Abstract
The tongue is paramount to natural speech and swallowing, and good tongue function is important in the overall quality of life. Autologous free-flap reconstruction of the tongue after glossectomy allows for adequate speech, swallow, and quality-of-life outcomes in a majority of patients. Herein, the authors review autologous free-flap reconstruction of the tongue with a focus on different flap options, speech and swallow outcomes, quality-of-life outcomes, and factors that affect how patients perform after tongue reconstruction.
Keywords: tongue reconstruction, free tissue transfer, head and neck cancer
The oral tongue is the most common site of oral cavity malignancy. 1 Oral and oropharyngeal tongue malignancies are commonly encountered by head and neck surgeons, and some lesions can present a significant reconstructive challenge. The oral tongue functions in speech and articulation, as well as mastication, oral hygiene, and the oral phase of swallowing. 2 The oropharyngeal tongue is important for the pharyngeal phase of swallowing and prevention of aspiration. The ideal tongue reconstructive method, therefore, closes large ablative defects in a watertight fashion that separates the oral cavity from the neck, avoids tethering tongue scars that limit tongue mobility, and restores the innate functions of speech and swallowing by providing sufficient tissue volume. 3
Free-flap reconstruction of intraoral defects was first introduced in 1983 with the radial forearm free flap (RFFF), 1 and it has since expanded to include a multitude of flap types and techniques with the goal of optimizing postoperative function. Herein, we review the current state of free-flap reconstruction of the tongue, focusing on defects requiring free-flap reconstruction, sources of free autologous tissue, and how different flaps and harvest/inset techniques affect postoperative speech, swallowing, and quality of life.
Defects
Causes and Classification
Tongue defects are commonly a result of oncological resection, but severe trauma can also lead to extensive defects. The most common oral site of malignancy is the tongue, and the site of most tongue malignancies is the lateral border of the anterior two-thirds of the tongue. 1 Malignancies occur with equal frequency on the left and right sides of the tongue, respectively, and nearly three-fourths of patients have unilateral disease that does not cross the midline. 4 Oncological margins of 1.5 to 2 cm are recommended for squamous cell carcinomas; therefore, even small cancers can lead to relatively large surgical defects.
Surgical resection of the tongue is termed glossectomy. In general, a partial glossectomy involves resection of less than one-third of the tongue, hemiglossectomy involves resection of one-third to half of the tongue, subtotal glossectomy involves resection of half to three-fourths of the tongue, and total glossectomy involves resection of the entire tongue. Glossectomy refers specifically to the tongue, but any of these resections can also involve resection of the floor of the mouth, soft palate, oropharynx, hypopharynx, mandible, or any other adjacent structures given the size and location of a malignancy. Some authors classify surgical defects slightly differently, as involving the oral tongue only, base-of-tongue only, or oral and base-of-tongue, as this classification has more specific prognostic and reconstructive implications. For example, many authors suggest that the greater the degree of tongue resection, the greater the swallow impairment after reconstruction. 5 6 Similarly, larger tongue defects are associated with worse tongue mobility. 7
Reconstruction
The goal of tongue reconstruction is to restore function, primarily speaking and swallowing, to as close to normal as possible. Typically, smaller defects (less than one-fourth of the tongue) are amenable to healing by secondary intention, primary closure, or reconstruction with a skin graft 4 with excellent functional outcomes. Larger defects such as from a subtotal glossectomy, total glossectomy, or those involving the floor of the mouth require reconstruction that includes vascularized tissue replacement to adequately restore bulk and prevent tethering scars and ankyloglossia. 4 In addition to tissue reconstruction, many patients may also require tracheostomy due to significant airway swelling immediately following the surgery, and placement of a gastrostomy tube (g-tube) or a temporary nasogastric tube feeding to facilitate adequate postoperative nutrition.
The decision on how to reconstruct a tongue defect depends on several factors: (1) size of the tongue defect, (2) availability of neck donor vessels, (3) floor-of-mouth involvement, or (4) presence of concurrent mandible bony defect and/or concurrent oropharyngeal defect. Our treatment algorithm for various tongue defect can be seen in Fig. 1 .
Fig. 1.
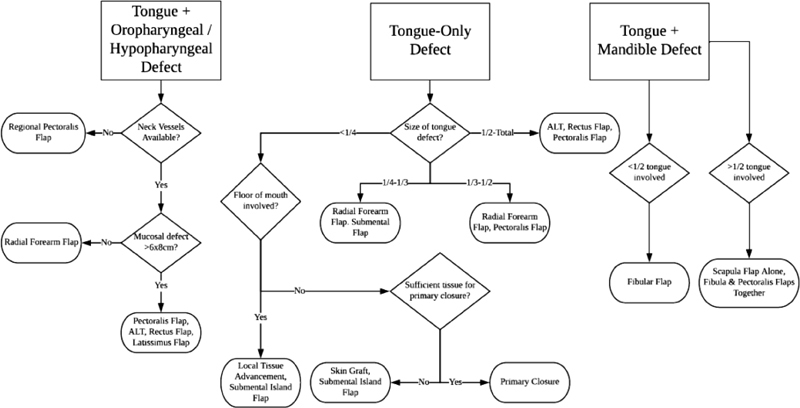
Treatment algorithm for defects of the tongue, tongue and pharynx, and tongue and mandible. ALT, anterolateral thigh flap.
