Abstract
Background:
New point-of-care (POC) assays for early infant HIV diagnosis (EID) are costlier than conventional total nucleic acid assays, but may increase access to testing, shorten time to result-return, and expedite ART initiation.
Methods:
We used the Cost Effectiveness of Preventing AIDS Complications (CEPAC)-Pediatric model to examine the clinical benefits, costs, and cost-effectiveness of replacing conventional EID assays with POC EID assays at 6 weeks of age in Zimbabwe. We simulated two EID strategies: conventional and POC. Modelled assays differed in sensitivity, specificity, time for and probability of result-return, and cost. Model outcomes included survival over time, life expectancy (LE), and average lifetime per-person treatment cost, reported separately for: 1) all HIV-exposed infants and 2) HIV-infected infants. We calculated incremental cost-effectiveness ratios (ICERs) using discounted (3%/year) costs and LE from a healthcare system perspective for all HIV-exposed infants, defining ICERs ≤$1,010/year of life saved (Zimbabwe annual per-capita GDP) as cost-effective.
Findings:
With conventional EID, projected undiscounted LE was 22·7 years (HIV-infected infants) and 62·5 years (all HIV-exposed infants), at a cost of $610/HIV-exposed infant. POC EID improved projected undiscounted LE (HIV-infected: 25·5 years, HIV-exposed: 62·6 years) at $690/HIV-exposed infant, and increased HIV-infected survival by 7·4% at 12 weeks of life. The ICER of POC vs. conventional EID was $680/year of life saved (YLS). Holding conventional EID characteristics constant, this ICER remained <$1,010/YLS as long as POC specificity was >92% or POC sensitivity was >65%. Substantial improvements in conventional assay result-return probability were needed to offset the lower sensitivity of the POC assay. Results were robust to plausible variations in POC assay cost, probability of ART initiation, and probability of POC result-return.
Interpretation:
POC EID assays for HIV-exposed infants in Zimbabwe will improve survival, extend life expectancy, and be cost-effective compared to conventional assays.
Funding:
EGPAF, NIAID, NICHD, UNITAID, and the Steve and Deborah Gorlin MGH Research Scholar Award.
Keywords: Early infant HIV diagnosis (EID), point-of-care (POC), 6-week testing, Zimbabwe, cost effectiveness
INTRODUCTION
There are nearly 1.4 million children bom to HIV-infected mothers annually worldwide.1 While 76% of pregnant women living with HIV now have access to antiretroviral therapy (ART) to prevent transmission of HIV to their infants, 160,000 children still became infected with HIV in 2016.1, 2 Without treatment, half of all children born with HIV die before age two;3 however, only 43% of children living with HIV received ART in 2016, falling short of global treatment targets.1 One of the greatest challenges to the pediatric HIV response is diagnosing HIV in early infancy. Although the World Health Organization (WHO) recommends early infant diagnosis (EID) testing at 6 weeks of age for all HIV-exposed infants, fewer than 50% of these infants undergo EID testing.4 This gap exists largely because HIV diagnosis in infants requires virologic (e.g., PCR-based) assays; conventional EID testing therefore requires advanced technology often only available at central laboratories. The logistical difficulties of transporting samples to these laboratories and returning results to health facilities often leave caregivers waiting several months to receive EID test results.5 Nearly half of infants tested never receive their results, and of those who test positive and receive results, only 50-80% are eventually linked to care and ART.6
New point-of-care (POC) infant HIV testing technologies are now available.5 If strategically integrated into national EID networks, these POC assays may both increase the number of HIV-exposed infants who are diagnosed and dramatically reduce times for result-return and ART initiation and thus, decrease infant mortality.5 POC platforms are simpler, faster, and do not require extensive training or complex infrastructure. However, the clinical impact and cost-effectiveness of these novel POC EID assays compared to conventional EID assays remain largely unknown. A recent EID testing initiative, launched by Unitaid and the Elizabeth Glaser Pediatric AIDS Foundation (EGPAF), has expanded access to these POC testing platforms in nine African countries.7 We used a validated computer model of pediatric HIV disease, populated with program evaluation data from Zimbabwe, to examine the clinical benefits and cost-effectiveness of POC EID assays in Zimbabwe.
METHODS
Study design
We used the Cost-Effectiveness of Preventing AIDS Complications (CEPAC)-Pediatric model to evaluate the clinical impact, costs, and cost-effectiveness of integrating POC EID assays into existing EID programs in Zimbabwe.8–11 We modelled a population of infants bom to HIV-infected mothers presenting to 6-week EID testing, and simulated two EID testing strategies: conventional and POC. Model outcomes included short- and long-term survival, HIV-related healthcare costs, and life expectancy. To reflect outcomes and resource requirements for an entire HIV program, we projected results for the full cohort of HIV-exposed infants (including both HIV-infected and HIV-uninfected children). We also evaluated clinical outcomes for the HIV-infected infants specifically. Using HIV-exposed outcomes, discounted at 3%/year, we calculated the incremental cost-effectiveness ratio (ICER) for the POC EID strategy compared to the conventional EID strategy in $/year of life saved (YLS), a useful metric for program planners because it is comparable across many health programs.12 Based on emerging literature, we defined an ICER less than lx Zimbabwe’s 2016 annual per capita GDP ($1,010) as cost-effective.13 In one-way and multi-way sensitivity analyses, we varied key model input data and assumptions, including parameters associated with diagnosis, ART initiation, assay characteristics, and costs for both the conventional and POC assays. While the base-case analysis focused on the Unitaid/EGPAF project, we considered all available relevant data for the range of evaluated sensitivity analyses (Table 1, Appendix, p. 13).
Table 1.
