Abstract
Purpose:
This study examined the relationship between self-reported symptom severity and oral intake in long-term head and neck cancer (HNC) survivors.
Methods:
An observational survey study with retrospective chart abstraction was conducted. HNC patients who had completed an MD Anderson Symptom Inventory-Head and Neck (MDASI-HN) questionnaire and also had clinician graded oral intake ratings (Functional Oral Intake Scale [FOIS]) were included. Correlation coefficients were computed. FOIS scores were regressed on MDASI-HN symptom items using stepwise backwards elimination for multivariate models.
Results:
152 survey pairings were included in the analysis (median: 44 months follow-up, range: 7-198). Per FOIS, 28% of survivors maintained a total oral diet with no restrictions, 67% reported a restricted oral diet (without tube), 3% were partially tube dependent with some oral intake, and 2% were NPO. Of the 22 symptom items, the most severe items in decreasing order were: dry mouth, difficulty swallowing\chewing, problems with mucus, tasting food, and choking/coughing. Significant bivariate correlations, after Bonferroni correction for multiple comparisons, were present for 8 of 22 symptoms with FOIS. On multivariate analysis, symptom severity for difficulty swallowing and problems with teeth/gums remained significantly associated with FOIS.
Conclusions:
Oral intake in HNC survivorship is a multidimensional issue and functional outcome that is not only impacted by dysphagia but also by dental status. Symptom drivers of oral intake likely differ in acute survivorship. Nonetheless, these findings highlight the lack of specificity in this endpoint and also the need for multidisciplinary supportive care to optimize oral intake in survivors.
Keywords: oral intake, head and neck cancer, survivors, symptom drivers, toxicity, functional outcomes
Introduction
Radiotherapy (RT), which may be delivered as a primary modality, concurrently with chemotherapy, or adjuvant to surgical resection, is a mainstay of treatment for head and neck cancer (HNC). Although HNC cases make up a small percentage of all new cancers in the United States (3%), the survival rates have been steadily increasing in recent years with 5-year survival estimated at 65% for oral and pharyngeal cancers and 60% for laryngeal cancers [1,2]. With an increase in survival and intensification of RT with concurrent chemotherapy, there is heightened awareness of the supportive care needs of HNC survivors [3]. A safe and efficient swallow is required for optimal quality of life, nutrition, and to prevent aspiration. There are a variety of validated clinical instruments to assess oral intake, and also validated patient-reported outcomes (PRO) instruments that assess symptoms affecting oral intake, but no single method is accepted as standard.
Oral intake of HNC survivors is often impaired due to effects of tumor as well as short- and long-term side effects associated with cancer therapy. Relevant acute toxicities of radiation therapy include dysguesia, mucositis, odynophagia, dysphagia, aspiration, fatigue, and pain [4,5]. Chemotherapy has been reported not only to cause nausea and vomiting, leading to dehydration but also to enhance the above side effects of RT [5]. While many acute toxicities improve in the months immediately following RT, a number of salivary and neuromuscular toxicities may linger, progress, or have delayed onset as consequential late effects of therapy. The most commonly cited chronic symptoms of multimodality therapy include xerostomia, trismus, soft tissue fibrosis, leading to pharyngo-esophageal strictures and stenosis, dysphagia, and aspiration [5,6].
Acute and chronic toxicities and their associated symptoms cause functional impairments, such as restrictions in oral intake in HNC survivorship. Swallowing disorders [7], mucosal sensitivity [8], and xerostomia [8] have been shown to significantly relate to oral intake, as have clinical factors such as age, tumor location, tumor staging, and treatment. Limited published data exist, however, on the relationship between clusters of symptoms as they relate to functional impairments in oral intake after treatment. The negative consequences of altered oral intake include treatment interruptions, increased recovery times, impaired immunity, weight loss, increased risk of complications, poor quality of life (QOL), and reduced survival rates [9,10], Up to 57% of HNC patients present with significant malnutrition, manifested by greater than 10% weight loss from baseline body mass [11], During multimodality therapy, the percentage of malnourished patients rises as high as 88% [12], After the completion of treatment, HNC survivors continue facing challenges with oral intake due to the long-term side effects of cancer therapy.
Various PRO and clinician-graded scales exist to measure oral intake in HNC patients. Although there is a lack of consensus on one predominant oral intake scale, the Performance Status Scale for Head and Neck Cancer Patients (PSS-HN) and Functional Oral Intake Scale (FOIS) are the most commonly reported validated scales in published literature. FOIS was originally developed and validated in stroke patients, and the psychometric properties were subsequently confirmed in other populations such as HNC patients [13], FOIS is reliable, valid, and sensitive to change in functional oral intake [12], PSS-HN was specifically designed to assess three areas of dysfunction in HNC patients including understandability of speech, normalcy of diet, and eating in public [13], Unlike FOIS, which takes into account the use of a feeding tube with oral intake, PSS-HN focuses more on the complexity of the patient’s diet regardless of feeding tube status. Other non-validated oral intake classifications (e.g., regular diet versus blended/pureed versus liquids) are also regularly reported as an outcome in HNC publications [14,15], The MDASI-HN is a PRO instrument that takes two minutes to complete to rate the severity of symptoms that may affect oral intake.
The objective of this pilot study is to examine the relationship between self-reported symptom severity (per MD Anderson Symptom Inventory-Head and Neck, MDASI-HN) and oral intake (per FOIS) in long-term HNC survivors. Primary symptoms of interest included surrogates of mucosal toxicity, salivary dysfunction, and dysphagia.