In the case of a concurrent, full-thickness mandible bony defect with a tongue defect, in which the plan is to restore mandible continuity at the same time as tongue reconstruction, a bony free flap is likely required (scapula flap, fibula flap, iliac flap). In the case of a concurrent sizable oropharyngeal or hypopharyngeal mucosal defect, there can be dangerous communication into the neck and great vessels from the oral cavity and pharynx. In these situations, sealing off the oral cavity can prevent salivary exposure in the neck and minimize the risk of carotid blowout and fistula formation. Smaller defects may be amenable to local tissue advancement or a submental island flap. For larger pharyngeal defects, however, transfer of bulky autologous tissue is often more ideal for better restoration of swallow function.
Microvascular free-flap reconstruction necessitates that the patient has available donor vessels in the neck. If no such neck vessels are available, a pectoralis flap or a pedicled latissimus flap may be a useful option. A regional composite pectoralis flap with an osteocutaneous component from the rib has been described. Alternatively, a vein graft can be used to reach vessels in the contralateral neck or to connect to the internal mammary vessels as the free-flap donor vessels.
Regional Flaps
Many regional flaps have been described for tongue reconstruction, and many are still employed when free tissue transfer is contraindicated. The infrahyoid fasciomycutaneous flap, infrahyoid myofascial flap, trapezius island myocutaneous flap, pedicled submental island flap, pedicled latissimus dorsi flap, and pectoralis major myocutaneous flap (PMMF) are examples of regional flaps that can be used to reconstruct the tongue. 3 4 8 Advantages of regional flaps include shorter operative times, strong reliability, and ability to harvest without a second surgical team. They can also be used as a salvage option after failed free tissue transfer. 4 Smaller regional flaps such as submental island flap can be useful for reconstructing a small tongue defect, whereas larger regional flaps such as a pectoralis myocutaneous flap are preferred for larger defects, especially in patients with poor neck donor vessels. Even large regional flaps, however, such as a pectoralis flap or a pedicled latissimus flap, may not be useful for total glossectomy defect reconstruction if the donor flap lacks sufficient tissue bulk. The adequacy of regional flaps depends on the size of the tongue defect and the donor site's tissue bulk. In obese patients, there will be much more soft tissue available for reconstruction than thin patients. Regional flaps can be contraindicated in the case of extensive neck disease or prior neck surgery as, often, the pedicle supplying flaps has already been compromised or will be sacrificed during oncological resection. 4
Free Tissue Transfer
Free tissue transfer has become the mainstay of reconstruction of large tongue defects. Multiple sources of autologous tissue have been investigated, and commonly used flaps include the radial forearm, anterolateral thigh, rectus abdominus, and latissimus dorsi. Other less commonly used free-flap options include tensor fascia lata, gracilis, scapula, iliac crest, and fibula. 3 9 In selecting free tissue for tongue reconstruction, surgeons should consider the defect size, the need to reconstruct surrounding structures in addition to the tongue (such as bone or oropharyngeal/hypopharyngeal mucosa), donor-site morbidity, potential for flap reinnervation and the reinnervation site, and whether simultaneous flap harvest and oncological resection are desirable. In general, immediate reconstruction at the time of tumor extirpation leads to optimal functional results. 3 Since a large portion of oncological patients will have postoperative radiation therapy, it is important to overcorrect the volume defect, as one can expect significant volume loss over time following radiation therapy.
The RFFF and anterolateral thigh free flap (ALTFF) are the two most commonly used flaps to reconstruct the tongue ( Figs. 2 and 3 ). The RFFF has the advantage of relative ease of harvest with very consistent anatomy, can be harvested simultaneously with oral ablation, has reinnervation potential, is thin and pliable, can be harvested with additional adipose tissue to increase its bulk, has a long pedicle, and has larger caliber vessels to allow easier microvascular anastomosis. 4 Furthermore, the RFFF allows for reinnervation through the coaptation of the lateral antebrachial cutaneous nerve to the lingual or inferior alveolar nerves. 9 The RFFF can leave an obvious scar on the wrist, however, and rarely can lead to hand numbness and weakness. In the literature, partial skin graft loss is observed at rates between 19 and 53%, donor-site flexor tendon exposure occurs in 13 to 33% of cases, and between 16 and 100% of patients' grip or pinch strength is reduced. 1 In addition, with RFFF harvest, there is a potential to disrupt the primary arterial supply to the hand if the patient has an unusual hand circulation pattern. Thus, preoperative assessment with Allen's test is imperative to avoid hand ischemia. 1 The RFFF typically provides less tissue than other free-flap sources, even when extra forearm flap is harvested; therefore, it may ultimately have insufficient bulk for the reconstruction of subtotal or total glossectomy defects. In general, the RFFF is preferred for smaller than hemiglossectomy defects.
