Abstract
A commercially available three-step (etch-and-rinse) adhesive was modified by adding chlorhexidine (CHX)-loaded nanotubes (Halloysite®, HNT) at two concentrations (CHX10% and CHX20%). The experimental groups were: SBMP (unmodified adhesive, control), HNT (SBMP modified with HNT), CHX10 (SBMP modified with HNT loaded with CHX10%), and CHX20 (SBMP modified with HNT loaded with CHX20%). Changes in the degree of conversion (DC%), Knoop hardness (KHN), water sorption (WS), solubility (SL), antimicrobial activity, cytotoxicity, and anti-matrix metalloproteinase [MMP-1] activity (collagenase-I) were evaluated. In regards to DC%, two-way ANOVA followed by Tukey’s post-hoc test revealed that only the factor “adhesive” was statistically significant (p<0.05). No significant differences were detected in DC% when 20 s light-curing was used (p>0.05). For Knoop microhardness, one-way ANOVA followed by the Tukey’s test showed statistically significant differences when comparing HNT (20.82±1.65) and CHX20% (21.71±2.83) with the SBMP and CHX10% groups. All adhesives presented similar WS and cytocompatibility. The CHX-loaded nanotube-modified adhesive released enough CHX to inhibit the growth of S. mutans and L. casei. Adhesive eluates were not able to effectively inhibit MMP-1 activity. The evaluation of higher CHX concentrations might be necessary to provide an effective and predictable MMP inhibition.
Keywords: Halloysite, nanotubes, chlorhexidine, dentin, adhesive, matrix metalloproteinase
INTRODUCTION
Among all resin-based restorative materials, dental adhesives play both an important role in the bonding process and the overall success of adhesively-bonded restorations.1 In essence, after phosphoric acid etching, the adhesive is able to create a sealed interface between the methacrylate-based resins and the dentin substrate, penetrating into collagen fibrils and creating the hybrid layer (HL).1,2 Regrettably, even with the continuing advances in the synthesis and development of novel dentin adhesives and improved bonding strategies,3 decreases in dentin bond strength are still reported, thus demonstrating that the established HL may not be as durable as previously assumed.4
The degradation of HL is a complex process resulting from resin material and collagen fibril hydrolysis.5 As a result, the collagen is exposed to external stresses (e.g., hydrolysis) and endogenous proteases, such as matrix metalloproteinases (MMPs), resulting in long-term degradation and clinical failure.5–8 Latent forms of MMPs can be activated under acidic pH (pH 4.5),9–11 which can occur at different stages of caries development and/or after restorative procedures.10 During the caries process, the acid generated from bacterial activity can dissolve the mineral phase of the teeth, and may leave the dentin organic matrix exposed to bacteria-derived enzymes and entombed MMPs.9 In terms of restorative procedures, phosphoric acid etching and the use of etch-and-rinse adhesives (pH ranging from 2.68-4.60) can activate MMPs.6 The slow-release activation (by phosphoric acid)6 or reactivation (by etch-and-rinse adhesives)6 of these host-derived proteases can result in loss of bonding effectiveness, even in the absence of bacterial colonization.12
To arrest enzymatic bond degradation and maintain long-term stability of resin-dentin bonds, that is, to prevent collagen matrix degradation, chlorhexidine (CHX) has been investigated as a potential strategy.13–16 However, dissolving CHX into the resin blend at concentrations higher than 1% can significantly compromise adhesive mechanical properties.17 Thus, only small volumes of CHX can be incorporated into the adhesive, limiting clinical outcome. According to recent studies, the incorporation of Halloysite® nanotubes (HNTs) into dentin adhesives improves the adhesive’s mechanical properties and also serves as a reservoir for the sustained release of therapeutic agents.18–22 Thus, the purpose of this study was to fine-tune the nanotube loading method by adding CHX at distinct concentrations and evaluate whether the addition of CHX-loaded nanotubes would affect the physicochemical, mechanical, and biological properties of the adhesives.
MATERIALS AND METHODS
Halloysite® nanotubes morphology
Transmission electron microscopy (TEM) analysis was carried out to examine the morphology of the aluminosilicate clay nanotubes (Al2Si2O5(OH)4.nH2O) [HNT], Dragonite 1415JM, Applied Minerals Inc., New York, NY, USA).22 Briefly, HNT powder was placed in the water bath of the microtome (Diatome, Electron Microscopy Sciences, Hatfield, PA, USA). HNTs were picked up on Cu grids under the microscope and then imaged at 80 kV using a transmission electron microscope (TEM, Tecnai BioTWIN, FEI, Hillsboro, OR, USA).22
Halloysite® nanotubes loaded with CHX
Chlorhexidine digluconate solution 20% in H2O (Lot #BCBM3595V, Sigma-Aldrich, St. Louis, MO, USA) was loaded into the nanotubes at two concentrations: 10 wt.% and 20 wt.%. To obtain CHX10%, the CHX solution was dissolved in distilled water. HNT loading was performed following a well-established protocol.19,22 For loading, 1.25 g of HNT and 5 ml of CHX solution (10% or 20%) were centrifuged, vortexed (20 s), and sonicated (2 h). To minimize any air between and within the HNTs, the solutions were placed in a vacuum (25 in. Hg) chamber for 1 h. Next, the solutions were mixed for 1 h and vacuum was reapplied. Finally, the HNT+CHX solutions were centrifuged (3000 rpm) for 10 min. The mixed material was stored at 37ºC for 7 days. After drying, the powder was sieved at 45 μm and the CHX+HNT powder was then obtained.20,22
