Abstract
Objective:
The ability to perceive the motion of biological objects, such as faces, is a critical component of daily function and correlates with the ability to successfully navigate social situations (“social cognition”). Deficits in motion perception in schizophrenia were first demonstrated ~20 years ago but remain understudied, especially within the early, potentially prodromal, stages of the illness. The authors examined the neural bases of visual sensory-processing impairments, including motion, in schizophrenia and attenuated psychosis (clinical high risk) patients relative to age-matched controls.
Methods:
Electrophysiological recordings during stimulus and motion processing were analyzed using oscillatory (“time frequency”) approaches that differentiated motion-onset evoked activity from stimulusonset sensory-evoked responses. These were compared to functional MRI measures of motion processing.
Results:
Significant deficits in motion processing were observed across the schizophrenia and attenuated psychosis populations and these deficits predicted impairments in both face-emotion recognition and cognitive function. In contrast to motion, sensory-evoked stimulus-onset responses were intact in attenuated psychosis individuals and, further, the relative deficit in motion-versus stimulus-onset responses predicted transition to schizophrenia. In schizophrenia patients, motion detection deficits mapped to impaired activation of motion-sensitive visual cortex (area MT+) during fMRI. Additional visual impairments in schizophrenia patients, not present in individuals with attenuated psychosis, implicated other visual regions, including the middle occipital gyrus and the pulvinar thalamic nucleus.
Conclusions:
The present study emphasizes the importance of sensory-level visual dysfunction in the etiology and personal experience of individuals with schizophrenia, and demonstrates that motion processing deficits may predate illness onset and contribute to impaired function even in clinical high risk, attenuated psychosis patients.
Introduction
The ability to perceive and process motion is central to everyday human function, and contributes strongly to the ability to decode a person’s emotion and intent based on facial expression (“face emotion recognition”) (1, 2). Motion discrimination ability is known to depend heavily on function of visual sensory regions located in mid-temporal cortex (area MT+), which can be investigated physiologically using electrophysiological (3) or functional neuroimaging (fMRI) approaches (4). In addition to its role in simple motion processing, area MT+ plays an important role in decoding facial expressions, suggesting that decoding of emotion even from static faces may depend, in part, on processing of the implied motion inherent in the facial display (5).
Deficits in motion-detection ability were first demonstrated in schizophrenia ~20 years ago (6). However, determination of the course of such deficits as well as their underlying neural mechanisms remains an area of active investigation. Here, we investigated motion processing ability, along with other aspects of visual function, in both schizophrenia patients and individuals meeting criteria for the attenuated psychosis syndrome, which has been included in the DSM-5 (7) as a condition for further study. In addition to showing attenuated symptoms of schizophrenia, such individuals show significant impairments in social and role function, and show markedly elevated risk for transitioning to schizophrenia (8).
In primates, visual sensory cortex receives convergent information from several distinct subcortical pathways. The magnocellular pathway is uniquely sensitive to low contrast, low spatial frequency (SF) stimuli and stimulus motion. In contrast, the parvocellular system is specialized for processing of high SF, high-contrast information (9). The retinotectal system projects from the retina to the superior colliculus and subsequently to the pulvinar nucleus of the thalamus and is proposed to account for the phenomenon of “blindsight” following lesions of the primary visual cortex, as well as for rapid activation of amygdala to fearful faces (reviewed in 10).
Consistent with motion processing dysfunction, robust deficits in magnocellular function have been documented in schizophrenia using behavioral, electrophysiological and fMRI methods, and have been tied to underlying dysfunction of N-methyl-D-aspartate-mediated glutamatergic neurotransmission (reviewed in 11, 12, 13). Impairments of visual processing are also concordant with post-mortem structural studies that show consistent reductions of volume and neuron number in both primary visual cortex (14) and the pulvinar (15). Occipital volumes are apparently unaffected in attenuated psychosis individuals as a whole (16), but may differentiate individuals who transition to schizophrenia from those who do not (17).
Here, we analyzed electrophysiological responses derived from an optimized visual-stimulation paradigm in both schizophrenia and attenuated psychosis patients. Spectral decomposition (“time frequency”) analysis was used to isolate components of interest. In this paradigm, oscillatory activity related to initial stimulus-onset occurs primarily at theta (4–7 Hz) frequency (18). By contrast, motiononset evoked slower activity (19) that mapped to the delta (1–4 Hz) frequency range. Steady-state visual evoked potentials (ssVEP) elicited by alpha-frequency (10 Hz) oscillatory stimuli were also measured. ssVEPs have been shown to be reliably reduced in schizophrenia (reviewed in 20) but have not been studied in attenuated psychosis patients. We additionally obtained fMRI measures of motion processing from a subset of schizophrenia patients.
Our aim was two-fold: first to investigate the integrity of visual processing dysfunction across schizophrenia and attenuated psychosis populations and second, to examine functional correlates of visual sensory impairments. Given recent findings that deficits in face emotion recognition precede illness onset and predict transition to schizophrenia (21, 22), we hypothesized that deficits in motion processing would likewise predate illness onset and correlate with impaired face emotion recognition and other aspects of cognitive processing within schizophrenia and attenuated psychosis patients.
Materials and Methods
Subjects
Clinical subjects were recruited from the Nathan Kline Institute for Psychiatric Research (NKI), Orangeburg, NY and the New York State Psychiatric Institute at Columbia University (NYSPI/CU), New York, NY. Control subjects were recruited from staff and surrounding communities. The study was approved by the Institutional Review Boards of the respective institutions. Written informed-consent was obtained from all subjects, who were paid to participate.