Selected input parameters for a model-based analysis of POC EID vs. conventional EID in Zimbabwe.
| Cohort characteristics | Value | Sources* |
|---|---|---|
| Age, months (SD) | 0 (0) | Assumption |
| Female/Male (%) | 51·2/48·8 | 10 |
| Mothers with maternal CD4 ≤ 350 cells/μL before ART (%) | 36 | 10 |
| Mothers receiving ART during pregnancy and breastfeeding (%) | 93 | 2 |
| Breastfeeding, proportion of all mother-infant pairs (%) | 80 | Assumption |
| Mean breastfeeding duration, months (SD) | 18 (2) | 14 |
| EID cascade parameters | Value | Sources* |
| EID uptake (%) | 100 | Modelled population |
| Probability of receiving test results (%) | ||
| Conventional EID | 80·0 | 5 |
| POC EID | 99·0 | |
| Delay between primary test and result receipt, months (SD) | ||
| Conventional EID | 2 (0) | 5 |
| POC EID | 0 (0) | |
| Delay between confirmatory test and result receipt, months (SD) | ||
| Conventional EID | 0 (1) | 5 |
| POC EID | 0 (0) | |
| Probability of linking to care/ART among those tested (%) | ||
| Conventional EID | 51·9 | 5 |
| POC EID | 98·5 | |
| Nucleic Acid Test (Conventional) assay characteristics | Value (%) | Sources* |
| Sensitivity for IU infection (all ages) | 100 | |
| Sensitivity for IP infection (month 1, later months) | 0, 100 | 17 |
| Sensitivity for PP infection (month of infection, later months) | 0, 100 | |
| Specificity (all ages) | 99·6 | |
| Error rate** | 1·4 | 32 |
| Point-of-Care (POC) assay characteristics | Value (%) | Sources* |
| Sensitivity for IU infection (all ages) | 96·9 | |
| Sensitivity for IP infection (month 1, later months) | 0, 96·9 | |
| Sensitivity for PP infection (month of infection, later months) | 0, 96·9 | 15 |
| Specificity (all ages) | 100 | |
| Error rate** | 6·0 | |
| Costs | Value (2016 USD) | Sources* |
| HIV care, per month; (range by age, CD4) | $32·75-33·69 | 18 |
| CD4 test | $4·79 | 19 |
| VL test | $17·50 | 16 |
| ART regimen costs, per month (range by regimen, dose, age/weight) | $8·50-44 | 20 |
| Cost of conventional assay | $24·18 (1·4% error rate*) | 16, 32 |
| Cost of POC assay | $27·61 (6·0% error rate*) | 15, 16 |
Abbreviations: POC: point-of-care; EID: early infant HIV diagnosis; SD: standard deviation; ART: antiretroviral therapy; IU: intrauterine; IP: intrapartum; PP: postpartum; HIV: human immunodeficiency virus; RNA: ribonucleic acid; LPV/r: lopinavir/ritonavir; ABC: abacavir; 3TC: lamivudine; EFV: efavirenz; AZT: zidovudine; USD: United States dollar; VL: viral load.
Due to limited number of references permitted, here we cite previous CEPAC papers that used the same primary data sources; full primary data sources are listed in the Appendix, p. 13.
Error in performing a POC test (due to a platform malfunction, human error, etc.) leads to an inconclusive test result and a repeat test, but does not affect result-return.
Model description
The CEPAC-Pediatric model is a validated individual-level, state-transition model of pediatric HIV disease, expanded to incorporate perinatal HIV transmission and infant EID testing.8–11 Infants enter the model at birth and are simulated until death. Maternal CD4 count and ART availability determine mother-to-child HIV transmission (MTCT) risks during three time periods: intrauterine (one-time risk), intrapartum (one-time risk), and postpartum (monthly risk until breastfeeding cessation, excluding HIV acquisition outside of MTCT). All infants face age-stratified monthly risks of non-HIV related mortality, and HIV-infected individuals face additional age- and CD4-stratified risks of opportunistic infections (OIs), OI-related mortality, and non-OI-related mortality. Planned EID testing can occur at any age from 0-24 months.
After HIV infection is confirmed, children experience a probability of initiating ART. Once on ART, children have an initial probability of early virologic suppression; children with early virologic suppression experience monthly risks of treatment failure. While HIV viral load is suppressed by effective ART, CD4% (or total CD4 count) increases, leading to reduced risks for OIs and mortality. Children engaged in care may also become lost to follow-up, and then subsequently return to care.
Modelled population and EID strategies
Because EID testing is currently recommended only for infants known to be HIV-exposed, we simulated a population of infants born to women identified during antenatal care as HIV infected.4 Based on current WHO recommendations and Zimbabwe national guidelines and data, we simulated 93% of women receiving ART during pregnancy and breastfeeding (WHO Option B+).2 Women who breastfed (80%) did so for a mean duration of 18 months (SD of 2 months).14
We focused our analysis on EID testing at 6 weeks to remain consistent with the Unitaid/EGPAF pilot project and the current structure of most EID programs in sub-Saharan Africa.5 For conventional and POC assays, we assigned different diagnostic characteristics (sensitivity and specificity), costs, and “EID cascade” characteristics (probability of result-return, time to result-return, and ART initiation rate). In the base case, any positive conventional or POC result was followed by a second, confirmatory assay of the same type and the opportunity for ART initiation if successfully linked to care. We varied ART initiation rates between the conventional and POC strategies, based on pre- and post-pilot study data from the EGPAF/Unitaid project;5 for those initiated on treatment, ART was stopped if the confirmatory assay and a third conventional assay (all sent pre-ART) were negative. For infants missed by EID or infected after 6 weeks of age, HIV infection was assumed to be diagnosed upon presenting to care later with a WHO stage 3 or 4 OI or at an 18-month clinic visit.