Experimental CHX-loaded nanotube adhesive fabrication
A 3-step etch-and-rinse dental adhesive (Adper Scotch Bond Multi-Purpose [SBMP]; 3M ESPE, St. Paul, MN, USA) was selected based on our previous knowledge.20,22 Four experimental groups were tested: SBMP (unmodified adhesive, control), HNT (SBMP modified with 15 wt.% HNT, a concentration previously optimized19), CHX10 (SBMP modified with 15wt.% of HNT loaded with CHX10%), and CHX20 (SBMP modified with 15wt.% of HNT loaded with CHX20%). Noteworthy, for the HNT-containing groups, dried HNT+CHX (10% and 20%) powder was incorporated into the adhesive and mixed for 24 h. The adhesive manipulation and all specimens were prepared under constant temperature and a filtered light system to minimize unintentional polymerization.18–22
Degree of conversion (DC)
DC was performed to determine whether the incorporation of CHX-loaded nanotubes could affect adhesive polymerization. Disks-shaped specimens (7 mm × 0.24 mm; n=3 for each group and time) were obtained and distinct curing times were tested: 10 s (manufacturer’s recommendation) and 20 s.19–22 A light-emitting diode curing system (DEMI LED, Kerr, Orange, CA, USA) was used. The irradiance was monitored ~ 1,500 mW/cm2 (Managing Accurate Resin Curing, MARC-RC Calibrator, BlueLight Analytics Inc., Halifax, Nova Scotia, Canada). DC was evaluated with Fourier transform infrared spectroscopy (FTIR) in attenuated total reflection mode.19–22 Three initial readings were taken for the unpolymerized adhesive and three readings per specimen were also taken for the cured sample. The absorbance bands at 1637 cm−1 (methacrylate group) and 1607 cm−1 (aromatic ring in Bis-GMA) were used to calculate the DC (%), according to the following equation (Eq. 1)19–23
Knoop Hardness (KHN)
Disk-shaped specimens (15 mm in diameter × 1 mm thick) were fabricated for each adhesive group (n = 5) using a Teflon® mold. The control and experimental adhesives were light-cured for 10 s on the top and bottom surfaces of the disks. Next, the specimens were embedded in epoxy resin (EpoxiCure™, Buehler, Lake Bluff, IL, USA) using an acrylic matrix (19 mm in diameter × 12.7 mm thick), and after curing, they were stored at 37°C for 24 h. The specimens were wet-finished with 600-grit SiC paper (Buehler), polished with a polycrystalline diamond suspension (Buehler), and sonicated in distilled water for 5 min. The specimens were subjected to hardness testing (M-400, LECO Corporation, St. Joseph, MI, USA) using a Knoop diamond indenter (50 kg load and 15 s dwell time). Five readings were obtained from each specimen. The diagonal lengths were measured immediately after each indentation. The values were converted to KHN numbers (kg/mm2).19–22
Water sorption and solubility
Five disk-shaped specimens (11.9 mm in diameter × 1 mm thick) per group were prepared based on ISO4049:2009(E) (International Organization for Standardization, ISO).24 Briefly, the adhesive was dispensed into a mold and a polyester strip was carefully placed on top to avoid bubbles. According to the ISO specification, a glass slide was placed on top of the mold with the polyester strip, and the disk was light-cured for 10 s at 9 different points (top and bottom).24 The disk was removed from the mold and the periphery was finished using 180-grit SiC paper. The disks were transferred to a desiccator and maintained at 37°C for 22 h (Heratherm Oven, Thermo Fisher Scientific Inc., Waltham, MA, USA). Next, the disks were placed in a vacuum chamber and maintained at room temperature for 2 h. They were weighed to an accuracy of 0.1 mg (m1) using an analytical balance (Mettler-Toledo International Inc., Columbus, OH, USA). Once constant mass was achieved, the diameter and thickness of each disk was measured and the area (mm2) and volume (mm3) were calculated. The specimens were individually immersed in 10 ml of distilled water at 37°C for 7 d. After the immersion period, the specimens were gently wiped (Kimwipes, Kimberly-Clark Professional, Roswell, GA, USA) until they presented no visible moisture and then weighed (m2). The disks were maintained at 37°C and weighed weekly until a constant mass (m3) was reached. To calculate water sorption (WS, μg/mm3) and solubility (SL, μg/mm3), the following equations (Eq. 2 and Eq. 3) were applied:
Antimicrobial properties of CHX-loaded nanotube-modified adhesives
The antimicrobial properties of the experimental CHX-loaded nanotube-modified dentin adhesives and control groups (SBMP and HNT) were determined against Streptococcus mutans (S. mutans, UA159) and Lactobacillus casei (L. casei, ATCC 393) through agar diffusion assays.20,22 Three disk-shaped specimens (6.2 mm diameter × 1 mm thick) were prepared for each group using a Teflon® mold and light-cured (10 s per each side).20,22 The specimens were kept at 37°C for 24 h and disinfected using UV light (30 min per each side). S. mutans and L. casei were cultured in tryptic soy broth (Difco Laboratories Inc., Detroit, MI, USA) that was freshly prepared in 5% CO2 at 37°C. After 24 h, 100 μl of the bacterial suspension was swabbed onto blood agar plates to create a bacterial lawn.20,25,26 The adhesive-disks were placed directly onto the plate. Each plate contained SBMP, HNT, CHX10%, CHX20% disks, and 10 μl of CHX 0.12% (positive control). The agar plates were incubated in 5% CO2 at 37°C for 24 h and the inhibition zones (mm) measured.
