whereas Takemoto et al. (15) perceived resolution of depression in patients previously diagnosed with this condition. Although these findings imply that symptomatic IST may play a role in exacerbating underlying psychiatric disease, it remains to be seen whether the psychiatric disease contributes to the pathophysiology of IST. As such, we have no evidence to support the theory that psychological screening and treatment alone can alter the course of IST disease.
In addition, the reports of Takemoto et al. of decreasing serum B-type natriuretic peptide concentration after RF catheter ablation (15) may hold clinical ramifications. Further validation in future trials is warranted for all aforementioned findings.
BAILOUT STRATEGY.
When symptom control could not be achieved, management protocols varied. Multiple studies stressed the significance of redoing the procedure to achieve proper symptomatic control (15 of 151 [9.9%]). Alternatively, some patients were treated with antiarrhythmic drugs, some with His ablation plus PCM implantation, and 1 patient was referred for combined minimally invasive surgical/catheter SN cryoablation.
Many therapeutic recommendations have been advocated in the management of IST. However, the literature lacks proper comparison between ablative therapy and alternate treatment modalities. Because ablation is typically reserved for drug-refractory patients, a direct comparison cannot be extrapolated from available published data.
We note, however, that the recently developed selective SN If channel inhibitor (ivabradine) has shown promising results in the treatment of IST. Ivabradine (21), in combination with a beta-blocker or as monotherapy, effectively reduced mean heart rates (from 84 ± 11 beats/min to 74 ± 8 beats/min) in patients with IST. This decrease is not dissimilar to the one herein reported consequent to ablation (104.54 ± 13.50 beats/min to 78.26 ± 13.63 beats/min). Moreover, ivabradine (22) also reportedly elicited significant reductions in median and maximal heart rates over 6 months, with subsequent improvement in exercise tolerance. Finally, ivabradine showed effects on resting heart rate comparable to metoprolol therapy, although it seemed to be more effective in relieving symptoms during exercise (23).
Efforts have been made to explore nonpharmacological, noninvasive treatment alternatives in POTS. A small study of 19 patients with POTS supported the use of exercise training to improve quality of life and to maintain upright cardiac output compared with the use of propranolol (24). Such data are unavailable for IST, however, and it remains to be seen whether exercise training could similarly benefit patients with IST.
COMPLICATIONS.
A broad spectrum of complications occurred with a fair amount of frequency. They included pericarditis (17), right diaphragmatic paralysis (3,4), SVC syndrome (3,16), PCM implantation (3–5,18), and cardiac tamponade (14). Naturally, the rate of occurrence was influenced by the application of a pericardial puncture. Although the initial studies did not include performance of pericardial puncture, this technique has lately been advocated first as an alternative approach for RF delivery and secondly for balloon inflation in patients who presented with PN capture at the desired ablation point. The anterior approach seems to associate with lower time to achieve optimal balloon positioning and fluoroscopy time (14). Figure 1 presents an example of mapping and ablation of IST with PN protection (through an anterior and posterior approach). Little information regarding the anterior approach has been reported to date, and further investigations are needed for its validation.
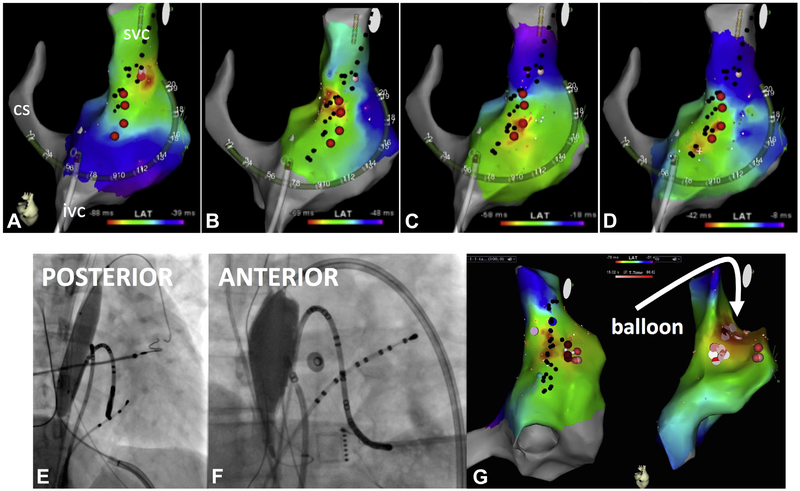
FIGURE 1. Mapping and Ablation of IST, and Phrenic Nerve Protection.