Methods
Study design and eligibility
An observational study was conducted at the University of Texas MD Anderson Cancer Center to identify symptom drivers of oral intake in long-term HNC survivors. HNC patients who completed MDASI-HN questionnaire and also had prospective clinician graded quantitative oral intake ratings using the Performance Scale of Head and Neck Cancer (PSS-HN) available for retrospective analysis were eligible for inclusion in the study. Inclusion criteria were: 1) diagnosis of oral cavity, oropharyngeal, nasopharyngeal, laryngeal, or unknown primary HNC; 2) history of RT with or without chemotherapy; 3) at least 18 years of age; 4) MDASI-HN questionnaire in the study database; and 5) prospective clinical oral intake ratings per PSSHN using the Normalcy of Diet scale in the study database or clinical record.
A total of 952 eligible patients with 2,422 MDASI-HN were identified in the MDASI-HN database. One hundred and sixty three HNC patients were excluded according to the following criteria: radical or salvage surgery; re-irradiation; skin, sinonasal or skull base cancers; active disease at time of MDASI-HN completion; and less than 18 years of age. Of the 789 eligible HNC patients with MDASI-HN scores, only 123 also had clinical PSS-HN assigned in proximity to the MDASI-HN survey, i.e., within 6 months. Thus, a final sample size of 152 survey pairings (MDASI-HN + PSS-HN) from 123 patients were included in this analysis.
Study instruments
MDASI-HN [16]
The MDASI-HN questionnaire permits patients to self-report and grade their symptom burden via 28 items: 22 items rate the severity of symptoms and 6 items rate how symptoms interfere with daily life. Symptoms are graded on a 0-10 scale, 0 representing “not present” and 10 indicating “as bad as you can imagine” Interference items are also graded on a 0-10 scale, 0 indicating the item “did not interfere” and 10 indicating the item “interfered completely.” MDASI-HN were prospectively collected by phone or mailing as part of a prospective HNC survivorship study. MDASI-HN symptom severity items were the primary independent variables of interest in the statistical analysis.
PSS-HN [17].
The PSS-HN is clinician-graded based on semi-structured interview with three subscales: normalcy of diet, understandability of speech, and eating in public. Only the normalcy of diet subscale was utilized in this study. The normalcy of diet subscale is arranged hierarchically where 100 indicates a regular, fully unrestricted diet and 0 indicates non-oral nutrition. This subscale takes into account the complexity of the patient’s diet according to various food textures. PSS-HN normalcy of diet scores >0 do not take into account the feeding tube status of the patient. Feeding tube status was prospectively documented at the time of PSS-HN completion using four standardized questions ascertaining presence, type of use (sole use [i.e., no or minimal oral intake], supplementation, liquids only, or not currently used), number of cans, and proportion of intake by tube. PSS-HN and feeding tube use were routinely documented using a standardized clinical form at clinical encounters with the speech pathology service. PSS-HN was considered as a secondary oral intake measure in sensitivity analyses.
FOIS [13].
The FOIS is a psychometrically validated clinician-graded ordinal scale of oral intake that consists of seven levels with level 1 representing nothing by mouth and level 7 representing total oral diet with no restrictions. As a distinction from PSS-HN, FOIS includes ordinal rankings to account for various degrees of partial oral intake with tube supplementation (levels 2 and 3), but specific food avoidances are not graded as in the PSS-HN normalcy of diet subscale (PSSHN 60-80). PSS-HN normalcy of diet scores and tube status were converted to the FOIS scale for this analysis, conversion criteria are listed in Supplementary Table 1. FOIS was considered as the primary measure of oral intake for statistical analyses.
Pairing measures (MDASI-HN and Oral Intake)
The source of MDASI-HN (symptom instrument) and PSS-HN/FOIS (oral intake instruments) differed. That is, MDASI-HN was collected from a prospective survey study administered longitudinally outside of clinic whereas PSS-HN (converted to FOIS) was collected at clinical encounters within the Head and Neck Center. The authors allowed a maximum 6-month window in which the patient was NED, with no cancer treatment or rehabilitation rendered to pair instruments. Six months was felt to reflect a fairly stable window for symptoms and functional status at this point given that most survivors included were years out from cancer treatment.
Statistical methods
Descriptive statistics were calculated. Mean MDASI-HN summary scores (total symptom burden, local symptom burden, systemic symptom burden, and interference) were computed. Statistical analyses of the categorical variables were performed using chi-square test and t-tests for continuous outcomes. Symptom clusters were defined by hierarchal cluster analysis and displayed via heat map, by which each patient’s individual item ratings were identified, to provide a pictorial representation of how symptoms clustered. Symptom clusters are groups of symptoms, which centered together and may share specific underlying dimensions relatively independent of other clusters. The symptoms that join with others were rated by patients more similarly and could be interrelated. The heat map for the hierarchal cluster analysis is accompanied with a dendrogram to show the relative distances between clusters, i.e., those symptoms that join with others earlier within small relative distance were rated by patients more similarly. Spearman correlation coefficients were computed for ordinal variables. For primary analysis, FOIS scores were regressed on MDASI-HN symptom items. To control for multiple comparison of each of the 22 MDASI-HN symptom items, a Bonferroni correction was performed with a p value of ≤ 0.002 (i.e., 0.05/22, accounting for n= 22 items) deemed significant. Eight MDASI items with significant p value (p≤0.002) were entered the multivariate models using stepwise backwards elimination per methods of Hosmer and Lemeshow, the final model retained confounders that were independently associated (p≤0.006 i.e., 0.05/8) with FOIS. Univariate generalized linear regression models were first examined for each MDASI symptom item and clinical variables (T-classification, N-classification, sex, age, treatment modalities, surgery, and primary site). Multivariable models retained confounders that were independently associated (p<0.05) with FOIS. A p value of <0.05 was considered statistically significant. Statistical analyses were performed using the STATA data analysis software, version 14.0 (StataCorp LP, College Station, TX, US) and JMP, version 12 (Pro, SAS Institute, Cary, NC, USA).