Cytotoxicity test
For the cytotoxicity test, a colorimetric assay was carried out.21,22 The adhesive disk specimens (n=3/group; 6.2 mm diameter × 1 mm thick) were made, kept at 37°C for 24 h, and disinfected using UV light (30 min per each side). Low-glucose Dulbecco modified Eagle medium (DMEM, Gibco Invitrogen Corporation, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone Laboratories Inc., Logan, UT, USA) and antibiotic mix (Penicillin G and Gentamicin) were used for the stored specimens and cells. The disinfected specimens were individually stored in an sterile glass container with 15.1 ml of the medium and kept in a shaker at 37°C under 45 rpm. The cytotoxicity of the CHX-loaded nanotube-modified adhesive and control groups (SBMP and HNT) were evaluated using human dental pulp stem cells (hDPSC, AllCells, Alameda, CA, USA). The positive control was a 0.3 vol% phenol solution and the negative control was the medium with and without cells. Aliquots of the specimens/medium were collected after 24 h.21,22 The specimens/medium were tested according to the dilution: 0 (no dilution), 32, 64, and 128 × dilution in the cell-culture medium, respectively. The cells were seeded at a density of 3 ×103/well in 96-well plates. Each well received 100 μl of the collected aliquot for each dilution and was tested in triplicate. The plate was incubated at 37°C in a 5% CO2 humidified atmosphere. After 48 h, the solution reagent (WST-1, Roche Diagnostics, Indianapolis, IN, USA) was added to the well and incubated for 1 h and then the absorbance was read at 450 nm in a microplate reader against blank wells.21,22
Anti-MMP activity of CHX-loaded nanotube-modified adhesives
Fluorescein isothiocyanate (FITC)-labeled type I collagen cleavage assay was used to determine the anti-MMP activity of CHX released from CHX-loaded nanotube-modified adhesives collected in a Tris buffer for 14 days. Disk-shaped specimens (n=4/group) were obtained, as previously detailed. The specimens were individually incubated at 37°C in 1 mL of PBS. Aliquots (150 μL) were collected at days 1, 7, and 14. An equal volume of buffer was added to keep the volume constant. Aliquots were stored at −20°C until use.22 Type I collagen derived from rat-tail tendon (RTT) was purified and labeled with FITC (Sigma-Aldrich).27 Human pro-matrix metalloproteinase 1 (0.05 mg/mL, Abcam, Cambridge, MA, USA) was activated with 4-aminophenylmercuric acetate (APMA, Sigma-Aldrich) prior to use.28
All eluates (150 μL, n=4/group) were incubated with APMA-activated MMP-1 for 30 min at room temperature. Tris buffer (200 μL) and FITC-labeled type I collagen (50 μL) were added and the mixture was incubated at 37°C for 120 min. The negative and positive controls were Tris buffer and a 0.1% DOX solution, respectively. Samples (70 μL) were periodically (0, 30, 60, 90, and 120 min) removed and the reaction was terminated by the addition of 1,10-phenanthroline (200 mM, 10 μL) to a final concentration of 25 mM. Reducing SDS–PAGE sample buffer was added (1:1); samples boiled for 10 min, and then resolved in 8% SDS–PAGE (150 V, 3 h). The fluorescent signals were captured using the Bio-Rad imaging system (Gel Doc™ XR imaging system). The fluorescence intensity at 0 min and 120 min incubation was analyzed with ImageJ software (National Institutes of Health, Bethesda, MD, USA). The percent of FITC-labeled RTT of buffer, 0.1% DOX (positive control), and the eluate collected from the adhesives was calculated as follows:22
The percent (%) of MMP inhibition was calculated using the following equation:
Statistical Analysis
The degree of conversion (curing time × group), cytotoxicity (dilution × group), and anti-MMP activity (group × period) data was submitted to Two-way Analysis of Variance (Two-way ANOVA). Knoop microhardness, water sorption and solubility, and antimicrobial data were submitted to One-way Analysis of Variance (One-way ANOVA). The Tukey’s test (p<0.05) was used for post-hoc comparisons.
RESULTS
Halloysite® nanotubes morphology
The TEM micrograph shows that the HNT powder consisted of cylinders with a diameter of approximately 70 nm and 400 nm in length (Fig. 1).
FIGURE 1.
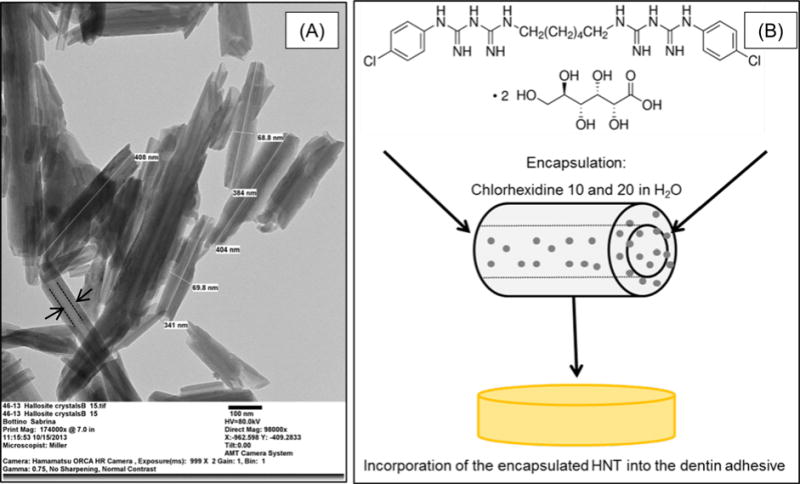
(A) Representative TEM micrograph of the Halloysite® aluminosilicate clay nanotubes (HNTs). The measurements indicate the estimated external length (384.5±30.21nm) and diameter (69.3±0.7nm). Note that it is possible to observe the HNT lumen (→) that was used as a reservoir for CHX. (B) Schematic illustration of the proposed system. HNT as a nanocontainer for CHX release.
