Abstract
People who inject drugs (PWID) with HIV experience an elevated risk of death. A potentially important determinant of survival is the high burden of depression. This study examined the relationship of depressive symptoms at HIV testing with two-year all-cause mortality among newly diagnosed HIV-positive PWID in Vietnam. At HIV testing, 141 PWID (42%) experienced severe depressive symptoms, and over the two years following diagnosis, 82 PWID (24%) died. Controlling for potential confounders, the two-year risk of death among those with depressive symptoms was 9.7% (95% CI: −1.2%-20.6%) higher than the risk among those without depressive symptoms. This increased risk of mortality for PWID with depressive symptoms was relatively consistent throughout the two-year period: at 6, 12, and 18 months, the risk difference was 12.6% (5.5%-19.7%), 13.9% (4.6%-23.2%), and 11.0% (0.9%-21.1%), respectively. HIV diagnosis may provide an important opportunity for depression screening and treatment, subsequently improving survival in this key population.
Keywords: HIV, depression, mortality, injection drug use, Vietnam
INTRODUCTION
Infection with human immunodeficiency virus (HIV) contributes to substantial morbidity and mortality worldwide (1,2), and there remain significant global disparities in controlling the epidemic. A particularly vulnerable group are people who inject drugs (PWID) (3–5). Parenteral exposure to infected blood is one of the most efficient means of HIV transmission (6–8), which has resulted in rapid and uncontrolled HIV epidemics among PWID (5). PWID with HIV experience poorer outcomes than their non-injecting peers at all stages of the HIV care continuum, resulting in persistently high rates of mortality (9–12). Injection drug use is the key driver of the HIV epidemic in Asia and Eastern Europe (3,4), and in Vietnam, HIV prevalence among PWID is above 30% in some provinces (13). There is an urgent need to improve care engagement and treatment adherence in this vulnerable population (5,13,14). However, achieving widespread use of antiretroviral therapy (ART) and improving survival among PWID present significant challenges (10,15–17).
One factor that may contribute to poor HIV outcomes among PWID is the high burden of depression. The prevalence of depressive symptoms is as high as 50% among PWID with HIV in Vietnam (18). A large body of research has linked depression to HIV disease progression and mortality (19–23). Compared to patients without depression, depressed HIV patients have lower rates of engaging in HIV care (24–26), adhering to ART (27,28), and achieving viral suppression (29), resulting in a higher risk of mortality (30–32). However, few studies on depression and HIV outcomes focus on PWID (33,34). It is unknown whether identifying and treating depression could improve HIV care engagement and treatment adherence among PWID. Given the high mortality experienced by this group, it is critical to understand the role of depression in worsening outcomes (5,10,14,35–37).
Due to our limited understanding of depression among PWID with HIV, coupled with their disproportionately poor outcomes, the objective of our study was to assess the relationship of depressive symptoms at the time of HIV testing with two-year all-cause mortality among PWID in Vietnam. We hypothesized that PWID with depressive symptoms at the time of HIV testing would have a higher risk of mortality at 6, 12, 18, and 24 months. We focused on the assessment of depressive symptoms at a clinically actionable decision point (i.e., time of HIV testing prior to new diagnosis). We could then examine how hypothetical interventions to reduce depressive symptoms at that time might subsequently impact mortality over the next 24 months.
METHODS
Study population
Our analysis used longitudinal data from a randomized controlled trial of an intervention to reduce HIV- and injection drug use-related stigma and high-risk injecting and sexual behaviors among PWID with HIV in Thai Nguyen from 2009-2013 (38). Thai Nguyen is a northeastern province in Vietnam with increases in injection drug use since the 1990s. At the time of the study, over 6,000 PWID lived in the province (39). Out of the 180 communes in Thai Nguyen, the trial enrolled participants in the 32 communes with the highest number of PWID. Participant recruitment was conducted using snowball sampling, in which recruiters who were former and current drug users approached members of drug networks in private places to discuss study enrollment. The trial enrolled 455 participants who met the following eligibility criteria: 1) HIV-positive diagnosis confirmed through study testing, 2) male (given that 97% of PWID in Thai Nguyen are male), 3) at least 18 years old, 4) had sex in the past 6 months, 5) injected drugs in the previous six months, and 6) planned to live in Thai Nguyen for the next 24 months. Of those 455 enrolled participants, 336 had no history of HIV testing or had not previously received a positive test result. These 336 participants were newly diagnosed with HIV at the trial’s baseline visit and are the focus of this analysis. We focused on new diagnoses to understand how depressive symptoms at the time of HIV testing were associated with mortality over the next two years following diagnosis.