(A to D) Sequential activation maps during inappropriate sinus tachycardia (IST). Black dots mark sites of phrenic nerve capture during high-output pacing. (A) Earliest activation site (red area) is usually mapped in the superior vena cava (SVC)–right atrial (RA) junction. (C to E) After ablation at that site, the earliest activation site shifts downward, requiring consecutive ablations at progressively lower sites. Epicardial balloon for phrenic nerve protection. (E) Balloon positioning via a posterior-inferior pericardial puncture. (F) Balloon positioning via an anterior puncture. (G) Activation maps after balloon positioning, showing indentation of the RA geometry (arrow) and no phrenic nerve capture (no black dots) after balloon inflation.
STUDY LIMITATIONS.
The total number of patients included was relatively small secondary to the limited published data on the topic. Moreover, assessment of long-term efficacy throughout the available studies was based on symptomatic relief, which may be subjective and patient specific. “Symptoms” can include palpitations, pre-syncope/syncope, chest pain, dizziness, shortness of breath, anxiety, and depression. However, it remains to be seen which of the these findings improve after ablation and which do not, and these data are not reflected in the existing literature. Furthermore, a formal assessment of the impact of the ablation procedure on quality of life was not performed in any of the studies discussed here; thus, conclusions regarding the clinical long-term efficacy of IST ablation should be interpreted with this limitation in mind.
Studies on IST are rare, and the temporal variability in published data makes it difficult to reliably aggregate the results. The use of different ablation techniques and mapping systems, as well as variable follow-up and outcome measures, further impedes comparison of results across studies. Notably, irrigated tip catheters were only used in 3 studies, and ivabradine in 1 study. Similarly, autonomic tone, use of sympathomimetic agents, and level and type of sedation all varied widely between and even within included studies. As such, these aspects must be taken into account when interpreting quantitative deductions.
Although the spectrum of IST ranges from persistent to paroxysmal such as daytime only and gradual-onset tachycardia, none of the studies we describe have made this distinction. Differentiating between these subsets of patients could be useful in analyzing treatment success/failure and ultimately in better allocating intervention.
No attempt was made to locate and incorporate unpublished data, which introduces the potential for publication bias. These sources are often difficult to identify and acquire when not indexed in databases such as Medline. Lack of indexing is a significant barrier to successfully incorporating unpublished data into the search methodology. For this reason, unpublished data were excluded from this review (19).
CONCLUSIONS
Procedural intervention is reserved for severe refractory cases of IST. The results of the present study indicate that IST ablation/modification achieves acute success in the vast majority of patients. However, on long-term follow-up, successful symptomatic relief drops substantially with a corresponding high recurrence rate. Complications are fairly common and diverse.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
SN ablation has shown promise in drug-refractory cases of inappropriate sinus tachycardia in multiple studies. Unfortunately, only limited population studies have been individually assessed, and no conclusive, generalizable inference has ever been made.
TRANSLATIONAL OUTLOOK:
Currently published data were reviewed, summarized, and analyzed in-depth. In light of this, investigators are requested to consider the sufficiency of these data, and determine whether more trials are needed in the establishment of optimal interventional guidelines.
ABBREVIATIONS AND ACRONYMS
- ICE
intracardiac echocardiography
- IST
inappropriate sinus tachycardia
- PN
phrenic nerve
- POTS
postural orthostatic tachycardia syndrome
- RA
right atrial
- RF
radiofrequency
- SN
sinus node
- SVC
superior vena cava
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Olshansky B What’s so inappropriate about sinus tachycardia? J Cardiovasc Electrophysiol 2008;19:977–8. [DOI] [PubMed] [Google Scholar]
- 2.Olshansky B, Sullivan RM. Inappropriate sinus tachycardia. J Am Coll Cardiol 2013;61: 793–801. [DOI] [PubMed] [Google Scholar]
- 3.Lee RJ, Kalman JM, Fitzpatrick AP, et al. Radiofrequency catheter modification of the sinus node for “inappropriate” sinus tachycardia. Circulation 1995;92:2919–28. [DOI] [PubMed] [Google Scholar]
- 4.Man KC, Knight B, Tse HF, et al. Radiofrequency catheter ablation of inappropriate sinus tachycardia guided by activation mapping. J Am Coll Cardiol 2000;35: 451–7. [DOI] [PubMed] [Google Scholar]
- 5.Callans DJ, Ren JF, Schwartzman D, Gottlieb CD, Chaudhry FA, Marchlinski FE. Narrowing of the superior vena cava-right atrium junction during radiofrequency catheter ablation for inappropriate sinus tachycardia: analysis with intracardiac echocardiography. J Am Coll Cardiol 1999;33:1667–70. [DOI] [PubMed] [Google Scholar]
- 6.Leonelli FM, Pisano E, Requarth JA, et al. Frequency of superior vena cava syndrome following radiofrequency modification of the sinus node and its management. Am J Cardiol 2000;85:771–4. [DOI] [PubMed] [Google Scholar]
- 7.Sheldon RS, Grubb BP 2nd, Olshansky B, et al. 2015. Heart Rhythm Society Expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015;12:e41–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panic N, Leoncini E, de Belvis G, Ricciardi W, Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PloS One 2013;8:e83138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen WK, Low PA, Jahangir A, Munger TM, Friedman PA, Osborn MJ, et al. Is sinus node modification appropriate for inappropriate sinus tachycardia with features of postural orthostatic tachycardia syndrome? Pacing Clin Electrophysiol 2001;24:217–30. [DOI] [PubMed] [Google Scholar]
- 10.Healthcare Improvement Scotland. Scottish Intercollegiate Guidelines Network (SIGN). Available at: http://www.sign.ac.uk/. Accessed November 2016.
