Abstract
With the advent of the Industrial Revolution, traumatic injuries of the upper extremity increased exponentially. As a result, surgeons began to reevaluate amputation as the standard of care. Following the Second World War, local and regional pedicled flaps became common forms of traumatic upper extremity reconstruction. Today, microsurgery offers an alternative when options lower on the reconstructive ladder have been exhausted or will not produce a desirable result. In this article, the authors review the use of free tissue transfer for upper extremity reconstruction. Flaps are categorized as fasciocutaneous, muscle, and functional tissue transfers. The thin pliable nature of fasciocutaneous flaps makes them ideal for aesthetically sensitive areas, such as the hand. The radial forearm, lateral arm, scapula, parascapular, anterolateral thigh, and temporoparietal fascia flaps are highlighted in this article. Muscle flaps are utilized for their bulk and size; the latissimus dorsi flap serves as a “workhorse” free muscle flap for upper extremity reconstruction. Other muscle flaps include the rectus abdominis and serratus anterior. Lastly, functional tissue transfers are used to restore active range of motion or bony integrity to the upper extremity. The innervated gracilis can be utilized in the forearm to restore finger flexion or extension. Transfer of vascularized bone such as the fibula may be used to correct large defects of the radius or ulna. Finally, replacement of “like with like” is embodied in toe-to-thumb transfers for reconstruction of digital amputations.
Keywords: upper extremity reconstruction, free flap, fasciocutaneous flap, muscle flap, functional tissue transfer
With the arrival of the Industrial Revolution, the frequency of traumatic upper extremity injuries exploded. Increasingly common, wounds derived from manned machines placed a premium on reconstructive options for the hand, forearm, and arm. This led surgeons to reevaluate amputation as the primary method of management of upper extremity trauma. With more sophisticated knowledge of anatomy and with the advent of antibiotics, reconstruction of the mangled upper extremity became a reasonable option. 1 During and after the Second World War, reconstruction of the hand and arm became more sophisticated, but remained limited by the availability of local tissue and ongoing issues with poorly vascularized pedicled flaps. Refinement in microsurgical techniques and increasing study of the blood supply to muscle and skin led to a whole new way to look at reconstructive tissue transfer. It was not until the 1960s, when the first toe-to-thumb transfer was performed, that upper extremity free flap reconstruction was introduced as a technique to provide tissue coverage and functional restoration. 2
Free flaps often offer advantages over local and regional pedicled flaps. Local and regional flaps may provide better color and texture matching; however, these flaps may be associated with increased morbidity to the afflicted limb and are limited by tissue availability and composition. 3 Free flaps can be designed to include multiple tissue types that better reconstruct all parts of a defect and are not limited by local tissue availability.
Preoperative Care
A thorough history and physical is the first step in operative planning for all reconstructive patients. Important details impacting the type of reconstruction include age, occupation, handedness, mechanism of injury (blast, avulsion, crush, sharp, etc.), medical comorbidities (peripheral vascular disease, diabetes), previous surgical procedures, history of smoking, and patient motivation. 4
Irrigation, removal of foreign material, and debridement of devitalized tissue after traumatic injury are critical initial steps in eventual upper extremity reconstruction. 5 6 Aggressive cleansing of the wound should be performed by the most senior surgeon in the room as more junior surgeons tend to be reluctant to perform adequate debridement. With the exception of nerves and blood vessels, all nonviable and contaminated tissue must be removed in order for any reconstruction to be successful. Intraoperative photographs should be taken to document the wound status for future surgical planning.
Preoperative planning should account for size of the defect, contamination/infection, vascular injury, types of tissue lost, and potential donor sites. Appropriate imaging should be obtained to evaluate the status of the bone and vasculature (if in question) within the zone of injury. 4
Timing of Reconstruction
The concept of primary reconstruction was first advocated by Marko Godina in his classic 1986 paper. He noted a greater than 99% free flap success rate in cases done within 72 hours, with decreased rates of infection and better outcomes in those patients who had coverage within this period. 7 Ninkovic et al described what is now the standard method for classifying reconstruction based on timing. 8 Three classes are defined: primary, delayed primary, and secondary reconstruction. Free flap coverage during the first 24 hours is defined as a primary reconstruction; coverage between 1 and 7 days following injury is delayed secondary reconstruction. 8 9
The optimal timing for free flap reconstruction remains largely inconclusive. 10 Primary reconstruction is associated with decreased length of hospitalization and cost. 3 9 10 In a more recent review of the literature, Harrison et al demonstrate no clear relationship between timing of reconstruction and postoperative complications. 10 Most surgeons today would advocate “early” coverage whenever possible, as this approach decreases infection rates, decreases wound fibrosis, and allows earlier mobilization of the limb and the patient.