Baseline depression and HIV testing and diagnosis
At the baseline visit, participants received HIV testing, and prior to receipt of HIV results, they were administered a face-to-face interview using a structured questionnaire. The questionnaire collected information on demographics, injecting behaviors and other substance use, quality of life, social support, and HIV and injection drug use-related stigma. The questionnaire included the Center for Epidemiologic Studies Depression Scale (CES-D), which is a 20-item screening tool and widely used measure of depressive symptoms experienced over the past week (40). Previous research has demonstrated that the CES-D is a valid and reliable measure of depressive symptoms in Vietnam (41). Importantly, this depression assessment took place before participants learned the positive result of their HIV test and therefore reflects pre-existing depressive symptoms, not a reaction to the diagnosis. HIV test results were provided within one week of the baseline visit, and all participants received post-test counseling in accordance with the WHO/CDC protocol for HIV testing and counseling.
Follow-up assessments and ascertainment of mortality
Follow-up visits for the trial took place at 6, 12, 18, and 24 months after HIV diagnosis. Participants were asked to provide a blood specimen to assess CD4 cell count and responded to the same questionnaire administered at baseline. Over follow-up, there were no study withdrawals, and for participants who missed a visit, study outreach workers attempted to contact participants using the contact information collected at baseline. During tracing procedures, family members of participants informed the study outreach worker if a participant had died. Therefore, vital status was known for all participants at all visits, through either their completion of the visit or their family member report of vital status at the visit time. Although mortality was ascertained at each 6-month visit, the exact time of death during the 6-month interval since the last visit was not known. For analysis, the time of death was defined as the first scheduled follow-up visit when mortality was ascertained (i.e., 6, 12, 18, or 24 months).
Statistical analysis
The prevalence of depressive symptoms at HIV diagnosis was assessed using the CES-D score measured at the baseline visit, with scores of 23 or greater classified as severe depressive symptoms (18,40,42). We examined the association between depression at HIV diagnosis and mortality over the following two years by using Kaplan-Meier cumulative risk curves to estimate the crude risk difference (RD) in mortality at 6, 12, 18, and 24 months.
We then used inverse probability weights to control for baseline levels of confounders at HIV testing (43). We used a propensity score model to predict the probability of baseline depressive symptoms as a function of potential confounders. Then, each participant was weighted by the inverse of the predicted probability of their observed depression status. In this weighted population, there is no longer an association between baseline depressive symptoms and potential confounding variables. Confounders were determined a priori based on variables that could affect both depression and mortality but did not mediate their relationship (44,45). Based on these criteria, confounders controlled for in the weighted analysis were age, marital status, education, employment, history of drug overdose, experience of HIV and injection drug use-related stigma, perceived social support, self-rated health, CD4 cell count, and trial intervention arm. Current frequency of injection drug use was assessed at baseline but was not included as a confounder in our primary analysis; we hypothesized that baseline depressive symptoms may affect frequency of injection drug use, with drug use then mediating the relationship between depression and subsequent overdose-related mortality. We performed a sensitivity analysis that included frequency of injection drug use as a confounder in the weighted model to determine how much influence this modeling decision had on our study results. Given that the parent trial’s intervention was previously shown to increase 24-month survival (46), we also assessed potential modification of the relationship between depression and mortality by including interaction terms in the model between depression and intervention arms.
Finally, we used the weighted risk estimates for 24-month mortality to calculate the population attributable risk difference (PARD) and the number needed to treat (NNT). The PARD is calculated by subtracting the risk of death for participants without baseline depression (R0) from the risk of death in the total population (RT): (RT − R0). It indicates the reduction in the 24-month risk of death in our study population if a hypothetical intervention had eliminated depression (without changing other baseline risk factors for mortality). The NNT is the reciprocal of the absolute value of the risk difference (1/|RD|) and corresponds to the number of people we would have to treat with a hypothetical intervention eliminating depression in order to reduce the expected number of deaths by 1 over the 24 months following HIV diagnosis.
For all estimates, our interpretation focuses on the strength of association and precision, rather than statistical significance. All analyses were conducted using R Version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
Ethics
The parent trial and this analysis were approved by the ethical review committees at all participating institutions. Written informed consent was obtained from all participants.