Degree of conversion and Knoop Hardness
The DC and KHN results are presented in Table 1. In regards to DC%, 2-way ANOVA followed by Tukey’s post-hoc test revealed that only the factor “adhesive” was significant (p<0.05). No statistically significant differences were detected in DC% when 20 s light-curing was used (p>0.05). Noteworthy, after collecting the data, the curing time was adjusted to 10 s on the top and bottom surfaces to prepare the adhesive samples for all additional studies, including hardness. One-way ANOVA followed by Tukey’s post-hoc test showed statistically significant differences when comparing HNT (20.82±1.65) and CHX20% (21.71±2.83) with the SBMP (14.81±1.49) and CHX10% (16.22±1.44) groups.
Table 1.
Data (Mean±SD) of Degree of conversion (DC), Knoop microhardness (KHN), Water sorption (WS) and Solubility (SL) of the adhesives.*
| Group | DC** (%) |
KHN (kg/mm2) |
Water Sorption (μg/mm3) |
Solubility (μg/mm3) |
|---|---|---|---|---|
| SBMP | [10 s] 67.00 (±0.94)B | 14.81 (±1.49)A | 95.37 (±5.39) | −4.91 (±2.41)b |
|
[20 s] 73.07 (±3.11)A,B
| ||||
| HNT | [10 s] 77.63 (±2.98)A | 20.82 (±1.65)B | 95.72 (±22.45) | −4.50 (±1.15)b |
|
[20 s] 78.41 (±3.12)A
| ||||
| CHX10% | [10 s] 72.78 (±3.81)A,B | 16.22 (±1.44)A | 109.27 (±12.20) | −1.07 (±2.17)ab |
|
[20 s] 70.18 (±2.48)B
| ||||
| CHX20% | [10 s] 68.22 (±0.48)B | 21.71 (±2.83)B | 110.80 (±3.04) | 1.87 (±4.29)a |
| [20 s] 68.97 (±1.55)B |
Different letters indicate statistical differences. The absence of letters means no significant difference between groups.
Distinct letters indicate statistical differences depicted by 2-Way (factors adhesive and time) ANOVA with Tukey’s post-hoc test. Note that only the factor “adhesive” was statistically significant.
Water sorption and solubility
The results of WS and SL are shown in Table 1. In regards to WS, no statistically significant differences among the groups were noted. Concerning SL, CHX20% (1.87 ± 4.29 μg/mm3) presented the highest mean value, which was statistically different from SBMP (−4.91 ± 2.41 μg/mm3) and HNT (−4.50 ± 1.15 μg/mm3).
Antimicrobial properties of CHX-loaded nanotube-modified adhesives
Figure 2 illustrates the antimicrobial activity of all adhesive groups against S. mutans and L. casei. The data showed that the groups CHX10% and CHX20% were the only groups capable of inhibiting bacterial growth around the adhesive disks, comparable to the positive control group (CHX 0.12%). The data collected from the groups CHX10% and CHX20% and the control group were submitted to statistical analysis. No statistically significant difference between CHX10% and CHX20% was detected, but both groups presented statistically significant differences (p˂0.05) when compared to CHX 0.12%.
FIGURE 2.
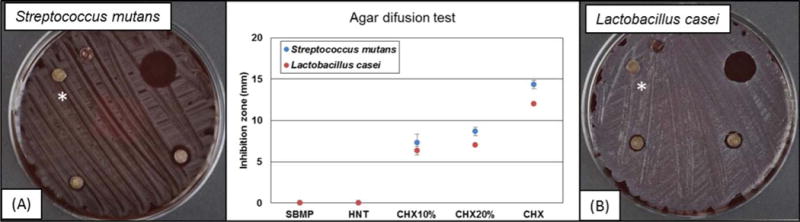
Antimicrobial activity: Representative macrophotographs of blood agar plates against (A) S. mutans and (B) L. casei. CHX (chlorhexidine at 0.12%) was used as the positive control. *Groups from left to right: HNT(*): nanotubes mixed with the dentin adhesive SBMP; SBMP: unmodified dentin adhesive (control group); CHX: 10 μl of CHX at 0.12% (positive control); CHX10%: CHX-loaded nanotube-modified adhesive; and CHX20%: CHX-loaded nanotube-modified adhesive. (Chlorhexidine – Sigma Aldrich)
Cytotoxicity test
The results of cytotoxicity for each adhesive group (SBMP, HNT, CHX10%, and CHX20%) and for the positive control (0.3 vol.% phenol solution) are shown in Figure 3. No statistically significant differences between the experimental adhesives (HNT, CHX10%, and CHX20%) and the control (SBMP) were noted.
FIGURE 3.
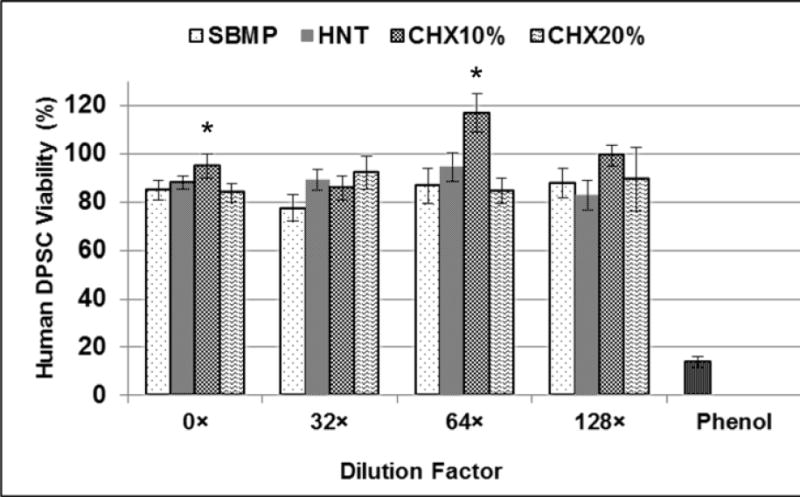
Cytotoxicity (Mean±SD) of adhesive resin eluates released from adhesive disk-shaped specimens tested on hDPSC (% viability). No significant differences (p=0.133) were found comparing all groups and dilutions tested (Two-way ANOVA).