- 11.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galvagno SM Jr., Thomas S, Stephens C, et al. Helicopter emergency medical services for adults with major trauma. Cochrane Database Systematic Rev 2013;(3):CD009228. [DOI] [PubMed] [Google Scholar]
- 13.Bonhomme CE, Deger FT, Schultz J, Hsu SS. Radiofrequency catheter ablation using non-contact mapping for inappropriate sinus tachycardia. J Interv Card Electrophysiol 2004;10: 159–63. [DOI] [PubMed] [Google Scholar]
- 14.Ibarra-Cortez SH, Rodríguez-Mañero M, Kreidieh B, Schurmann P, Dave AS, Valderrábano M. Strategies for phrenic nerve preservation during ablation of inappropriate sinus tachycardia. Heart Rhythm 2016;13: 1238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takemoto M, Mukai Y, Inoue S, et al. Usefulness of non-contact mapping for radio-frequency catheter ablation of inappropriate sinus tachycardia: new procedural strategy and long-term clinical outcome. Intern Med 2012;51: 357–62. [DOI] [PubMed] [Google Scholar]
- 16.Marrouche NF, Beheiry S, Tomassoni G, et al. Three-dimensional nonfluoroscopic mapping and ablation of inappropriate sinus tachycardia. Procedural strategies and long-term outcome. J Am Coll Cardiol 2002;39:1046–54. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson JT, Kraus A, Lee R, Goldberger JJ. Epicardial/endocardial sinus node ablation after failed endocardial ablation for the treatment of inappropriate sinus tachycardia. J Cardiovasc Electrophysiol 2014;25:236–41. [DOI] [PubMed] [Google Scholar]
- 18.Frankel DS, Lin D, Anastasio N, et al. Frequent additional tachyarrhythmias in patients with inappropriate sinus tachycardia undergoing sinus node modification: an important cause of symptom recurrence. J Cardiovasc Electrophysiol 2012;23: 835–9. [DOI] [PubMed] [Google Scholar]
- 19.Banks M Connections between open access publishing and access to gray literature. J Med Libr Assoc 2004;92:164–6. [PMC free article] [PubMed] [Google Scholar]
- 20.Killu AM, Syed FF, Wu P, Asirvatham SJ. Refractory inappropriate sinus tachycardia successfully treated with radiofrequency ablation at the arcuate ridge. Heart Rhythm 2012;9:1324–7. [DOI] [PubMed] [Google Scholar]
- 21.Zellerhoff S, Hinterseer M, Felix Krull B, et al. Ivabradine in patients with inappropriate sinus tachycardia. Naunyn Schmiedebergs Arch Pharmacol 2010;382:483–6. [DOI] [PubMed] [Google Scholar]
- 22.Calo L, Rebecchi M, Sette A, et al. Efficacy of ivabradine administration in patients affected by inappropriate sinus tachycardia. Heart Rhythm 2010;7:1318–23. [DOI] [PubMed] [Google Scholar]
- 23.Ptaszynski P, Kaczmarek K, Ruta J, Klingenheben T, Wranicz JK. Metoprolol succinate vs. ivabradine in thetreatment of inappropriate sinus tachycardia in patients unresponsive to previous pharmacological therapy. Europace 2013;15:116–21. [DOI] [PubMed] [Google Scholar]
- 24.Fu Q, Vangundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Exercise training versus propranolol in the treatment of the postural orthostatic tachycardia syndrome. Hypertension 2011;58:167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]