RESULTS
The characteristics of the 336 PWID enrolled in the trial and newly diagnosed with HIV at baseline are shown in Table 1. Based on the trial inclusion criteria, all participants were male. The average age of participants was 35 years, and half were married or cohabitating (49%). One-third had at least a high school education (33%), and the majority were employed full-time (70%). Most participants had a CD4 cell count between 200 and 500 cells/μL (47%) or less than 200 cells/μL (40%). The majority of participants (72%) rated their general health as fair or good, and the remaining 28% of participants rated their general health as poor. All participants reported injecting heroin in the past three months, half reported injecting heroin daily (51%), and a minority reported a history of overdose (18%). Most participants reported current alcohol use (72%) and cigarette smoking (94%). All 336 participants did not know their HIV-positive status at the time of HIV testing, and 141 participants (42%) reported severe depressive symptoms at testing (prior to diagnosis). Over the following 24 months, 82 participants (24%) died.
Table 1.
Baseline characteristics of 336 PWID newly diagnosed with HIV in Thai Nguyen, Vietnam 2009-2013.
| Characteristic | N (%) or Mean (SD) | |
|---|---|---|
| Baseline depressive symptoms (n=141) | No baseline depressive symptoms (n=195) | |
| Age in years (range 19-60) | 35 (6) | 35 (6) |
| Education | ||
| Primary school or less | 15 (11) | 15 (8) |
| Secondary school | 76 (54) | 118 (61) |
| High school | 43 (31) | 51 (26) |
| University/college | 7 (5) | 11 (6) |
| Employment status | ||
| Full-time | 97 (69) | 138 (71) |
| Part-time | 30 (21) | 35 (18) |
| Unemployed | 14 (10) | 22 (11) |
| Marital status | ||
| Single | 59 (42) | 66 (34) |
| Widowed, divorced, or separated | 56 (40) | 107 (55) |
| Married or cohabitating | 26 (18) | 22 (11) |
| Self-rated health | ||
| Poor | 66 (47) | 28 (14) |
| Fair or Good | 75 (53) | 167 (86) |
| CD4 cell count (cells/μL)a | ||
| ≥500 | 16 (12) | 24 (13) |
| 200-499 | 67 (49) | 89 (47) |
| <200 | 55 (40) | 78 (41) |
| Current frequency of injection drug usea | ||
| 1 time/month | 4 (3) | 12 (6) |
| 2-3 times/month | 9 (6) | 20 (11) |
| 1 time/week | 7 (5) | 20 (11) |
| 2-3 times/week | 14 (10) | 25 (13) |
| 4-6 times/week | 18 (13) | 34 (18) |
| Everyday | 89 (63) | 80 (42) |
| History of overdose | 34 (24) | 27 (14) |
| Current alcohol use | 81 (57) | 161 (83) |
| Current cigarette smoking | 133 (94) | 182 (93) |
| HIV stigma score (range 14-44)b | 30 (5) | 30 (4) |
| Injection drug use stigma score (range 9-28)b | 19 (3) | 18 (3) |
| Social support score (range 0-400)c | 256 (83) | 287 (84) |
| Trial intervention arm | ||
| Control | 24 (17) | 33 (17) |
| Community | 40 (28) | 65 (33) |
| Individual | 30 (21) | 37 (19) |
| Combined (Community+Individual) | 47 (33) | 60 (31) |
| 24-month mortality | 44 (31) | 38 (20) |
CD4 cell count was missing for 7 participants (2%), and frequency of injection drug use was missing for 4 participants (1%).
Stigma scores were calculated with stigma scales on perceived, internalized, and experienced stigma previously developed for the study population.
Social support was calculated using a modified version of the MOS social support scale.
Table 2 shows the crude and weighted risk differences in mortality at 6, 12, 18, and 24 months following HIV diagnosis for participants with and without severe depressive symptoms at baseline. In the crude analysis, those with depressive symptoms faced a higher absolute risk of death at 24 months (RD = 11.7%, 95% CI: 2.3%, 21.2%). Using a weighted model to control for potential confounders attenuated the 24-month risk difference from 11.7% to 9.7% (95% CI: −1.2%, 20.6%). This increased risk of mortality for PWID with depressive symptoms was fairly consistent in magnitude throughout the two-year period: at 6, 12, and 18 months after HIV diagnosis, the weighted RD was 12.6% (95% CI: 5.5%, 19.7%), 13.9% (95% CI: 4.6%, 23.2%), and 11.0% (95% CI: 0.9%, 21.1%), respectively (Fig. 1). Table 2 also shows results from our sensitivity analysis where we included frequency of injection drug use in the weighted model. All estimates in the sensitivity analysis were slightly attenuated but remained largely consistent in magnitude and direction. Across all analyses and time points, there was an increased risk of mortality for PWID with depressive symptoms between 8 and 14 percentage points (Fig. 2). We did not find evidence of modification of the depression-mortality relationship by trial intervention arm (likelihood ratio test p-value > 0.5).
