Abstract
Objective
We sought to determine the prevalence, molecular epidemiology, and factors associated with Staphylococcus aureus environmental surface and pet colonization in households of children with community-associated methicillin-resistant S. aureus (CA-MRSA) infection.
Methods
Between 2012 and 2015, 150 children with CA-MRSA infections and their household contacts and pets were enrolled in this cross-sectional study in metropolitan Saint Louis, MO. Cultures to detect S. aureus were collected from 3 anatomic sites of household members, 2 dog/cat sites, and 21 environmental surfaces in each household. Molecular epidemiology of S. aureus isolates was determined via repetitive-sequence PCR. Generalized linear models were developed to identify factors associated with S. aureus/MRSA household contamination.
Results
MRSA was recovered from environmental surfaces in 69 (46%) households (median 2 surfaces [range 1–18]). The enrollment infecting strain type was the most common strain recovered from the environment in most (64%) households. In generalized linear models, factors associated with a higher proportion of MRSA-contaminated environmental surfaces were household member MRSA colonization burden, MRSA as the dominant S. aureus strain colonizing household members, more strain types per household member, index case African-American race, and renting (vs. owning) the home. Of 132 pets, 14% were colonized with MRSA. Pets whose primary caretaker was MRSA-colonized were more likely to be MRSA-colonized than pets whose primary caretaker was not MRSA-colonized (50% vs. 4%, p<0.001).
Conclusions
Household environments and pet dogs and cats serve as reservoirs of MRSA. Household member MRSA colonization burden predicts environmental MRSA contamination. Longitudinal studies will inform the directionality of household transmission.
Keywords: community-associated methicillin-resistant Staphylococcus aureus, household reservoirs, environmental contamination, pets
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) is an important cause of skin and soft tissue infection (SSTI) in children [1]. Incomplete understanding of the epidemiology of carriage and transmission of these strains has contributed to their widespread dissemination in communities and, recently, into hospitals, where healthcare-associated (HA)-MRSA strains once predominated [2, 3]. While preventive measures in hospitalized patients have curtailed the incidence of HA-MRSA infections [4, 5], similar strides have not been achieved in the community setting. Indeed, after performing decolonization, up to 50% of SSTI patients develop recurrent infections over the ensuing year [6].
CA-MRSA is a disease of households, facilitated by complex transmission dynamics yet to be fully understood [2, 7]. Dogma holds that S. aureus colonization poses risk for recurrent SSTI and spread among household contacts [8]. Instances of discordance between infecting S. aureus strains and those colonizing index cases and their household contacts [9, 10], and varied effectiveness of decolonization trials [6, 11], indicate that exclusive focus on human colonization may be inadequate. The household environment is increasingly recognized as a community reservoir of S. aureus. Indeed, household MRSA environmental contamination is significantly more prevalent in homes of MRSA-infected patients than in control households [12, 13]. Additionally, household companion animals can carry S. aureus and may contribute to S. aureus household transmission [7, 14]. Studies to date [9, 12, 13, 15, 16] have been limited by examining only a subset of household members, discounting socioeconomic status, sampling a small number of surfaces, or disregarding pet colonization.
Understanding these complex community MRSA reservoirs is imperative to mitigate transmission. The objectives of this study were to determine the prevalence, molecular epidemiology, and factors associated with S. aureus, and specifically MRSA, environmental surface contamination and pet carriage in households of children with CA-MRSA infection.
METHODS
Participants
Between January 2012 and October 2015, 377 children with SSTI were screened for the HOME: Household Observation of MRSA in the Environment study. Screening took place at St. Louis Children’s Hospital (SLCH), Cardinal Glennon Children’s Hospital, and community pediatric practices affiliated with the Washington University Pediatric and Adolescent Ambulatory Research Consortium. Children with HA-MRSA infections [17] (e.g., recent hospitalization, invasive medical device, residing in long-term care facility) were excluded from screening. One hundred thirty subjects did not meet eligibility criteria (e.g., SSTI not verified as MRSA, time interval from infection to screening, distance from medical center). Of 247 eligible patients with CA-MRSA infection, 150 index cases (149 with SSTI, 1 invasive infection) were enrolled, along with their household contacts (individuals sleeping in the home ≥4 nights/week) and indoor pet dogs and cats. The Washington University Institutional Review Board and Institutional Animal Care and Use Committee approved study procedures. Written, informed consent/assent was obtained for all household members (by participant and/or guardian) and pets (by primary caretaker).
Data collection
An enrollment visit was conducted in the index case’s primary home. Participants were queried about demographics, socioeconomic surrogates (e.g., insurance status, home ownership, household crowding), prior S. aureus infections, personal hygiene practices, activities, pet characteristics, and household layout and cleaning practices. To control for potential bias introduced by participants misrepresenting their cleaning habits, the research team assigned each household an objective ‘home cleanliness score’ from 1 (above average) to 4 (very dirty), which considered odor, clutter, and grime, modified from the Environmental Cleanliness and Clutter Scale [18].