Soft Tissue Coverage
Fascia and Fasciocutaneous Flaps
Soft tissue reconstructive options can generally be categorized into two groups: fascial/fasciocutaneous flaps and muscle-containing flaps. 2 Fasciocutaneous flaps are the preferred method of reconstruction for aesthetically sensitive areas such as the hand. These flaps can be thinned to provide defect contouring and are of primary importance in patients with exposed tendon as they provide a gliding plane for tendon excursion. 11
The radial forearm flap is the primary “workhorse” fasciocutaneous flap. 12 Most commonly utilized as a pedicled flap to the ipsilateral hand, it can also be used as a free flap to the opposite hand. 2 Because the radial artery must be harvested with this flap, it is important to perform an Allen's test to ensure adequate blood flow to the hand via the ulnar artery as part of preoperative planning. 13 Advantages of the radial forearm free flap include its reliable anatomy, large feeding vessels, long vascular pedicle, and great versatility to be molded to the defect. The primary drawback of the radial forearm free flap is the noncosmetic donor site. 12 13 14 A skin graft is required to close all but the smallest donor sites from this flap. 13 In general, the radial forearm free flap is reserved for coverage of other parts of the body, and we do not advocate using the contralateral uninjured forearm as a donor site to cover the injured hand. If, for reasons of anatomy or due to the type of injury, an ipsilateral pedicled radial forearm flap cannot be utilized, we would propose that a different flap be utilized for coverage of the injured hand.
The lateral arm free flap is adaptable in its composition as a fascial, fasciocutaneous, or composite flap, similar to the radial forearm flap. 15 16 Benefits of this flap include its dependable anatomy and dedicated blood supply from the radial collateral artery branching from the profunda brachii ( Fig. 1 ). Additionally, the lateral arm flap can frequently be closed primarily. 2 17 This flap can be quite thick due to the pedicle being between the brachialis and triceps, and while the margins can be thinned, the thickest part of the flap cannot be thinned without potentially disturbing its blood supply. 17 18 While the vascular pedicle is dependable, the lateral arm flap is limited somewhat by the short length of its relatively small pedicle. 2 19
Fig. 1.
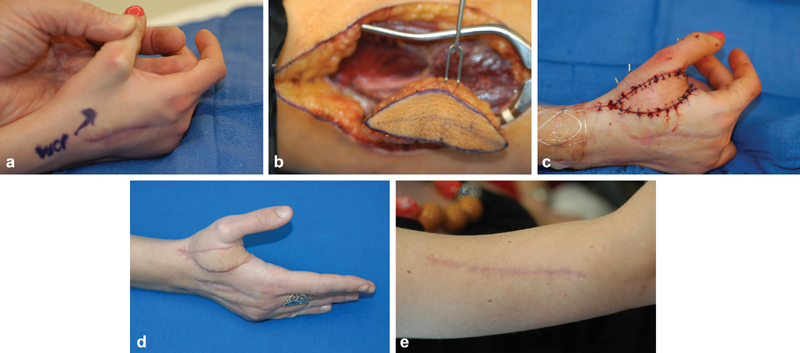
( a ) An 18-year-old female with tight first web space after crushing injury. ( b ) View of lateral arm flap in situ. ( c ) Flap inset in web space. ( d ) Result at 4 months. ( e ) Donor site at 4 months; note small scar after primary closure.
The scapular and parascapular free flaps blood supply is from branches of the subscapular arterial system. These flaps are arranged transversely and obliquely in relationship to the midline of the back. 2 20 They can be utilized for large extremity wounds as they afford readily available soft tissue. 2 20 21 Benefits of the scapular and parascapular free flaps include their long pedicle and low donor site morbidity. 2 20 21 22 23 The donor site is generally closed primarily. 21 Disadvantages include the necessity for lateral intraoperative positioning and a lack of available sensory nerves to be harvested rendering them insensate. 23
The temporoparietal fascial free flap is a pliable flap most notably used in hand and finger reconstruction in the upper extremity. When covered with a skin graft, it can provide the thinnest flap available for hand coverage, and is ideal for reconstruction of the palm of the hand. It is supplied and drained via the superficial temporal vessels which provide a prolific vascular supply to the flap. 2 20 24 25 26 Its uniqueness stems from its ability to be highly vascularized while also remaining thin and flexible. 24 The smooth areolar connective tissue overlying the temporoparietal fascia may serve as a surface for tendon gliding. 2 20 24 25 Lastly, the temporoparietal flap has a donor site hidden within the hairline. 2 20 24 25 26 Drawbacks of this flap are that it requires a skin graft for flap coverage and superficial dissection may lead to iatrogenic alopecia at the donor site, which can be particularly problematic in males with receding hairlines. It can certainly provide the thinnest flap available for hand coverage and is ideal for reconstruction of the palm. Most cutaneous flaps are too bulky in the palm and make grasp difficult.