Table 2.
Association of depressive symptoms at HIV testing with cumulative risk of all-cause mortality at 6, 12, 18, and 24 months among 336 PWID newly diagnosed with HIV in Vietnam.
| Months since HIV diagnosis | Cumulative events | Crude analysis | Weighted analysisa | Sensitivity analysisb | |
|---|---|---|---|---|---|
| N (%) | RD (95% CI) | RD (95% CI) | RD (95% CI) | ||
| 6 | Depression | 25 (18) | 14.1 (7.3, 21.0) | 12.6 (5.5, 19.7) | 12.5 (3.7, 21.2) |
| No depression | 7 (4) | Ref | Ref | Ref | |
| 12 | Depression | 35 (25) | 14.1 (5.7, 22.4) | 13.9 (4.6, 23.2) | 12.1 (2.1, 22.0) |
| No depression | 21 (11) | Ref | Ref | Ref | |
| 18 | Depression | 38 (27) | 10.5 (1.6, 19.5) | 11.0 (0.9, 21.1) | 9.2 (−1.8, 20.2) |
| No depression | 32 (16) | Ref | Ref | Ref | |
| 24 | Depression | 44 (31) | 11.7 (2.3, 21.2) | 9.7 (−1.2, 20.6) | 8.3 (−3.3, 20.0) |
| No depression | 38 (19) | Ref | Ref | Ref | |
The weighted model in the main analysis controlled for baseline confounding by age, marital status, education, employment, history of drug overdose, experience of HIV and injection drug use-related stigma, perceived social support, self-rated health, CD4 cell count, and trial intervention arm.
The weighted model in the sensitivity analysis included frequency of injection drug use, in addition to the variables listed above.
Figure 1.
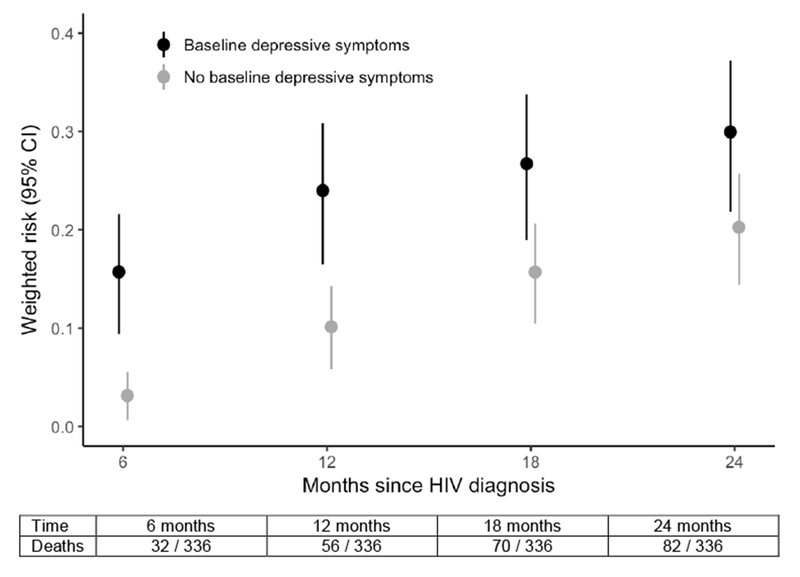
Weighted cumulative risk of death (with 95% CI) by baseline depression (at HIV testing) at 6, 12, 18, and 24 months after HIV diagnosis for 336 PWID in Vietnam.
Figure 2.
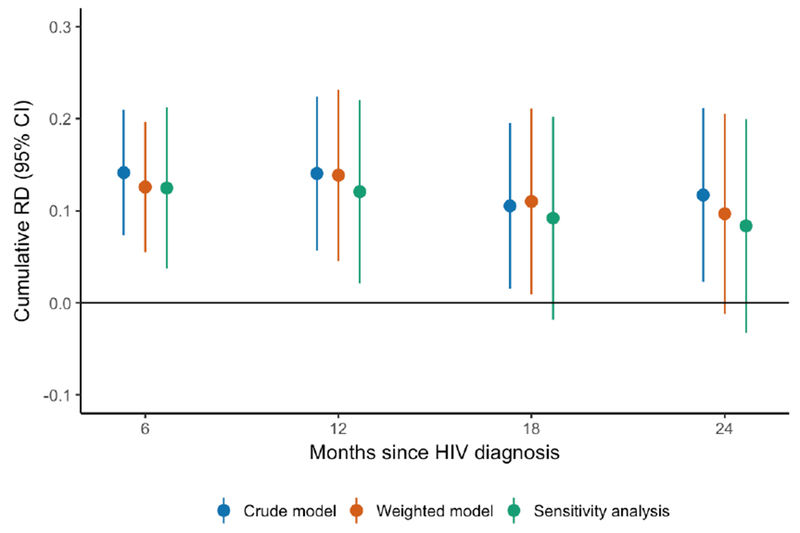
Crude and weighted cumulative RDs (with 95% CI) for mortality at 6, 12, 18, and 24 months after HIV diagnosis, comparing PWID with and without depressive symptoms at HIV testing.