Adhesive eluates against MMP-1 activity
No statistical differences in MMP-1 inhibition were detected between the experimental (CHX) and control (SBMP) groups when compared to the positive control (0.1% DOX) (Figure 4 and Table 2).
FIGURE 4.
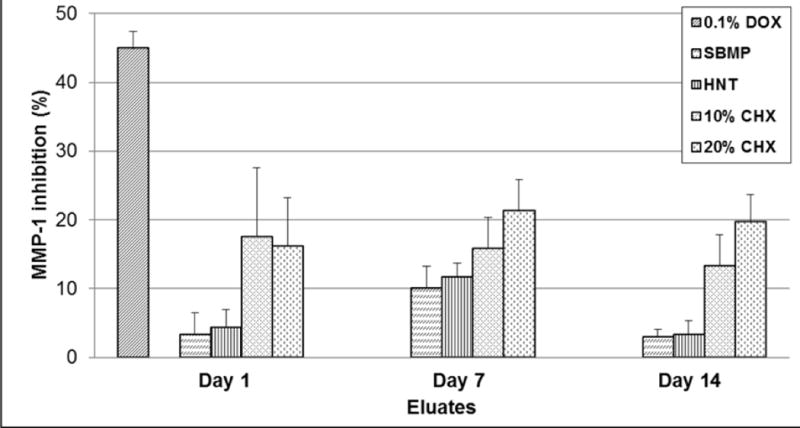
Anti-MMP-1 activity (Mean±SE) of eluates collected from CHX-loaded nanotube-modified adhesives and control groups determined by the FITC-labeled type I collagen assay. No significant differences were found comparing all groups.
Table 2.
Anti MMP-1 (collagenase 1) activities of the CHX-loaded nanotube-modified adhesive eluates (Mean±SE). No statistically significant difference was detected among the groups (p˃0.05).
| Average inhibition of adhesive eluates (%) | |||
|---|---|---|---|
| Group | Day 1 | Day 7 | Day 14 |
| 0.1%DOX | 44.92 ± 2.46 | ||
| SBMP | 3.39 ± 3.12 | 10.15 ± 3.13 | 3.00 ± 1.11 |
| HNT | 4.39 ± 2.60 | 11.73 ± 1.95 | 3.41 ± 1.98 |
| CHX10% | 17.60 ± 10.02 | 15.91 ± 4.46 | 13.35 ± 4.53 |
| CHX20% | 16.18 ± 7.07 | 21.31 ± 4.58 | 19.74 ± 3.97 |
The absence of letters means no significant difference between groups
DISCUSSION
Our research group has been investigating the application of Halloysite® nanotubes (HNTs) in the field of adhesive dentistry over the past several years.18–22 As previously stated, HNTs present nano-scale features, with a nano-lumen that can be loaded for controlled release of therapeutic agents.18–22, 29–32 The TEM micrograph clearly illustrates these features: HNT powder showed a similarity to cylinders with an external diameter and a length ranging from 68.8–69.8 nm and 384-408 nm, respectively. The lumen diameter was not measured, but similar aluminosilicate clay nanotubes have been reported to present an internal diameter of ca. 10-15 nm.29–32
Fourier transform infrared spectroscopy (FTIR) was used to evaluate possible detrimental effects on polymerization after incorporation of the CHX-loaded nanotube into the dentin adhesive by determining the degree of conversion. According to the statistical analysis, only the factor “adhesive” was significant. Taken together, the data suggest that during adhesive fabrication, a homogeneous dispersion of the nanotubes into the adhesive resin was obtained, thus enabling the curing light to pass through the adhesive sample in the same fashion as the control group and HNT-modified adhesives. Moreover, the results showed that the CHX volume (10% or 20%) incorporated into HNTs did not compromise the adhesive color, an important issue that was previously reported when the nanotube was loaded with doxycycline.20,22
As an inorganic reinforcing agent, selected physico-mechanical properties and cytocompatibility of the adhesive were found to not deteriorate when 10-20 wt.% of HNT powder was added.19–21 As shown in Table 1, the incorporation of 15 wt.% HNT improved the adhesive hardness (HNT>SBMP). When HNT was added to the commercial adhesive (SBMP, KHN=14.81±1.49) for both the HNT (KHN=20.82±1.65) and CHX20% (KHN=21.71±2.83) groups, hardness was improved. Nevertheless, the dispersion of the HNT powder into the adhesive might be critical to ensure that all groups present the same behavior, since CHX10% was the only group modified with HNT that presented statistically similar hardness values (KHN=16.22±1.44) when compared to the control (SBMP).
Water sorption and solubility of resin-based materials can affect some mechanical properties, such as the modulus of elasticity, yield strength, flexural strength, and roughness.33,34 Therefore, it would be desirable for the adhesive physical properties to not be jeopardized by the incorporation of CHX-loaded nanotubes. Although numerically higher, no statistical differences in WS were found between the control (95.37 μg/mm3) and experimental groups (95.72 - 110.80 μg/mm3). A previous report has shown greater values of WS and SL for the 3-step etch-and-rinse adhesive (SBMP) when compared to 2-step and self-etch adhesives.35 Higher solubility (wt.% loss) was presented by the CHX20% group. Collectively, our data suggest that, under the conditions tested, the addition of CHX-loaded nanotubes appears not to change the adhesive integrity, since low or no interference in the solubility and water sorption was found.
