Abstract
Loss of dystrophin expression in Duchenne Muscular Dystrophy (DMD) causes progressive degeneration of skeletal muscle, which is exacerbated by reduced self-renewing asymmetric divisions of muscle satellite cells. This in turn impacts production of myogenic precursors and impairs regeneration, and suggests that increasing such divisions may be beneficial. Here, through a small molecule screen we identified epidermal growth factor receptor (EGFR) and Aurora kinase A (Aurka) as regulators of asymmetric satellite cell divisions. Inhibiting EGFR causes a substantial shift from asymmetric to symmetric division modes, while EGF treatment increases asymmetric divisions. EGFR activation acts through AurkA to orient mitotic centrosomes, and inhibiting AurkA blocks EGF stimulation-induced asymmetric division. In vivo EGF treatment markedly activates asymmetric divisions of dystrophin-deficient satellite cells in mdx mice, thereby increasing progenitor numbers, enhancing regeneration, and restoring muscle strength. Therefore, activating an EGFR-dependent polarity pathway promotes functional rescue of dystrophin-deficient satellite cells and enhances muscle force generation.
Keywords: Skeletal muscle, Satellite cell, muscle stem cell, Asymmetric cell division, Apicobasal polarity, EGF, EGFR, Aurka, Duchenne Muscular Dystrophy
eTOC blurb

Wang et al., found that EGFR–Aurka signaling in muscle stem cells acts to direct apicobasally oriented mitoses and asymmetric cell division. EGF treatment rescues the reduction of asymmetric divisions in dystrophin-deficient satellite cells in mdx mice, resulting in increased numbers of progenitors and enhanced regeneration.
INTRODUCTION
The balance between stem cell self-renewal and differentiation impacts the kinetics and efficiency of tissue regeneration. Rather than directly undergoing differentiation, stem cells can give rise to progenitors through asymmetric cell divisions. This creates a layer of regulation that allows stem cells to self-renew, as well as imprint the identity of their progeny by asymmetrically segregating fate determinants through polarity, protein trafficking, and cell cycle-dependent mechanisms (Knoblich, 2008; Morin and Bellaïche, 2011). While many intrinsic mechanisms of asymmetric divisions are conserved across evolution and in different cell types, extrinsic determinants are dependent on the tissue organization and spatial localization of cell fate determinants (Arsenio et al., 2015; Matsuzaki and Shitamukai, 2015).
Muscle stem cells, or satellite cells, are essential for the growth and regeneration of skeletal muscle (reviewed in Dumont et al., 2015a). The majority of satellite cells represent a short term repopulating cell (Kuang et al., 2007), while a subset are capable of long-term self-renewal and can give rise to committed progenitors through asymmetric cell divisions (Gurevich et al., 2016; Kuang et al., 2007; Rocheteau et al., 2012). We term these cells satellite stem cells. A key feature of satellite stem cells is the lack of the myogenic transcription factor Myf5, which can be used to distinguish stem cells from committed progenitors (Kuang et al., 2007).
Stimulating satellite stem cell symmetric expansion results in augmented muscle regeneration with a dramatic increase in satellite cell numbers (Le Grand et al., 2009). Conversely, promiscuous activation of JAK2/STAT3 mediates the decline of satellite cell self-renewal in aging by biasing satellite stem cells toward asymmetric divisions (Price et al., 2014; Tierney et al., 2014). Moreover, cell-autonomous defects leading to loss of polarized p38MAPK signaling in aged cells attenuates self-renewal, whereby pharmacological rejuvenation of aged stem cells can restore muscle function (Bernet et al., 2014; Cosgrove et al., 2014). Thus, regulation of satellite stem cell asymmetric division is a key control point that impacts the efficiency of the muscle regenerative program.
We recently discovered deficits in muscle stem cell asymmetric divisions is part of the underlying mechanism that results in progressive wasting of skeletal muscle found in Duchenne Muscular Dystrophy (DMD), an X-linked genetic disease caused by mutations in the dystrophin gene (Dumont et al., 2015b). Whereas dystrophin-deficiency in muscle fibers make them susceptible to membrane damage (Anderson and Kunkel, 1992; Cohn and Campbell, 2000), dystrophin-deficiency in satellite stem cells results in loss of polarity determination and reduced asymmetric divisions, ultimately leading to diminished production of myogenic progenitors and hindered regeneration. The compounding effect of diminished regeneration with chronic degeneration of fragile myofibers accounts for the eventual replacement of muscle by adipose and fibrotic infiltrates in mouse (Cohn et al., 2002; Irintchev et al., 1997) and human muscle (Bell and Conen, 1968).