Based on the weighted risk estimates for 24-month mortality from our main analysis, we calculated the PARD and NNT to understand how eliminating depression could impact survival. We estimated the PARD to be 4.0% (95% CI: 3.0%, 5.1%), indicating that the two-year risk of death in our study population would have been approximately 4 percentage points lower if a hypothetical intervention had eliminated depression (without changing other baseline risk factors for mortality). The NNT is 10.3, indicating that for every 10 people treated with a hypothetical intervention to eliminate depression, we would reduce the number of deaths by 1 over the 24 months following HIV diagnosis.
DISCUSSION
Our study found that depressive symptoms at the time of HIV testing were strongly associated with all-cause mortality at 6, 12, 18, and 24 months following HIV diagnosis in a sample of 336 PWID in Thai Nguyen, Vietnam. The differences in risk of death across time points were fairly consistent in magnitude, but tended to decrease over time from diagnosis at baseline. Our estimates attenuated slightly when we controlled for confounding using a weighted model and when we included frequency of injection drug use as a confounder in a sensitivity analysis. There was a loss of precision at later time points (18 and 24 months) due to higher mortality; the increase in the underlying risks of death resulted in larger variance. Overall, PWID with depressive symptoms experienced a risk of death between 8 and 14 percentage points higher than the risk among PWID without depressive symptoms throughout the 24 months following HIV diagnosis. Consistent with prior work (30–32), this study shows that people living with HIV and depressive symptoms face a markedly higher risk of mortality than those without depression, and it is the first to demonstrate this relationship among PWID with HIV in the two years following diagnosis. Of note, the overall mortality over two years (24%) was very high among participants. Similar to other provinces in Vietnam, the supply of ART was limited in Thai Nguyen during the study period (2009-2013) and was generally not provided for patients with CD4 cell count above 250 cells/μL (51).
This study focused on the total effect of depressive symptoms at time of HIV testing on mortality over 24 months. There are several possible mechanisms through which depressive symptoms may impact mortality among PWID with HIV. Depression could hinder health-seeking behaviors and interfere with HIV treatment, leading to AIDS-related mortality. Prior studies have shown that depressed HIV patients have lower rates of initiation and retention in HIV care (24–26), ART adherence (27,28), and viral suppression (29). Depression may also increase injection drug use and result in overdose-related mortality, although the time-ordering of the relationship between depression and injection drug use is not clear. There is evidence that depression is a risk factor for injection drug use, preceding its initiation (47,48), and also that depressive symptoms can be substance-induced (49) or a consequence of stigma experienced by PWID (50). In our study data, both depressive symptoms and frequency of injection drug use were assessed at the same time, preventing us from disentangling whether injection drug use led to or resulted from depressive symptoms. As shown in the sensitivity analysis, the RD estimates were slightly reduced when frequency of injection drug use was included in the weighted model. This could be due to removing confounding (if injection drug use resulted in depression) or due to inappropriately adjusting away a mediating path from depression to mortality (if depression resulted in injection drug use). Although we cannot determine the directionality from these data alone, there remains a clear overall association between depression and mortality in this population.
Based on these results, HIV testing and diagnosis may present an important opportunity to screen and treat depressive symptoms. For PWID diagnosed with HIV, post-test counseling and ART clinic visits could include depression screening and referral to care. In Vietnam, mental health services are a national health priority, and there is growing attention and funding for increasing local services and availability of treatment (52). Hypothetically, if depression were successfully treated, this study’s estimates of the PARD and NNT indicate the possible impact on survival in this population. Identifying depressive symptoms at the time of HIV diagnosis and treating those symptoms as part of HIV care has the potential to improve outcomes for this vulnerable population.