Data sources
Clinical data
We derived MTCT risks from clinical trials and cohort studies in Africa (Table 1).9–11 Mortality rates for HIV-exposed, uninfected infants were from pooled UNAIDS analyses. Because detailed clinical data to inform HIV disease progression with and without ART were not available from Zimbabwe, we used clinical data inputs calibrated to other Southern African settings. For children aged 0-13, we used International Epidemiologic Database to Evaluate AIDS (IeDEA) East African data to derive rates of CD4% and CD4 count decline, opportunistic infections, and death. After age 13, we used data from the Cape Town AIDS Cohort to derive these event risks. For children initiated on ART, we derived 24- and 48-week rates of RNA suppression, CD4% gains on suppressive ART, and risk of late virologic failure after early suppression, from the P1060 trial (Table 1). CEPAC outcomes were calibrated to the longest-term empiric OI risk and survival data available from various trials and cohort studies for children and adults with and without ART (Appendix, p. 4).
Operational test characteristics and care cascade
Based on WHO systematic reviews, published data, and Unitaid/EGPAF pilot study data across eight Unitaid/EGPAF countries, we assigned conventional EID characteristics (assay sensitivity: 100%, specificity: 99·6%, error rate: 1·4%, result-return time: 2 months, result-return probability: 80·0%, ART initiation for those with samples drawn: 51·9%) and POC EID characteristics (assay sensitivity: 96·9%, specificity: 100%, error rate: 6·0%, result-return time: immediate, result-return probability: 99·0%, ART initiation for those with samples drawn: 98·5%; Table 1, Appendix, p. 13).5, 15–17 Test errors (i.e. user error or operational error) led to an inconclusive test result and additional costs for a repeat test, but did not affect result-return. Although lower result-return probabilities and longer result-return times have been reported for some conventional EID programs, we have modelled the conventional EID strategy in this analysis to be conservative with regard to the benefit of POC testing, and to use publicly available Unitaid/EGPAF data as of July 2018.5
Test and care costs
Conventional ($24·18) and POC ($27·61) EID test costs were from the Global Fund’s total cost of ownership (TCO) estimates.16 TCO estimates include reagents, controls and other consumables, apportioned costs of equipment, logistics, training and service and maintenance costs. We derived HIV care costs from Zimbabwe HIV treatment facilities, as reported in Zimbabwe’s 2012 National AIDS Spending Assessment.18 These costs included clinical care, laboratory monitoring, and 01 prophylaxis. Costs for ART regimens, CD4 count and viral load (VL) tests were from the Global Fund and Clinton Health Access Initiative.16,19,20 All costs were converted to 2016 US dollars.
Scenario and sensitivity analyses
In one-way sensitivity analyses, we varied result-return probability, result-return time, and likelihood of ART initiation, to reflect setting-specific availability of pediatric ART services as well as patient- and caregiver-level behavior. We also evaluated conventional and POC assay characteristics through wide ranges of sensitivity, specificity, and assay cost. Additionally, we varied parameters which apply equally to both strategies, including MTCT risks for ART-treated and untreated women, breastfeeding duration, and PMTCT coverage. In multi-way sensitivity analyses, we simultaneously varied clinically relevant parameters that have prompted the most concern surrounding successful field implementation of POC EID (conventional EID result-return time and probability, POC EID sensitivity)4 Data from other countries in the EGPAF/Unitaid project informed plausible parameter ranges for all sensitivity analyses.7
In four scenario analyses, we examined: A) optimistic, intermediate, and pessimistic conditions of uptake along the EID cascade for both the conventional and POC EID strategies; B) a “prioritized POC” testing strategy in which infants of women who did not receive ART during pregnancy received POC EID while all others received conventional EID; C) poorer ART outcomes following POC EID testing; and D) poorer ART outcomes following both POC and conventional EID testing (Appendix, p. 10).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
In the base case analysis, we projected a total MTCT risk of 5·2% for the entire HIV-exposed cohort (1·4% of infants had intrauterine HIV infection, 1·0% had intrapartum infection, and 2·8% had postpartum infection), leaving 94·8% HIV-exposed/uninfected. The clinical impact of POC EID considering the entire HIV-exposed cohort was limited, with 1-year survival increasing from 93·1% with conventional to 93·4% with POC, and projected undiscounted HIV-exposed life expectancy increasing from 62·5 years with conventional to 62·6 years with POC (Table 2). For HIV-infected infants, however, projected 1-year survival was 69·0%, and undiscounted life expectancy was 22·7 years with conventional EID; 1-year survival increased to 78·0% and undiscounted life expectancy to 25·5 years with POC EID. Notably, POC EID increased survival by 7·4%, from 76·1% to 83·5%, by 12 weeks of life compared to conventional EID (Figure 1).
Table 2.
Base case results for a model-based analysis of POC EID vs. conventional EID in Zimbabwe.
| I. Economic and clinical outcomes | ||||||
|---|---|---|---|---|---|---|
| HIV-infected infants | HIV-exposed infants | |||||
| EID strategy | One-year survival (%) | Life expectancy (years, undiscounted) | Lifetime costs (2016 USD, per person) | One-year survival (%) | Life expectancy (years, undiscounted) | Lifetime costs (2016 USD, per person) |
| Conventional | 69·0 | 22·7 | $11,830 | 93· 1 | 62·5 | $610 |
| POC | 78·0 | 25·5 | $13,460 | 93·4 | 62·6 | $690 |
| II. Incremental cost-effectiveness ratios (ICERs) | ||||||
| EID strategy | HIV-exposed life expectancy (years, discounted) | HIV-exposed lifetime costs (USD per person, discounted) | Incremental cost-effectiveness ratio ($/YLS) | |||
| Conventional | 25·69 | $370 | Comparator | |||
| POC | 25·77 | $420 | $680 | |||
Abbreviations: POC: point-of-care; EID: early infant HIV diagnosis; HIV: human immunodeficiency virus; USD: United States dollar; ICER: incremental cost-effectiveness ratio; YLS: year of life saved.