Results
Patients
Table 1 lists the demographic and disease-related characteristics for the patients contributing 152 surveys included in this study. The median age was 60 years (range: 41-83) and 75% were male. The most common disease sites were the oropharynx (55%) and larynx/hypopharynx (40%). Only 13 surveys were collected after surgery (8.5%), which included procedures such as selective neck dissection, neck skin wide local excision, transoral robotic surgery, and marginal mandibulectomy. All 152 surveys post-dated radiation therapy: 30% radiation alone, and 70% with chemotherapy.
Table 1.
Patient, tumor and treatment characteristics.
| No. | % | |
|---|---|---|
| Sex | ||
| Male | 94 | 75 |
| Female | 29 | 24 |
| Age | ||
| Median (range) | 60 (32-83) | |
| Tumor Site | ||
| Oropharynx | 70 | 57 |
| Nasopharynx | 7 | 6 |
| Larynx or hypopharynx | 46 | 37 |
| T classification | ||
| TX/1/2 | 79 | 64 |
| T3/4 | 44 | 36 |
| N classification | ||
| NX/0 | 48 | 39 |
| N1/2 | 72 | 59 |
| N3 | 3 | 2 |
| Therapeutic Combination | ||
| RT alone | 36 | 29 |
| IC --> RT alone | 10 | 8 |
| Concurrent ChemoRT | 43 | 35 |
| IC --> ChemoRT | 33 | 27 |
| RT --> Adjuvant chemotherapy | 1 | 1 |
Abbreviations: IC, induction chemotherapy; RT, radiotherapy.
MDASI-HN
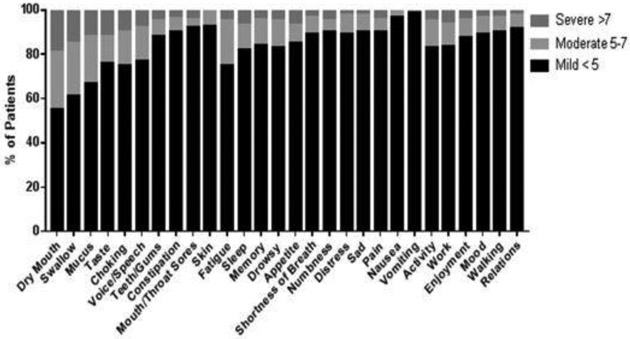
The average time from end of treatment to completion of MDASI-HN was 44 months (range 7-198 months). The mean individual and composite MDASI-HN symptom and symptom interference item ratings are shown in Table 2. Of the 10 local symptom items, the three most severe items in order of decreasing severity were dry mouth, difficulty swallowing\chewing, and problems with mucus. Of the 12 systemic items, the three most severe items in order of decreasing severity were fatigue, sleep, and memory. The total mean of both local and systemic symptom severity items (22 items) was 1.74, representing low symptom burden among long-term survivors who participated in the survey study. For symptom interference, the three most severe items in order of decreasing severity were activity, work, and enjoyment. The total mean of symptom interference items (6 items) was 1.40. The percentage of patients experiencing each level of symptom severity reported as mild, moderate, or severe is shown in Figure 1 & Supplementary Table 2. Overall, the majority of patients reported mild symptom severity for all 28 symptom and interference items included in the MDASI-HN questionnaire.
Table 2.
Mean individual MDASI-symptom item and symptom interference ratings by order of decreasing severity
| Regression coefficient- Univariate analysis* |
P value | Regression coefficient- Multivariate analysis** |
P value | |||
|---|---|---|---|---|---|---|
| Symptom | Entire study cohort n = 152 |
|||||
| Local Items | Mean | SD | ||||
| Dry Mouth* | 4.14 | 3.16 | −0.121 | 0.000* | ||
| Swallow** | 3.56 | 3.28 | −0.149 | 0.000* | 0.000** | |
| Mucus | 3.09 | 3.06 | ||||
| Taste* | 2.43 | 3.23 | −0.102 | 0.000* | ||
| Choking* | 2.40 | 3.00 | −0.099 | 0.001* | ||
| Voice/Speech* | 2.36 | 2.73 | −0.111 | 0.001* | ||
| Teeth/Gum** | 1.27 | 2.37 | −0.182 | 0.000* | 0.004** | |
| Constipation | 1.17 | 2.13 | ||||
| Sores | 0.90 | 2.15 | ||||
| Skin | 0.56 | 1.51 | ||||
| Systemic Items | ||||||
| Fatigue | 2.40 | 2.62 | ||||
| Sleep | 2.02 | 2.69 | ||||
| Memory | 1.99 | 2.48 | ||||
| Drowsy | 1.95 | 2.49 | ||||
| Appetite* | 1.61 | 2.66 | −0.159 | 0.000* | ||
| Breath | 1.34 | 2.31 | ||||
| Numbness | 1.33 | 2.38 | ||||
| Distress | 1.28 | 2.07 | ||||
| Sad | 1.14 | 2.05 | ||||
| Pain* | 1.13 | 2.15 | −0.128 | 0.002* | ||
| Nausea | 0.28 | 1.14 | ||||
| Vomiting | 0.15 | 0.87 | ||||
| Total | ||||||
| (all 22 symptom items) | 38.17 | 33.85 | ||||
| Symptom | ||||||
| Interference | ||||||
| Activity | 1.78 | 2.50 | ||||
| Work | 1.68 | 2.54 | ||||
| Enjoyment | 1.46 | 2.25 | ||||
| Mood | 1.36 | 2.20 | ||||
| Walking | 1.13 | 2.28 | ||||
| Relations | 0.97 | 2.00 | ||||
| Total | ||||||
| (6 symptom interference items) | 1.40 | 1.96 | ||||
Significant correlations between MDASI-HN symptom items and the FOIS dietary scale in univariate analysis after Bonferroni correction for multiple comparisons.