Index case infecting isolates were obtained from the clinical microbiology laboratory when available. At enrollment, colonization cultures were collected by trained study personnel from the anterior nares, axillae, and inguinal folds of each household member (Eswab, Becton Dickinson [BD], Franklin Lakes, NJ) and from the nares (minitip Eswab, BD) and dorsal fur (Eswab) of indoor pet dogs and cats. Twenty-one environmental surfaces [19] were sampled (Table 1); standardized environmental sampling employed the Baird Parker Agar contact plate (Hardy, Santa Maria, CA) and the Eswab [19, 20].
Table 1.
Prevalence of S. aureus on Household Environmental Surfaces and Pets
| Household surfacea or petb | S. aureus at site,N (%) | MRSA at site,N (%) | MSSA at site,N (%) |
|---|---|---|---|
| Any environmental surface | 95 (63) | 69 (46) | 56 (37) |
| Living room | 49 (33) | 32 (21) | 22 (15) |
| TV remote control (n=148) | 33 (22) | 22 (15) | 11 (7) |
| Computer keyboard and mousec (n=129) | 22 (17) | 15 (12) | 8 (6) |
| Videogame controller (n=109) | 17 (16) | 9 (8) | 8 (7) |
| Telephoned (n=148) | 16 (11) | 11 (7) | 5 (3) |
| Bathroom | 72 (48) | 50 (33) | 38 (25) |
| Countertop (n=148) | 30 (20) | 17 (11) | 13 (9) |
| Toilet seat (n=150) | 22 (15) | 11 (7) | 11 (7) |
| Bathtub (n=150) | 22 (15) | 11 (7) | 11 (7) |
| Sink (n=150) | 21 (14) | 14 (9) | 7 (5) |
| Sink faucet handle (n=150) | 18 (12) | 12 (8) | 6 (4) |
| Light switch (n=150) | 18 (12) | 10 (7) | 8 (5) |
| Soap bar and dish (n=81) | 10 (12) | 8 (10) | 2 (2) |
| Hand towel (n=98) | 11 (11) | 9 (9) | 2 (2) |
| Index case bath towel (n=86) | 8 (9) | 3 (3) | 5 (6) |
| Toilet handle (n=150) | 14 (9) | 10 (7) | 4 (3) |
| Door handle (n=150) | 13 (9) | 7 (5) | 6 (4) |
| Kitchen | 51 (34) | 34 (23) | 20 (13) |
| Refrigerator door handle (n=149) | 28 (19) | 16 (11) | 12 (8) |
| Table top (n=145) | 20(14) | 17 (12) | 3 (2) |
| Hand towel (n=102) | 11 (11) | 7 (7) | 4 (4) |
| Sponge or cloth (n=136) | 13 (10) | 10 (7) | 3 (2) |
| Sink faucet handle (n=150) | 10 (7) | 8 (5) | 2 (1) |
| Bedroom | 33 (22) | 22 (15) | 12 (8) |
| Index case bed linensc(n=149) | 33 (22) | 22 (15) | 12 (8) |
| Any dog or cat cultured in householdb (N=71) | 22 (31) | 15 (21) | 9 (13) |
| Dog (N=100) | 24 (24) | 16 (16) | 10 (10) |
| Nares | 19 (19) | 12 (12) | 7 (7) |
| Dorsal fur | 9 (9) | 6 (6) | 3 (3) |
| Cat (N=32) | 4 (13) | 3 (9) | 1 (3) |
| Nares | 4 (13) | 3 (9) | 1 (3) |
| Dorsal fur | 0 (0) | 0 (0) | 0 (0) |
Abbreviations. MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus Note. Culturing technique used for each surface: Eswab (Living room - TV remote control, computer keyboard and mouse, videogame controller, telephone; Bathroom - sink faucet handle, light switch, hand towel, index case bath towel, toilet handle, door handle; Kitchen - hand towel, sponge or cloth, sink faucet handle; Bedroom - index case bed linens) or Baird Parker Agar contact plate (Bathroom - countertop, toilet seat, bathtub, sink, soap bar and dish; Kitchen - refrigerator door handle, table top).
All objects and surfaces were not present in all homes; if present, the object/surface was sampled. N represents the number of households in which this surface was present.
A dog or cat was present in 81 (54%) households; 132 dogs and cats were cultured in 71 (47%) households.
Both MRSA and MSSA were recovered from 1 computer keyboard/mouse and 1 index case bed linens.
If a landline was not present, the mobile phone of the index case or his/her mother was cultured.
Laboratory procedures
From Eswabs, S. aureus was recovered using broth-enrichment. From the contact plate, colonies were selected based on morphology. S. aureus identification and antibiotic susceptibility testing were performed in accordance with established techniques [21] (Supplementary Methods). Molecular typing was performed on all recovered S. aureus isolates by repetitive-sequence PCR (repPCR) [22, 23]. Isolates with unusual repPCR patterns and all isolates recovered from pets were confirmed as S. aureus by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (VITEK MS v2.0) [24].