An important limitation of our study is that we focus on baseline depressive symptoms. Depression is known to be episodic, with variability in symptom severity over time. We chose to consider only the baseline measure because the timing of this assessment corresponded to a clinically actionable decision point: the time of HIV testing for patients who are newly diagnosed with HIV. Despite not accounting for subsequent changes in depression status, we observed a persistent relationship between baseline depression and mortality over 24 months. Even though the RD in mortality decreased in magnitude at 18 and 24 months, baseline depressive symptoms remained relevant as a determinant of mortality. Another limitation of the depression assessment is that the CES-D corresponds to probable depression, not a clinical diagnosis. However, validation studies have found that the CES-D has high reliability and validity when compared with clinical assessments made by psychiatrists (40,41).
Our study conclusions are specific to this sample and should not be inferred as representative of the population. Due to the snowball sampling approach, participants were not randomly sampled for this study and may differ from other PWID with HIV in Vietnam who were not recruited. In addition, our findings may not be applicable to other groups, such as women or settings where the HIV epidemic is not concentrated among PWID. However, male PWID in Vietnam are a critical population to study. They are the driver of the HIV epidemic in Vietnam, and one-third of PWID in Thai Nguyen are HIV-positive. Our findings may apply to other Asian and Eastern European countries where the HIV epidemic is concentrated among similar groups of men who inject drugs. There is also increasing relevance to the United States, as the current opioid epidemic has the potential to result in explosive HIV outbreaks among PWID.
In conclusion, our study found that PWID with depressive symptoms at time of HIV testing faced a markedly higher risk of death over the next two years. This is the first study to examine the relationship between depression and mortality following HIV diagnosis among PWID. The time of HIV diagnosis offers an important opportunity to screen and treat depressive symptoms and could subsequently improve survival in this key population.
Acknowledgments
Funding: Traineeship support was provided by the National Institute of Allergy and Infectious Diseases (https://www.niaid.nih.gov/): T32 AI070114-10 [SNL]. The parent trial for this study was funded by the National Institute on Drug Abuse (http://www.drugabuse.gov): R01 DA022962-01 [VFG TVH NLM TS CAL PTV VMQ CF]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest: None.
Ethical approval: This research was approved by the ethical review committees at the Thai Nguyen Center for Preventive Medicine, the Johns Hopkins Bloomberg School of Public Health, and the University of North Carolina at Chapel Hill Gillings School of Global Public Health. All procedures performed were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Written informed consent was obtained from all participants.
Clinical trial registration (parent trial): ClinicalTrials.gov NCT01689545
REFERENCES
- 1.Wang H, Wolock TM, Carter A, Nguyen G, Kyu HH, Gakidou E, et al. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015. Lancet HIV. 2016; 3:e361–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS. How AIDS changed everything - MDG6: 15 years, 15 lessons of hope from the AIDS response. 2015. Available from: http://www.unaids.org/en/resources/documents/2015/MDG6_15years-15lessonsfromtheAIDSresponse
- 3.Dutta A, Wirtz A, Stanciole A, Oelrichs R, Semini I, Baral S, et al. The global HIV epidemics among people who inject drugs. Washington DC: World Bank; 2013. Available from: http://search.ebscohost.com/login.aspx?direct=true&db=ecn&AN=1386909&site=ehost-live&scope=site [Google Scholar]
- 4.Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008; 372: 1733–45. [DOI] [PubMed] [Google Scholar]
- 5.UN Joint Programme on HIV/AIDS (UNAIDS). The Gap Report. 2014. Available from: http://www.unaids.org/en/resources/documents/2014/20140716_UNAIDS_gap_report
- 6.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk. AIDS. 2014; 28: 1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quan VM, Go VF, Nam L Van, Bergenstrom A, Thuoc NP, Zenilman J, et al. Risks for HIV, HBV, and HCV infections among male injection drug users in northern Vietnam: a case-control study. AIDS Care. 2009; 21:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas M, Sahu D, Raj Y, Pandey A. A probability model for estimating the force of transmission of HIV infection and its application. Am J Math Stat. 2014; 4(3): 171–7. [Google Scholar]