Significant correlations between MDASI-HN symptom items and the FOIS dietary scale in multivariate analysis.
Abbreviations: FOIS, Functional Oral Intake Scale; MDASI-HN, MD Anderson Symptom Inventory-Head and Neck questionnaire.
Figure 1.
Percentage of patients with MDASI-HN symptoms.
Abbreviations: MDASI-HN, MD Anderson Symptom Inventory-Head and Neck
PSS-HN
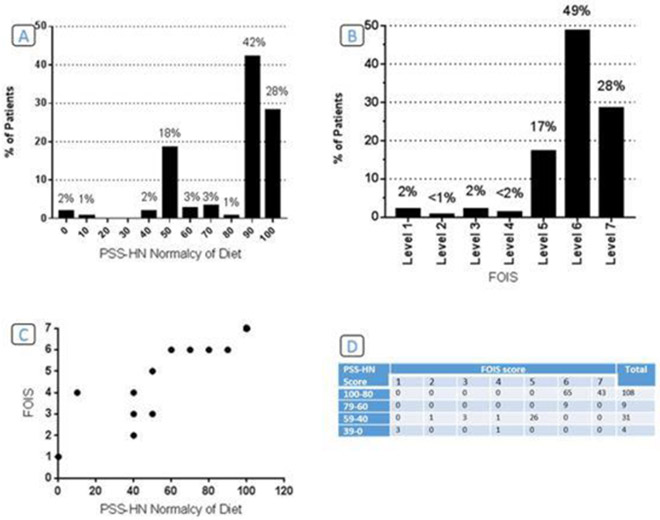
The average time from end of treatment to completion of PSS-HN was 43 months (range 6-197 months). The distribution of diet ratings and median for the normalcy of diet subscale is noted in Figure 2. Twenty eight percent were eating a regular, unrestricted diet, 42% required liquid assistance to wash down solid foods, and 28% were eating a restricted diet. Complete feeding tube dependence was rare (2%).
Figure 2.
A. PSS-HN Normalcy of Diet Subscale. B. FOIS Outcome Summary. C. Comparison of FOIS and PSS-HN Dietary Scales. D. Number of patients in each group per FOIS & PSS-HN Normalcy of Diet Subscale.
In Figure 2C. The dot size is weighting by the number of patients in each group.
Abbreviations: PSSHN, the Performance Scale of Head and Neck Cancer; FOIS, Functional Oral Intake Scale.
FOIS
FOIS distribution of diet ratings is noted in Figure 2. Twenty eight percent had a total oral diet with no restrictions, 67% had a total oral diet with restrictions, and 3% were tube dependent with some oral intake. As expected, the FOIS scale and the PSS-HN normalcy of diet scale were highly correlated (Spearman’s rho = 0.9760, p<0.001). The distribution of patients, per FOIS and diet score of PSS-HN is shown in Figure 2.
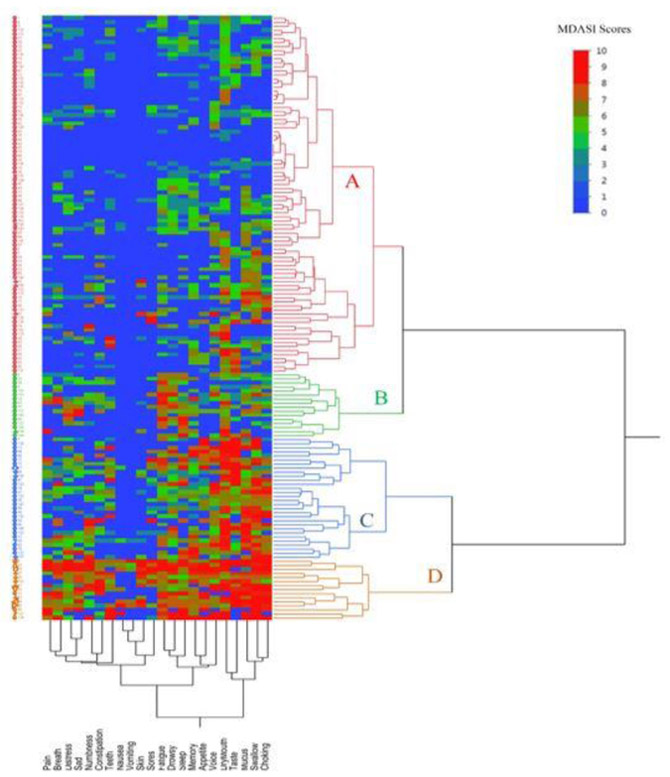
Cluster analysis (symptoms)
Hierarchical cluster analysis results are presented in Figure 3, categorizing the patients into four district groups. In general, group D and group A experienced the highest and lowest symptom burden, respectively. The dendrogram, on the X axis, illustrating the clustering of various symptoms and showing the relative distance between the clusters. Those symptoms that join with others were rated by patients more similarly. For example, the symptoms; choking, swallow, mucous, taste and dry mouth joined together quickly, indicating that patients perceived and rated these symptoms similarly. Groups C and D had poor oral intake per FOIS, with mean scores of 4.8 and 5.7, respectively. The other two groups (A&B) had near normal intake with mean FOIS score of 6.12±0.9 compared to groups C&D (p<0.01). Groups C and D had 30 (20%) and 15 (10%) patients with a median age of 60 and 63 years, respectively. In group C, difficulty swallowing/chewing, dry mouth, problem with tasting food, and problem with mucus represent the highest symptom burdens, per MDASI-HN. Difficulty swallowing\chewing, problems with mucus, choking/coughing and dry mouth were the highest MDASI-HN scores in group D, similar to group C with dysphagia and salivary symptoms but not experiencing high dysgeusia symptoms. In groups C and D, 50% and 33% patients received concurrent chemotherapy (CCRT), while 27% and 40% had combined induction (IC)+CCRT, respectively, additionally 80% and 67% presented with nodal positive disease. The patient groups with poor oral intake (group D&C) shared the same symptom profile/severity and the oral morbidity symptoms clustered together. This finding suggests, a link between the symptom cluster grouping and oral intake status, and at the same suggests interrelated oral morbidity symptoms found within the same cluster.