Fig. 2.
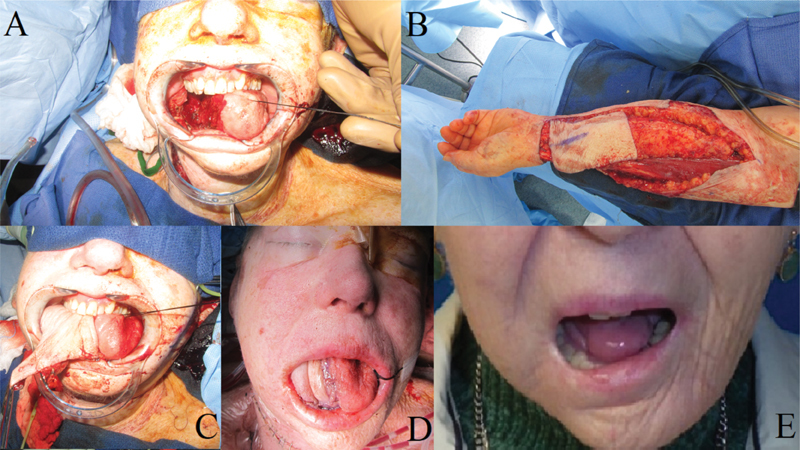
Patient with a lateral oral tongue squamous cell carcinoma. ( A ) Defect remaining after partial glossectomy. ( B ) Left radial forearm fasciocutanous free-flap harvest. ( C ) Free-flap inset and initial intra-oral free-flap appearance. ( D ) Tongue appearance 2 years after reconstruction and adjuvant radiotherapy. Notice the significant flap volume loss.
Fig. 3.
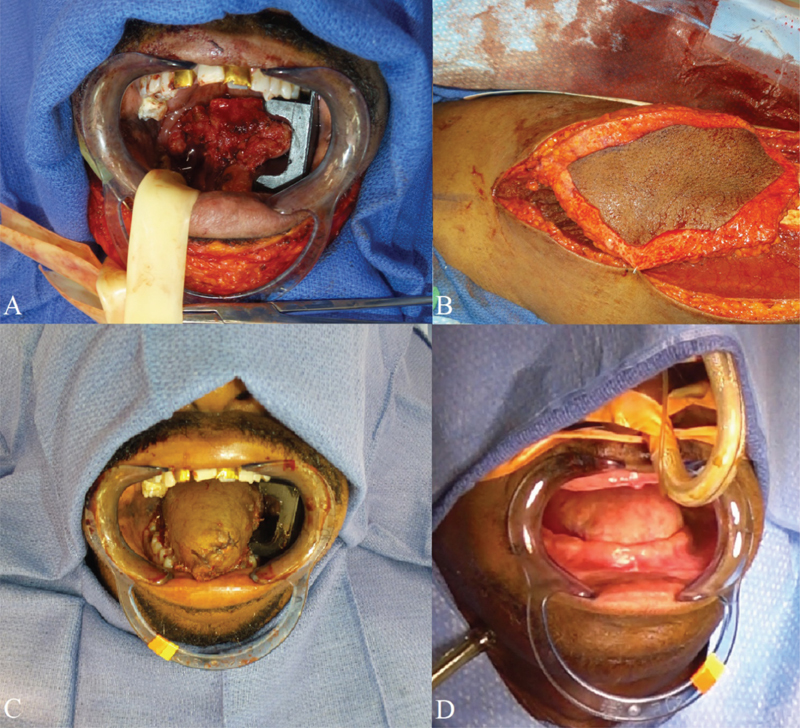
( A ) Total glossectomy defect. ( B ) Harvest of the anterolateral thigh musculocutaneous free flap. ( C ) Neotongue immediately after flap inset. ( D ) Neotongue 1 year postoperatively.
The ALTFF has the advantage of minimal donor-site morbidity and provides significant bulk of tissue. However, its anatomy is less consistent, leading to a more difficult harvest in some cases. Furthermore, its pedicle is typically shorter than that of an RFFF. 4 By designing the skin flap more distally (closer to the knee), one can increase the harvested pedicle length. Marginal necrosis of the inset ALTFF is the most common flap-specific complication, but total flap failure is rare. 1 10 Some authors have suggested that the bulkiness of an ALTFF leads to less mobility of the neotongue. 10 In a direct comparison of tongue defects reconstructed with RFFF versus ALTFF, however, de Vicente et al found no significant differences in postoperative speech intelligibility, tongue mobility, or deglutition and concluded that because the ALTFF has less donor morbidity, it is a superior choice to the RFFF for tongue reconstruction. 1 Of note, a confounding factor in this study is that, given ALTFF is typically a much larger flap than RFFF, ALTFF is generally only used for larger glossectomy defects (typically larger than half of the tongue).