Participants were 63 DSM-5 schizophrenia patients, 32 attenuated psychosis patients diagnosed using the Structured Interview for Prodromal Syndromes (SIPS) (23); 44 healthy volunteers of similarage to the schizophrenia group (“controls”) and 23 healthy volunteers of similar age to the attenuated psychosis group (“young controls”) (Table 1 and Supplementary Table 1). All schizophrenia patients were on a stable dose of medication. A subset of 21 schizophrenia patients and 16 controls participated in the fMRI study (Supplementary Table 2).
Table 1: Mean (sd) demographics and clinical measures of study participants.
SES, socioeconomic status; CPZ, chlorpromazine equivalents; ER40, Penn Emotion Recognition Task-40 Faces, PANSS, Positive and Negative Scale for Schizophrenia; SIPS/SOPS, Structured Interview for Prodromal Syndromes/Scale of Prodromal Symptoms; MATRICS domains: SoP, Speed of Processing; AV, Attention Vigilance; WM, Working Memory. VerL, Verbal Learning, VisL, Visual Learning; RPS, Reasoning and Problem Solving.
| Schizophrenia | Control | Attenuated psychosis | Young control | |
|---|---|---|---|---|
| (N=63) | (N=44) | (N=32) | (N=23) | |
| Age | 41.2 (11.2) | 33.3 (7.5) | 21.9 (3.7) | 24.6 (4.4) |
| Gender (F/M) | 13/50 | 14/30 | 12/20 | 12/11 |
| Years of Education | 12.5 (2.3) | 14.7 (1.9) | 13.1 (2.2) | 15.4 (2.1) |
| Quick IQ | 97.7 (8.8) | 102.5 (11.1) | 97.3 (13.1) | |
| Participant SES | 25.1 (9.3) | 36.5 (12.0) | 31.8 (12.4) | 40.7 (12.8) |
| Parental SES | 47.0 (13.7) | 44.3 (14.8) | 45.6 (11.7) | 49.4 (12.8) |
| Illness Duration (yrs) | 20.5 (9.5) | -- | -- | -- |
| CPZ Equiv. | 754.5 (717.8) | -- | -- | -- |
| ER40 | 28.0 (5.6) | 34.9 (2.3) | 34.0 (2.9) | 34.6 (2.5) |
| PANSS (positive) | 12.0 (4.3) | -- | -- | -- |
| PANSS (negative) | 15.3 (5.3) | -- | -- | -- |
| SIPS/SOPS (positive) | -- | -- | 14.1 (4.1) | -- |
| SIPS/SOPS (negative) | -- | -- | 17.6 (6.3) | -- |
| SIPS/SOPS (disorganization) | -- | -- | 12.5 (4.6) | -- |
| SIPS/SOPS (general) | -- | -- | 11.1 (3.6) | -- |
| MATRICS (SoP) | 33.0 (11.9) | -- | 43.2 (11.9) | -- |
| MATRICS (AV) | 35.4 (13.8) | -- | 42.6 (9.7) | -- |
| MATRICS (WM) | 35.9 (12.8) | -- | 43.9 (9.4) | -- |
| MATRICS (VerL) | 36.7 (7.5) | -- | 45.8 (8.3) | -- |
| MATRICS (VisL) | 36.6 (13.4) | -- | 45.6 (8.8) | -- |
| MATRICS (RPS) | 40.2 (11.2) | -- | 41.3 (11.6) | -- |
Attenuated psychosis patients were recruited from the Center of Prevention and Evaluation at Columbia University. Exclusion criteria included history of threshold psychosis or family history of psychosis, risk of harm to self/others incommensurate with outpatient care, major medical or neurological disorder and IQ < 70. Additionally, attenuated psychosis symptoms could not occur solely in the context of substance use or withdrawal or be better accounted for by another disorder. Two years after initial identification, six subjects (19%) had received a diagnosis of schizophrenia. Informed consent was obtained from all subjects following full explanation of the procedures.
Symptom and neuropsychological measures
Psychiatric symptoms were evaluated using the Positive and Negative Syndrome Scale (PANSS) (24). Attenuated psychosis symptoms were assessed using the Scale of Prodromal Symptoms (SOPS). General neuropsychological function was assessed using the MATRICS consensus cognitive battery (MCCB) (25) minus social cognition. The MCCB was available for 52 schizophrenia and 17 attenuated psychosis patients. Overall MCCB score was calculated as the average of the six MATRICS domains. Face emotion recognition was assessed with the Penn Emotion Recognition Task-40 Faces (ER40) (26).
Coherent Motion Discrimination Task (Behavior)
Individual thresholds for coherent motion detection were determined using random-dot kinetograms comprised of a square patch (7.5° x 7.5° visual angle) containing 120 randomly arranged gray dots (0.5°x 0.5°, 65% contrast) on a darker (40% contrast) background (Figure 1A). The motion coherence for each trial was determined by the QUEST procedure which computes a Bayesian estimate based on all previous trials and a pre-defined prior to determine the optimal coherence level for threshold estimation for the following trial. Coherence thresholds (at 82% correct) were based on 100 trials. Analyses were carried out on motion sensitivity scores which was calculated as 1 over the estimated coherence threshold for each subject.
Figure 1: Behavioral, EEG and fMRI paradigms.
A. Behavioral motion-discrimination task. On each trial, a proportion of dots moved coherently (left/right) for 1000ms. Following, participants were cued to indicate the direction of coherent motion.
B. Stimulus sequence for EEG task. Each trial began with presentation of one of three stimuli which remained static for 400 ms, drifted rightward (14°/s) for 200ms then (after an 800ms delay), counterphase reversed at a rate of 10 Hz for 3000ms, thereby generating an ssVEP. Each subject took part in 420 trials.