Here we report the identification of epidermal growth factor receptor (EGFR) and aurora kinase A (Aurka) pathways as determinants of asymmetric satellite stem cell divisions through an in-niche muscle stem cell screen. EGF stimulation activates EGFR localized at the basal surface of muscle stem cells and recruits the mitotic spindle assembly protein Aurka to induce apicobasal asymmetric divisions. siRNA mediated knockdown of Aurka abolishes EGF induced asymmetric divisions. Importantly, the EGFR polarity pathway acts independently of dystrophin and can rescue the deficit in asymmetric division in dystrophin-deficient satellite cells. Treatment with exogenous EGF in mdx mice, a mouse model of DMD, enhances the formation of new myofibers resulting in better muscle function while delaying fibrotic accumulation. Therefore, we conclude the EGFR pathway can be exploited to restore muscle stem cell polarity and function in DMD.
RESULTS
In-Niche Screen for Regulators of Satellite Cell Self-Renewal
The satellite cell microenvironment is required to provide necessary signals for asymmetric divisions (Bentzinger et al., 2013a). Therefore, we designed a scalable method to quantify satellite stem cell fate decisions without removing them from their native niche. Using Myf5-Cre (Tallquist et al., 2000) and R26R-eYFP (Srinivas et al., 2001) alleles, Cre-mediated recombination at the R26R-eYFP allele and expression of yellow fluorescent protein following Myf5 activation discriminate Myf5Neg satellite stem cells and Myf5Pos committed satellite cells. Culturing single myofibers from Myf5-Cre/ROSA26-eYFP mice for 42h, where 80% of satellite cells have undergone a single round of cell division, we can quantify symmetric and asymmetric satellite stem cell divisions, as well as committed satellite cell divisions through the expression of eYFP (Figure 1A).
Figure 1. Identification of Small Molecules that drive Satellite Stem Cell Symmetric Division.
(A) Symmetric satellite stem cell division, asymmetric satellite stem cell division, and committed satellite cell division on single Myf5-Cre/R26R-eYFP myofibers after 42h culture stained with Pax7 (red), eYFP (green) and DAPI (blue).
(B) Graphic overview of myofiber screening protocol.
(C) Relative changes to satellite stem cell numbers with small molecule treatment sorted by changes to eYFPNeg satellite stem cell numbers compared to vehicle (DMSO) controls. Wnt7a was a positive control. Screening hits are listed in Table S1.
(D) Microarray heatmap representing genes from the EGFR/Erbb and Aurk family from prospectively isolated satellite cells, cultured myoblasts in vitro, and 2- and 5-day-differentiated myotubes.
Recombination at the R26R-eYFP allele results in individual genotypes for satellite stem cells and committed satellite cells. Therefore, primer combinations designed against the recombination status of the R26R-eYFP allele can be used to quantify numbers of satellite stem cells and committed satellite cells by quantitative real-time PCR (Figure S1A and B). Consistent with manual enumeration of satellite stem cell by immunofluorescence, qRT-PCR quantification of the recombination state of genomic DNA isolated from myofiber cultures accurately detected a 1.5-fold increase in satellite stem cell numbers after Wnt7a stimulation (Figure S1C). Thus, this method enables us to analyze changes in the stem cell population. We adapted this system to a 96-well format using myofibers isolated from Flexor digitorum brevis (FDB) muscles, which have a higher satellite cell to myonuclei ratio and are amenable for transfer by pipetting (Shefer and Yablonka-Reuveni, 2005), to screen well-characterized small molecule inhibitors.
Altogether, 640 well-characterized pharmacological compounds were screened against Wnt7a as a positive control (Le Grand et al., 2009). We identified 43 candidate compounds as inducers of satellite stem cell expansion (Table S1). Consistent with p38MAPK driving satellite cell commitment (Bernet et al., 2014; Charville et al., 2015; Cosgrove et al., 2014), several inhibitors of the p38MAPK pathway, including SB203580, were confirmed to increase satellite stem cell numbers in the screen (Figure 1C), thus validating the screening platform reliably identified compounds capable of driving symmetric expansion of satellite stem cells.