The rectus abdominus free flap (RAFF) has also been commonly employed for tongue reconstruction. It provides a significant amount of soft tissue bulk, similar to ALTFF. It can also provide thin, pliable tissue and can be harvested with the 10th, 11th, and 12th intercostal nerves, allowing reinnervation. 11 As with all other flap reconstruction, the RAFF should be harvested larger than the measured defect size to allow for expected atrophy during the postoperative healing process. 11
Reinnervation
Many studies have investigated whether reinnervation of an autologous free tissue transfer allows better outcomes. In the case of tongue reconstruction, harvested nerves can be coapted to the lingual or inferior alveolar nerves. Other recipient nerves described include the cervical plexus, hypoglossal nerve, and posterior auricular nerve. 9 Studies have demonstrated that reinnervated flaps show improved two-point discrimination compared with their nonreinnervated counterparts but no return of taste function. 12 Furthermore, RFFF and ALTFF have been shown to regain better sensation than other flap types. 9 Many studies conflict on whether overall speech and swallow outcomes are improved. Some studies, for example, suggest that a sensate ALTFF significantly improves swallowing compared with an insensate ALTFF. 13 14 Others have found that sensate free flaps used to reconstruct subtotal or total oral glossectomy defects lead to significantly improved speech and swallow. 15 16 17 Most reinnervated free flaps have the goal of sensory improvement, but the tongue has also been reconstructed with the goal of muscular reinnervation and improved movement of the neotongue. 18 Studies have demonstrated that, in large part, neotongues demonstrate no autologous motion but rather move based on movements of surrounding muscles of the pharynx. 19 Yoleri and Mavioğlu reported a case of tongue reconstruction using the gracilis muscle and obturator nerve, a flap often employed for facial reanimation. In their report, the neotongue regained some autologous movement; however, the patient unfortunately passed away before long-term evaluation could be completed.
Outcomes
In general, patients with high motivation, good family support, and regular follow-up with both a physician and a speech-language pathologist develop better speech quality, swallowing ability, and overall quality-of-life after free-flap reconstruction of the tongue. 19 20 Speech and swallow therapy are also important in the preoperative setting. 20 In the search for the ideal reconstructive flap after glossectomy, multiple studies have evaluated speech, swallow, and quality-of-life outcomes.
Speech
Intelligibility
Free-flap reconstruction of the tongue often allows for intelligible speech production after surgery. In a series by Chien et al, 13 (89%) of 15 patients with subtotal or total glossectomy defects that were reconstructed with RFFF or ALTFF went on to develop intelligible speech. 10 Similarly, in a series by Liao et al, 100% of patients after RAFF for partial glossectomy defects retained intelligible speech. 11 Malignancies of the tongue often impair speech in the preoperative setting due to bulkiness of the tumor and associated pain; for example, Dziegielewski et al found that preoperative speech intelligibility in their patient population was 78%. 20 At 1 year postoperatively, sentence intelligibility in their population dropped to 66%, whereas single word intelligibility was 44%. They noted that patients who could attend more than 80% of speech therapy sessions, both before and after surgery, had higher scores overall. 20 Matsui et al compared speech outcomes among 126 patients who underwent RFFF, PMMF, or RAFF reconstruction of the anterior tongue. They found that patients reconstructed using an RFFF performed significantly better than those using PMMF or RAFF in some articulatory sites for plosives. Overall intelligibility was not different among the three groups, however. 21 Again, a confounding factor in this study is that RFFF is generally used for smaller glossectomy defects, whereas PMMF and RAFF are more commonly used for larger glossectomy defects. Thus, improved function after RFFF may simply reflect the smaller defect being reconstructed. Furthermore, PMMF can sometimes lead to tongue tethering; the pedicle can only be mobilized so much before risking flap compromise, and this, in turn, can limit the final flap position and, subsequently, tongue movement.
Lam and Samman, and Manrique et al performed systematic reviews of patients undergoing free-flap reconstruction after glossectomy. 2 19 They report that patients undergoing anterior tongue resection with free-flap reconstruction had an average preoperative single word intelligibility of 90% across all studies. Intelligibility dropped to 69% at 1 month postoperatively, but it recovered to 79% at 6 months. Sentence intelligibility for patients undergoing anterior tongue resection and reconstruction was 77% at 1 month after surgery, improving to 91% at 6 months. Speech outcomes were better for patients undergoing base-of-tongue resection and reconstruction; sentence intelligibility remained higher than 90% before and after surgery, but single word intelligibility declined from 95% in the preoperative setting to 77 to 82% postoperatively. Finally, outcomes were worst for patients undergoing concurrent anterior and base-of-tongue resection with reconstruction. Intelligibility 10 months after reconstruction was reported at “good” in 29% of patients, “acceptable” in 53% of patients, and “poor” in 18% of patients. Of note, essentially all patients undergoing anterior and base-of-tongue resection will also likely undergo adjuvant radiotherapy (RT) or adjuvant chemoradiotherapy, both of which may lead to undesirable tissue contracture and fat atrophy affecting tongue function.