Statistical analysis
To compare the proportion of household environmental surfaces contaminated with S. aureus or MRSA, as well as the number of unique strains (by repPCR) recovered from households, across various attributes, nonparametric Mann-Whitney U and Kruskal-Wallace tests were performed. The relationship between environmental surface contamination prevalence and household colonization pressure was determined by linear regression. Pearson’s χ2 tests compared pet carriage with primary caretaker colonization and various pet factors. Data analyses were performed with SPSS 23 for Windows (IBM SPSS, Chicago, IL).
Generalized linear Poisson models were developed (in R language [25]; Supplementary Methods) in a Bayesian framework to define the impact of household member colonization pressure and individual and household-level cleanliness variables on the proportion of household environmental surfaces contaminated with S. aureus or MRSA. The ‘Personal Colonization and Pet Carriage’ model considered how colonization status and strain distribution of household members and pets and socioeconomic factors influence household contamination. The ‘Household Practices and Personal Behaviors’ model examined how hygiene and epidemiological factors influence household contamination. See Supplementary Tables 1 and 2 for primary and secondary covariates included.
RESULTS
Study population and S. aureus colonization prevalence
The 150 index cases (53% female) had 546 household contacts, of which 521 (52% female) enrolled (96% participation). The median age of index cases and household contacts was 3 years (range 0.1–18) and 27 years (range 0.1–82), respectively. Index cases were primarily Caucasian (68%) or African-American (25%); 6% were of Hispanic/Latino ethnicity. The majority of participants lived in houses (81%, vs. apartments/townhomes) that they owned (66%, vs. rented) in urbanized areas (87%, vs. urban clusters or rural areas) [26, 27]. Median household size was 4 (range 2–13). Enrollment visits were conducted a median of 20 days (range 3–95) following MRSA infection. The study area encompassed 4,035 miles2 (household distance from SLCH: median 17 miles, range 1–76). Fifty-seven (38%) index cases and 218 (43%) household contacts were colonized with S. aureus at enrollment (30% and 23% with MRSA, respectively). Sixty-two (41%) index cases and 87 (17%) household contacts had a history of confirmed S. aureus infection (excluding the infection prompting study enrollment); 59% and 27%, respectively, reported an SSTI (of any etiology) in the year prior to enrollment.
Prevalence of S. aureus environmental contamination and pet colonization
S. aureus was recovered from ≥1 environmental surface in 95 (63%) of 150 households (median 3 surfaces [range 1–18]); MRSA was recovered from surfaces in 69 (46%) households (median 2 surfaces [range 1–18]) (Table 1). Of households with S. aureus contamination, 39 (41%) were contaminated exclusively with MRSA, 26 (27%) exclusively with methicillin-susceptible S. aureus (MSSA), and 30 (32%) with both.
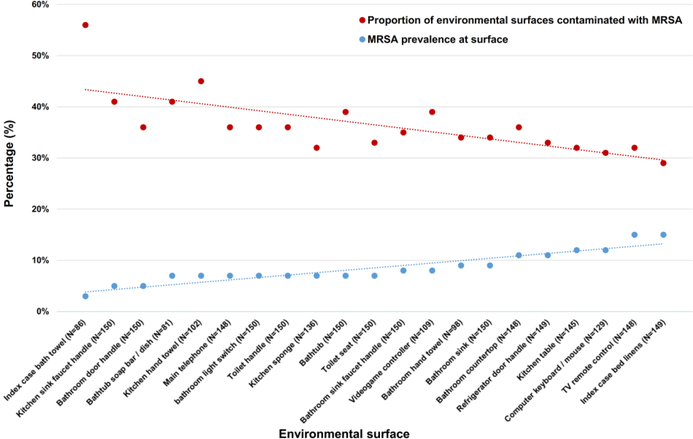
The most common sites of environmental MRSA contamination were the index case’s bed linens (15%), television remote control (15%), kitchen table (12%), computer keyboard/mouse (12%), bathroom countertop (11%), and refrigerator door handle (11%) (Table 1). Surfaces that were less frequently MRSA-contaminated overall (index case bath towel, kitchen sink faucet handle or hand towel, and bathtub soap bar/dish) were more likely to be contaminated in households with a higher proportion of MRSA-contaminated environmental surfaces (Figure 1).
Figure 1.
Prevalence of MRSA contamination at specific surfaces (blue dots/trendline) is inversely related to the proportion of environmental surfaces contaminated with MRSA in households when given surface is contaminated with MRSA (red dots/trendline). Environmental surfaces are listed from left to right in order of increasing MRSA prevalence. When infrequently colonized surfaces (e.g., the index case bathroom towel) are contaminated with MRSA, the proportion of environmental surfaces contaminated with MRSA is high, suggesting these surfaces require a high burden of MRSA in the household prior to becoming colonized. Conversely, the most frequently contaminated surfaces (e.g., the index case bed linens) are contaminated in the context of a low proportion of environmental surfaces contaminated with MRSA, suggesting these surfaces are the first fomites to be colonized (inverse relationship between colonization prevalence and the proportion of environmental surfaces contaminated with MRSA demonstrated via linear regression analysis; β=-0.696, p<0.001).