C. fMRI paradigm. Low-contrast concentric rings presented at fixation alternated between stationary and moving (expanding/contracting) in twenty-second epochs.
Electrophysiology
Stimuli and tasks:
Stimuli were grayscale vertical sinusoidal gratings (3° x 3° visual angle) composed of either 1) LSF (0.8 cpd) at high (75%) luminance contrast, 2) LSF at low (8%) luminance contrast, or 3) HSF (5 cpd) at high luminance contrast (Figure 1B). Each trial began with presentation of one of the three stimuli, which remained static for 400 ms then drifted rightward (14°/s) for 200ms. After an 800ms delay, during which the grating was static, the grating counterphase reversed at a rate of 10Hz for 3000ms, thereby generating an ssVEP. Subjects’ task was to fixate the central cross and respond with a button press when the central cross dimmed slightly (every 3–12 s). On average, schizophrenia patients correctly detected 87.5%, attenuated psychosis 91.3%, young controls 90.7% and older controls 92.5% of the fixation dimming events. Event-related activity was analyzed separately following: 1) stimulus onset 2) the onset of apparent motion and 3) onset of ssVEP (counterphase reversals). This interleaved design and 5-s intertrial interval was designed to minimize habituation of motion-evoked activity while maximizing number of trials.
Recordings and data analysis:
EEG activity was recorded continuously from 64 electrodes using either an ANT (Advanced Neuro Technology) recording system with a Waveguard cap (NKI) (27) or Brainvision system and 10/10 system cap (NYSPI/CU) (28). Data were digitized online at 512Hz, and recorded relative to a common-reference during acquisition. Data were re-referenced offline to the average of all electrodes. In both cases, impedances of all electrodes were kept below 5 kΩ, throughout the recording and eye movements were monitored with bipolar electrodes on the left and right outer canthi.
An independent component analysis (ICA) was performed for removal of blink-related artifacts. Epochs with amplitudes exceeding ±100μV at any electrode were also excluded. On average, 9.2% (controls), 17.8% (schizophrenia), 10.6% (young-controls) and 11.8% (high-risk) of trials were excluded resulting in 381, 345, 375 and 370 total number of accepted trials, respectively, for each subject group. Scalp topography and amplitudes of event-related neural activity were similar across recording sites (NKI, NYSPI/CU) for all populations (Supplementary Figure 1).
ERPs were obtained by time-locking to the onset of all stimuli and averaging across trials. The averaged ERPs were digitally low-pass filtered with a Gaussian finite impulse function (3 dB attenuation at 46 Hz) to remove high-frequency noise produced by muscle activity and external electrical sources. Time-frequency (evoked power) measures were obtained by convolving the time-domain averaged ERPs with 3-cycle Morlet wavelets over a 3000ms window beginning 1000ms before the initial onset of each stimulus. Evoked power was extracted at each time point over 74 frequency scales from 0.48 to 27.6 Hz, incremented logarithmically. ssVEP data were analyzed as a function of the driving stimulation frequency (10Hz), thus analysis of the ssVEP was performed using Fast-Fourier (FFT) transform. FFT power at 10Hz was calculated using artifact-corrected epochs extending from 500 to 3000ms following ssVEP onset. All analyses were performed using MATLAB (Mathworks, Natick, MA) with the EEGLAB and ERPLAB toolboxes (29).
Stimulus-onset and motion-onset activity was calculated across 4 bilateral electrode sites corresponding to the peak amplitude region, and quantified in the theta (4–7 Hz) and delta (1–4 Hz) frequency bands, respectively (Supplementary Figure 1). Measurement windows were centered around the peak delta and theta amplitude latencies based on combined data from patients/controls for each age group. This yielded theta-windows of 150–250ms post-stimulus onset and delta-windows of 100–300ms and 80–280ms post-motion onset for older and younger subjects, respectively. Corresponding timedomain ERP components (P1, N2) are shown in Supplementary Figure 2. FFT power of the ssVEP response was maximal in the alpha frequency band at 10Hz, the driving/stimulation frequency, and was measured across three midline parieto-occipital sites.
Functional MRI
Stimuli and task:
Stimulus/motion-sensitive cortical areas were identified using a previously described paradigm (4). Briefly, stimuli were low-contrast (12%) concentric rings presented at fixation, (extending throughout a circular region measuring 15° in diameter) and expanding/contracting at a rate of 7°/s for 20-s followed by 20-s during which the stimuli were static. A fixation cross was continuously present at the center of the display and its luminance was slightly decreased every 4–10 seconds. Subjects were instructed to indicate detection of this dimming by button-press (Figure 1C).
Acquisition and data analysis:
T2*-weighted echo-planar images (TR(s)/TE(ms)/flip angle(degrees)=2/38/90) were acquired on a Siemens Tim Trio 3T 80cm bore head-only MRI system housed at the Nathan Kline Institute Center for Advanced Brain Imaging. Functional images (voxel size=4 mm3) were acquired on 32 contiguous axial slices. The Analysis of Functional NeuroImages software (AFNI; https://afni.nimh.nih.gov) was used for all pre-processing and statistical testing. For anatomical localization, high-resolution (1mm3) whole-brain structural images were acquired from each subject using an MPRAGE sequence. Functional images were registered to each participant’s anatomy and transformed into standardized Talairach stereotaxic space.
Individual subject’s data were analyzed using general linear model (GLM) procedures that contrasted the hemodynamic response to epochs of moving vs. stationary stimuli. Individual motion parameter estimates were included in the model as regressors. The resulting beta-weight maps were analyzed by ANOVA across all subjects to identify motion-sensitive regions of interest (ROI). Groupdifferences in cortical activation were then evaluated within each of these ROIs by separate ANOVA’s utilizing individual beta-weight maps. Significance levels and minimum cluster sizes were calculated using Monte-Carlo simulations with a corrected value of p<.01.