Figure 1. Early HIV-infected infant survival.
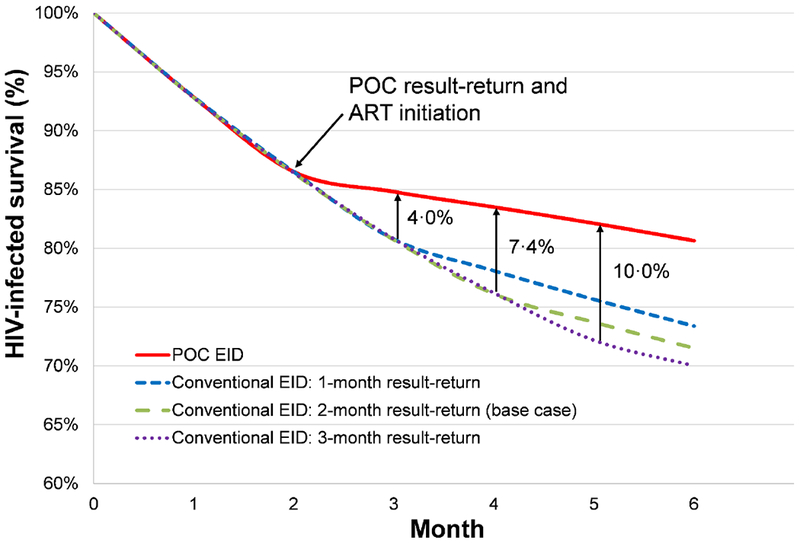
Survival for HIV-infected infants through the first 6 months of life, with HIV-infected infant survival percentage along the vertical axis and time, in months, along the horizontal axis. Survival curves for HIV-infected infants undergoing EID at 6 weeks of age are shown: POC EID (red solid), and conventional EID with a 1-month (blue short dash), 2-month (green long dash, base case value), and 3-month (purple dotted) result-return time. The point at which infants in the POC EID strategy receive results and initiate ART is marked with an arrow at the start of Month 2. Subsequent arrows mark the points at which infants receive results and initiate ART in each of the conventional EID strategies. The absolute difference in survival between each of the conventional EID strategies and the POC EID strategy at respective times of result-return and ART initiation are shown to the right of each arrow.
Abbreviations: HIV: human immunodeficiency virus; POC: point-of-care; EID: early infant HIV diagnosis.
Conventional EID yielded the lowest projected HIV-related healthcare costs for the HIV-exposed cohort, with a lifetime cost of $610/HIV-exposed infant (Table 2). Lifetime costs with POC EID were higher at $690/HIV-exposed infant. Lifetime costs for HIV-infected infants were also higher in the POC EID strategy, at $13,460/HIV-infected infant, compared to conventional EID at $11,830/HIV-infected infant, reflecting improved access to ART and longer survival while receiving care and ART. The ICER of POC EID compared to conventional EID was $680/YLS (67% of Zimbabwe’s annual per capita GDP).
With longer delays in result-return time for conventional EID, POC EID had an increasing impact on reducing early mortality (Figure 1). In cost-effectiveness analysis, the ICER of POC EID compared to conventional EID exceeded $1,010/YLS if HIV-related care costs doubled across both strategies or if ART costs increased 3-fold across both strategies (Figure 2, dashed vertical line). The cost-effectiveness of POC EID remained robust (ICER <$1,010/YLS) throughout plausible variations in parameters along the POC EID cascade, POC assay sensitivity and specificity, and POC assay cost (Figure 2). When ranged to extreme values, POC EID was no longer cost-effective if POC EID assay cost exceeded $60, fewer than 50% of infants undergoing POC EID testing received test results, POC EID assay sensitivity was <65%, POC EID assay specificity was <92%, or fewer than 45% of infants initiated ART after receiving POC EID results. In contrast, the ICER of POC EID compared to conventional EID remained <$1,010/YLS despite plausible variations in parameters applied to both strategies (breastfeeding duration and practices, PMTCT coverage, presentation for EID testing (50-100%), and conventional assay sensitivity (70-100%), specificity (90-100%), and cost ($1-10). Longer result-return times and lower result-return probabilities for conventional EID (Appendix, p. 23) did not change policy conclusions.
Figure 2. Tornado diagram: key parameters and thresholds that change the cost-effectiveness of POC EID compared to conventional EID.