Pet dogs or cats were present in 81 (54%) households (median 2 [range 1–9] pets per household). Overall, 100 dogs and 32 cats from 71 households were swabbed; 16 (16%) dogs and 3 (9%) cats carried MRSA (Table 1). While 50% of pets whose primary caretaker (i.e., the human primarily responsible for their feeding, exercising, and waste cleanup) was MRSA-colonized also carried MRSA, just 4% of pets whose primary caretaker was not MRSA-colonized carried MRSA (p<0.001); for 3 of the 4 pets who carried MRSA without an MRSA-colonized caretaker, an alternate household member was colonized. Pets with MRSA carriage lived in households with higher household member MRSA colonization pressure (median 0.50, interquartile range [IQR] 0.25–0.75) than pets without carriage (median 0.08, IQR 0.00–0.27; p<0.001). No relationship was observed between pet MRSA carriage and pet daycare/boarding attendance, sleeping with colonized household members, overall health, or infection history.
Factors associated with environmental contamination
In univariate analyses, a higher proportion of MRSA-contaminated environmental surfaces was associated with the index case being African-American or multiracial (vs. Caucasian, p=0.01), renting the home (vs. owning, p=0.001), index case MRSA colonization (p<0.001), and any household member MRSA colonization (p<0.001) (Supplementary Table 3). In addition to the covariates significantly associated with a higher proportion of MRSA-contaminated environmental surfaces, a higher proportion of S. aureus-contaminated environmental surfaces was associated with Medicaid or no health insurance (vs. private or Tricare, p=0.03) and presence of more unique S. aureus strains in the household (p<0.001). Neither prior S. aureus infections nor past-year SSTIs in household members were associated with S. aureus or MRSA surface contamination.
Predicting environmental contamination: multivariable models
Personal Colonization and Pet Carriage Model
Covariates significantly predictive of a higher proportion of MRSA-contaminated environmental surfaces included increased household member MRSA colonization pressure, MRSA as the dominant S. aureus strain colonizing household members, increased number of unique S. aureus strains (by repPCR) per household member, and renting (vs. owning) the home (Table 2).
Table 2.
Factors Associated with Proportion of Environmental Surfaces Contaminated with S. aureus and MRSA, Multivariable Model
| S. aureus | MRSA | |||
|---|---|---|---|---|
| Factor | Rate Ratio (95% CrI) | pMCMC | Rate Ratio (95% CrI) | pMCMC |
| Personal Colonization and Pet Carriage Model | ||||
| Household member S. aureus colonization pressurea | 14.8 | <0.001 | - | - |
| (5.2–41.2) | ||||
| Household member MRSA colonization pressurea | - | - | 21.6 | <0.001 |
| (5.3–102.8) | ||||
| MRSA dominant S. aureus colonizing household members | 1.4 | 0.002 | 2.6 | <0.001 |
| (vs. MSSA) | (1.1–1.7) | (1.8–3.6) | ||
| Number of unique strains (by repPCR) per household member | 3.4 | 0.002 | 5.2 | <0.001 |
| (1.5–7.4) | (2.1–12.8) | |||
| Renting (vs. owning) home | 1.5 | 0.05 | 1.9 | 0.009 |
| (1.01–2.4) | (1.2–3.1) | |||
| Proportion of household members reporting SSTI in prior | - | - | 0.4 | 0.03 |
| yearb | (0.1–0.9) | |||
| Individuals per square foot of home | 1.12 | 0.06 | - | - |
| (0.99–1.3) | ||||
| Household Practices and Personal Behaviors Modelc | ||||
| Renting (vs. owning) home | 2.5 | <0.001 | 3.1 | 0.003 |
| (1.5–4.2) | (1.5–7.0) | |||
| African-American or multiraciald (vs. Caucasian) race of | 1.7 | 0.05 | 2.7 | 0.02 |
| index case | (1.0–2.9) | (1.2–6.5) | ||
| Public (vs. private) insurance status of index case | - | - | 0.4 | 0.06 |
| (0.2–1.1) | ||||
| Proportion of household members reporting SSTI in prior | 0.6 | 0.33 | 0.5 | 0.30 |
| yearb | (0.3–1.6) | (0.1–1.9) | ||
| Proportion of household members reporting bathing once | - | - | 1.03 | 0.96 |
| daily or more | (0.4–2.8) | |||
| Proportion of household members reporting washing bed | - | - | 0.8 | 0.63 |
| linens once weekly or more | (0.3–2.0) | |||
Note. Poisson generalized linear mixed models constructed in the R library ‘MCMCglmm’. See Supplementary Methods for runtime parameters and Supplementary Tables 1 and 2 for primary and secondary covariates used in model selection. “-” denotes variable not included in applicable model.
Abbreviations: MRSA, methicillin-resistant S. aureus; CrI, credible interval; SSTI, skin and soft tissue infection.
Colonization pressure calculated as (number of S. aureus [MRSA] colonized anatomic sites) / [(number of sampled household members)*(number of sampled anatomic sites)].
This does not include the infection that prompted enrollment into the study.
Excludes personal colonization and pet carriage information.