Figure 3.
Heat map representing the symptom burden of 22 individual MDASI-HN symptom items, for each individual patient, and grouped by hierarchal cluster analysis of patients with dendrogram. X-axis represent symptom clusters and Y-axis represent the patients’ grouping. Solid red squares denote high MDASI-HN scores (high symptom burden), while solid blue squares indicate low MDASI-HN scores. Four patient clusters labelled A, B, C and D and four symptom clusters have been categorized; 1st cluster include choking, swallow, mucous, taste and dry mouth, 2nd include voice, appetite, sleep, drowsy and fatigue, 3rd include oral sores, skin, vomiting, nausea, constipation and numbness, 4th include teeth, sad, breath, distress and pain The dendrogram, on the X axis, illustrating the clustering of various symptoms and showing the relative distances between clusters, i.e., those items that join with others earlier within small relative distance scale were rated by patients more similarly and could be interrelated. For example, the symptoms choking, swallow, mucous, taste and dry mouth joined together quickly, indicating that patients perceived and rated these symptoms similarly. Of note, the red squares are more abundant, and representing the severity of 1st symptom cluster which is centered around and representing oral morbidity symptoms across patient group D, C, B and A in order.
Abbreviations: MDASI-HN, MD Anderson Symptom Inventory-Head and Neck.
Correlation of clinical characteristics with oral intake
Advanced T-category (i.e., T3-4) (p=0.0004), presentation with positive lymph nodes (p=0.0001), location of primary site (oropharynx rather than larynx or nasopharynx) (p=0.0005), intensified treatment modalities (IC±CCRT) (p=0.007) were significantly associated with poor oral intake, per FOIS. While age (p=0.649), gender (p=0.177), and surgery (p=0.591) failed to demonstrate an association with oral intake status. Larger T stage and intensified treatment maintained significantly associated with poor oral intake in the multivariate analysis (p=0.0089 and 0.004, respectively).
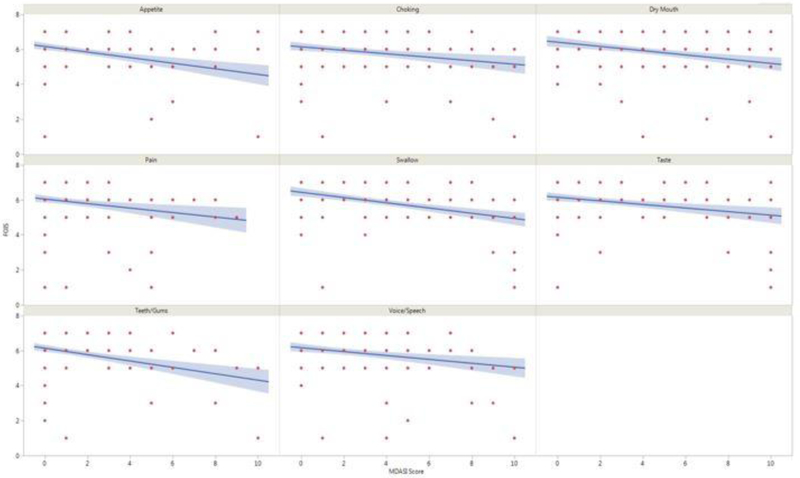
Correlation of MDASI-HN symptom items with oral intake
On bivariate analysis, significant correlations, after Bonferroni correction for multiple comparisons, were identified between 8 MDASI-HN patient-reported symptom items and the FOIS clinician-graded dietary scale ratings as shown in Table 2 and Figure 4. Multivariate models found severity of difficulty swallowing/chewing and problem with gums/teeth were independently associated with oral intake as measured by FOIS. Sensitivity analysis examining symptom severity and oral intake as measured by PSS-HN found similar relationships, Supplementary Figure 1.
Figure 4.
MDASI-HN symptom items with significant correlations to FOIS dietary scale
Abbreviations: FOIS, Functional Oral Intake Scale; MDASI-HN, MD Anderson Symptom Inventory-Head and Neck questionnaire.