Several compounds effective in stimulating satellite stem cell expansion were inhibitors of EGFR/Erbb or Aurk pathways (Figure 1C). Notably, Lapatinib, an FDA approved and clinically experienced EGFR/Erbb2 inhibitor, was identified as a lead compound. Furthermore, the top hit from the screen ZM 449829 and its prodrug ZM 39923 hydrochloride, have known inhibitory actions on EGFR (Brown et al., 2000). Of the Aurk inhibitors, ZM 447439 and JNJ-7706621 are extensively studied inhibitors against both aurora kinase A (Aurka) and B (Aurkb) (Kollareddy et al., 2012), whereas TC-A2317 is a VX-680 variant that exhibits higher specificity for Aurka (Ando et al., 2010).
Toward identifying specific gene targets of the EGFR/Erbb and Aurk inhibitors, we examined gene expression using microarray data (Bentzinger et al., 2013b) to correlate expression pattern of EGFR/Erbb and Aurk family to possible regulatory function in satellite cell self-renewal. Only EGFR from the EGFR/Erbb family was highly expressed in freshly-isolated satellite cells, whereas Erbb2, Erbb3, and Erbb4 were expressed at low levels (Figure 1D and S1D). This agrees with published data where EGFR protein is detectable in satellite cells and myoblasts but is lost in differentiation (Charville et al., 2015; Olwin and Hauschka, 1988). Aurka, and Aurkb were expressed moderately in satellite cells. However, they were highly expressed in proliferating myoblasts, consistent with their function in regulating cell cycle (Meraldi et al., 2004) (Figure 1D and S1E). Aurkc was only expressed late in differentiation (Figure 1D). This information suggested EGFR, Aurka, and Aurkb are likely targets of the inhibitors identified in our screen.
Validation of Lead Compound Activity
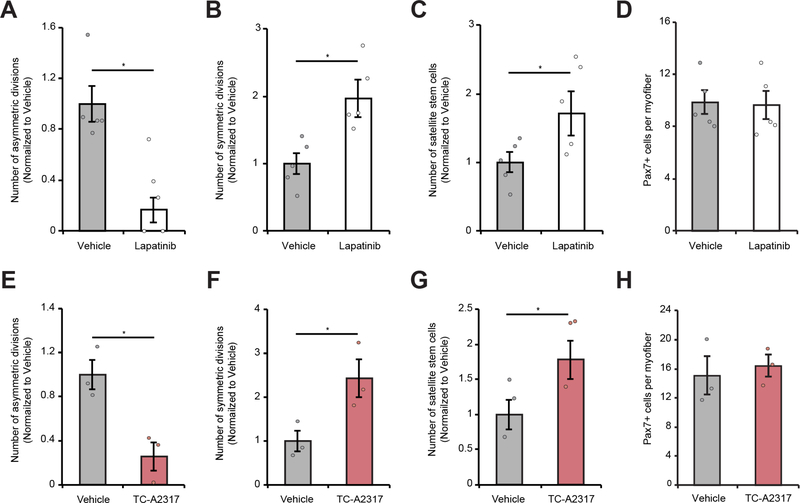
To confirm our lead compound activity on satellite cell asymmetric division and validate that our findings are not specific to FDB muscle satellite stem cells, we cultured satellite cells on myofibers isolated from Extensor digitorum longus (EDL) muscles from Myf5-Cre/R26R-eYFP mice and monitored changes to cell proliferation and symmetric or asymmetric cell division. Inhibition of EGFR signaling resulted in a shift towards satellite stem cell symmetric division as evidenced by a 83% decrease in number of asymmetric divisions observed (Figure 2A–B, and S2A). This change in satellite stem cell division mode resulted in a 71% increase in the number of satellite stem cells (Figure 2C). Importantly, EGFR inhibition did not change total numbers of satellite cells (Figure 2D), indicating EGFR signaling does not impact cell proliferation.
Figure 2. Lapatinib and TC-A2317 Inhibit Asymmetric Satellite Stem Cell Divisions.
(A) Number of asymmetric and (B) symmetric satellite stem cell divisions per myofiber at 42h of culture in the presence of Lapatinib normalized to DMSO control (vehicle).