The anterolateral thigh flap (ALT) is a versatile free flap for upper extremity reconstruction. 6 14 20 27 28 It can be harvested as a fascial, fasciocutaneous, or musculocutaneous flap incorporating the tensor fascia latae or vastus lateralis, or as a composite flap including the iliac crest. 6 20 27 29 The ALT is perfused by the descending branch of the lateral femoral circumflex artery and veins. 6 14 20 27 28 29 30 Its donor site can be closed primarily in most cases; however, a skin graft may be needed when the skin paddle measures greater than 8 cm in width. 27 29 30 The lateral femoral cutaneous sensory nerve can be harvested as well to provide a sensate flap. 28 This flap offers a large piece of skin, and can be taken with fascia if necessary ( Fig. 2 ). It has the advantage that the patient does not have to be turned to harvest the flap and it can be harvested while work is done on the hand if this is appropriate.
Fig. 2.
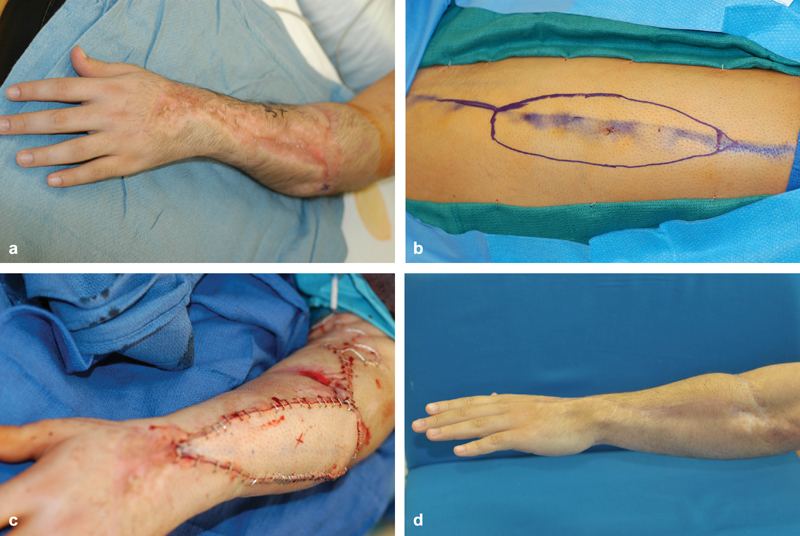
( a ) View of forearm after crush injury and severe scarring of tendons. ( b ) Anterolateral thigh flap marked on leg. ( c ) Flap after vascular anastomoses and inset. ( d ) Flap at 6 months with active digital extension.
Muscle Flaps for Coverage
While cutaneous flaps have come into broad use in hand and forearm coverage, many still prefer muscle flaps covered with split-thickness skin grafts. While they are initially bulky, with time they atrophy and can have a reasonable cosmetic appearance on the hand and arm. They are indicated in dirty/contaminated wounds as they have been shown to clear infection and are better in deep wounds than fasciocutaneous flaps due to their ability to obliterate dead space. 2 8 20 In general, muscle flaps are simpler to harvest and inset compared to the fasciocutaneous flaps. 8 Here, we will discuss three commonly used muscle free flaps for upper extremity injuries.
The latissimus dorsi flap is considered a “workhorse flap” for free muscle transfer because of its reliability, predictable anatomy, and relatively easy disection. 8 Based on the thoracodorsal vessels, the latissimus flap provides a wide pliable muscular sheet with impressive versatility. 2 8 31 If required, the serratus anterior can be raised as well to extend flap length or total volume. 2 8 32 Additionally, the thoracodorsal nerve which innervates the latissimus dorsi can be harvested to offer motor function, but the latissimus is not the best choice in most instances for functional reconstruction. 2 8 Synergistic muscles at the donor site prevent significant functional deficit in all except athletes and weight lifters. The most common complication of this flap is seroma formation at the donor site. 2 8 31 32 Drawbacks include the necessity for placing the patient in the lateral decubitus position intraoperatively for harvest and position change depending on the location of the defect. For hand and arm coverage, a skin paddle is rarely utilized and thus a skin graft will be needed to cover the muscle. 2 32 The latissimus dorsi is probably the best option for coverage of exposed tendon and bone in severe degloving injuries of the forearm and hand. It is the largest muscle available and can cover most defects in this region.