The chemical agent selected for loading into the nanotubes, CHX, is an important synthetic antimicrobial from the biguanide family; it has high broad-spectrum efficacy and substantivity.36 CHX works fast on bacteria by damaging the outer cell layers, crossing the outer membrane (by passive diffusion), and subsequently attacking the bacteria’s inner membrane.36 In adhesive dentistry, this biguanide has been used on dentin prior to the bonding protocol to prevent bonding degradation.37–40
Our data show that CHX-loaded nanotube-modified adhesive contains enough CHX to inhibit, under direct contact, the growth of S. mutans and L. casei. The data is in agreement with a previous report, where doxycycline (DOX) was loaded into HNTs and mixed into the adhesive and tested with S. mutans.20,22 Moreover, all the adhesives tested were considered non-toxic to DPSCs (Figure 3). This finding is in agreement with a previous study, which confirmed that HNTs in concentrations up to 75 μg/mL are cytocompatible.41
To preserve the HL, studies have applied CHX as a potential MMP inhibitor.39,40,42,43 A previous study,43 reported that when dentin was exposed to a 0.2% CHX solution after the etching protocol and before application of the dentin adhesive, no improvement or adverse effects on bond strength were noted; however, another group,39 found that the use of 2% CHX, associated or not with phosphoric acid preserved resin-dentin bond durability.
MMPs are a family of zinc-dependent proteinases44 that are synthesized and secreted as proenzymes into the extracellular cellular matrix by endogenous tissue cells and some types of hematopoietic cells9,44 for tissue remodeling.9,45,46 Among metalloproteinases, MMP-1, as used in the present study, is found in high and low concentrations in the deep and superficial layers of human coronal dentin, respectively.47 The anti-MMP-1 activity of eluates from dentin adhesive, as determined by the FITC-labeled type I collagen assay, presented no statistically significant difference when HNTs were loaded with CHX at 10 or 20 wt.%. These findings suggested that the levels of CHX released from the experimental CHX-loaded modified-adhesive were not sufficient to suppress collagenase-I activity. Previous findings48 have suggested that CHX could inhibit MMP-2, -8, and -9 under certain concentrations, possibly acting via a cation-chelating mechanism. An important concern from our MMP results was that all the experimental groups presented a higher MMP-inhibition for eluates collected after seven days of incubation. One possible explanation is based on the suggestion that the concentration of MMP-2, -8, and -9 could decrease after dentin-coronal treatment with different adhesive types and after aging.49 Conceivably, some commercially available dentin adhesives could present a selective anti-MMP activity when in contact with coronal-dentin. In the present study, this activity was detected only on eluates collected after 7 days of incubation. Anti-MMP activity (Figure 4 and Table 2) showed that the HNT-CHX-containing groups achieve higher means than the control group. We speculate that the amount of CHX released by the sample into the medium was not consistent for all the evaluated specimens. Increasing concentrations of CHX might be needed to provide an effective and predictable inhibition of MMPs.
In sum, it is fair to state that Halloysite® nanotubes are a suitable reservoir for the loading and releasing of CHX, since its release was confirmed with the display of an antimicrobial action (agar diffusion assays). Our group has demonstrated how to successfully load CHX into Halloysite® nanotubes. However, the findings of the present study cannot support whether the amount of CHX released was enough to consistently inhibit MMP-1. The statistical analysis could not detect differences among the groups, mostly as a result of the high standard error. Although adhesive eluates showed promising MMP-1 inhibition, the actual amount of CHX concentration was not determined. The kinetics of CHX release from the adhesives will be quantified using high performance liquid chromatography (HPLC) or Mass spectrometry in future studies to provide a solid basis for the design of more clinically relevant assays, such as on the dentinal MMPs activity using zymographic analysis, as well as long-term evaluation of resin-dentin bond strength stability.
CONCLUSION
Our data suggested that:
No detrimental differences were found in the degree of conversion, Knoop microhardness, water sorption, and cytotoxicity when the HNT-modified adhesives were compared to the unmodified (SBMP) commercial adhesive;
The CHX-loaded nanotube-modified adhesive presented sufficient release of CHX to inhibit the growth of S. mutans and L. casei;
Halloysite® is a suitable reservoir for the loading and release of CHX; however, they did not consistently promote MMP-1 inhibition.
Acknowledgments
Sabrina A. Feitosa would like to thank the International Association for Dental Research and G.C. Corporation for the Toshio Nakao Fellowship 2015 and also Dr. Ivan Balducci for his assistance with statistical analyses.