(C) Number of eYFPNeg and (D) total Pax7-expressing satellite stem cells per myofiber at 42h of culture in the presence of Lapatinib normalized to DMSO control (vehicle).
(E) Number of asymmetric and (F) symmetric satellite stem cell divisions per myofiber at 42h of culture in the presence of TC-A2317 normalized to DMSO control (vehicle).
(G) Number of eYFPNeg and (H) total Pax7-expressing satellite stem cells per myofiber at 42h of culture in the presence of TC-A2317 normalized to DMSO control (vehicle).
(A–H) Error bars represent means ± SEM; p-values: *=<0.05; **=<0.01; ***=<0.005. (A–D) n=5 mice; (E–H) n=3 mice.
Similarly, we tested Aurka inhibition using TC-A2317 on single EDL myofibers isolated from Myf5-Cre/R26R-eYFP mice. Comparable to EGFR inhibition, TC-A2317 decreased the rate of asymmetric divisions by 74% (Figure 2E and F). This shift to symmetric divisions increased the number of satellite stem cells by 77% (Figure 2G). Consistently, inhibition of Aurka with TC-A2317 did not impact cell proliferation (Figure 2H). These results suggest EGFR and Aurka signaling regulate the mode of satellite stem cell division.
To investigate if inhibition of EGFR or Aurka prevents myogenic differentiation generally, we treated differentiating primary myoblasts with Lapatinib, TC-A2317 or vehicle controls. Interestingly, unlike satellite cells on myofibers, inhibition of EGFR signaling in differentiating myoblasts led to a slight increase in activation of the differentiation marker Myog (Figure S2B), consistent with reports regarding siRNA knockdown of EGFR in mouse and human myoblasts (Leroy et al., 2013; Olwin and Hauschka, 1988). Likewise, Aurka inhibition by TC-A2317 modestly increased the percentage of Myog-expressing cells (Figure S2B). These results suggest EGFR and Aurka have different roles in satellite cell self-renewal and progenitor differentiation.
EGFR is polarized in satellite cells and promotes asymmetric division in satellite stem cells
To gain insight to the function of EGFR in satellite stem cell asymmetric division, we examined EGFR localization and activation in vitro and in vivo. Immunofluorescence on muscle sections revealed EGFR protein is polarized to the basal surface of quiescent satellite cells (Figure 3A). To examine the signaling status of EGFR, we immunostained satellite cells on myofibers cultured in serum free medium with and without EGF stimulation. In quiescent satellite cells, EGFR is inactive by immunostaining activated EGFR phosphorylated on Tyr1068 (p-EGFR) (Figure 3B). Following 1h stimulation with EGF, activated p-EGFR was detected in most satellite cells (Figure 3B and C).
Figure 3. Polarized Localization and Activation of EGFR in Satellite Cells.
(A) Localization of EGFR (green) in Pax7-expressing (red) satellite cells on an immunostained muscle section. The basal surface of the satellite cell is attached to a basal lamina that surrounds both the cell and its host fiber. Dashed lines are based on autofluorescence of the myofiber sarcolemma.
(B) Signaling status of p-EGFR (green) in Pax7-expressing (red) and DAPI positive (blue) cells on EDL myofibers at 1h culture in vehicle or EGF containing media.
(C) Quantification of p-EGFR staining in satellite cells on EDL myofibers at 1h culture in vehicle or EGF containing media.
(D) Polarized p-EGFR (green) staining in mitotic p-H3-expressing (white) Pax7-expressing (red) satellite cells on EDL myofibers at 36h. DAPI (blue).
(E) Analysis of EGFR and p-EGFR localization from injured EDL muscle fixed 2 days post injury and manually dissociated then stained with DAPI (blue), EGFR or p-EGFR (green) and Pax7 (red).
(F) Immunoblotting analysis of p-EGFR, EGFR, Aurka, Pax7, Myog expression in TA muscles of uninjured, or 3, 7, 14 and 21 days post-saline or cardiotoxin (CTX) injection.
(G) Number of asymmetric satellite stem cell divisions per myofiber at 42h of culture in EGF containing media normalized to vehicle.
(H) Number of apicobasally oriented mitotic satellite cell divisions at 36h of culture in EGF containing media normalized to vehicle stained with p-Aurk (green). The host myofiber is outlined with a dashed line. DAPI (blue).