- 9.United Nations Office on Drugs and Crime. World Drug Report. New York; 2015. Available from: https://www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf [Google Scholar]
- 10.Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: a systematic review and meta-analysis. Bull World Health Organ. 2013; 91:102–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lappalainen L, Hayashi K, Dong H, Milloy MJ, Kerr T, Wood E. Ongoing impact of HIV infection on mortality among people who inject drugs despite free antiretroviral therapy. Addiction. 2015; 110:111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber R, Huber M, Battegay M, Stahelin C, Castro Batanjer E, Calmy A, et al. Influence of noninjecting and injecting drug use on mortality, retention in the cohort, and antiretroviral therapy, in participants in the Swiss HIV Cohort Study. HIV Med. 2015; 16(3): 137–51. [DOI] [PubMed] [Google Scholar]
- 13.Ministry of Health of Vietnam. Results from the HIV/STI integrated biological and behavioral surveillance (IBBS) in Vietnam, Round II, 2009. Vietnam: Ministry of Health; 2011. [Google Scholar]
- 14.Mathers BM, Degenhardt L, AN H, Wiessing L, Hickman M, Mattick RP, et al. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet. 2010; 375:1014–28. [DOI] [PubMed] [Google Scholar]
- 15.Fraser H, Mukandavire C, Martin NK, Hickman M, Cohen MS, Miller WC, et al. HIV treatment as prevention among people who inject drugs – a re-evaluation of the evidence. Int J Epidemiol. 2016;dyw180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet. 2010; 376:355–66. [DOI] [PubMed] [Google Scholar]
- 17.Parashar S, Collins AB, Montaner JSG, Hogg RS, Milloy M- J. Reducing rates of preventable HIV/AIDS-associated mortality among people living with HIV who inject drugs. CurrOpin HIV AIDS. 2016; 11:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levintow SN, Pence BW, Ha TV, Minh N Le, Sripaipan T, Latkin CA, et al. Prevalence and predictors of depressive symptoms among HIV-positive men who inject drugs in Vietnam. PLoS One. 2018;13(1):e0191548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Treisman G, Angelino A. Interrelation between psychiatric disorders and the prevention and treatment of HIV infection. Clin Infect Dis. 2007; 45: S313–7. [DOI] [PubMed] [Google Scholar]
- 20.Bing EG, Burnam M a, Longshore D, Fleishman JA, Sherbourne CD, London a S, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001; 58:721–8. [DOI] [PubMed] [Google Scholar]
- 21.Leserman J Role of depression, stress, and trauma in HIV disease progression. Psychosom Med. 2008; 70:539–45. [DOI] [PubMed] [Google Scholar]
- 22.Nanni MG, Caruso R, Mitchell AJ, Meggiolaro E, Grassi L. Depression in HIV infected patients: A review. Curr Psychiatry Rep. 2015; 17:1–11. [DOI] [PubMed] [Google Scholar]
- 23.Ironson G, Balbin E, Klimas N, Schneiderman N, Solomon G, I BMG. Psychosocial factors predict CD4 and viral load change in men and women with human immunodeficiency virus in the era of highly active antiretroviral treatment. Psychosom Med. 2005; 67:1013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tegger MK, Crane HM, Tapia KA, Uldall KK, Holte SE, Kitahata MM. The effect of mental illness, substance use, and treatment for depression on the initiation of highly active antiretroviral therapy among HIV-infected individuals. AIDS Patient Care STDS. 2008; 22: 233–43. [DOI] [PubMed] [Google Scholar]
- 25.Ramirez-Avila L, Regan S, Giddy J, Chetty S, Ross D, Katz JN, et al. Depressive symptoms and their impact on health-seeking behaviors in newly-diagnosed HIV-infected patients in Durban, South Africa. AIDS Behav. 2012; 16: 2226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuniga JA, Yoo-Jeong M, Dai T, Guo Y, Waldrop-Valverde D. The role of depression in retention in care for persons living with HIV. AIDS Patient Care STDS. 2016; 30:34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordillo V, del Amo J, Soriano V, González-Lahoz J. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS. 1999; 13:1763–9. [DOI] [PubMed] [Google Scholar]
- 28.Uthman OA, Magidson JF, Safren SA, Nachega JB. Depression and adherence to antiretroviral therapy in low-, middle- and high-income countries: a systematic review and meta-analysis. Curr HIV/AIDS Rep. 2014; 11:291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pence BW, Miller WC, Gaynes BN, Eron JJ. Psychiatric illness and virologic response in patients initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007; 44:159–66. [DOI] [PubMed] [Google Scholar]
- 30.Cook JA, Grey D, Burke J, Cohen MH, Gurtman AC, Richardson JL, et al. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health. 2004; 94:1133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001; 285:1466–74. [DOI] [PubMed] [Google Scholar]