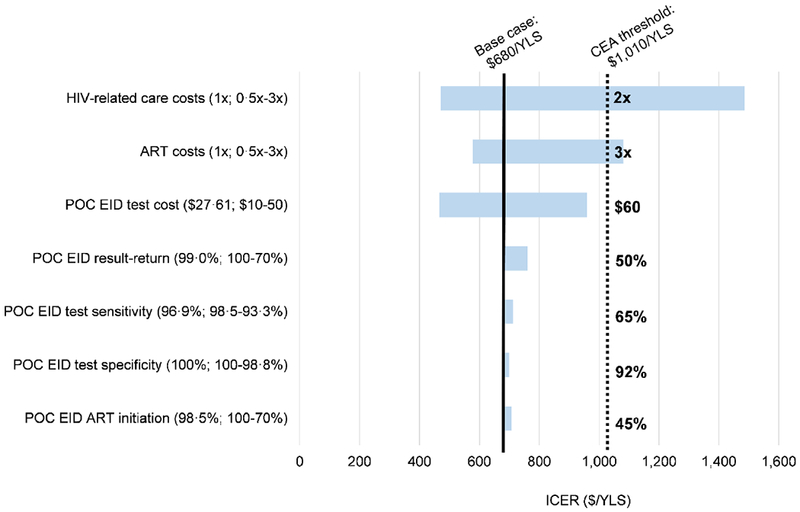
Key parameters varied in one-way model sensitivity analyses are shown on the left. Values in parentheses indicate the range examined (from the value leading to the lowest ICER to the value leading to the highest ICER with base case values before the semicolon). ICERs for the comparison of POC EID vs. conventional EID are shown on the horizontal axis, in 2016 USD/YLS. The range of ICER values for each varied parameter is indicated by the blue horizontal bars; longer bars indicate parameters to which the model results were more sensitive. The solid, black vertical line indicates the ICER for POC EID vs. conventional EID using all base case parameters ($680/YLS). The dotted, black vertical line indicates Zimbabwe’s 2016 annual per capita GDP cost-effectiveness threshold ($1,010). The value for each parameter at which the ICER of POC EID vs. conventional EID crosses the cost-effectiveness threshold is shown to the right of the black, dotted vertical line.
Abbreviations: CEA: cost-effectiveness analysis; ICER: incremental cost-effectiveness ratio; POC: point of care; EID: early infant HIV diagnosis; USD: United States dollar; YLS: year of life saved; GDP: gross domestic product.
In multi-way sensitivity analysis, even if conventional result-return probability improved to 90% (from base case 80%), POC tests with sensitivity >65% remained cost-effective (Figure 3a). Furthermore, using the lowest PCR-based POC sensitivity point-estimate reported in published literature (93·3%),21 POC EID remained the preferred strategy even if conventional result-return improved to 100%. With conventional result-return time shortened to 1 month, POC EID was cost-effective if POC test sensitivity exceeded 75% (Figure 3b).
Figure 3. Effect of varying POC EID assay sensitivity and conventional EID result-return probability on the ICER of POC EID vs. conventional EID.
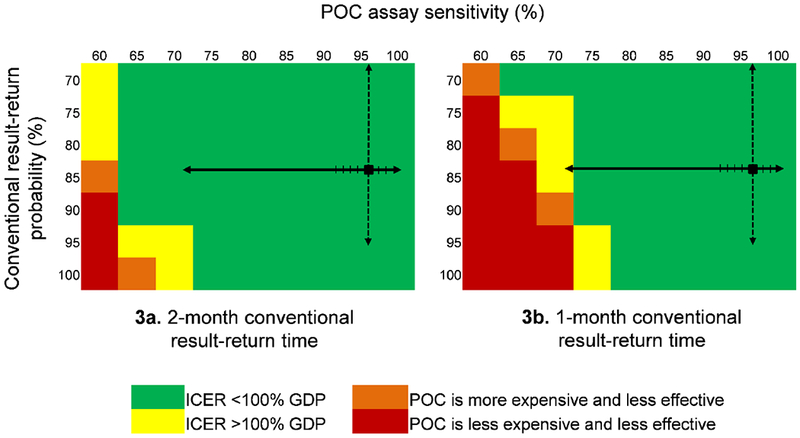
The cost-effectiveness of POC EID changes when POC assay sensitivity, on the top horizontal axis, and the probability of receiving a conventional EID test result, on the vertical axis, are varied simultaneously. In each panel, the black square marks the base case value in the upper right. The solid black arrows on each panel indicate the range of reported POC assay sensitivities: the lowest reported value, 71·9%, is from a p24 antigen POC assay;33 all reported POC PCR assay sensitivities are >93% (hash marks on solid black line).21 The dashed black arrows indicate the range of reported conventional result-return probabilities. Green cells indicate where the ICER of POC EID vs. conventional EID is <100% of Zimbabwe’s annual per capita GDP ($1,010). Yellow cells indicate where the POC EID vs. conventional EID ICER exceeds the $1,010 cost-effectiveness threshold. Orange cells show where POC EID is more expensive and less clinically effective than conventional EID, and red cells show the range over which POC EID is less expensive, but also less effective than conventional EID. The green cells indicate where POC EID is the preferred strategy, and yellow, orange, and red cells indicate where conventional EID is the preferred strategy. The left panel (3a) shows how varying POC assay sensitivity and conventional result-return probability affect policy conclusions when conventional EID result-return time is 2 months; the right panel (3b) shows this same analysis when conventional EID result-return time is 1 month.
Abbreviations: POC: point-of-care; ICER: incremental cost-effectiveness ratio; GDP: gross domestic product.
In Scenario A, POC EID led to greater life expectancy than conventional EID under each of the optimistic, intermediate, and pessimistic conditions (Appendix, p. 24). POC EID was also cost-effective compared to conventional EID in 7 of 9 combinations of conditions (Table 3). In Scenario B, Scenario C, and Scenario D, POC EID remained cost-effective (Appendix, p. 24).
Table 3.
Scenario analysis: ICERs for POC EID compared to conventional EID.
| POC | ||||
| Conventional | Pessimistic | Intermediate | Optimistic | |
| Pessimistic | $730 | $760 | $750 | |
| Intermediate | $590 | $720 | $720 | |
| Optimistic | Less effective, less expensive* | Less effective, less expensive* | $650 | |
Abbreviations: ICER: incremental cost-effectiveness ratio, in $/year of life saved; POC: point-of-care; EID: early infant HIV diagnosis.
POC EID is less effective and less expensive compared to conventional EID.