The free rectus abdominis muscle flap is smaller than the latissimus dorsi and ideally suited for moderate to large deep wounds in need of bulky coverage. 8 32 It is supplied by the deep inferior epigastric vascular pedicle. 2 8 32 33 34 35 While the bulky nature of the flap at initial inset may be worrisome, Horch and Stark demonstrated that over a time period of months the flap atrophies, becoming more flush with the surrounding tissue. 34 Disadvantages of its use relate mostly to donor site morbidity, with abdominal hernia and bulge formation being seen infrequently. 2 5 Despite the wide choice of skin flaps available, the rectus remains an excellent choice for smaller defects, and has the advantage that the patient does not need to be turned to harvest the muscle. The serratus anterior muscle flap is generally used for smaller defects of the upper extremity. 2 32 36 The serratus anterior muscle originates from the first nine ribs and inserts to the medial border of the scapula. Only the three most inferior muscular slips, supplied by the serratus branch of thoracodorsal artery, are harvested for this flap. The lateral thoracic artery supplies the superior two-thirds of the muscle. 2 8 32 36 37 The primary benefit of this flap is its ability to serve as a small muscle flap with a long, dependable pedicle, making it ideal for patients with hand wounds without adjacent recipient vessels. 37 A branch of the long thoracic nerve can be transferred with the flap to power motor function, but this is rarely utilized. 2 32 36 37 Disadvantages includes the need for lateral decubitus intraoperative positioning and difficulty of dissection of the serratus branch of the thoracodorsal artery from the long thoracic nerve to avoid winged scapula. 2 9 32 36 37 38 39 Donor site morbidity is minimal assuming that the long thoracic nerve remains uninjured. 37 38 39
Functional Tissue Transfer
Functional muscle transfer for restoration of upper extremity flexion/extension of the fingers, wrist, or elbow can be accomplished by transfer of an innervated latissimus or gracilis. 40 41 The gracilis flap has a width and length similar in size to the flexor and extensor muscles of the forearm. Its tendinous distal third is ideal for attachment to flexor and extensor tendons of the digits. These characteristics make this flap a workhorse for restoration of finger function. This flap is indicated in the setting of significant tissue loss with functional deficit and for reconstruction in cases of muscle necrosis (Volkmann's contracture). The gracilis flap's primary pedicle is a branch of the profunda femoris which penetrates the muscle at its deep surface 6 to 12 cm from its origin at the pubis. 2 The primary pedicle length is typically 5 to 6 cm and its width is 1 to 2 mm. 2 The anterior branch of the obturator nerve enters the muscle proximally with the pedicle and supplies motor function. There is minimal functional donor site deficit. The gracilis offers an ideal muscle for reconstruction of the flexor muscles of the forearm, with an adequate pedicle and a single nerve. This operation is a complex one, but can offer excellent results in cases of loss of the flexors ( Fig. 3 ).
Fig. 3.
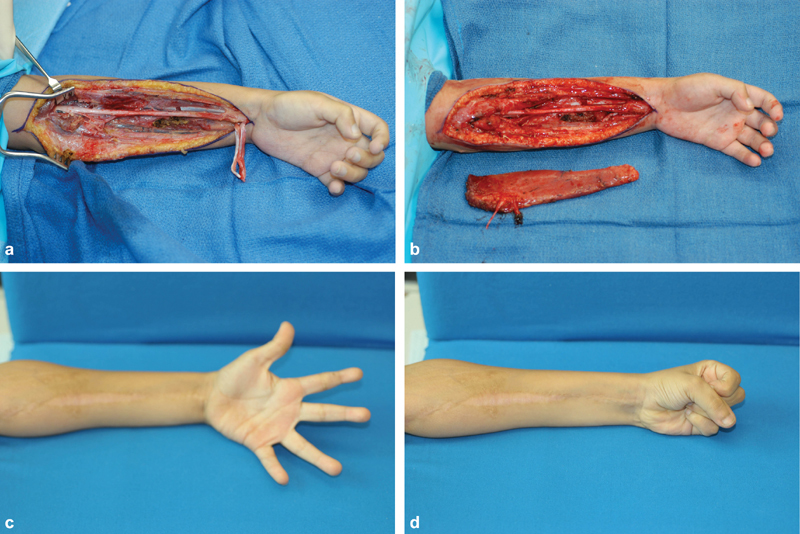
( a ) View of a 7-year-old boy with Volkmann's contracture after exploration of the forearm. ( b ) Gracilis muscle beside forearm after harvest. ( c ) 1-year post-operative extension. ( d ) 1-year post-operative flexion.
Composite tissue transfer offers the ability to reconstruct deficits of multiple tissue types by incorporating bone, joint, nerve, muscle, and skin. 2 32 Bony deficits of the upper extremity, including those that are greater than 6 cm in length, resulting from tumor extirpation in an irradiated field, or in a contaminated bed may best be managed with vascularized bone transfer.