Statistical analyses of electrophysiological data
Between-group analyses were performed by MANOVA with between-subject factors of clinical status (patient/control), age group (older/younger) and testing site. Within-subject factors of stimulus-type (low SF low contrast; low SF high contrast; high SF) and (where appropriate) hemisphere (left, right) were also included. Within group correlations were performed using Pearson r. Between-group correlations were calculated using partial-r (rp) adjusted for group. Effect sizes (d) are interpreted according to conventions of Cohen.
Results
Time-frequency plots by clinical status (patient/control) and age group (older/younger) are shown in (Figure 2). Separate analyses were conducted for delta, theta and ssVEP responses.
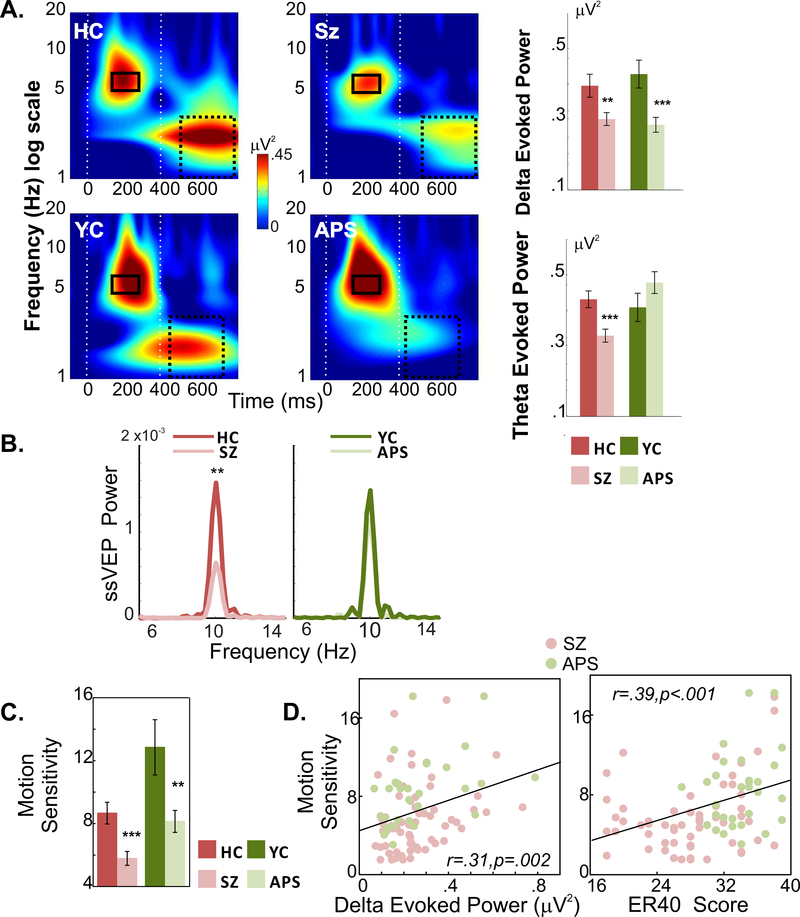
Figure 2: Behavioral and EEG responses by group.
A. Evoked power to stimulus and motion onset. Time-frequency plots (average across stimuli) for healthy (older) controls (HC), schizophrenia patients (SZ), younger control (YC) and attenuated psychosis (APS) subjects. Latency/frequency testing intervals for delta (1–4Hz) and theta (4–7Hz) are indicated by solid and dashed rectangles, respectively. Mean delta/theta power for each group is shown in bar plots. (***p<.00; **p<.01; *p<.05)
B. ssVEP responses. Tracings of group-averaged FFT power derived from ssVEP, collapsed across stimulus types.
C. Motion Sensitivity. Motion sensitivity measures for each group and its correlation with delta power and face emotion recognition (ER40) scores across clinical (SZ/APS) subjects.
Motion-evoked (delta) activity
Motion-related delta power differed significantly between patients and controls (F(1,152)=17.66, p<.00001, d=.68) with no significant main effect of age group (F(1,152)=.006, p=.938, d=.02) or clinical status X age group interaction (F(1,152)=.779, p=.379). Highly significant between group differences were observed for both schizophrenia (t(105)=2.71, p=.008, d=.54) and attenuated psychosis (t(53)=3.45, p=.001, d=.96) patients relative to their respective control groups (Figure 2A, Supplementary Table 3).
There was also a significant stimulus-type X clinical status interaction (F(2,151)=3.45, p=.034), reflecting greater deficits for low SF stimuli of both high (t(160)=4.50,p<.0001, d=.72) and low (t(160)=4.50,p<.0001, d=.53) luminance contrast, relative to high SF stimuli (t(160)=4.50,p<.0001, d=.43), supporting differential magnocellular system involvement.
Finally, there was small effect-size, non-significant effect of testing site (F(1,152)=3.75, p=.055, d=.31) that did not significantly interact with clinical status (F(1,152)=.012, p=.915) nor age group (F(1,152)=.221, p=.639).
Stimulus-onset (theta) activity
As opposed to motion-onset responses, stimulus-onset (theta) responses were not significantly different between patients and controls overall (F(1,152)=0.27, p=.607, d=.08). However, there was a highly significant clinical status X age-group interaction (F(1,152)=9.14, p=.003), reflecting significantly reduced mean theta activity in schizophrenia patients compared to their controls (t(105)=3.48, p=.0007, d=.69), but preserved stimulus-onset responses in attenuated psychosis individuals (t(53)=−1.41, p=.164, d=.39) (Figure 2A).