Factors Affecting Speech
Most studies on speech after free-flap tongue reconstruction report either acoustic or perceptual evaluations of speech, and there is a wide variability in how studies measure speech quality both before and after reconstruction. 2 Many studies suggest that intelligibility is positively correlated with the volume and degree of protuberance of the neotongue. Also, not surprisingly, a positive relationship has been reported between tongue mobility and both subjective and objective speech evaluations. 19 Multiple studies have found that larger tumors (T3 or T4), resections including the tongue tip, and postoperative RT significantly negatively affect speech. 2 19 22 Furthermore, studies have repeatedly demonstrated that patients who have floor-of-mouth resections have poorer postoperative speech. 19 Studies vary greatly on the type of free tissue used for reconstruction. When considering the literature as a whole, there is no definitive evidence suggesting that one type of flap leads to superior speech outcomes compared with another. There is also no definitive evidence to suggest that a reinnervated flap has better speech. 19
Swallow Outcomes
Free-flap reconstruction of the tongue often allows for resumption of an oral diet without the need for permanent reliance on a g-tube or similar, but not surprisingly, larger resections are associated with poorer swallow ability after free-flap reconstruction. 2 19 In general, patients who regularly attend appointments with a trained speech-language pathologist, both before and after surgery, develop better swallow function.
Recovery Time
Many patients with tongue cancers will have some difficulty with swallowing preoperatively, but they should expect worse swallowing immediately after surgery that gradually improves. In one study, preoperative liquid laryngeal penetration was estimated to be 50%, but long-term follow-up after anterior tongue resection and free-flap reconstruction showed that liquid penetration rates had improved to 40%, whereas 0% demonstrated penetration with pudding or solids. 2 Swallow ability has been shown to improve after surgery often over the course of months, with patients achieving their best possible postreconstructive swallow ability by around 1 year after surgery. Patients have been found to regain mobility of the tongue immediately at 4 to 6 months after surgery, with subsequent improvement in swallow ability. 23 In a series by Brown et al of 15 patients who underwent resection of 50 to 75% of the anterior tongue and RFFF reconstruction, there was no significant difference found in the ability to swallow liquids or tongue mobility at 6 and 12 months after surgery. Furthermore, in studies that have reported on swallowing with videofluoroscopic swallow studies (VFSSs), patients have demonstrated a return to preoperative swallow parameters by 1 year postoperatively, regardless of the flap type. 2 In general, the greater the amount of tongue resected, the poorer the postoperative swallow ability patients achieve. 3
Postoperative Diet
Many studies have compared flaps based on postoperative swallow achieved; however, no significant benefit of any particular flap over other types has ever consistently been demonstrated. 3 Similarly, while studies consistently demonstrate that many patients resume an oral diet, the type of oral diet and the number of patients who are not permanently reliant on enteral feeding methods vary greatly. In a series by Chien et al, for example, 12 of 15 patients reconstructed with an ALTFF achieved an oral diet; 3 patients had persistent aspiration. 10 Rates of requiring a g-tube at 6 months postoperatively range in the literature from 0 to 87%, whereas rates at more than 1 year postoperatively range from 0 to 75%. 20 In a series by Dziegielewski et al, preoperative VFSSs were compared with those performed 1 year after ALTFF reconstruction of the tongue. The authors found that there was no difference in rates of aspiration or penetration, but there was a significant increase in bolus transit times after surgery. 20 In another study by Hartl et al, two of nine patients had clinical evidence of aspiration at 15-month follow-up, whereas one patient required enteral feeding. 3
Recent systematic reviews have reported on studies comparing patients with oral tongue resection and reconstruction, base-of-tongue resection and reconstruction, and combined oral and base-of-tongue resection and reconstruction. 2 19 For the first group, studies have demonstrated that swallow parameters measured on VFSSs have returned to baseline by 1 year after reconstruction, regardless of flap type. Furthermore, clinical swallowing evaluations after oral tongue reconstruction have shown that 75% of patients, on average, achieve an unrestricted oral diet by 6 months after surgery.
For patients undergoing base-of-tongue resection and free-flap reconstruction, no one had preoperative aspiration; however, 14% of the patients went on to demonstrate aspiration at 1-year follow-up. 2 19 Despite the high aspiration rate, 97% of patients were able to safely consume a thickened oral diet; 3% of patients had persistent g-tube dependence. Finally, for patients undergoing combined oral and base-of-tongue resection and free-flap reconstruction, 82% achieved an oral diet by 1 year, and nearly all achieved some form of an oral diet by 2 years postoperatively.