Multiracial participants include African-American/Caucasian (N=9), Caucasian/American Indian (N=1), and African-American/Caucasian/American Indian (N=1).
Household Practices and Personal Behaviors Model
Given the strong influence of personal colonization on environmental contamination in the ‘Personal Colonization and Pet Carriage’ model, we developed models that excluded human colonization and pet carriage to discern the influence of personal behaviors on household environmental contamination. Renting (vs. owning) the home and index case African-American (or multiracial) race (vs. Caucasian) were predictive of a higher proportion of MRSA-contaminated environmental surfaces (Table 2, Supplementary Figure 1).
Molecular epidemiology of S. aureus strains in households
Overall (including index cases, household contacts, environmental surfaces, and pets), up to 7 (median 2) unique strains (by repPCR) were recovered from a single household. Up to 4 strains (median 1) were recovered from the environmental surfaces of a single household. Crowded households (>2 people per bedroom) harbored more strains overall (median 2, interquartile range [IQR] 2–4) than non-crowded households (median 2, IQR 1–2; p=0.004). Households assigned a home cleanliness score of below average or very dirty harbored more strains (median 2, IQR 1–3) than average or above average households (median 1, IQR 1–2; p=0.01). Households with more strains also had a higher proportion of S. aureus-contaminated environmental surfaces (p=0.001, Supplementary Table 3, Supplementary Figure 1).
The MRSA isolate from the enrollment infection was available for 91 (61%) index cases, and a concordant strain was recovered from an environmental surface in 44 (48%) of these households. The enrollment infecting strain was the most common strain recovered in 37 (64%) of 58 MRSA-contaminated household environments. An environmental surface was contaminated with a S. aureus strain concordant with an index case colonizing or infecting strain in 60 (52%) of 116 households with an available index case strain (Supplementary Figure 2A); an environmental surface was contaminated with a household contact-concordant strain in 68 (45%) of 150 households. Strains discordant with index case strains were recovered from the environment in 18 (23%) of 78 households with both an available index case strain and environmental contamination (Supplementary Figure 2B); only 4 (5%) of these 78 household environments yielded a strain not recovered from a human household member. Among household contacts, siblings of index cases were the most likely to be colonized with a strain recovered from an environmental surface (31%), while adults other than the index case’s parents were least likely to be colonized with environmental strains (11%, p=0.03).
Overall, the index case’s bed linens, TV remote control, and refrigerator door handle were most often colonized with a S. aureus strain concordant with an index case strain (Supplementary Figure 2A), reflective of environmental sites with high colonization prevalence (Table 1). Focusing on contaminated surfaces, in households with an available index case strain, the bathroom soap bar/dish, bathroom hand towel, and bathroom door handle were most often colonized with a strain concordant with an index case strain (Supplementary Figure 2B). Certain high-touch surfaces shared by all household members, such as the toilet handle, kitchen hand towel, and videogame controller were more often colonized with a household contact strain than an index case strain (Supplementary Figure 2B).
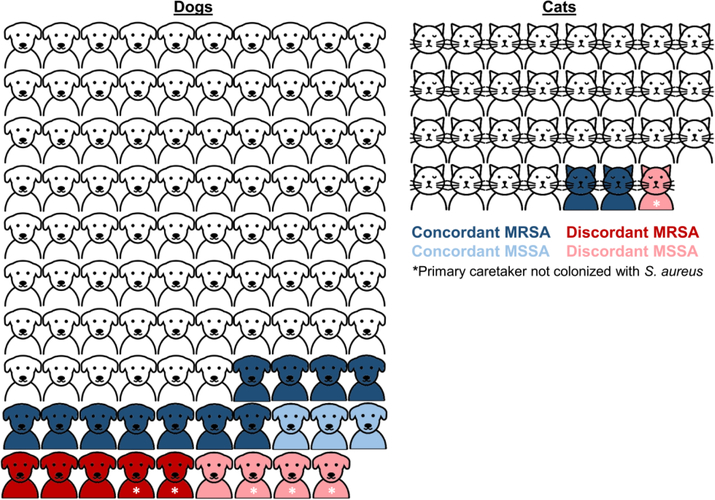
Of 130 swabbed pets with a swabbed primary caretaker, 104 (80%) did not carry S. aureus. Sixteen (12%) pets carried a S. aureus strain concordant with a primary caretaker strain, while 4 (3%) pets carried a strain discordant from primary caretaker strains; the primary caretaker was not colonized for 6 (5%) pets carrying S. aureus (Figure 2). Overall, 6 (5%) of 132 swabbed pets carried a distinct strain not recovered from a human household member. Likelihood of strain concordance did not differ between dogs and cats or between pets who did and did not sleep with a household member. In 14 households with both environmental contamination and pet carriage, 13 (93%) displayed strain concordance between these sites.
Figure 2.