The effect of age-group was significant (F(1,152)=4.99, p=.027, d=.36), reflecting larger responses in younger versus older individuals irrespective of clinical status. The stimulus-type X clinical/non-clinical interaction was non-significant (F(2,151)=.757, p=.470). There was no main effect of testing site (F(1,152)=.013, p=.911, d=.02) nor site X age-group (p=.173) or site X clinical status (p=.898) interaction (Supplementary Table 3).
ssVEP (FFT) Power (Alpha)
As with theta, ssVEP power was not significantly different between patients and controls overall (F(1,152)=2.20, p=.140, d=.24), but did show a significant clinical status X age-group interaction (F(1,152)=6.20, p=.014) reflecting significant deficits in schizophrenia patients (t(105)=3.16, p=.002, d=.63), but not attenuated psychosis (t(53)=−.68, p=.495, d=.19), compared to their respective controls (Figure 2B). The main effect of testing site (F(1,152)=.007, p=.932, d=.01) and all interactions with testing site were non-significant (p>.25 for all).
Motion sensitivity (behavior)
As with delta, behavioral motion sensitivity (1/coherence threshold) differed significantly between the patients and controls (F(1,152)=20.02, p<.0001, d=.72), reflecting significant reductions in both schizophrenia (p<.001) and attenuated psychosis (p<.01) patients vs. their respective controls (Figure 2C). There was a significant main effect of age-group (F(1,152)=17.401, p<.0001, d=.66), reflecting better performance in younger vs. older individuals, however, clinical status and age did not significantly interact (F(1,152)=1.05, p=.305).
Though motion sensitivity differed as a function of testing site, (F(1,152)=5.03, p=.026, d=.36), there was no interaction between site and clinical status (F(1,152)=.273, p=.602) nor site and age (F(1,152)=2.32, p=.130).
Across schizophrenia and attenuated psychosis subjects, the amplitude of both delta (r=.309, p=.002) and theta (r=.290, p=.004) evoked power correlated significantly with impaired motion sensitivity. Reduced motion sensitivity in clinical subjects additionally correlated with impaired face emotion (ER40) recognition (r=.385, p<.0001) (Figure 2D).
fMRI results
During scanning, correct detections of fixation dimming events did not significantly differ between schizophrenia (79%) and control subjects (87%) (t(35)=.57,p=.581, d=.19).
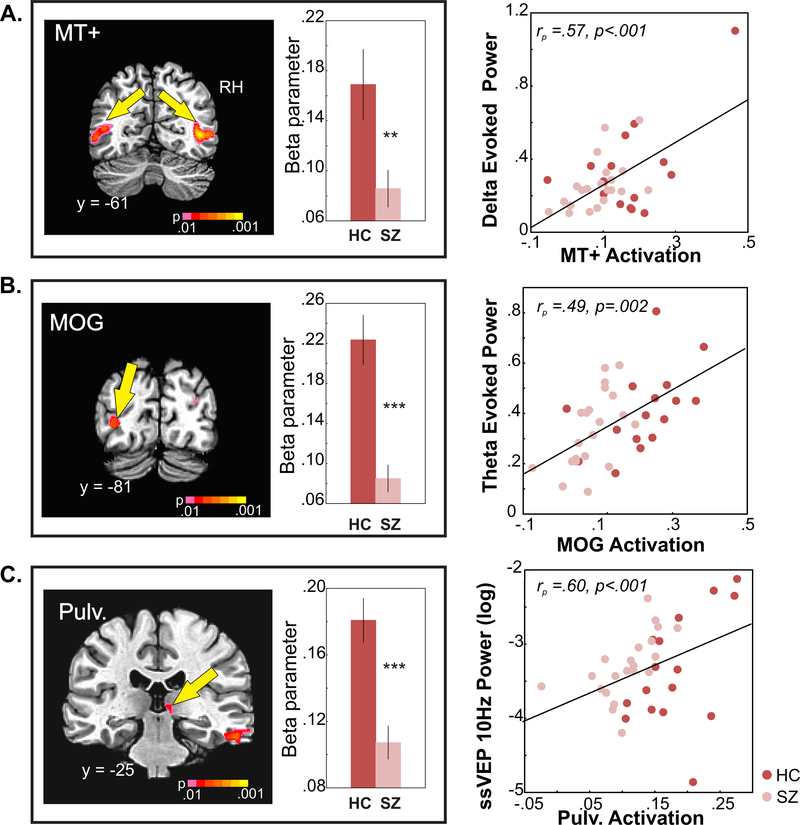
MT+ activation to moving, versus stationary, stimuli was reduced in schizophrenia patients overall (F(1,35)=6.54, p=.015, d=.87). The main effect of hemisphere was also significant (F(1,35)=18.09, d=1.45, p=.0001), with greater activation on the right, however, the group X hemisphere interaction was non-significant (F(1,35)=.757, p=.390). Finally, mean MT+ activation correlated significantly with motion-evoked delta power both across groups (rp=.573, p<.001) and within the schizophrenia (r=.642, p=.002) and HC (r=.583, p=.018) groups (Figure 3A).
Figure 3: fMRI measures of motion perception.
A. MT+ activation. ROIs in middle temporal cortex corresponding to MT+ ( RH is on the right) and bar graphs are of mean activation (beta parameter) elicited by the contrast of moving vs. static across hemispheres in SZ and HC groups. Reduced MT+ activation correlated with diminished delta power (low SF low contrast stimuli) to motion onset (scatterplot on right).
B. MOG activation. Mean activation of MOG (LH) and correlation with diminished mean theta power in response to stimulus onset.