The vascularized fibula free flap is indicated for long bony defects of the radius, ulna, and humerus. 42 43 44 45 It is similar in shape and length to the diaphysis of the radius and ulna, making it ideal for reconstruction. 42 44 The vascular supply of the fibula is from the peroneal artery which branches from the tibioperoneal trunk distal to the takeoff of the anterior tibial artery. It travels along the deep surface of the fibula until reaching the ankle. The vessel sends a rich blood supply to the medullary canal and periosteum as well as septocutaneous and musculocutaneous perforators to the overlying skin. The pedicle length is short but can be extended by taking more bone than needed proximally and dissecting the artery from the extra bone, effectively lengthening the pedicle. 2 43 44 45 The neck of the fibula is in close proximity to the common peroneal nerve and the distal bone is the lateral malleolus of the ankle joint. To avoid damage to the nerve, osteotomies should be avoided near the fibular neck and 6 cm of bone stock should be preserved distally to avoid instability of the ankle. If a paddle of skin is harvested with the flap, a small cuff of muscles should be taken to avoid damage to perforators wrapping deep to superficial around the bone. Functional deficits postoperatively are rare, with the most common morbidity being ankle pain. 43 46 The fibula is an ideal piece of vascularized bone to reconstruct long defects of the arm and forearm.
The medial femoral condyle can be used to correct nonunion or avascular necrosis of the carpal bones, primarily the scaphoid. 42 47 48 49 This flap receives its vascular supply from the descending genicular artery and superomedial genicular artery. 42 47 48 49 50 Advantages of this flap include its large component of vascularized cancellous bone and dependable pedicle. 42 47 50 These characteristics in conjunction with its small size make it very useful for the treatment of scaphoid nonunion. 48 Risks of donor site knee pain and seroma formation are the primary morbidites. 47 Postoperatively, patients regain full mobility within 6 weeks. 49
The plastic surgery principle to “replace like with like” is embodied by toe transfer for digital reconstruction. Transfers range from pulp only ( Fig. 4 ) to harvest of the entire great toe. The great toe and second toe are both acceptable methods for thumb reconstruction. The vascular pedicle of either flap is usually based on the dorsalis pedis and dorsal metatarsal arterial systems. The dorsal superficial veins of the foot provide drainage. The proper plantar digital nerves and the deep peroneal nerves are also included in the flap for sensation. The flexor and extensor tendons are dissected with the flap for recipient site coaptation. 2 51 52 53
Fig. 4.
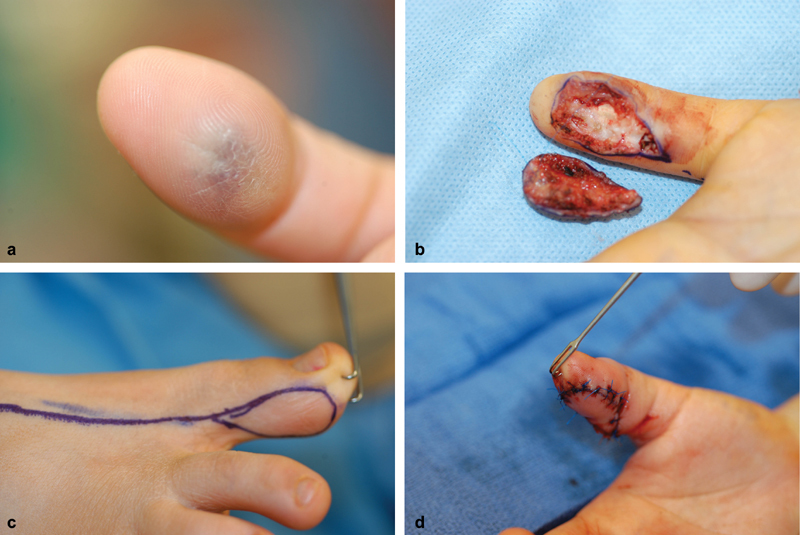
( a ) View of thrice-recurrent hemangioma on ulnar pulp of thumb. ( b ) Area resected and resulting defect. ( c ) Markings for toe pulp transfer based on dorsal circulation. ( d ) Toe pulp after inset into thumb.
The great toe usually provides better functionality and cosmesis in the hand in adults; however, the donor site can be aesthetically unappealing. 51 52 53 54 The second toe is often chosen in children as it has lower donor site morbidity and it matches a child's thumb better than the great toe. Donor site morbidity for thumb reconstruction is generally low; however, Sosin et al demonstrated gate disturbance to be highest in patients who underwent great toe transfer. 55 In amputations distal to the interphalangeal joint, a pulp-only transfer is a good option for reconstruction, 54 and for injuries distal to the mid proximal phalanx, a great toe wrap-around transfer can be performed. This procedure reduces morbidity at the donor site, and although it does not reconstruct the interphalangeal joint of the thumb, it provides the best aesthetic match of the normal thumb. Reconstruction of total thumb amputation requires entire great toe transfer. The primary disadvantage of this procedure is its complexity. 2
It is important to note that while toe-to-thumb transfer help patients recover functionality of the hand, full strength and sensation is not regained. 51 53 54 55 If two toes are required for reconstruction of the “metacarpal hand,” the second toe of each feet should be utilized to lower donor site morbidity.
Conclusion
Upper extremity reconstruction is an evolving field with numerous free flap options available. This discussion serves as a brief introduction to free flap reconstruction of the upper extremity. Workhorse flaps were of primary focus with the goal of providing reconstructive options to the most common upper extremity deficits requiring free flap reconstruction. Further reading and self-education is necessary to become an expert in this complex field.