Molecular epidemiology of S. aureus strains recovered from pet dogs (N=99) and cats (N=31) and their primary caretakers (i.e., the human who is primarily responsible for their feeding, exercising, and waste cleanup). Pets carrying strains concordant or discordant with their primary caretaker are shaded in blue and red, respectively; pets shaded in white did not carry S. aureus. Darker and lighter shades represent MRSA and MSSA pet carriage, respectively. Asterisk (*) denotes that the pet carried S. aureus while the primary caretaker was not colonized with S. aureus (and thus, colonization status was discordant). Figure excludes one cat and one dog who each carried S. aureus but whose primary caretakers were not sampled. Artwork created by Llisole from Noun Project (https://thenounproject.com)
DISCUSSION
In the present study characterizing households of children with MRSA SSTI, nearly half of homes were found to harbor environmental MRSA contamination. In addition to the index case’s bed linens, commonly touched and shared surfaces (TV remote control, computer keyboard/mouse, and kitchen table) were the fomites most likely to be MRSA-contaminated. In most households, the index case infecting strain was the predominant environmental strain. Moreover, while >20% of households with pets had dogs and/or cats with MRSA carriage, such carriage rarely occurred in the absence of a MRSA-colonized household member. While the cross-sectional nature of our study precludes defining directionality of transmission, our data support the premise that infected/colonized household members contaminate their home environment and subsequently, environmental reservoirs perpetuate the cycle of reacquisition and transmission.
The prevalence of household environmental MRSA contamination (46%) was higher in the present study compared to two prior US studies in homes of patients with MRSA SSTI. Studies conducted in Chicago/Los Angeles and New York recovered MRSA from environmental surfaces in 27% and 30% of homes, respectively [12, 28]. Our higher measured prevalence of environmental contamination may reflect inclusion of more surfaces (21 vs. ~8–11) and solely pediatric index cases (vs. households without children), who may be in closer contact with their environment; indeed, strains recovered from children had the highest concordance with environmental strains. While several surfaces with high MRSA prevalence (e.g., TV remote control, refrigerator door handle, computer, index case bed linens) were sampled across all studies, the present study identified other frequently contaminated sites (e.g., kitchen table, bathroom countertop) that represent potential targets for intervention.
It has been demonstrated that occupants leave an identifiable bacterial signature on household surfaces. Lax and colleagues monitored household microbial communities before and after a physical move; bacterial communities of participants’ new homes promptly converged on the communities of their original homes, implicating household members as the vector [29]. Collectively, our predictive models indicate that household member colonization pressure is the most significant predictor of the burden of MRSA environmental contamination. In the majority of households in our study, the index case infecting strain was the predominant environmental strain; in prior studies, environmental contamination with clinical S. aureus strains has been associated with household transmission, reacquisition, and recurrent infection [12, 28, 30]. In total, the available data indicate that household contamination with S. aureus is a marker of household member colonization, which itself is driven by complex influences of epidemiological, behavioral, and genetic factors. When household member MRSA colonization (the strongest influence on environmental contamination) was removed in our models, home renting (vs. ownership) was the most informative covariate in predicting environmental MRSA contamination. This relationship may be influenced by crowding and lower home cleanliness, possible surrogates of lower socioeconomic status in our study [31].
The present study of household MRSA contamination is strengthened by the sampling of companion animals. While S. aureus has been recovered from pets, such carriage can resolve without antimicrobial treatment, suggesting that pets are not a natural S. aureus reservoir [7]. A study in the United Kingdom sequenced genomes of 46 isolates from MRSA-infected pets, all of which clustered with human colonizing strains from unrelated patients, representing the circulating epidemic clade [32]. These data suggest S. aureus transmission from human to pet, though the study did not include colonizing isolates of human household contacts. In the present study of households of children with MRSA SSTI, more than 20% of sampled companion animals carried S. aureus (14% carried MRSA). Importantly, pets were more likely to carry MRSA if their primary caretaker was MRSA-colonized and if household member MRSA colonization pressure was higher. Additionally, pets almost exclusively carried strains concordant with both the index case infecting strain and strains found on environmental surfaces. Collectively, these studies indicate that while pets may participate in household S. aureus transmission, humans represent the primary source of S. aureus in pets.
Compared to other recent studies [9, 12], the present work systematically sampled a much higher proportion of household members and more environmental surfaces, and included companion animals, to provide the most comprehensive available picture of household S. aureus dynamics. Our conclusions are based on substantial epidemiologic data and advanced statistical modeling techniques. We quantified the proportion of contaminated surfaces rather than recording only presence or absence of environmental S. aureus, though we did not measure the density of S. aureus at each surface. As the primary objective of this study was to identify household reservoirs of MRSA to inform interventions to interrupt MRSA transmission and prevent recurrent infections, we only sampled households of children with MRSA SSTI. Strain concordance between the MRSA enrollment infection isolate and other recovered isolates could only be evaluated in the 91 households where it was available. A further limitation of the study is that due to its cross-sectional nature, directionality of transmission among humans, pets, and the environment were not definitively established. Finally, while whole-genome sequencing would provide the highest strain resolution, repPCR has been shown to be highly discriminatory, especially in the household context [23].