References
- 1.Nakabayashi N, Nakamura M, Yasuda N. Hybrid layer as a dentin-bonding mechanism. J Esthet Dent. 1991;3(4):133–138. doi: 10.1111/j.1708-8240.1991.tb00985.x. [DOI] [PubMed] [Google Scholar]
- 2.Frankenberger R, Pashley DH, Reich SM, Lohbauer U, Petschelt A, Tay FR. Characterization of resin-dentine interfaces by compressive cyclic loading. Biomaterials. 2005;26(14):2043–2052. doi: 10.1016/j.biomaterials.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Breschi L, Mazzoni A, Nato F, Carrilho M, Visintini E, Tjäderhane L, Ruggeri A, Jr, Tay FR, Dorigo E, de S, Pashley DH. Chlorhexidine stabilizes the adhesive interface: a 2-year in vitro study. Dent Mater. 2011;26(4):320–325. doi: 10.1016/j.dental.2009.11.153. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Tjäderhane L, Breschi L, Mazzoni A, Li N, Mao J, Pashley DH, Tay FR. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res. 2011;90(8):953–968. doi: 10.1177/0022034510391799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frassetto A, Breschi L, Turco G, Marchesi G, Di Lenarda R, Tay FR, Pashley DH, Cadenaro M. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability-A literature review. Dent Mater. 2016;32(2):41–53. doi: 10.1016/j.dental.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Mannello F, Tjäderhane L, Toledano M, Pashley EL, Tay FR. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials. 2006;27(25):4470–4476. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 7.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83(3):216–221. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 8.Pashley DH, Tay FR, Breschi L, Tjäderhane L, Carvalho RM, Carrilho M, Tezvergil-Mutluay A. State of the art etch-and-rinse adhesives. Dent Mater. 2011;27(1):1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S. The role of matrix metalloproteinases (MMPs) in human caries. J Dent Res. 2006;85(1):22–32. doi: 10.1177/154405910608500104. [DOI] [PubMed] [Google Scholar]
- 10.Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J Dent Res. 1998;77(8):1622–1629. doi: 10.1177/00220345980770081001. [DOI] [PubMed] [Google Scholar]
- 11.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 12.Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol. 2007;52(2):121–127. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol ILS, Geraldeli S, Tezvergil-Mutluay A, Carrilho M, Carvalho RM, Tay FR, Pashley DH. Optimizing dentin bond durability: strategies to prevent hydrolytic degradation of the hybrid layer. Dent Mater. 2013;29(10):999–1011. doi: 10.1016/j.dental.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng P, Chen H. Evaluate the effect of different mmps inhibitors on adhesive physical properties of dental adhesives, bond strength and mmp substarte activity. Sci Rep. 2017;7(1):4975. doi: 10.1038/s41598-017-04340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.da Silva EM, de Sá Rodrigues CU, de Oliveira Matos MP, de Carvalho TR, dos Santos GB, Amaral CM. Experimental etch-and-rinse adhesive systems containing MMP-inhibitors: Physicochemical characterization and resin-dentin bonding stability. J Dent. 2015;43(12):1491–7. doi: 10.1016/j.jdent.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 16.De Munck J, Van den Steen PE, Mine A, Van Landuyt KL, Poitevin A, Opdenakker G, Van Meerbeek B. Inhibition of enzymatic degradation of adhesive-dentin interfaces. J Dent Res. 2009;88(12):1101–6. doi: 10.1177/0022034509346952. [DOI] [PubMed] [Google Scholar]
- 17.Cadenaro M, Pashley DH, Marchesi G, Carrilho M, Antoniolli F, Mazzoni A, Tay FR, Di Lenarda R, Breschi L. Influence of chlorhexidine on the degree of conversion and E-modulus of experimental adhesive blends. Dent Mater. 2009;25(10):1269–74. doi: 10.1016/j.dental.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Alkatheeri MS, Palasuk J, Eckert GJ, Platt JA, Bottino MC. Halloysite nanotube incorporation into adhesive systems-effect on bond strength to human dentin. Clin Oral Investig. 2015;19(8):1905–1912. doi: 10.1007/s00784-015-1413-8. [DOI] [PubMed] [Google Scholar]
- 19.Bottino MC, Batarseh G, Palasuk J, Alkatheeri MS, Windsor LJ, Platt JA. Nanotube-modified dentin adhesive–physicochemical and dentin bonding characterizations. Dent Mater. 2013;29(11):1158–1165. doi: 10.1016/j.dental.2013.08.211. [DOI] [PubMed] [Google Scholar]
- 20.Feitosa SA, Palasuk J, Kamocki K, Geraldeli S, Gregory RL, Platt JA, Windsor LJ, Bottino MC. Doxycycline-encapsulated nanotube-modified dentin adhesives. J Dent Res. 2014;93(12):1270–1276. doi: 10.1177/0022034514549997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feitosa SA, Münchow EA, Al-Zain AO, Kamocki K, Platt JA, Bottino MC. Synthesis and characterization of novel halloysite-incorporated adhesive resins. J Dent. 2015;43(11):1316–1322. doi: 10.1016/j.jdent.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Palasuk J, Windsor LJ, Platt JA, Lvov Y, Geraldeli S, Bottino MC. Doxycycline-loaded nanotube-modified adhesives inhibit MMP in a dose-dependent fashion. Clin Oral Investig. 2017 doi: 10.1007/s00784-017-2215-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bourbia M, Ma D, Cvitkovitch DG, Santerre JP, Finer Y. Cariogenic bacteria degrade dental resin composites and adhesives. J Dent Res. 2013;92(11):989–994. doi: 10.1177/0022034513504436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International Organization for Standardization ISO 4049:2009(E): Dentistry – Polymer-based restorative materials. 2009 [Google Scholar]
- 25.Gregson KS, Shih H, Gregory RL. The impact of three strains of oral bacteria on the surface and mechanical properties of a dental resin material. Clin Oral Investig. 2012;16(4):1095–1103. doi: 10.1007/s00784-011-0613-0. [DOI] [PubMed] [Google Scholar]