C. Pulvinar activation. Mean pulvinar (RH) activation and correlation with ssVEP power at 10Hz.
Significant main effects of group were also observed in the middle occipital gyrus (MOG) of the left hemisphere (F(1,35)=27.44, p<.0001, d=1.79), which correlated with reduced mean theta power across (rp=.493, p=.002) and within (schizophrenia: r=.485, p=.026; controls: r=.518, p=.040) groups (Figure 3B); and in the right pulvinar of the thalamus (F(1,35)=20.62, p<.0001, d=1.55) which correlated with reduced ssVEP power in both schizophrenia patients (r=.511, p=.018) and HC (r=.740, p=.001) subjects as well as across groups (rp=.60, p<.001, (Figure 3C).
Reduced activations were observed in additional occipital and frontal brain regions (Supplementary Table 4), however, no significant correlations were observed with any electrophysiological measures for these regions in either group (all p>.25).
Correlations with neurocognitive measures
Across the patient groups, reduced motion-evoked delta activity correlated with lower scores on the MCCB Overall (r=.397, p=.001), Visual Learning (r=.408, p=.0005) (Figure 4A), Attention Vigilance (r=.271, p=.024) and Speed of Processing domains (r=.392, p=.001). Similarly, mean theta power evoked by stimulus onset correlated with scores on the Visual Learning (r=.296, p=.014) and Speed of Processing domains (r=.406 p=.001) across all patients (not shown).
Figure 4: Correlation of delta power with neurocognitive measures and measure of transition.
A. Correlation of delta power to MATRICS. Across clinical groups (SZ, APS) higher delta evoked power to the magnocellular-biased low SF, low contrast stimulus correlated with higher scores (better performance) on the overall (left) and Visual Learning (right) domains of the MATRICS MCCB. Correlation values and regression lines shown are across all subjects. Within-group correlations were: Overall score-SZ: r=.336, p=.015, APS: r=.476,p=.053; Visual Learning-SZ: r=.341,p=.014, APS: r=.542, p=.025.
B. delta/theta ratio in APS subjects. Bar plots of the ratio of delta to theta evoked power to low SF, low contrast stimuli for APS subjects who transitioned to schizophrenia (outlined bar) and those who did not.
Transition to schizophrenia
Of the 32 attenuated psychosis individuals, 6 transitioned to psychosis (all schizophrenia) over a 2-year follow up period. Within attenuated psychosis individuals, the ratio between delta and theta activity differed significantly between those who did and did not convert to schizophrenia (t(30)=2.91,p=.007, d=1.06) (Figure 4B) with a cutoff value of .7 (equivalent to 99% confidence interval for remaining subjects) correctly predicting 100% of converters, but excluding 9/26 (35%) of nonconverters (LR χ2=4.48, p=.034).
Furthermore, across subjects, the delta/theta ratio correlated significantly with positive (r=−.32, p=.036), negative (r=−.32, p=.036) and general (r=−.33, p=.029) symptoms as ascertained by the SOPS. Finally, face emotion recognition scores (ER40) also significantly differentiated subjects that transitioned to schizophrenia from those that did not (t(30)=2.25,p=.045, d=.82). By contrast, no significant differences were observed for either neurocognitive (MCCB) or symptom (SOPS) measures.
Control analyses
When separate ANOVA’s were conducted by testing site, both motion-evoked delta activity (NKI: p=.001; NYSPI/CU: p=.008) and behavioral motion-sensitivity (NKI: p<.001; NYSPI/CU: p=.026) were independently significant for schizophrenia/attenuated psychosis patients versus their respective controls at each site. For theta (NKI: p=.011; Columbia/NYSPI: p=.01) and ssVEP power (NKI: p=.009; Columbia/NYSPI: p=.018), differences between schizophrenia and control patients were also independently significant at each site.
No significant differences by sex were observed either across- or within-site. No significant correlations were observed between any measure and medication dosage (CPZ equivalents) (30).
Discussion
This study investigated the integrity of early visual processing in both schizophrenia and attenuated psychosis patients and its linkage to cognitive function. The findings confirm the hypothesis that deficits in motion perception are present even prior to schizophrenia onset and are associated with impaired activation of motion-sensitive visual cortex (area MT+). Moreover, deficits in motion processing correlate significantly with impairments in face emotion recognition as well as with multiple domains of cognition in schizophrenia and attenuated psychosis patients (Figure 2). Overall, these findings highlight the importance of sensory-level dysfunction in the personal experience and neuronal machinery not only of schizophrenia patients (31), but also of individuals with attenuated psychosis who are at high risk of transition to schizophrenia.
As opposed to deficits in motion-processing, attenuated psychosis patients showed preservation of other aspects of early visual function, demonstrating functional differences as well as similarities between the schizophrenia and attenuated psychosis syndromes. Specifically, the theta-frequency response to stimulus onset was unimpaired in attenuated psychosis subjects but was significantly reduced in schizophrenia patients and correlated with impaired activation of the middle occipital gyrus (Figure 3B). Likewise, whereas the amplitude of the alpha-frequency ssVEP to steady-state stimuli was intact in attenuated psychosis, in schizophrenia patients it was significantly diminished and correlated with reduced functional activation of the pulvinar nucleus (Figure 3C). This finding is consistent with the known role of the pulvinar in the generation of occipital alpha rhythms (32–34) as well as with studies showing that lesions of the pulvinar produce schizophrenia-like positive symptoms and language impairments in otherwise healthy individuals (35). Taken together, the discrepancy in deficit patterns between attenuated psychosis and schizophrenia patients suggests that further deterioration in visual cortical processing may occur during transition to schizophrenia, potentially leading to additional visual impairments and social cognitive decline.(10, 11, 12, 13, 20)
Although sensory processing was once considered an “intact simple function”, research over the past decades has increasingly documented the importance of both auditory and visual sensory disturbances in the pathophysiology of schizophrenia. Impaired motion perception, in particular, has been associated with deficits in several perceptual/cognitive processes including eye tracking, biological motion detection and potential for “theory of mind” (reviewed in 1). The present findings further suggest that motion processing deficits are present, and may contribute to disability, even in individuals with only attenuated psychotic symptoms.