Discussion
The current study is a part of a continued interdisciplinary effort at MD Anderson Cancer Center to simultaneously improve survival and functional outcomes in HNC survivors. In the scenario in which disease control and organ preservation can be achieved, maintaining optimal function becomes the ultimate endpoint of HNC treatment. Thus, we aim to involve patients in clinical decisions and rehabilitation strategies by integrating symptom reporting into clinical care. This requires understanding of the functional relevance of various symptom items. In a sample of 123 HNC survivors, we identified the functional relevance of self-reported severity of swallowing and oral morbidity symptoms to impaired oral intake in long-term survivorship. On univariate analysis, symptom severity for difficulty swallowing and oral-related morbidities; diminished appetite, dry mouth, problem with teeth/gums, choking, voice/speech changes, diminished appetite and pain were correlated with poor oral intake. While many symptoms significantly correlated with oral intake on univariate analysis, swallowing impairment and injured gums/missing teeth were identified as independent drivers for poor oral intake per FOIS among long-term HNC survivors in multivariate analysis.
Oral intake profiles during the survivorship period have been previously studied. However, the multi-symptom focus of this work represents a departure from the previously reported single symptom focus. The long-term promise of this work is that multi-symptom tracking may help clinicians and patients to develop more standardized, proactive toxicity management paradigms that accelerate the recovery of oral intake during the surveillance period after RT. By considering the functional impact of multiple symptoms acting in clusters, our approach represents a departure from the status quo of single symptom methodology that does not align with the complexity of the clinical reality either during HNC treatment or the survivorship period.
Abnormal oral intake is a critical manifestation of unavoidable radiotherapy (RT) effects to mucosal, salivary, and soft tissue, previously published to persist in up to 33% of HNC survivors after RT. In long-term survivorship, restricted oral intake, avoidance of social eating, and weight loss have a detrimental influence on the QOL [18] and can also have a negative influence on survival [19], Using standardized clinical tracking of oral intake, we identified only 28% of survivors reporting fully unrestricted oral intake (i.e., 72% with impaired oral intake) suggesting potentially greater prevalence of this problem than previously published. Oral intake was also significantly associated with symptom severity. Thus, standardized tracking of oral intake-related symptoms in HNC survivors may help to provide appropriate interventions to maintain the patient’s health and improve QOL.
These data and others demonstrate that persistent dysphagia is one of the primary symptom drivers of impaired oral intake after RT in HNC survivors. For instance, in cross-sectional study, at a median of 44 months, the severity of patient-reported dysphagia significantly correlated with greater oral intake restrictions (r=0.41) [20], Late dysphagia is mainly thought to impair the range of motion of swallowing structures either by fibrosis [21] or neuropathy [22], These results and others [23] also suggest that oral morbidities significantly influence restricted oral intake in long-term survivorship. Acute mucositis is commonly accepted as a functionally limiting toxicity. However, there is less known about functional relevance of long-term oral morbidities on oral intake.
Severity of oral symptoms (the MDASI-HN item “difficulty with my teeth/gums”) was one of two symptoms that retained in the multivariate model for poor oral intake. This symptom item is rather non-specific, so it is not entirely clear what physical oral morbidity it reflects. Indeed, tooth loss, which could be related to these perceived symptoms as well as periodontal or mucosal oral toxicities that damage the gum [23] may significantly decrease functional mastication [24] and lead to deterioration of the efficiency of food absorption [25] and nutritional status. Patients with injured gums or missing teeth are often forced to modify their diet to include moist, soft foods to facilitate mastication and swallowing. A limitation of this study is inability to specify the most functionally relevant oral morbidity.
A somewhat surprising outcome was that severity of xerostomia symptoms did not significantly associate with oral intake in multivariate models. RT-induced xerostomia (RIX) has been associated with dysphagia, dysphonia, alterations in taste, poor dental health [23,26], Additionally, RIX is commonly implicated as the source of altered intake in survivorship. Xerostomia leads to desiccated mucosal tissues and exacerbates thick mucus secretions. Salivary enzymes initiate the process of digestion of starches and fats during oral intake. When food enters the oral cavity, it is softened and lubricated by salivation and mastication, permitting the food bolus to be propelled easily into the oropharynx. While not an independent driver of oral intake in this model, results of the cluster analysis supported the common clinical observation that HNC patients have substantial individual differences in the severity of their treatment-related symptoms, and they could be grouped [27] as those with either high or low symptom severity based on the symptom cluster profiles [28], Dry mouth symptoms retained in clusters associated with poor oral intake.
The limitations of the current study include those inherent to biases of a single-institution, cross-sectional study in a tertiary cancer hospital. Notable limitations include potential selection biases of our convenience sample of survivors with available data in existing databases, as well as lack of baseline data regarding symptom burden and nutrition status. Historically, prophylactic gastrostomy was often considered mandatory to maintain the nutritional status of patients during and after RT and to minimize treatment interruptions [29,30], Although beneficial for nutritional status, the use of gastrostomy tubes may have a negative impact on long-term swallowing recovery [30], prompting more frequent adoption of reactive feeding tube models in many practices. Simultaneously, many have also adopted proactive swallowing therapy [31] (i.e., “use it or lose it”) models of service. These data support also the functional relevance of integrating dental services not only to oral health but also to oral intake. While not variables in the analysis, all patients were treated in a time in which a reactive feeding tube model as well as proactive speech pathology and dental oncology referrals, were standard in HNC RT at the authors’ institution. Finally, symptoms and functional outcomes are recognized as dose-dependent toxicities of RT in HNC. The probability of restricted oral intake depends on the dose delivered to the non-target irradiated normal structures, especially with the modern RT techniques [32] that allow radiation oncologists to meet normal tissue constraints without compromising the target coverage. While critical covariates, regional doses to normal structures were not available for covariate adjustment.