In this comprehensive investigation of households affected by CA-MRSA, we have demonstrated that household member colonization pressure is the most significant predictor of household environmental contamination. These findings suggest that environmental reservoirs, particularly those shown here to harbor a high burden of MRSA, may perpetuate the cycle of reacquisition and transmission. Thus, in addition to personal decolonization, integrating environmental hygiene measures (which have been effective in healthcare settings [33]) may prove beneficial in household infection prevention efforts. Longitudinal studies are needed to further elucidate S. aureus household transmission dynamics, prevalence and factors associated with persistent colonization, and how these phenomena relate to recurrent infection, ultimately informing the design of interventions to disrupt transmission and prevent infection.
Supplementary Material
Highlights.
MRSA contaminated 46% of environments in households of children with MRSA SSTI
The most prevalent sites of MRSA were index case bed linens and TV remote control
Risk factors included household member MRSA colonization burden and home rental
14% of pet dogs and cats were colonized with MRSA
The infecting strain type was the most common strain recovered from the environment
ACKNOWLEDGEMENTS
We appreciate assistance in patient recruitment by Rachel Orscheln, MD, Washington University; Jennifer Seigel, RN, PNP and the Saint Louis Children’s Hospital Pediatric Ambulatory Wound Service; Mary Bixby, RN, BSN, Cardinal Glennon Children’s Hospital; and Jane Garbutt, MB, ChB, Sherry Dodd, and the physicians and staff of the participating Washington University Pediatric and Adolescent Ambulatory Research Consortium practices, including Mercy Clinic Pediatrics–Union and Washington, Johnson Pediatric Center, Heartland Pediatrics, Forest Park Pediatrics, Tots Thru Teens, Pediatric Healthcare Unlimited, Northwest Pediatrics–St Charles, Esse Health Pediatric & Adolescent Medicine – Watson Road, Fenton Pediatrics, LLC, Blue Fish Pediatrics, and Southwest Pediatrics. We thank Meghan Wallace, BS and Angela Shupe, BS, for assistance with molecular typing of S. aureus isolates. We also thank Mike Talcott, DVM and Mary Ellenberger, DVM, MS, for providing training in animal specimen collection and David Hunstad, MD, for thoughtful review of the manuscript.
FUNDING
This work was supported by the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital; National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases [grant number K23-AI091690 to S.A.F.]; NIH/National Center for Advancing Translational Sciences [grant number UL1-TR002345 to S.A.F.]; and the Agency for Healthcare Research and Quality (AHRQ) [grant numbers R01-HS021736, R01-HS024269 to S.A.F.]; and the Burroughs Wellcome Foundation Investigators in the Pathogenesis of Infectious Disease Award [to J.B.W.]. The computational analysis was partially funded by Defense Advanced Research Projects Agency Big Mechanism program under ARO contract W911NF1410333 [to A.R.]; NIH grants R01HL122712, 1P50MH094267, U01HL108634 [to A.R.]; and a gift from Liz and Kent Dauten [to A.R.]. These funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or AHRQ.
Footnotes
CONFLICTS OF INTEREST
All authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kaplan SL. Community-acquired methicillin-resistant Staphylococcus aureus infections in children. Semin Pediatr Infect Dis, 2006; 17: 113–119. [DOI] [PubMed] [Google Scholar]
- 2.Miller LG, Diep BA. Clinical practice: Colonization, fomites, and virulence: rethinking the pathogenesis of community-associated methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis, 2008; 46: 752–760. [DOI] [PubMed] [Google Scholar]
- 3.Diekema DJ, Richter SS, Heilmann KP, et al. Continued emergence of USA300 methicillin-resistant Staphylococcus aureus in the United States: results from a nationwide surveillance study. Infect Control Hosp Epidemiol, 2014; 35: 285–292. [DOI] [PubMed] [Google Scholar]
- 4.Kallen AJ, Mu Y, Bulens S, et al. Health care-associated invasive MRSA infections, 2005–2008. JAMA, 2010; 304: 641–648. [DOI] [PubMed] [Google Scholar]
- 5.Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med, 2013; 368: 2255–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fritz SA, Hogan PG, Hayek G, et al. Household versus individual approaches to eradication of community-associated Staphylococcus aureus in children: a randomized trial. Clin Infect Dis, 2012; 54: 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis MF, Iverson SA, Baron P, et al. Household transmission of meticillin-resistant Staphylococcus aureus and other staphylococci. Lancet Infect Dis, 2012; 12: 703–716. [DOI] [PubMed] [Google Scholar]
- 8.Fritz SA, Epplin EK, Garbutt J, Storch GA. Skin infection in children colonized with community-associated methicillin-resistant Staphylococcus aureus. J Infect, 2009; 59: 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller LG, Eells SJ, Taylor AR, et al. Staphylococcus aureus colonization among household contacts of patients with skin infections: risk factors, strain discordance, and complex ecology. Clin Infect Dis, 2012; 54: 1523–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez M, Hogan PG, Burnham CA, Fritz SA. Molecular epidemiology of Staphylococcus aureus in households of children with community-associated S. aureus skin and soft tissue infections. J Pediatr, 2014; 164: 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan SL, Forbes A, Hammerman WA, et al. Randomized trial of "bleach baths" plus routine hygienic measures vs. routine hygienic measures alone for prevention of recurrent infections. Clin Infect Dis, 2014; 58: 679–682. [DOI] [PubMed] [Google Scholar]