Impaired motion sensitivity, measured behaviorally, correlated with neurophysiological (delta) measures of poor motion processing in attenuated psychosis and schizophrenia patients, as well as with deficits in the ability to detect emotion based upon facial expression (Figure 2D), a deficit that we (21) and others (36) have previously shown to be highly predictive of transition to schizophrenia. In the present study, static faces were used to evaluate correlations between visual sensory function and faceemotion recognition, nonetheless, the results encourage development of validated dynamic emotion paradigms with greater ecological validity (5) than currently available measures such as the ER40, and further suggests that application of such batteries may help differentiate both attenuated psychosis and schizophrenia individuals from healthy controls. Finally, deficits in the neurophysiological response to motion onset also predicted impaired scores in the MCCB domains of visual learning (Figure 4A), processing speed and attention vigilance in attenuated psychosis and schizophrenia patients, suggesting a significant contribution to impaired present function. Visual learning, for example, is significantly correlated with employability in patients with schizophrenia (37).
Consistent with the differential impairments in delta-vs. theta-frequency responses in attenuated psychosis patients, the dissociation between these measures (delta/theta ratio) differed significantly between attenuated psychosis individuals who transitioned to schizophrenia and those who did not (Figure 4B). Although this finding requires replication in a larger sample, the effect-size of the difference (d=1.06) compares favorably with that of previously described biomarkers such as the duration (d=.71) or frequency (d=.32) MMN (38, 39). Moreover, other clinical measures such as MCCB scores or symptoms did not significantly predict conversion in the present sample. Further studies are needed to evaluate the degree to which the predictive value of the delta/theta ratio adds to previously identified predictors such as impairments in face emotion recognition, MMN, negative symptoms, and MRI-based measures.
In summary, visual as well as auditory sensory deficits have become increasingly established in schizophrenia over recent years, especially involving the ability to detect motion, which in turn affects both cognitive and emotion-detection processes. Here, we show that such deficits are present even in individuals with an attenuated psychosis syndrome, and contribute to functional impairments and symptoms. Although we have obtained initial evidence that visual processing deficits may also be predictive of transition to schizophrenia, the findings must be confirmed in larger prospective cohorts.
Limitations:
Several limitations of the present study must be acknowledged. First, the study was conducted across two recruitment sites, which may have introduced variance. Nevertheless, the main finding of reduced motion sensitivity and reduced motion-related delta activity across attenuated psychosis and schizophrenia cohorts remained strongly significant even if the sites were considered independently, suggesting relative cross-site reliability of the measure.
Second, schizophrenia patients participating in the study were all receiving antipsychotic medication, which might have affected our behavioral and neurophysiological measures. Nevertheless, no correlations with medication dose were observed. Furthermore, the finding of equivalent deficits in motion processing in at-risk and schizophrenia patients argues strongly against a significant medication effect. Finally, given the small number of individuals who transitioned to psychosis, the prediction analyses must be considered exploratory. Nevertheless, effect sizes were comparable to those of other well-established measures, encouraging further confirmatory studies.
Supplementary Material
Acknowledgments
The authors thank Gail Silipo, M.A. for assistance in subject recruitment, Raj Sangoi (RT)(R)(MR) and Caxia Hu, M.S., for assistance in MRI scanning and Isabel and Herb Stusser for their generous support. This research was supported by NIMH grant MH084031 (MJH) DA03383 (DCJ).
References
- 1.Chen Y Abnormal visual motion processing in schizophrenia: a review of research progress. Schizophr Bull. 2011;37:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry DS. What can a moving face tell us? J Pers Soc Psychol. 1990;58:1004–1014. [DOI] [PubMed] [Google Scholar]
- 3.Kuba M, Kubova Z, Kremlacek J, Langrova J. Motion-onset VEPs: characteristics, methods, and diagnostic use. Vision Res. 2007;47:189–202. [DOI] [PubMed] [Google Scholar]
- 4.Tootell RB, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, Rosen BR, Belliveau JW. Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. Journal of Neuroscience. 1995;15:3215–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein M, Yovel G. Two neural pathways of face processing: A critical evaluation of current models. Neurosci Biobehav Rev. 2015;55:536–546. [DOI] [PubMed] [Google Scholar]
- 6.Holzman PS. Recent studies of psychophysiology in schizophrenia. Schizophr Bull. 1987;13:49–75. [DOI] [PubMed] [Google Scholar]
- 7.Association AP: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA, American Psychiatric Publishing; 2013. [Google Scholar]
- 8.Carrion RE, Cornblatt BA, McLaughlin D, Chang J, Auther AM, Olsen RH, Javitt DC. Contributions of early cortical processing and reading ability to functional status in individuals at clinical high risk for psychosis. Schizophr Res. 2015;164:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milner AD, Goodale MA. Two visual systems re-viewed. Neuropsychologia. 2008;46:774–785. [DOI] [PubMed] [Google Scholar]