Factors Affecting Postoperative Swallow
Many studies have found that patients who undergo postoperative RT have worse long-term swallow outcomes than those who do not undergo RT. 2 19 22 It is theorized that radiation causes increased shrinkage of tissue, leading to decreased bulk of the oropharyngeal tongue and, thus, poor swallowing function. 22 Other factors such as the flap type and whether the flap was reinnervated have not been demonstrated to significantly affect swallowing outcomes. 2 19 22
Overall, the literature demonstrates a divergence of findings when comparing patient-reported outcomes and clinical tests of dysphagia, pointing to the need to use both approaches when measuring outcomes. 24 25
Quality of Life
Many studies have evaluated quality of life after free-flap reconstruction of the tongue, with most reporting the outcome measurements with the European Organization for Research and Treatment of Cancer Head and Neck 35 (EORTC H&N 35) or the M.D. Anderson Dysphagia Index. Both are validated patient-response surveys. 19 20 26
Overall quality of life measured by the EORTC H&N 35 has been shown to be similar when measured before and 1 year after tongue resection and free-flap reconstruction. 20 Not surprisingly, quality-of-life scores worsened in the immediate postoperative period. 20 Hartl et al found that resection of the tongue base was specifically and significantly associated with worse swallowing function, worse quality of life, and patient depression. 3 Next, a review by Manrique et al found that among patients undergoing subtotal or total glossectomy without laryngectomy, 90% achieved decannulation, often within the first 2 weeks after surgery with subsequently improved quality of life. 19 Patients with high motivation, good family support, and regular follow-up (both with a physician and a speech-language pathologist) have reported better EORTC H&N 35 scores, whereas patients with persistent postoperative pain and those who underwent adjuvant chemotherapy reported worse scores, reflecting poorer quality of life. 19
Treatment Algorithm
It is the belief of the authors that when reconstructing a tongue defect, one should always overcorrect the tissue volume, as the flap will shrink significantly with radiation and flap tissue atrophy. In the initial reconstruction, we aim to place sufficient flap bulk so that the neotongue can touch the palate as long as it will not lead to excessive flap compression. Overtime, one can expect around 50% volume loss, especially if the patients undergo radiation therapy afterward.
In patients who have persistent dysarthria and dysphagia despite initial free-flap tongue reconstruction, one must determine if the neotongue is tethered, and if so, one may consider releasing the scar with subsequent placement of a skin graft to minimize tethering. More commonly, however, we have found that dysarthria and dysphagia are because of insufficient volume of the neotongue. In these cases, the senior author (Y.D.) considers placement of a second flap in a delayed fashion to provide additional tongue bulk. We have treated 11 patients after total glossectomy with secondary tongue augmentation through an onlay free-flap (RFFF or RAFF) and have found that 63% had improved swallowing and 45.4% achieved g-tube independence. 27 All patients noted improved speech and achieved tracheostomy decannulation.
Considering all the factors described previously, we propose the following treatment algorithm ( Fig. 1 ). When there are concurrent tongue and full-thickness bony mandible defects, the surgeon should first decide if the mandible continuity is to be restored and then select either a scapula flap or fibula flap based on (1) the size of the skin paddle needed for the reconstruction and (2) the length of the bone needed for the mandible reconstruction. A fibular flap can be combined with a pectoralis flap if there is a sizable tongue defect (subtotal glossectomy) for which the fibula skin paddle will not be adequate. Alternatively, a scapula flap provides significant soft tissue bulk that can easily reconstruct a subtotal glossectomy defect, but it is limited by the mandible bone defect length. If there is a concurrent oropharyngeal or hypopharyngeal defect, coverage of great vessels and the cervical spine is imperative along with the tongue reconstruction. Smaller tongue and pharyngeal mucosal defects are amenable to RFFF reconstruction. However, a larger mucosal defect will require a larger flap, such as a pectoralis flap, an ALTFF, or an RAFF.
For defects involving the tongue only, one should consider if there is a floor-of-mouth defect or not, as primary closure of defects involving the floor of the mouth are at a high risk for developing ankyloglossia and significant tongue tethering. For patients who undergo a partial glossectomy in which less than one-third of the tongue is removed without floor-of-mouth involvement, conservative reconstruction is appropriate to include healing by secondary intention, primary closure, or coverage with a skin graft. Patients in this category often do not require other procedures at the time of surgery, though one may consider careful airway observation postoperatively.
For patients who undergo a partial glossectomy with floor-of-mouth involvement, one may consider smaller locoregional flaps such as a submental island flap. This will minimize the risk of tongue tethering to the floor of the mouth and optimize tongue mobility. Of note, however, a submental island flap cannot be used if the flap pedicle is resected during neck dissection for oncological reasons.