(I) Quantification of EdU labelled satellite cells on myofibers from Myf5-Cre/R26R-eYFP mice cultured in vehicle or EGF containing media supplemented with EDU for 20h and with a 20h chase prior to fixation.
(J) Number of asymmetric satellite stem cell divisions, (K) symmetric satellite stem cell divisions and (L) number of eYFPNeg satellite stem cells per myofiber at 42h of culture after transfection with siRNA against EGFR (siEGFR) normalized to scrambled siRNA (siSCR).
(H, G, and J–L) Error bars represent means ±SD; p-values: *=<0.05; **=<0.01; ***=<0.005; (C) n=3 mice; (E) n=43 and n=46 cells respectively; (F) n=2 mice per timepoint; (G) n=6 mice; (H) n=5 mice; (I) n=3 mice; (J–L) n=4 mice.
In sublaminar satellite cells, p-EGFR was polarized in 63% of satellite cells (Figure 3C) localized to the basal surface on the opposite cortex to the myofiber (Figure S3B). Surprisingly, p-EGFR was restricted to a constrained streak-like domain (Figure 3B) and this polarized localization was maintained as satellite cells enter M-phase (Figure 3D). P-EGFR is basal-laterally localized in satellite cells on myofibers isolated and immediately fixed following injury-induced activation in vivo (Figure 3E). In injured muscle, p-EGFR and Aurka follow the expression pattern of Pax7 peaking between 3–7 days of regeneration (Figure 3F). Early activation of EGFR and Aurka correlates with expansion of the proliferating satellite cell population (Dumont et al., 2015b), and expression of Myog in differentiating myogenic progenitors (Figure 3F). Together, this suggests basally localized EGFR primes satellite stem cells for asymmetric divisions.
To investigate whether EGFR activation is a driver of asymmetric division, EGF was added to cultures of single EDL myofibers isolated from Myf5-Cre/R26R-eYFP mice. Strikingly, EGF treatment increased asymmetric satellite stem cell division rate 2.5-fold (Figure 3G).
EGFR signaling can orient the mitotic axis of polarized epithelial MDCK cells along the apicobasal axis (Bañón ‐ Rodríguez et al., 2014). Moreover, asymmetric satellite cell divisions occur in an apicobasal orientation (Kuang et al., 2007). Therefore, single EDL myofibers were cultured for 36h and satellite cell centrosomes were detected by immunostaining for phosphorylated aurora kinase (p-Aurk). Importantly, EGF increased the proportion of total mitotic satellite cells along the apicobasal axis by 50% (Figure 3H). We did not observe changes to the proliferation rate of satellite cells at 36h with EGF, around 5% of cells undergoing mitosis marked by p-Aurk staining (Figure S3C–D). Moreover, there was no change in rate of S-phase entry in eYFPPos or eYFPNeg satellite cell populations following EGF treatment as determined by EdU incorporation over the first 20h of culture (Figure 3I).
To confirm the specific effect of EGFR signaling, siRNA against EGFR (siEGFR) was transfect into satellite cells on single EDL myofibers from Myf5-Cre/R26R-eYFP mice. Similar to inhibition with Lapatinib, siEGFR reduced the rate of asymmetric division by 65% compared to scrambled siRNA (siSCR) (Figure 3J). Transfection of siEGFR also increased the rate of symmetric division and increased satellite stem cell numbers by 76% (Figure 3K and L). This suggests EGF can change mitotic orientation by recruitment of centrosomes along polarized p-EGFR in satellite cells.
To test if EGF is acting independently of cell polarity by activating Myf5 expression or enforcing myogenic commitment, we studied the effect of EGF on satellite stem cell-derived myoblasts in culture. qRT-PCR showed no change in Myf5 (eYFP) activation in cultured myoblasts derived from eYFPPos or eYFPNeg satellite cells from Myf5-Cre/R26R-eYFP mice (Figure S3H-I). Importantly, EGF did not promote eYFP protein expression in eYFPNeg myoblast (Figure S3J). These data support our hypothesis that polarized EGFR in satellite cells promotes apicobasal division within the myofiber niche.