Conclusions
The current research effort aimed to identify the functional relevance of self-reported symptoms burden on the oral intake status of long-term HNC survivors. We hypothesized that multiple symptoms could share specific underlying dimensions and act as a cluster that could drive the functional outcomes of the cancer survivors. Our approach is aligned with the complexity of the clinical reality either during HNC treatment or the survivorship period. Our study showed that oral intake in HNC survivorship is a multidimensional issue and functional outcome that is not only impacted by dysphagia but also by dental symptoms. Symptom drivers of oral intake likely differ in acute survivorship. Nonetheless, these findings highlight the lack of specificity of oral intake as a dysphagia endpoint as well as the need for multidisciplinary supportive care to optimize oral intake in survivors.
Supplementary Material
MDASI-HN symptom items correlations to Diet score of PSS-HN. Abbreviations: PSSHN, the Performance Scale of Head and Neck Cancer; MDASI-HN, MD Anderson Symptom Inventory-Head and Neck questionnaire.
ACKNOWLEDGEMENT (Funding):
This work was completed with support of the MD Anderson Oro pharynx Program Patient-Reported Outcomes/Function Core. Dr. Hutcheson receives grant support from the Patient-Centered Outcomes Research Institute (PCORI), the MD Anderson Institutional Research Grant Program, and the National Cancer Institute (R03 CA188162). Ors. Fuller and Hutcheson receive funding support from the National Institute for Dental and Craniofacial Research (ROl DE025248 and 1R56DE025248-0l). Dr. Fuller received/receives grant and/or salary support from: the National Institutes of Health/National Cancer lnstitute’s Paul Calabresi Clinical Oncology Award Program (K12 CA088084-06) and Clinician Scientist Loan Repayment Program (L30 CA136381-02); the SWOG/Hope Foundation Dr. Charles A. Coltman, Jr., Fellowship in Clinical Trials; a General Electric Healthcare/MD Anderson Center for Advanced Biomedical Imaging In-Kind Award; an Elekta AB/MD Anderson Department of Radiation Oncology Seed Grant; the Center for Radiation Oncology Research at MD Anderson Cancer Center; and the MD Anderson Institutional Research Grant Program. This work was supported in part by infrastructure support from the National Institutes of Health Cancer Center Support (Core) Grant CA016672 to The University of Texas MD Anderson Cancer Center. These listed funders/supporters played no role in the study design, collection, and analysis, interpretation of data, manuscript writing, or decision to submit this manuscript for publication.
†-. Co-author specific contributions:
All listed co-authors performed the following:
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work;
Drafting the work or revising it critically for important intellectual content;
Final approval of the version to be published;
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Specific additional individual cooperative effort contributions to study/manuscript design/execution/interpretation, in addition to all criteria above are listed as follows:
MK, MPB, AE -Drafted manuscript, undertook supervised analysis and interpretation of data. KH - Corresponding author; primary investigator; conceived, coordinated, and directed all study activities, responsible for data collection, project integrity, data collection infrastructure, programmatic oversight, direct oversight of classified personnel, manuscript content and editorial oversight and correspondence.
SYL, GBG, JSL, DIR, QS, TH - Co-investigators; direct patient care provision, direct toxicity assessment and clinical data collection; interpretation and analytic support MPB, MK, AE - Data coordination, collection, curation, and analysis.
Footnotes
CONFLICT OF INTEREST DISCLOSURES: The authors made no disclosures.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018 (2018) CA Cancer J Clin 68:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2014, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017. [Google Scholar]
- [3].Mason H, DeRubeis MB, Burke N, Shannon M, Karsies D, Wolf G, et al. (2016) Symptom management during and after treatment with concurrent chemoradiotherapy for oropharyngeal cancer: a review of the literature and areas for future research. World J Clin Oncol 7:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Samim F, Epstein JB, Zumsteg ZS, Ho AS, Barasch A (2016) Oral and dental health in head and neck cancer survivors. Cancer Head Neck 1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xiao C, Hanlon A, Zhang Q, Ang K, Rosenthal DI, Nguyen-Tan PF, et al. (2013) Symptom clusters in patients with head and neck cancer receiving concurrent chemoradiotherapy. Oral Oncol 49:360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kubrak C, Olson K, Jha N, Jensen L, McCargar L, Seikaly H, et al. (2010) Nutrition impact symptoms: key determinants of reduced dietary intake, weight loss, and reduced functional capacity of patients with head and neck cancer before treatment. Head Neck 32:290–300. [DOI] [PubMed] [Google Scholar]
- [7].Pauloski BR, Rademaker AW, Logemann JA, Newman L, MacCracken E, Gaziano J, et al. (2006) Relationship between swallow motility disorders on videofluorography and oral intake in patients treated for head and neck cancer with radiotherapy with or without chemotherapy. Head Neck 28:1069–1076. [DOI] [PubMed] [Google Scholar]
- [8].Ganzer H, Touger-Decker R, Parrott JS, Murphy BA, Epstein JB, Huhmann MB (2013) Symptom burden in head and neck cancer: impact upon oral energy and protein intake. Support Care Cancer 21:495–503. [DOI] [PubMed] [Google Scholar]