For defects approaching one-third to half of the tongue, an RFFF is an ideal thin pliable flap for enhanced neotongue mobility with the possibility of reinnervation ( Fig. 2 ). A tracheostomy should be considered in this group as one can expect significant flap swelling immediately following the surgery that may linger for several weeks. If the surgeon decides not to perform a tracheostomy, careful airway observation is paramount. Often, a temporary nasogastric tube is also helpful for these patients. If the patient requires postoperative RT, concurrent placement of g-tube feed may be helpful to transition through the radiation-related mucositis.
For patients undergoing base-of-tongue resections and subtotal or total glossectomy (larger than half of the tongue), RAFF and ALTFF are ideal ( Fig. 1 ). In this patient population, the surgeon's goal is to restore as much tongue volume as possible and to initially overcorrect the volume of the defect. It is imperative that the volume loss is overcorrected, as one can expect flap muscle atrophy and radiation-related tissue atrophy. These patients should get concurrent tracheostomy with consideration of g-tube placement, as they are likely to have significant airway swelling postoperatively and have a prolonged recovery of swallow function. Regional flap reconstruction of the tongue using pectoralis flap is preferred when free flaps are significantly contraindicated or after a free-flap failure.
Conclusion
Autologous free-flap reconstruction of the tongue allows for good speech, swallowing, and quality-of-life outcomes in a majority of patients. There are many flap options available to surgeons, and no particular flap has consistently been demonstrated to be superior to others. Careful selection of flap reconstruction based on the tongue defect size, donor-site flap bulk, and the presence of concurrent subunit defects is critical for successful reconstruction. Overcorrection of tissue volume defect is paramount for successful postoperative speech and swallow functions. Furthermore, reinnervation of a flap has demonstrated mixed results in the literature in terms of speech and swallow outcomes. Postoperative RT negatively affects both speech and swallowing ability. Motivated patients with good family support who comply with regular follow-up perform better and report better quality of life overall. In general, there is a need for standardization of measurements, both objective and subjective, of speech, swallowing, and quality-of-life outcomes in the patient population to allow for improved future studies.
Acknowledgments
The authors have no financial or other disclosures.
Footnotes
Conflict of Interest None declared.
References
- 1.de Vicente J C, de Villalaín L, Torre A, Peña I. Microvascular free tissue transfer for tongue reconstruction after hemiglossectomy: a functional assessment of radial forearm versus anterolateral thigh flap. J Oral Maxillofac Surg. 2008;66(11):2270–2275. doi: 10.1016/j.joms.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Lam L, Samman N. Speech and swallowing following tongue cancer surgery and free flap reconstruction--a systematic review. Oral Oncol. 2013;49(06):507–524. doi: 10.1016/j.oraloncology.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Hartl D M, Dauchy S, Escande C, Bretagne E, Janot F, Kolb F. Quality of life after free-flap tongue reconstruction. J Laryngol Otol. 2009;123(05):550–554. doi: 10.1017/S0022215108003629. [DOI] [PubMed] [Google Scholar]
- 4.Bokhari W A, Wang S J. Tongue reconstruction: recent advances. Curr Opin Otolaryngol Head Neck Surg. 2007;15(04):202–207. doi: 10.1097/MOO.0b013e3281fbd406. [DOI] [PubMed] [Google Scholar]
- 5.Nicoletti G, Soutar D S, Jackson M S, Wrench A A, Robertson G. Chewing and swallowing after surgical treatment for oral cancer: functional evaluation in 196 selected cases. Plast Reconstr Surg. 2004;114(02):329–338. doi: 10.1097/01.prs.0000131872.90767.50. [DOI] [PubMed] [Google Scholar]
- 6.Diz Dios P, Fernández Feijoo J, Castro Ferreiro M, Alvarez Alvarez J. Functional consequences of partial glossectomy. J Oral Maxillofac Surg. 1994;52(01):12–14. doi: 10.1016/0278-2391(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 7.Hara I, Gellrich N C, Düker J et al. Evaluation of swallowing function after intraoral soft tissue reconstruction with microvascular free flaps. Int J Oral Maxillofac Surg. 2003;32(06):593–599. doi: 10.1054/ijom.2002.0436. [DOI] [PubMed] [Google Scholar]