DISCUSSION
In our analysis of the value of POC EID compared to conventional EID, we had four key findings. First, POC EID’s operational characteristics, improving result-return time, result-return probability, and ART initiation rates, markedly improved short-term survival for HIV-infected infants compared to conventional EID. This benefit extended to long-term survival as well; projected life expectancy for HIV-infected infants was 2·8 years longer with POC EID than with conventional EID, a substantial increase.22 POC EID was more costly ($80 more per HIV-exposed infant), due to greater numbers of children in care and on ART as well as longer life expectancies during which care and ART costs were accrued. Despite these slightly higher costs, POC EID was a cost-effective intervention by international standards for Zimbabwe, with an ICER of $680/YLS, well below annual per capita GDP.
Second, a key driver of the benefit of POC EID is reduced result-return time, which can be as high as 3 or 4 months in current conventional EID settings.7 We found that reduced result-return times increased the proportion of infants who received results and linked to HIV care, and substantially decreased mortality in the early months of life. In settings with longer delays in result-return time for conventional EID, POC EID conferred an even greater reduction in early mortality. While current trials and implementation studies to examine the clinical impact of POC EID testing have not yet generated data on long-term survival outcomes, the association between shorter POC EID result-return times and increased ART initiation rates remains consistent across studies throughout sub-Saharan Africa.5, 23, 24 In our model-based analysis with a conventional EID result-return time of 1 month - a threshold that has been difficult to reach in most settings - POC EID decreased infant mortality even at lower-than-reported result-return and ART initiation rates.7 This suggests that timely return of EID test results is one of the predominant mechanisms by which EID programs avert early infant mortality.
Third, there have been concerns about low POC assay sensitivity relative to conventional assays.4 Assigning even the lowest reported values for the sensitivity of PCR-based POC assays did not change our model-projected policy conclusions. Although reductions in POC sensitivity lead to small increases in false negative results and missed diagnoses, these outcomes should be balanced against the missed diagnoses due to suboptimal result-return with conventional EID. In our analysis, large improvements in conventional assay result-return were needed to offset the slightly lower sensitivity of the POC assay. A systematic review of POC CD4 testing in Africa identified this same trend, highlighting that improvements in retention of patients along the testing and treatment cascade with POC CD4 testing outweighed the superior sample processing, quality control, and technical characteristics of laboratory-based CD4 testing.25 The POC EID assay sensitivity values needed to make conventional EID the preferred strategy (<65%) fall significantly outside the range of reported sensitivities for PCR-based POC assays such as Abbott RDx mPima and Cepheid GeneXpert, which range from 93·3-98·5%.21, 26
Fourth, POC EID remained cost-effective under a range of assumptions, despite plausible variations in breastfeeding practices, PMTCT coverage, and improvements in the conventional EID cascade. These findings are consistent with studies that have examined the cost-effectiveness of other POC technologies, such as POC CD4 and viral load assays.27–31 Studies in sub-Saharan Africa found POC CD4 testing and VL monitoring to be cost-effective compared to laboratory-based testing and monitoring in adults despite wide variations in cost, sensitivity/specificity, and care cascade characteristics. 27, 28, 30, 31 However, if POC total cost of ownership (TCO) increased from the base case value of $28 to $60/test, POC EID was no longer the preferred strategy. TCO reflects potential fluctuations in throughput or increased service and maintenance costs that may be associated with service delivery in rural or low-serviced settings; a cost of $60/test has been reported for Abbott RDx mPima when throughput is reduced to <0·5 tests/day. Average daily utilization of POC EID machines in the Unitaid/EGPAF project in Zimbabwe is 1.51 tests/day. Currently, only 5% of POC EID sites in Zimbabwe fall below the 0.5 tests/day threshold; but this threshold may not be true/relevant for other countries. Our analysis assumes replacement of currently available conventional testing with POC testing, but does not examine the most efficient placement of a limited number of POC machines. There are likely several ways to implement POC EID machines that do not require one machine at every site providing EID. In Zimbabwe, the Unitaid/EGPAF project has successfully implemented a hub-and-spoke model, in which samples are sent from “spoke sites” to central “hub sites” with POC EID machines for processing. Additionally, if POC machines may be used for additional purposes, such as TB diagnostics or viral load monitoring, this would lead to substantial changes in utilization, costs, and clinical benefit.
There are several limitations to our analysis. First, while modelling is a useful tool for projecting future outcomes in the absence of long-term, empiric data, changes in treatment availability, clinical care, and healthcare costs are likely to occur over infants’ lifetimes, and long-term model-based projections for children are uncertain. We addressed this uncertainty by calibrating our model to ensure that results matched current survival, MTCT risk, and 01 data9 and then varying factors and policies likely to change overtime, such as PMTCT rates, ART availability, CD4 and viral load monitoring frequencies, and costs. Except where noted, plausible changes in these parameters did not change our policy conclusions. Next, our base case analysis simulated a population of HIV-exposed infants undergoing EID testing (100% EID uptake) for both POC and conventional EID to describe the full potential of these programs. This overestimates the clinical benefit of both modelled strategies, especially conventional EID, for which low values of uptake have been widely reported throughout sub-Saharan Africa.6 We addressed this issue through a scenario analysis, in which we evaluated each EID strategy using the highest and lowest values found in published literature for steps along the EID care cascade and for conventional and POC assay characteristics. While the model used costing inputs for conventional and POC EID drawn from the Global Fund’s TCO, these estimates do not include health worker costs and infrastructure upgrades that may be needed for centralized laboratories or health facilities. A detailed costing analysis of the POC EID program in Zimbabwe, which will add to the TCO estimates by refining logistics and training costs and including costs for site monitoring, quality assurance, and sample transport, is currently underway. Data about comprehensive POC EID costs in other settings are also critical; in the absence of these data, we have conducted extensive sensitivity analyses and identified cost thresholds where POC EID would no longer be cost-effective.