- 12.Knox J, Uhlemann AC, Miller M, et al. Environmental contamination as a risk factor for intra-household Staphylococcus aureus transmission. PLoS ONE, 2012; 7: e49900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uhlemann AC, Knox J, Miller M, et al. The environment as an unrecognized reservoir for community-associated methicillin resistant Staphylococcus aureus USA300: a case-control study. PLoS ONE, 2011; 6: e22407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris DO, Loeffler A, Davis MF, Guardabassi L, Weese JS. Recommendations for approaches to meticillin-resistant staphylococcal infections of small animals: diagnosis, therapeutic considerations and preventative measures. Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet Dermatol, 2017; 28: 304–e69. [DOI] [PubMed] [Google Scholar]
- 15.Shahbazian JH, Hahn PD, Ludwig S, et al. Multidrug and mupirocin resistance in environmental methicillin-resistant Staphylococcus aureus (MRSA) collected from the homes of people diagnosed with a community-onset (CO-) MRSA infection. Appl Environ Microbiol, 2017; 83: e01369–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng W, Faheem A, McGeer A, et al. Community- and healthcare-associated methicillin-resistant Staphylococcus aureus strains: an investigation into household transmission, risk factors, and environmental contamination. Infect Control Hosp Epidemiol, 2017; 38: 61–67. [DOI] [PubMed] [Google Scholar]
- 17.Klevens RM, Morrison MA, Fridkin SK, et al. Community-associated methicillin-resistant Staphylococcus aureus and healthcare risk factors. Emerg Infect Dis, 2006; 12: 1991–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halliday G, Snowdon J. The environmental cleanliness and clutter scale (ECCS). Int Psychogeriatr, 2009; 21: 1041–1050. [DOI] [PubMed] [Google Scholar]
- 19.Fritz SA, Hogan PG, Singh LN, et al. Contamination of environmental surfaces with Staphylococcus aureus in households with children infected with methicillin-resistant S. aureus. JAMA Pediatr, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogan PG, Burnham CA, Singh LN, et al. Evaluation of environmental sampling methods for detection of Staphylococcus aureus on fomites. Ann Public Health Res, 2015; 2: 1013. [PMC free article] [PubMed] [Google Scholar]
- 21.Cockerill F Performance standards for antimicrobial susceptibility testing; twenty-third informational supplement; M100-S23. Wayne, PA: Clinical and Laboratory Standards Institute; 2013. [Google Scholar]
- 22.Del Vecchio VG, Petroziello JM, Gress MJ, et al. Molecular genotyping of methicillin-resistant Staphylococcus aureus via fluorophore-enhanced repetitive-sequence PCR. J Clin Microbiol, 1995; 33: 2141–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez M, Hogan PG, Satola SW, et al. Discriminatory indices of typing methods for epidemiologic analysis of contemporary Staphylococcus aureus strains. Medicine, 2015; 94: e1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rychert J, Burnham CA, Bythrow M, et al. Multicenter evaluation of the Vitek MS matrix-assisted laser desorption ionization-time of flight mass spectrometry system for identification of Gram-positive aerobic bacteria. J Clin Microbiol, 2013; 51: 2225–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: 2016. Available from: https://www.R-project.org/ [Google Scholar]
- 26.National Archives and Records Administration, Department of Commerce, Bureau of the Census. Qualifying Urban Areas for the 2010 Census. Federal Register, 2012; 77: 18651–18669. [Google Scholar]
- 27.U.S. Department of Commerce USCB. Tiger/Line Shapefiles, 2012; Available from: ftp://ftp2.census.gov/geo/tiger/TIGER2012/UAC/.
- 28.Eells SJ, David MZ, Taylor A, et al. Persistent environmental contamination with USA300 methicillin-resistant Staphylococcus aureus and other pathogenic strain types in households with S. aureus skin infections. Infect Control Hosp Epidemiol, 2014; 35: 1373–1382. [DOI] [PubMed] [Google Scholar]
- 29.Lax S, Smith DP, Hampton-Marcell J, et al. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science, 2014; 345: 1048–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller LG, Eells SJ, David MZ, et al. Staphylococcus aureus skin infection recurrences among household members: an examination of host, behavioral, and pathogen-level predictors. Clin Infect Dis, 2015; 60: 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galobardes B, Shaw M, Lawlor DA, Lynch JW, Smith GD. Indicators of socioeconomic position (part 1). J Epidemiol Community Health, 2006; 60: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrison EM, Weinert LA, Holden MT, et al. A shared population of epidemic methicillin-resistant Staphylococcus aureus 15 circulates in humans and companion animals. mBio, 2014; 5: e00985–00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Datta R, Platt R, Yokoe DS, Huang SS. Environmental cleaning intervention and risk of acquiring multidrug-resistant organisms from prior room occupants. Arch Intern Med, 2011; 171: 491–494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.