Conflict of Interest None.
Sources of Support
None.
Products/Devices/Drugs
None.
References
- 1.Fang F, Chung K C.An evolutionary perspective on the history of flap reconstruction in the upper extremity Hand Clin 20143002109–122., v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pederson W C.Upper extremity microsurgery Plast Reconstr Surg 2001107061524–1537., discussion 1538–1539, 1540–1543 [PubMed] [Google Scholar]
- 3.Herter F, Ninkovic M, Ninkovic M. Rational flap selection and timing for coverage of complex upper extremity trauma. J Plast Reconstr Aesthet Surg. 2007;60(07):760–768. doi: 10.1016/j.bjps.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Smith A A, Duncan S FM. Preoperative planning for free-tissue transfer. Microsurgery. 2005;25(05):365–372. doi: 10.1002/micr.20133. [DOI] [PubMed] [Google Scholar]
- 5.Chim H, Ng Z Y, Carlsen B T, Mohan A T, Saint-Cyr M.Soft tissue coverage of the upper extremity: an overview Hand Clin 20143004459–473., vi [DOI] [PubMed] [Google Scholar]
- 6.Merritt K. Factors increasing the risk of infection in patients with open fractures. J Trauma. 1988;28(06):823–827. doi: 10.1097/00005373-198806000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78(03):285–292. doi: 10.1097/00006534-198609000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Ninkovic M, Mooney E K, Ninkovic M, Kleistil T, Anderl H.A new classification for the standardization of nomenclature in free flap wound closure Plast Reconstr Surg 199910303903–914., discussion 915–917 [DOI] [PubMed] [Google Scholar]
- 9.Schaverien M V, Hart A M.Free muscle flaps for reconstruction of upper limb defects Hand Clin 20143002165–183., v–vi [DOI] [PubMed] [Google Scholar]
- 10.Harrison B L, Lakhiani C, Lee M R, Saint-Cyr M. Timing of traumatic upper extremity free flap reconstruction: a systematic review and progress report. Plast Reconstr Surg. 2013;132(03):591–596. doi: 10.1097/PRS.0b013e31829ad012. [DOI] [PubMed] [Google Scholar]
- 11.Ng Z Y, Salgado C J, Moran S L, Chim H. Soft tissue coverage of the mangled upper extremity. Semin Plast Surg. 2015;29(01):48–54. doi: 10.1055/s-0035-1544170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedrich J B, Pederson W C, Bishop A T, Galaviz P, Chang J. New workhorse flaps in hand reconstruction. Hand (N Y) 2012;7(01):45–54. doi: 10.1007/s11552-011-9385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mühlbauer W, Herndl E, Stock W. The forearm flap. Plast Reconstr Surg. 1982;70(03):336–344. doi: 10.1097/00006534-198209000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Griffin M, Hindocha S, Malahias M, Saleh M, Juma A. Flap decisions and options in soft tissue coverage of the upper limb. Open Orthop J. 2014;8:409–414. doi: 10.2174/1874325001408010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yousif N J, Warren R, Matloub H S, Sanger J R.The lateral arm fascial free flap: its anatomy and use in reconstruction Plast Reconstr Surg 199086061138–1145., discussion 1146–1147 [PubMed] [Google Scholar]
- 16.Ulusal B G, Lin Y, Ulusal A E, Lin C H. Free lateral arm flap for 1-stage reconstruction of soft tissue and composite defects of the hand: a retrospective analysis of 118 cases. Ann Plast Surg. 2007;58(02):173–178. doi: 10.1097/01.sap.0000232832.18894.2b. [DOI] [PubMed] [Google Scholar]
- 17.Graham B, Adkins P, Scheker L R. Complications and morbidity of the donor and recipient sites in 123 lateral arm flaps. J Hand Surg [Br] 1992;17(02):189–192. doi: 10.1016/0266-7681(92)90086-h. [DOI] [PubMed] [Google Scholar]
- 18.Chen H C, el-Gammal T A. The lateral arm fascial free flap for resurfacing of the hand and fingers. Plast Reconstr Surg. 1997;99(02):454–459. doi: 10.1097/00006534-199702000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Akinci M, Ay S, Kamiloglu S, Erçetin O.Lateral arm free flaps in the defects of the upper extremity--a review of 72 cases Hand Surg 200510(2–3):177–185. [DOI] [PubMed] [Google Scholar]
- 20.King E A, Ozer K.Free skin flap coverage of the upper extremity Hand Clin 20143002201–209., vi [DOI] [PubMed] [Google Scholar]