- 32.Leserman J, Pence BW, Whetten K, Mugavero MJ, Thielman NM, Swartz MS, et al. Relation of lifetime trauma and depressive symptoms to mortality in HIV. Am J Psychiatry. 2007; 164:1707–13. [DOI] [PubMed] [Google Scholar]
- 33.Bouhnik A-D, Preau M, Vincent E, Carrieri MP, Gallais H, Lepeu G, et al. Depression and clinical progression in HIV-infected drug users treated with highly active antiretroviral therapy. AntivirTher. 2005; 10:53–61. [PubMed] [Google Scholar]
- 34.Springer SA, Chen S, Altice F. Depression and symptomatic response among HIV-infected drug users enrolled in a randomized controlled trial of directly administered antiretroviral therapy. AIDS Care. 2009; 21:976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.May MT, Justice AC, Birnie K, Ingle SM, Smit C, Smith C, et al. Injection drug use and hepatitis C as risk factors for mortality in HIV-infected individuals: The Antiretroviral Therapy Cohort Collaboration. J Acquir Immune Defic Syndr. 2015; 69:348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buckingham E, Schrage E, Cournos F. Why the treatment of mental disorders is an important component of HIV prevention among people who inject drugs. Adv Prev Med. 2013; 2013:690386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeLorenze GN, Satre DD, Quesenberry CP, Tsai A- L, Weisner CM. Mortality after diagnosis of psychiatric disorders and co-occurring substance use disorders among HIV-infected patients. AIDS Patient Care STDS. 2010; 24:705–12.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Go VF, Frangakis C, Minh N Le, Latkin C, Ha TV, Mo TT, et al. Efficacy of a multi-level intervention to reduce injecting and sexual risk behaviors among HIV-infected people who inject drugs in Vietnam: a four-arm randomized controlled trial. PLoS One. 2015; 10:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thai Nguyen Provincial AIDS Center Division of Social Evils Control and Prevention. Quarterly Report of the Statistics Office of Thai Nguyen Province, Thai Nguyen Provincial AIDS Center and the Division of Social Evils Control and Prevention, Department of Labor, Invalids and Social Affairs of Thai Nguyen, Vietnam: 2007. [Google Scholar]
- 40.Radloff LS. A self-report depression scale for research in the general population. Appl Psychol Meas. 1977; 1:385–401. [Google Scholar]
- 41.Thai TT, Jones MK, Harris LM, Heard RC. Screening value of the Center for Epidemiologic Studies - Depression Scale among people living with HIV/AIDS in Ho Chi Minh City, Vietnam: a validation study. BMC Psychiatry. 2016; 16:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huynh V-AN, To KG, Van Do D, To QG, Nguyen MTH. Changes in depressive symptoms and correlates in HIV+ people at An Hoa Clinic in Ho Chi Minh City, Vietnam. BMC Psychiatry. 2017; 17:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011; 46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robins J The control of confounding by intermediate variables. Stat Med. 1989; 8:679–701. [DOI] [PubMed] [Google Scholar]
- 45.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000; 11(5):550–60. [DOI] [PubMed] [Google Scholar]
- 46.Go VF, Frangakis C, Le Minh N, Fla TV, Latkin CA, Sripaipan T, et al. Increased survival among FllV-infected PWID receiving a multi-level hiv risk and stigma reduction intervention. J Acquir Immune Defic Syndr. 2017;74(2): 166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Latkin CA, Mandell W. Depression as an antecedent of frequency of intravenous drug use in an urban, nontreatment sample. Int J Addict. 1993;28(14): 1601–12. [DOI] [PubMed] [Google Scholar]
- 48.Blum J, Gerber H, Gerhard U, Schmid O, Petitjean S, Riecher-Rossler A, et al. Acute effects of heroin on emotions in heroin-dependent patients. Am J Addict. 2013;22(6):598–604. [DOI] [PubMed] [Google Scholar]
- 49.Samet S, Fenton MC, Nunes E, Greenstein E, Aharonovich E, Hasin D. Effects of independent and substance-induced major depressive disorder on remission and relapse of alcohol, cocaine and heroin dependence. Addiction. 2013; 108(1): 115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Latkin C, Davey-Rothwell M, Yang J, Crawford N. The relationship between drug user stigma and depression among inner-city drug users in Baltimore, MD. J Urban Health. 2013;90(1): 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ministry of Health of Vietnam. Guidelines for HIV/AIDS Diagnosis and Treatment. Hanoi, Vietnam; 2009. Available from: https://www.ilo.org/dyn/natlex/docs/ELECTRONIC/84198/93450/VNM84198.pdf [Google Scholar]
- 52.Vuong DA, Van Ginneken E, Morris J, Ha ST, Busse R. Mental health in Vietnam: Burden of disease and availability of services. Asian J Psychiatr. 2011. ;4(1):65–70. [DOI] [PubMed] [Google Scholar]