EGFR promotes asymmetric division in satellite stem cells in vivo
To explore if EGFR signaling affects Myf5Neg satellite stem cells, Myf5Pos committed satellite cells or myoblasts in vivo, we crossed the Myf5-Cre allele with a ROSA26R-nTnG allele (Prigge et al., 2013), consisting of a CMV/β-actin promoter, a loxP flanked nuclear TdTomato (nTdT) and nuclear GFP (nGFP) cassette, to generate a Myf5-Cre nTnG transgenic reporter model where all Myf5Neg cells express nTdT and Myf5Pos cells express nGFP. Isolation of nTdTPos satellite cells (Myf5Neg) and nGFPPos satellite cells (Myf5Pos) allows us to determine the outcome of asymmetric division following transplantation where nTdT satellite cells undergoing asymmetric division give rise to one nGFPPos myogenic progenitor and one nTdTPos satellite stem cell (Figure S4A–E).
We observed nTdTPos satellite cells gave rise to roughly three-fold higher engraftment as Pax7-expressing satellite cells compared to nGFPPos satellite cells (Figure 4B-C), consistent with our previous report (Kuang et al., 2007). By contrast, nGFPPos satellite cell-derived myoblasts from the same donors lost all self-renewal capacity and failed to engraft as Pax7-expressing satellite cells, despite injecting 10-fold higher number of cells (Figure 4D). Strikingly, treatment of nTdTPos satellite cells with EGF for 3 hours following isolation increased nGFPPos progeny and facilitated engraftment as nGFPPos satellite cells (Figure 4B). This enhanced differentiation as myonuclei by roughly 3-fold while maintaining stem cell self-renewal. Furthermore, consistent with our findings in ex vivo myofiber and primary myoblast culture, EGF had no effect on transplanted nGFPPos satellite cells or nGFPPos myoblasts (Figure 4C-D). This evidence supports that the role of EGF is specific to Myf5Neg satellite stem cells where it promotes generation of Myf5Pos progenitors that amplify and differentiate to muscle fibers.
Figure 4. EGF promotes asymmetric division in satellite stem cells.
(A) Graphic overview of Myf5-Cre/R26R-nTnG satellite cell transplantation into injured NSG mice.
(B-D) Representative images of transplanted satellite cells stained with DAPI (blue), GFP (green), TdTomato (red) and Pax7 (gray).
(E) Graphic overview of cardiotoxin-induced injury and treatment with recombinant EGF in EGFR cKO mice.
(F) Quantification of Pax7-expressing cells on sections from non-injured and regenerating EGFR cKO or Pax7-CreERT2 TA muscles 10 days post injury.
(G) Quantification of Pax7-expressing and (H) Myog-expressing cells on sections from regenerating EGFR cKO or Pax7-CreERT2 TA muscles 10 days post cardiotoxin-induced injury treated with control (saline), or EGF protein.
(B-D and F-H) Error bars represent means ±SEM; p-values: *=<0.05; (B, D) n=3 donor; (C) n=4 donor; (F) n=4 mice; (G-H) n=3 mice.
To understand the effect of EGFR signaling in satellite cells and other cell types that express EGFR in muscle, we developed a satellite cell-specific EGFR conditional knockout (EGFR cKO) mouse model by crossing the Pax7-CreERT2 allele with floxed alleles of EGFR (Lee and Threadgill, 2009). Excision of EGFR in satellite cells by tamoxifen treatment did not change the number of Pax7-expressing satellite cells in resting muscle, however we observed a decreased number of Pax7-expressing cells 10 days post injury compared to tamoxifen treated Pax7-CreERT2 littermates (Figure 4F). Correspondingly, treatment with EGF at early timepoints after injury (day 0 and 2) increased number of Pax7-expressing cells and Myog-expressing cells in muscles of Pax7-CreERT2 mice at day 10 post-injury (Figure 4G–H), while EGFR cKO satellite cells did not respond to exogenous EGF stimulation (Figure 4G–H). These experiments suggest EGFR signaling in satellite cells is crucial during regeneration and supplementation with recombinant EGF can enhance the pool of myogenic progenitors during muscle regeneration.
To assess the effect of EGF treatment on other cell types in skeletal muscle, we performed immunofluorescence staining for α-smooth muscle actin (αSMA) and VEGFR2 in EGF-treated TA muscles to quantify changes in muscle vasculature. Neither αSMA nor VEGFR2 showed change with EGF treatment or when comparing EGFR cKO mice or Pax7-CreERT2 littermates (Figure S3F-G). These findings support that differences in Pax7-expressing cells and Myog-expressing cells observed with EGF treatment is not due to EGF promoting vascularization within the muscle.