- 21.Klinkenberg M, Fischer S, Kremer T, Hernekamp F, Lehnhardt M, Daigeler A. Comparison of anterolateral thigh, lateral arm, and parascapular free flaps with regard to donor-site morbidity and aesthetic and functional outcomes. Plast Reconstr Surg. 2013;131(02):293–302. doi: 10.1097/PRS.0b013e31827786bc. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton S G, Morrison W A. The scapular free flap. Br J Plast Surg. 1982;35(01):2–7. doi: 10.1016/0007-1226(82)90075-3. [DOI] [PubMed] [Google Scholar]
- 23.Izadi D, Paget J T, Haj-Basheer M, Khan U M. Fasciocutaneous flaps of the subscapular artery axis to reconstruct large extremity defects. J Plast Reconstr Aesthet Surg. 2012;65(10):1357–1362. doi: 10.1016/j.bjps.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 24.Brent B, Upton J, Acland R D et al. Experience with the temporoparietal fascial free flap. Plast Reconstr Surg. 1985;76(02):177–188. doi: 10.1097/00006534-198508000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Carty M J, Taghinia A, Upton J. Fascial flap reconstruction of the hand: a single surgeon's 30-year experience. Plast Reconstr Surg. 2010;125(03):953–962. doi: 10.1097/PRS.0b013e3181cc964c. [DOI] [PubMed] [Google Scholar]
- 26.Flügel A, Kehrer A, Heitmann C, Germann G, Sauerbier M. Coverage of soft-tissue defects of the hand with free fascial flaps. Microsurgery. 2005;25(01):47–53. doi: 10.1002/micr.20070. [DOI] [PubMed] [Google Scholar]
- 27.Hsu C C, Lin Y T, Lin C H, Lin C H, Wei F C. Immediate emergency free anterolateral thigh flap transfer for the mutilated upper extremity. Plast Reconstr Surg. 2009;123(06):1739–1747. doi: 10.1097/PRS.0b013e3181a65b00. [DOI] [PubMed] [Google Scholar]
- 28.Hanasono M M, Skoracki R J, Yu P. A prospective study of donor-site morbidity after anterolateral thigh fasciocutaneous and myocutaneous free flap harvest in 220 patients. Plast Reconstr Surg. 2010;125(01):209–214. doi: 10.1097/PRS.0b013e3181c495ed. [DOI] [PubMed] [Google Scholar]
- 29.Saint-Cyr M, Gupta A. Indications and selection of free flaps for soft tissue coverage of the upper extremity. Hand Clin. 2007;23(01):37–48. doi: 10.1016/j.hcl.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Adani R, Tarallo L, Marcoccio I, Cipriani R, Gelati C, Innocenti M.Hand reconstruction using the thin anterolateral thigh flap Plast Reconstr Surg 200511602467–473., discussion 474–477 [DOI] [PubMed] [Google Scholar]
- 31.Gordon L, Buncke H J, Alpert B S. Free latissimus dorsi muscle flap with split-thickness skin graft cover: a report of 16 cases. Plast Reconstr Surg. 1982;70(02):173–178. doi: 10.1097/00006534-198208000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Wolfe S W, Pederson W C, Kozin S W, Cohen M S. Philadelphia, PA: Elsevier; 2017. Green's Operative Hand Surgery; pp. 1574–1609. [Google Scholar]
- 33.Rao V K, Baertsch A. Microvascular reconstruction of the upper extremity with the rectus abdominis muscle. Microsurgery. 1994;15(10):746–750. doi: 10.1002/micr.1920151015. [DOI] [PubMed] [Google Scholar]
- 34.Horch R E, Stark G B. The rectus abdominis free flap as an emergency procedure in extensive upper extremity soft-tissue defects. Plast Reconstr Surg. 1999;103(05):1421–1427. doi: 10.1097/00006534-199904050-00010. [DOI] [PubMed] [Google Scholar]
- 35.Taylor G I, Corlett R J, Boyd J B. The versatile deep inferior epigastric (inferior rectus abdominis) flap. Br J Plast Surg. 1984;37(03):330–350. doi: 10.1016/0007-1226(84)90076-6. [DOI] [PubMed] [Google Scholar]
- 36.Whitney T M, Buncke H J, Alpert B S, Buncke G M, Lineaweaver W C.The serratus anterior free-muscle flap: experience with 100 consecutive cases Plast Reconstr Surg 19908603481–490., discussion 491 [PubMed] [Google Scholar]
- 37.Logan S E, Alpert B S, Buncke H J. Free serratus anterior muscle transplantation for hand reconstruction. Br J Plast Surg. 1988;41(06):639–643. doi: 10.1016/0007-1226(88)90174-9. [DOI] [PubMed] [Google Scholar]
- 38.Dumont C E, Domenghini C, Kessler J. Donor site morbidity after serratus anterior free muscular flap: a prospective clinical study. Ann Plast Surg. 2004;52(02):195–198. doi: 10.1097/01.sap.0000096378.59694.54. [DOI] [PubMed] [Google Scholar]