- 8.Hanna T C, Lubek J E. The hybrid submental flap for tongue reconstruction. J Oral Maxillofac Surg. 2015;73(09):18760–1.876E9. doi: 10.1016/j.joms.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Baas M, Duraku L S, Corten E ML, Mureau M AM. A systematic review on the sensory reinnervation of free flaps for tongue reconstruction: does improved sensibility imply functional benefits? J Plast Reconstr Aesthet Surg. 2015;68(08):1025–1035. doi: 10.1016/j.bjps.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Chien C Y, Su C Y, Hwang C F, Chuang H C, Jeng S F, Chen Y C. Ablation of advanced tongue or base of tongue cancer and reconstruction with free flap: functional outcomes. Eur J Surg Oncol. 2006;32(03):353–357. doi: 10.1016/j.ejso.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Liao G, Su Y, Zhang J, Hou J, Chen Y, Li M. Reconstruction of the tongue with reinnervated rectus abdominis musculoperitoneal flaps after hemiglossectomy. J Laryngol Otol. 2006;120(03):205–213. doi: 10.1017/S002221510600017X. [DOI] [PubMed] [Google Scholar]
- 12.Biglioli F, Liviero F, Frigerio A, Rezzonico A, Brusati R. Function of the sensate free forearm flap after partial glossectomy. J Craniomaxillofac Surg. 2006;34(06):332–339. doi: 10.1016/j.jcms.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Yu P, Robb G L.Reconstruction for total and near-total glossectomy defects Clin Plast Surg 20053203411–419., vii [DOI] [PubMed] [Google Scholar]
- 14.Yu P. Reinnervated anterolateral thigh flap for tongue reconstruction. Head Neck. 2004;26(12):1038–1044. doi: 10.1002/hed.20106. [DOI] [PubMed] [Google Scholar]
- 15.Longo B, Pagnoni M, Ferri G, Morello R, Santanelli F. The mushroom-shaped anterolateral thigh perforator flap for subtotal tongue reconstruction. Plast Reconstr Surg. 2013;132(03):656–665. doi: 10.1097/PRS.0b013e31829acf84. [DOI] [PubMed] [Google Scholar]
- 16.Chang E I, Yu P, Skoracki R J, Liu J, Hanasono M M. Comprehensive analysis of functional outcomes and survival after microvascular reconstruction of glossectomy defects. Ann Surg Oncol. 2015;22(09):3061–3069. doi: 10.1245/s10434-015-4386-6. [DOI] [PubMed] [Google Scholar]
- 17.Ozkan O, Ozkan O, Derin A T et al. True functional reconstruction of total or subtotal glossectomy defects using a chimeric anterolateral thigh flap with both sensorial and motor innervation. Ann Plast Surg. 2015;74(05):557–564. doi: 10.1097/SAP.0b013e3182a6add7. [DOI] [PubMed] [Google Scholar]
- 18.Yoleri L, Mavioğlu H. Total tongue reconstruction with free functional gracilis muscle transplantation: a technical note and review of the literature. Ann Plast Surg. 2000;45(02):181–186. doi: 10.1097/00000637-200045020-00016. [DOI] [PubMed] [Google Scholar]
- 19.Manrique O J, Leland H A, Langevin C J et al. Optimizing outcomes following total and subtotal tongue reconstruction: a systematic review of the contemporary literature. J Reconstr Microsurg. 2017;33(02):103–111. doi: 10.1055/s-0036-1593772. [DOI] [PubMed] [Google Scholar]
- 20.Dziegielewski P T, Ho M L, Rieger J et al. Total glossectomy with laryngeal preservation and free flap reconstruction: objective functional outcomes and systematic review of the literature. Laryngoscope. 2013;123(01):140–145. doi: 10.1002/lary.23505. [DOI] [PubMed] [Google Scholar]
- 21.Matsui Y, Shirota T, Yamashita Y, Ohno K. Analyses of speech intelligibility in patients after glossectomy and reconstruction with fasciocutaneous/myocutaneous flaps. Int J Oral Maxillofac Surg. 2009;38(04):339–345. doi: 10.1016/j.ijom.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Shin Y S, Koh Y W, Kim S H et al. Radiotherapy deteriorates postoperative functional outcome after partial glossectomy with free flap reconstruction. J Oral Maxillofac Surg. 2012;70(01):216–220. doi: 10.1016/j.joms.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Brown L, Rieger J M, Harris J, Seikaly H. A longitudinal study of functional outcomes after surgical resection and microvascular reconstruction for oral cancer: tongue mobility and swallowing function. J Oral Maxillofac Surg. 2010;68(11):2690–2700. doi: 10.1016/j.joms.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Namin A W, Varvares M A. Functional outcomes of sensate versus insensate free flap reconstruction in oral and oropharyngeal reconstruction: a systematic review. Head Neck. 2016;38(11):1717–1721. doi: 10.1002/hed.24494. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen A, Wilson J, McColl E, Carding P, Patterson J. Swallowing outcome measures in head and neck cancer--how do they compare? Oral Oncol. 2016;52:104–108. doi: 10.1016/j.oraloncology.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Chen A Y, Frankowski R, Bishop-Leone J et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127(07):870–876. [PubMed] [Google Scholar]
- 27.Rihani J, Lee T, Ducic Y. Secondary onlay free flap reconstruction of glossectomy defects following initial successful flap restoration. Otolaryngol Head Neck Surg. 2013;149(02):232–234. doi: 10.1177/0194599813486882. [DOI] [PubMed] [Google Scholar]