- [9].Cacicedo J, Dal Pra A, Alongi F, Navarro A (2015) Impact of weight loss in patients with head and neck carcinoma undergoing radiotherapy: is it an underestimated phenomenon? A radiation oncologist’s perspective. Eur J Clin Nutr 69:757. [DOI] [PubMed] [Google Scholar]
- [10].Varkey P, Tang W-R, Tan N (2010) Nutrition in head and neck cancer patients. Semin Plast Surg 24:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ravasco P, Monteiro-Grillo I, Marques Vidal P, Camilo ME (2005) Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck 27:659–668. [DOI] [PubMed] [Google Scholar]
- [12].Alshadwi A, Nadershah M, Carlson ER, Young LS, Burke PA, Daley BJ (2013) Nutritional considerations for head and neck cancer patients: a review of the literature. J Oral Maxillofac Surg 71:1853–860. [DOI] [PubMed] [Google Scholar]
- [13].Crary MA, Mann GDC, Groher ME (2005) Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil 86:1516–520. [DOI] [PubMed] [Google Scholar]
- [14].Righini CA, Timi N, Junet P, Bertolo A, Reyt E, Atallah I (2013) Assessment of nutritional status at the time of diagnosis in patients treated for head and neck cancer. Eur Ann Otorhinolaryngol Head Neck Dis 130:8–14. [DOI] [PubMed] [Google Scholar]
- [15].Newman LA, Vieira F, Schwiezer V, et al. (1998) Eating and weight changes following chemoradiation therapy for advanced head and neck cancer. Arch Otolaryngol Head Neck Surg 124:589–592. [DOI] [PubMed] [Google Scholar]
- [16].Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. (2000) Assessing symptom distress in cancer patients. Cancer 89:1634–1646. [DOI] [PubMed] [Google Scholar]
- [17].List MA, Ritter-Sterr C, Lansky SB (1990) A performance status scale for head and neck cancer patients. Cancer 66:564–569. [DOI] [PubMed] [Google Scholar]
- [18].Jager-Wittenaar H, Dijkstra PU, Vissink A, van der Laan BF, van Oort RP, Roodenburg JL (2011) Malnutrition and quality of life in patients treated for oral or oropharyngeal cancer. Head Neck 33:490–496. [DOI] [PubMed] [Google Scholar]
- [19].Ravasco P, Monteiro-Grillo I, Marques Vidal P, Camilo ME (2005) Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck 27:659–668. [DOI] [PubMed] [Google Scholar]
- [20].van den Berg MGA, Rutten H, Rasmussen-Conrad EL, Knuijt S, Takes RP, van Herpen CML, et al. (2014) Nutritional status, food intake, and dysphagia in long-term survivors with head and neck cancer treated with chemoradiotherapy: a cross-sectional study. Head Neck 36:60–65. [DOI] [PubMed] [Google Scholar]
- [21].Martin M, Lefaix J, Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? (2000) Int J Radiat Oncol Biol Phys 47:277–290. [DOI] [PubMed] [Google Scholar]
- [22].Awan MJ, Mohamed ASR, Lewin JS, Baron CA, Gunn GB, Rosenthal DI, et al. (2014) Late radiation-associated dysphagia (Late-RAD) with lower cranial neuropathy after oropharyngeal radiotherapy: a preliminary dosimetric comparison. Oral oncol 50:746–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Beumer J 3rd, Curtis T, Harrison RE (1979) Radiation therapy of the oral cavity: sequelae and management, part 1. Head Neck Surg 1:301–312. [DOI] [PubMed] [Google Scholar]
- [24].Kimura Y, Ogawa H, Yoshihara A, Yamaga T, Takiguchi T, Wada T, et al. (2013) Evaluation of chewing ability and its relationship with activities of daily living, depression, cognitive status and food intake in the community-dwelling elderly. Geriatr Gerontol Int 13:718–725. [DOI] [PubMed] [Google Scholar]
- [25].Ranawana V, Clegg ME, Shafat A, Henry CJ (2011) Postmastication digestion factors influence glycemic variability in humans. Nutr Res 31:452–459. [DOI] [PubMed] [Google Scholar]
- [26].Bjordal K, Ahlner-Elmqvist M, Hammerlid E, Boysen M, Evensen JF, Biorklund A, et al. (2001) A prospective study of quality of life in head and neck cancer patients. Part II: Longitudinal data. Laryngoscope 111:1440–452. [DOI] [PubMed] [Google Scholar]
- [27].Shi Q, Mendoza TR, Gunn GB, Wang XS, Rosenthal DI, Cleeland CS (2013) Using group-based trajectory modeling to examine heterogeneity of symptom burden in patients with head and neck cancer undergoing aggressive non-surgical therapy. Qual Life Res 22:2331–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rosenthal DI, Mendoza T, Cleeland C (2009) Identifying head and neck cancer patients at risk for high symptom burden during treatment. J Clin Oncol 27:15_suppl, 6066. [Google Scholar]
- [29].Beaver ME, Matheny KE, Roberts DB, Myers JN (2001) Predictors of weight loss during radiation therapy. Otolaryngol Head Neck Surg 125:645–648. [DOI] [PubMed] [Google Scholar]
- [30].Mick R, Vokes EE, Weichselbaum RR, Panje WR (1991) Prognostic factors in advanced head and neck cancer patients undergoing multimodality therapy. Otolaryngol Head Neck Surg 105:62–73. [DOI] [PubMed] [Google Scholar]
- [31].Carroll WR, Locher JL, Canon CL, Bohannon IA, McColloch NL, Magnuson JS (2008) Pretreatment swallowing exercises improve swallow function after chemoradiation. Laryngoscope 118:39–43. [DOI] [PubMed] [Google Scholar]
- [32].MD Anderson Head and Neck Cancer Symptom Working Group (2016) Beyond mean pharyngeal constrictor dose for beam path toxicity in non-target swallowing muscles: dose-volume correlates of chronic radiation-associated dysphagia (RAD) after oropharyngeal intensity modulated radiotherapy. Radiother Oncol 118:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MDASI-HN symptom items correlations to Diet score of PSS-HN. Abbreviations: PSSHN, the Performance Scale of Head and Neck Cancer; MDASI-HN, MD Anderson Symptom Inventory-Head and Neck questionnaire.