In summary, we found that incorporating POC assays into EID programs at 6 weeks of age in Zimbabwe markedly improved survival and life expectancy for HIV-exposed infants, and was cost-effective compared to conventional EID. Results were robust across a wide range of sensitivity and scenario analyses, indicating that they may be largely generalizable to other sub-Saharan African countries, except when EID utilization is sparse. Ensuring the timely return of EID test results and increasing the proportion of infants who receive results are of crucial importance in averting infant mortality in the early months of life. Policymakers should incorporate POC assays into EID programs to optimize outcomes along the EID care cascade and thereby improve clinical outcomes for infants undergoing EID testing at 6 weeks of age.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
Although the World Health Organization recommends EID testing at 6 weeks of age for all HIV-exposed infants, fewer than 50% of these infants have access to EID testing. New point-of-care (POC) assays for EID are costlier than conventional total nucleic acid assays, but may increase access to diagnostic results, shorten time to result-return, and expedite ART initiation. While recent trials and implementation studies have characterized the operational improvements and clinical benefits of POC EID, the cost-effectiveness of these novel POC EID assays compared to conventional EID assays remains largely unknown. We searched PubMed for studies published from inception up to September 25, 2018, combining search terms for POC EID (“point-of-care,” “early infant HIV diagnosis”) with health economic terms (“cost-effectiveness,” “cost benefit,” “ICER”). We did not identify any cost-effectiveness studies evaluating POC EID in comparison to conventional EID.
Added value of this study
We report the first cost-effectiveness modelling study informed by real-world data from a large-scale POC EID implementation initiative in Zimbabwe. We include testing costs from the Global Fund reflecting real-time price-breakpoint negotiations and POC EID resource utilization data from Unitaid and EGPAF. We present novel outcomes to the POC EID literature, including projected survival overtime, life expectancy, lifetime per-person costs, and cost-effectiveness.
Implications of all the available evidence
Incorporating POC assays into EID programs at 6 weeks of age in Zimbabwe will improve survival, extend life expectancy, and be cost-effective compared to conventional EID. Results were robust across a wide range of sensitivity analyses, indicating that they may be largely generalizable to other sub-Saharan African countries. Policymakers should incorporate POC assays into EID programs to optimize outcomes along the EID care cascade and thereby improve clinical outcomes for infants undergoing EID testing.
ACKNOWLEDGMENTS
We gratefully acknowledge our collaborators at the Elizabeth Glaser Pediatric AIDS Foundation and Unitaid for their insightful contributions to the analysis, as well as their support in procuring and interpreting relevant data from their large-scale POC EID testing initiative, and we thank all of the study participants and study staff. We also thank Nicole McCann for her assistance with manuscript preparation.
Funding: This work was funded by Unitaid [EB21/R08], the Elizabeth Glaser Pediatric AIDS Foundation [0017A], the Eunice Kennedy Shriver National Institute of Child Health and Human Development [R01HD079214], the National Institute of Allergy and Infectious Diseases [R01AI058736, R37AI093269, T32AI007422], and the Steve and Deborah Gorlin MGH Research Scholar Award. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, EGPAF, or Unitaid.
DECLARATION OF INTERESTS
ALC, KAF, CMD, and RPW have received funding to their institutions from the National Institutes of Health (NIH). Additionally, CMD has received funding from the Harvard University Center for AIDS Research and RPW has received funding from the Massachusetts General Hospital Steve and Deborah Gorlin Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
These data were presented in part at the 9th International AIDS Society Conference (IAS 2017) July 2326, 2017 in Paris, France.
REFERENCES
- 1.UNICEF. Children and AIDS 2017. Available at: https://www.unicef.org/health/files/Children_and_AIDS_2017.pdf. Accessed 25 May 2018.
- 2.UNAIDS. UNAIDS Data 2017. Available at: http://www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf. Accessed 25 May 2018.
- 3.Newell ML, Coovadia H, Cortina-Boga M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 2004;364(9441):1236–43. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. June 2016. [PubMed]
- 5.Bianchi F, Machecano R, Lemaire J, Sacks E, Bailey R, Nzima V, et al. Diagnosing and treating more infants, faster: Findings from the first multi-country evaluation of routine point-of-care early infant diagnosis in eight sub-Saharan countries (abstract). Presented at the 10th International Workshop on HIV Pediatrics; Amsterdam, The Netherlands; 21-22 July 2018. [Google Scholar]
- 6.Ciaranello AL, Park JE, Ramirez-Avila L, Freedberg KA, Walensky RP, Leroy V. Early infant HIV-1 diagnosis programs in resource-limited settings: opportunities for improved outcomes and more cost-effective interventions. BMC Med 2011. May 20;9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elizabeth Glaser Pediatric AIDS Foundation. Point-of-Care Early Infant Diagnosis Data Dashboard. Available at: http://www.pedaids.org/impact/data-dashboard/point-care-early-infant-diagnosis-data-dashboard/. Accessed 25 May 2018.
- 8.Using the CEPAC Model to Simulate HIV Progression and Outcomes. Medical Practice Evaluation Center, Massachusetts General Hospital: http://www.massgeneral.org/mpec/cepac/. [Google Scholar]
- 9.Ciaranello AL, Morris BL, Walensky RP, Weinstein MC, Ayaya S, Doherty K, et al. Validation and calibration of a computer simulation model of pediatric HIV infection. PLoS One 2013;8(12):e83389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francke JA, Penazzato M, Hou T, Abrams EJ, MacLean RL, Myer L, et al. Clinical impact and cost-effectiveness of diagnosing HIV infection during early infancy in South Africa: test timing and frequency. J Infect Dis 2016. November 01;214(9):1319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunning L, Francke JA, Mallampati D, MacLean RL, Penazzato M, Hou T, et al. The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: A cost-effectiveness analysis. PLoS Med 2017. November;14(11):e1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunink M, Glasziou P, Siegel J, et al. Decision making in health and medicine: Integrating evidence and values. Cambridge University Press, 2003. [Google Scholar]
- 13.Woods A, Revill P, Sculpher M, Claxton K. Country-Level Cost-Effectiveness Thresholds: Initial Estimates and the Need for Further Research. Value in Health 2016. December;19(8):929–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimbabwe Demographic and Health Survey 2015. Zimbabwe National Statistics Agency; Harare, Zimbabwe. 2015. Available at: https://dhsprogram.com/pubs/pdf/FR322/FR322.pdf. Accessed 25 May 2018.