- 10.Tamietto M, Morrone MC. Visual Plasticity: Blindsight Bridges Anatomy and Function in the Visual System. Curr Biol. 2016;26:R70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Javitt DC. Neurophysiological models for new treatment development in schizophrenia: early sensory approaches. Ann N Y Acad Sci. 2015;1344:92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez A, Revheim N, Butler PD, Guilfoyle DN, Dias EC, Javitt DC. Impaired magnocellular/dorsal stream activation predicts impaired reading ability in schizophrenia. NeuroImage Clinical. 2012;2:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez A, Hillyard SA, Dias EC, Hagler DJ Jr., Butler PD, Guilfoyle DN, Jalbrzikowski M, Silipo G, Javitt DC. Magnocellular pathway impairment in schizophrenia: evidence from functional magnetic resonance imaging. J Neurosci. 2008;28:7492–7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorph-Petersen KA, Pierri JN, Wu Q, Sampson AR, Lewis DA. Primary visual cortex volume and total neuron number are reduced in schizophrenia. J Comp Neurol. 2007;501:290–301. [DOI] [PubMed] [Google Scholar]
- 15.Byne W, Fernandes J, Haroutunian V, Huacon D, Kidkardnee S, Kim J, Tatusov A, Thakur U, Yiannoulos G. Reduction of right medial pulvinar volume and neuron number in schizophrenia. Schizophr Res. 2007;90:71–75. [DOI] [PubMed] [Google Scholar]
- 16.Zhao C, Zhu J, Liu X, Pu C, Lai Y, Chen L, Yu X, Hong N. Structural and functional brain abnormalities in schizophrenia: A cross-sectional study at different stages of the disease. Prog Neuropsychopharmacol Biol Psychiatry. 2018;83:27–32. [DOI] [PubMed] [Google Scholar]
- 17.Ziermans TB, Schothorst PF, Schnack HG, Koolschijn PC, Kahn RS, van Engeland H, Durston S. Progressive structural brain changes during development of psychosis. Schizophr Bull. 2012;38:519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra J, Martinez A, Schroeder CE, Hillyard SA. Spatial attention boosts short-latency neural responses in human visual cortex. Neuroimage. 2012;59:1968–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitzalis S, Strappini F, De Gasperis M, Bultrini A, Di Russo F. Spatio-temporal brain mapping of motion-onset VEPs combined with fMRI and retinotopic maps. PloS one. 2012;7:e35771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenner CA, Krishnan GP, Vohs JL, Ahn WY, Hetrick WP, Morzorati SL, O’Donnell BF. Steady state responses: electrophysiological assessment of sensory function in schizophrenia. Schizophr Bull. 2009;35:1065–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corcoran CM, Keilp JG, Kayser J, Klim C, Butler PD, Bruder GE, Gur RC, Javitt DC. Emotion recognition deficits as predictors of transition in individuals at clinical high risk for schizophrenia: a neurodevelopmental perspective. Psychol Med. 2015;45:2959–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Healey KM, Penn DL, Perkins D, Woods SW, Keefe RSE, Addington J. Latent Profile Analysis and Conversion to Psychosis: Characterizing Subgroups to Enhance Risk Prediction. Schizophr Bull. 2018;44:286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–715. [DOI] [PubMed] [Google Scholar]
- 24.Kay S, Opler L, Fiszbein A: The Positive and Negative Syndrome Scale (PANSS) Manual. Toronto, Multi-Health Systems, Inc.; 1992. [Google Scholar]
- 25.Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. [DOI] [PubMed] [Google Scholar]
- 26.Taylor SF, MacDonald AW 3rd, Cognitive Neuroscience Treatment Research to Improve Cognition in S. Brain mapping biomarkers of socio-emotional processing in schizophrenia. Schizophr Bull. 2012;38:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez A, Gaspar PA, Hillyard SA, Bickel S, Lakatos P, Dias EC, Javitt DC. Neural oscillatory deficits in schizophrenia predict behavioral and neurocognitive impairments. Front Hum Neurosci. 2015;9:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee M, Sehatpour P, Dias EC, Silipo GS, Kantrowitz JT, Martinez AM, Javitt DC. A tale of two sites: Differential impairment of frequency and duration mismatch negativity across a primarily inpatient versus a primarily outpatient site in schizophrenia. Schizophr Res. 2017. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Calderon J, Luck SJ. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front Hum Neurosci. 2014;8:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose VL. APA practice guideline for the treatment of patients with schizophrenia. American family physician. 1997;56:1217–1220. [PubMed] [Google Scholar]
- 31.Javitt DC, Freedman R. Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am J Psychiatry. 2015;172:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou H, Schafer RJ, Desimone R. Pulvinar-Cortex Interactions in Vision and Attention. Neuron. 2016;89:209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldman RI, Stern JM, Engel J Jr., Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002;13:2487–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Z, de Zwart JA, Yao B, van Gelderen P, Kuo LW, Duyn JH. Finding thalamic BOLD correlates to posterior alpha EEG. Neuroimage. 2012;63:1060–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byne W, Hazlett EA, Buchsbaum MS, Kemether E. The thalamus and schizophrenia: current status of research. Acta Neuropathol. 2009;117:347–368. [DOI] [PubMed] [Google Scholar]
- 36.Addington J, Saeedi H, Addington D. Facial affect recognition: a mediator between cognitive and social functioning in psychosis? Schizophr Res. 2006;85:142–150. [DOI] [PubMed] [Google Scholar]
- 37.August SM, Kiwanuka JN, McMahon RP, Gold JM. The MATRICS Consensus Cognitive Battery (MCCB): clinical and cognitive correlates. Schizophr Res. 2012;134:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez VB, Woods SW, Roach BJ, Ford JM, McGlashan TH, Srihari VH, Mathalon DH. Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biol Psychiatry. 2014;75:459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bodatsch M, Brockhaus-Dumke A, Klosterkotter J, Ruhrmann S. Forecasting psychosis by eventrelated potentials-systematic review and specific meta-analysis. Biol Psychiatry. 2015;77:951–958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.