- 39.Derby L D, Bartlett S P, Low D W. Serratus anterior free-tissue transfer: harvest-related morbidity in 34 consecutive cases and a review of the literature. J Reconstr Microsurg. 1997;13(06):397–403. doi: 10.1055/s-2007-1006419. [DOI] [PubMed] [Google Scholar]
- 40.Terzis J K, Kostopoulos V K. Free muscle transfer in posttraumatic plexopathies: part III. The hand. Plast Reconstr Surg. 2009;124(04):1225–1236. doi: 10.1097/PRS.0b013e3181b5a322. [DOI] [PubMed] [Google Scholar]
- 41.Kay S, Pinder R, Wiper J, Hart A, Jones F, Yates A. Microvascular free functioning gracilis transfer with nerve transfer to establish elbow flexion. J Plast Reconstr Aesthet Surg. 2010;63(07):1142–1149. doi: 10.1016/j.bjps.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 42.Houdek M T, Wagner E R, Wyles C C, Nanos G P, III, Moran S L. New options for vascularized bone reconstruction in the upper extremity. Semin Plast Surg. 2015;29(01):20–29. doi: 10.1055/s-0035-1544167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soucacos P N, Korompilias A V, Vekris M D, Zoubos A, Beris A E. The free vascularized fibular graft for bridging large skeletal defects of the upper extremity. Microsurgery. 2011;31(03):190–197. doi: 10.1002/micr.20862. [DOI] [PubMed] [Google Scholar]
- 44.Kremer T, Bickert B, Germann G, Heitmann C, Sauerbier M.Outcome assessment after reconstruction of complex defects of the forearm and hand with osteocutaneous free flaps Plast Reconstr Surg 200611802443–454., discussion 455–456 [DOI] [PubMed] [Google Scholar]
- 45.Yajima H, Tamai S, Ono H, Kizaki K.Vascularized bone grafts to the upper extremities Plast Reconstr Surg 199810103727–735., discussion 736–737 [DOI] [PubMed] [Google Scholar]
- 46.Bodde E W, de Visser E, Duysens J E, Hartman E H. Donor-site morbidity after free vascularized autogenous fibular transfer: subjective and quantitative analyses. Plast Reconstr Surg. 2003;111(07):2237–2242. doi: 10.1097/01.PRS.0000060086.99242.F1. [DOI] [PubMed] [Google Scholar]
- 47.Jones D B, Jr, Rhee P C, Bishop A T, Shin A Y. Free vascularized medial femoral condyle autograft for challenging upper extremity nonunions. Hand Clin. 2012;28(04):493–501. doi: 10.1016/j.hcl.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 48.Jones D B, Jr, Bürger H, Bishop A T, Shin A Y. Treatment of scaphoid waist nonunions with an avascular proximal pole and carpal collapse. A comparison of two vascularized bone grafts. J Bone Joint Surg Am. 2008;90(12):2616–2625. doi: 10.2106/JBJS.G.01503. [DOI] [PubMed] [Google Scholar]
- 49.Kakar S, Duymaz A, Steinmann S, Shin A Y, Moran S L. Vascularized medial femoral condyle corticoperiosteal flaps for the treatment of recalcitrant humeral nonunions. Microsurgery. 2011;31(02):85–92. doi: 10.1002/micr.20843. [DOI] [PubMed] [Google Scholar]
- 50.Rao S S, Sexton C C, Higgins J P. Medial femoral condyle flap donor-site morbidity: a radiographic assessment. Plast Reconstr Surg. 2013;131(03):357e–362e. doi: 10.1097/PRS.0b013e31827c6f38. [DOI] [PubMed] [Google Scholar]
- 51.Buncke G M, Buncke H J, Lee C K. Great toe-to-thumb microvascular transplantation after traumatic amputation. Hand Clin. 2007;23(01):105–115. doi: 10.1016/j.hcl.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Henry S L, Wei F C. Thumb reconstruction with toe transfer. J Hand Microsurg. 2010;2(02):72–78. doi: 10.1007/s12593-010-0017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin P Y, Sebastin S J, Ono S, Bellfi L T, Chang K W, Chung K C. A systematic review of outcomes of toe-to-thumb transfers for isolated traumatic thumb amputation. Hand (N Y) 2011;6(03):235–243. doi: 10.1007/s11552-011-9340-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei F C, Chen H C, Chuang C C, Chen S H.Microsurgical thumb reconstruction with toe transfer: selection of various techniques Plast Reconstr Surg 19949302345–351., discussion 352–357 [DOI] [PubMed] [Google Scholar]
- 55.Sosin M, Lin C H, Steinberg J et al. Functional donor site morbidity after vascularized toe transfer procedures: a review of the literature and biomechanical consideration for surgical site selection. Ann Plast Surg. 2016;76(06):735–742. doi: 10.1097/SAP.0000000000000591. [DOI] [PubMed] [Google Scholar]