- 15.Hsiao NY, Dunning L, Kroon M, Myer L. Laboratory evaluation of the Alere q point-of-care system for early infant HIV diagnosis. PLoS One 2016;11(3):e0152672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.HIV Viral Load and Early Infant Diagnosis Selection and Procurement Information Tool. The Global Fundto Fight AIDS, Tuberculosis and Malaria; April 2017. Available at: https://www.theglobalfund.org/media/5765/psm_viralloadearlyinfantdiagnosis_content_en.pdf. Accessed 25 May 2018. [Google Scholar]
- 17.Mallampati D, Ford N, Hannaford A, Sugandhi N, Penazzato M. Performance of virological testing for early infant diagnosis: A systematic review. J Acquir Immune Defic Syndr 2017. July 1;75(3):308–14. [DOI] [PubMed] [Google Scholar]
- 18.Mabugu T Zimbabwe National Aids Spending Assessment: Consolidated Report 2011 and 2012. UNAIDS; 2012. [Google Scholar]
- 19.HIV/AIDS Diagnostic Pricing Outlook. Clinton Health Access Initiative; 2009. [Google Scholar]
- 20.2016 Antiretroviral (ARV) CHAI Reference Price List. Clinton Health Access Initiative; 2016. Available at: https://clintonhealthaccess.org/content/uploads/2016/11/2016-CHAI-ARV-Reference-Price-List_FINAL.pdf. Accessed 25 May 2018. [Google Scholar]
- 21.Ibrahim M, Moyo S, Mohammed T, Mupfumi L, Gaseitsiwe S, Maswabi K, et al. Brief report: High sensitivity and specificity of the Cepheid Xpert HIV-1 qualitative point-of-care test among newborns in Botswana. J Acquir Immune Defic Syndr 2017. August 15;75(5):e128–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright JC, Weinstein MC. Gains in life expectancy from medical interventions--standardizing data on outcomes. N Engl J Med 1998. Aug 6;339(6):380–6. [DOI] [PubMed] [Google Scholar]
- 23.Mwenda R, Fong Y, Magombo T, Saka E, Midian D, Mwase C, et al. Significant patient impact observed upon implementation of point-of-care early infant diagnosis technologies in an observational study in Malawi. Clin Infect Dis 2018. February 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jani IV, Meggi B, Loquiha O, Tobaiwa O, Mudenyanga C, Zitha A, et al. Effect of point-of-care early infant diagnosis on antiretroviral therapy initiation and retention of patients: a cluster-randomised trial. AIDS 2018. May 8. [DOI] [PubMed] [Google Scholar]
- 25.Vojnov L, Markby J, Boeke C, Harris L, Ford N, Peter T. POC CD4 testing improves linkage to HIV care and timeliness of ART initiation in a public health approach: A systematic review and meta-analysis. PLoS One 2016;11(5):e0155256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jani IV, Meggi B, Mabunda N, Vubil A, Sitoe NE, Tobaiwa O, et al. Accurate early infant HIV diagnosis in primary health clinics using a point-of-care nucleic acid test. J Acquir Immune Defic Syndr 2014. Sep 1;67(1):e1–4. [DOI] [PubMed] [Google Scholar]
- 27.Heffernan A, Barber E, Thomas R, Fraser C, Pickles M, Cori A. Impact and cost-effectiveness of point-of-care CD4 testing on the HIV epidemic in South Africa. PLoS One 2016;11(7):e0158303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hyle EP, Jani IV, Lehe J, Su AE, Wood R, Quevedo J, et al. The clinical and economic impact of point-of-care CD4 testing in mozambique and other resource-limited settings: a cost-effectiveness analysis. PLoS Med 2014. September;11(9):e1001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyle EP, Jani IV, Rosettie KL, Wood R, Osher B, Resch S, et al. The value of point-of-care CD4+ and laboratory viral load in tailoring antiretroviral therapy monitoring strategies to resource limitations. AIDS 2017. September 24;31(15):2135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips AN, Cambiano V, Nakagawa F, Ford D, Apollo T, Murungu J, et al. Point-of-care viral load testing for Sub-Saharan Africa: informing a target product profile. Open Forum Infect Dis 2016. September;3(3):ofw161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Estill J, Egger M, Blaser N, Vizcaya LS, Garone D, Wood R, et al. Cost-effectiveness of point-of-care viral load monitoring of antiretroviral therapy in resource-limited settings: mathematical modelling study. AIDS 2013. June 1;27(9):1483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Creek T, Tanuri A, Smith M, Seipone K, Smit M, Legwaila K, et al. Early diagnosis of human immunodeficiency virus in infants using polymerase chain reaction on dried blood spots in Botswana’s national program for prevention of mother-to-child transmission. Pediatr Infect Dis J 2008. January;27(1):22–6. [DOI] [PubMed] [Google Scholar]
- 33.Meggi B, Bollinger T, Mabunda N, Vubil A, Tobaiwa O, Quevedo JI, et al. Point-of-care p24 infant testing for HIV may increase patient identification despite low sensitivity. PLoS One 2017;12(1):e0169497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