- 26.Huang R, Li M, Gregory RL. Effect of nicotine on growth and metabolism of Streptococcus mutans. Eur J Oral Sci. 2012 Aug;120(4):319–325. doi: 10.1111/j.1600-0722.2012.00971.x. [DOI] [PubMed] [Google Scholar]
- 27.Birkedal-Hansen H, Yamada S, Windsor J, Poulsen AH, Lyons G, Stetler-Stevenson W, Birkedal-Hansen B. Matrix metalloproteinases. Curr Protoc Cell Biol. 2003 doi: 10.1002/0471143030.cb1008s17. Chapter 10: Unit 10.8. [DOI] [PubMed] [Google Scholar]
- 28.Windsor LJ, Steele DL, LeBlanc SB, Taylor KB. Catalytic domain comparisons of human fibroblast-type collagenase, stromelysin-1, and matrilysin. Biochim Biophys Acta. 1997;1334(2–3):261–272. doi: 10.1016/s0304-4165(96)00102-x. [DOI] [PubMed] [Google Scholar]
- 29.Fu Y, Zhao D, Yao P, Wang W, Zhang L, Lvov Highly aging-resistant elastomers doped with antioxidant-loaded clay nanotubes. ACS Appl Mater Interfaces. 2015 Apr 22;7(15):8156–8165. doi: 10.1021/acsami.5b00993. [DOI] [PubMed] [Google Scholar]
- 30.Lvov YM, Shchukin DG, Möhwald H, Price RR. Halloysite clay nanotubes for controlled release of protective agents. ACS Nano. 2008;2(5):814–820. doi: 10.1021/nn800259q. [DOI] [PubMed] [Google Scholar]
- 31.Tully J, Yendluri R, Lvov Y. Halloysite Clay Nanotubes for Enzyme Immobilization. Biomacromolecules. 2016;17(2):615–621. doi: 10.1021/acs.biomac.5b01542. [DOI] [PubMed] [Google Scholar]
- 32.Lvov Y, Wang W, Zhang L, Fakhrullin R. Halloysite Clay Nanotubes for Loading and Sustained Release of Functional Compounds. Adv Mater. 2016;28(6):1227–1250. doi: 10.1002/adma.201502341. [DOI] [PubMed] [Google Scholar]
- 33.Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, Rueggeberg FA, Foulger S, Saito T, Nishitani Y, Yoshiyama M, Tay FR, Pashley DH. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials. 2005;26(33):6449–6459. doi: 10.1016/j.biomaterials.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 34.Vitória LA, Aguiar TR, Santos PR, Cavalcanti AN, Mathias P. Changes in water sorption and solubility of dental adhesive systems after cigarette smoke. ISRN Dent. 2013 Jul 28; doi: 10.1155/2013/605847. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fabre HS, Fabre S, Cefaly DF, de Oliveira Carrilho MR, Garcia FC, Wang L. Water sorption and solubility of dentin bonding agents light-cured with different light sources. J Dent. 2007;35(3):253–258. doi: 10.1016/j.jdent.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 36.McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999 Jan;12(1):147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ali AA, El Deeb HA, Badran O, Mobarak EH. Bond durability of self-etch adhesive to ethanol-based chlorhexidine pretreated dentin after storage in artificial saliva and under intrapulpal pressure simulation. Oper Dent. 2013 Jul-Aug;38(4):439–446. doi: 10.2341/12-251-L. [DOI] [PubMed] [Google Scholar]
- 38.Sacramento PA, de Castilho AR, Banzi EC, Puppi-Rontani RM. Influence of cavity disinfectant and adhesive systems on the bonding procedure in demineralized dentin - a one-year in vitro evaluation. J Adhes Dent. 2012;14(6):575–583. doi: 10.3290/j.jad.a28730. [DOI] [PubMed] [Google Scholar]
- 39.Stanislawczuk R, Amaral RC, Zander-Grande C, Gagler D, Reis A, Loguercio AD. Chlorhexidine-containing acid conditioner preserves the longevity of resin-dentin bonds. Oper Dent. 2009;34(4):481–490. doi: 10.2341/08-016-L. [DOI] [PubMed] [Google Scholar]
- 40.Stanislawczuk R, Pereira F, Muñoz MA, Luque I, Farago PV, Reis A, Loguercio AD. Effects of chlorhexidine-containing adhesives on the durability of resin-dentine interfaces. J Dent. 2014 Jan;42(1):39–47. doi: 10.1016/j.jdent.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Vergaro V, Abdullayev E, Lvov YM, Zeitoun A, Cingolani R, Rinaldi R, Leporatti S. Cytocompatibility and uptake of halloysite clay nanotubes. Biomacromolecules. 2010;11(3):820–826. doi: 10.1021/bm9014446. [DOI] [PubMed] [Google Scholar]
- 42.da Silva EM, de Sá Rodrigues CU, de Oliveira Matos MP, de Carvalho TR, Dos Santos GB, Amaral CM. Experimental etch-and-rinse adhesive systems containing MMP-inhibitors: Physicochemical characterization and resin-dentin bonding stability. J Dent. 2015;43(12):1491–1497. doi: 10.1016/j.jdent.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Perote LC, Kamozaki MB, Gutierrez NC, Tay FR, Pucci CR. Effect of Matrix Metalloproteinase-inhibiting Solutions and Aging Methods on Dentin Bond Strength. J Adhes Dent. 2015;17(4):347–352. doi: 10.3290/j.jad.a34594. [DOI] [PubMed] [Google Scholar]
- 44.Murphy G. Matrix metalloproteinases and their inhibitors. Acta Orthop Scand Suppl. 1995;266:55–60. [PubMed] [Google Scholar]
- 45.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Sambandam V, Neelakantan P. Matrix metalloproteinases (MMP) in restorative dentistry and endodontics. J Clin Pediatr Dent. 2014;39(1):57–59. doi: 10.17796/jcpd.39.1.x452446251r1q428. [DOI] [PubMed] [Google Scholar]
- 47.Wang D, Zhang L, Li F, Ma K, Chen J. Localization and quantitative detection of matrix metalloproteinase in human coronal dentine. Zhonghua Kou Qiang Yi Xue Za Zhi. 2014a;49:688–692. [PubMed] [Google Scholar]
- 48.Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8 and 9 by chlorhexidine. Clin Diagn Lab Immunol. 1999;6(3):437–9. doi: 10.1128/cdli.6.3.437-439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang D, Zhang L, Li F, Xu S, Chen J. Study of the types of matrix metalloproteinases involved in dentin bonding interface degradation. Hua Xi Kou Qiang Yi Xue Za Zhi. 2014b;32:394–399. doi: 10.7518/hxkq.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