Aurka Acts Downstream of EGFR to Orient Asymmetric Divisions
Our observation that EGF regulates mitotic orientation of satellite cells suggests EGFR signaling induces recruitment of centrosome regulators along the apicobasal axis. We hypothesized aurora kinases, identified as regulators of asymmetric stem cell divisions in our screen, are effectors of EGFR signaling.
Aurora kinases are a family of kinases that regulate mitosis (Meraldi et al., 2004). Aurka and/or Aurkb are suggested to act with upstream regulators in determination of mitotic orientation of symmetric and asymmetric cell divisions (Johnston et al., 2009; Wirtz-Peitz et al., 2008). Consistent with involvement in organizing mitotic centrosomes, Aurka protein is localized at centrosomes in M-phase myoblasts (Figure 5A). Challenging the idea that Aurka is essential for mitosis, presence of Aurka at the centrosomes of cycling myoblasts is heterogeneous (Figure 5A).
Figure 5. EGFR Signals through Aurka to Stimulate Asymmetric Divisions.
(A) Immunofluorescence localization of Aurka (red) at centrosomes in mitotic metaphase (left) and anaphase (right) myoblasts. Pax7 (green); DAPI (blue).
(B) Immunoblotting of reciprocal co-immunoprecipitation of Aurka and p-EGFR in serum-starved myoblasts refed 1h in vehicle or EGF containing growth media.
(C) Proximity ligation assay for interactions between Aurka and p-EGFR (red) in serum-starved Pax7-nGFP (green) myoblasts refed 1h in vehicle or EGF containing growth media. DAPI (blue).
(D) Number of asymmetric and (E) symmetric satellite stem cell divisions per myofiber at 42h of culture after transfection of siRNA against Aurka (siAurka) normalized to scrambled control siRNA (siSCR).
(F) Number of eYFPNeg satellite stem cells per myofiber at 42h of culture after transfection with siRNA against Aurka (siAurka) normalized to scrambled control siRNA (siSCR).
(G) Number of asymmetric satellite stem cell divisions per myofiber at 42h of culture in EGF containing media after transfection with scrambled control siRNA (siSCR) or siRNA against Aurka (siAurka) normalized to control media after transfection with scrambled siRNA (siSCR). (D–F and G) Error bars represent means ±SEM; p-values: *=<0.05; **=<0.01; ***=<0.005. (D– F) n=3 mice; (G) n=3 mice.
In support of our hypothesis, Aurka was identified as an EGF-dependent interactor of EGFR in lung cancer cells (Chen et al., 2013). We observed by reciprocal co-IP western blot an EGF-dependent binding between p-EGFR and Aurka in myoblasts (Figure 5B). In addition, proximity ligation assay (PLA) detected a strong interaction between p-EGFR and Aurka in cultured myoblasts that was increased by EGF (Figure 5C and Figure S5A).
Similar to pharmacological inhibition of Aurka, knockdown of Aurka by siRNA (siAurka) decreased asymmetric divisions by 59% and increased satellite stem cell numbers by 36% (Figure 5D–F and S5B). Notably, siAurka did not decrease the rate of cell cycle as determined by Ki67 staining (data not shown) or number of total satellite cells (Figure S5C). Importantly, EGF stimulation of satellite cell asymmetric division was abolished following siAurka transfection (Figure 5G and S5D–E). Therefore, we conclude Aurka is a key effector of EGFR regulation of asymmetric division.
EGF Treatment Rescues the Polarity Deficits in Dystrophin-Deficient Satellite Cells
Loss of dystrophin in Duchenne muscular dystrophy (DMD) causes a polarity deficit in satellite cells of mdx mice (Dumont et al., 2015b). Satellite stem cells lacking dystrophin exhibit reduced asymmetric divisions resulting in diminished generation of progenitors and delayed regeneration.
To establish if EGFR activation is affected by the loss of dystrophin, single EDL myofibers from mdx mice were immunostained for p-EGFR after 1h EGF stimulation. P-EGFR was stimulated in mdx fibers treated with EGF (Figure 6A-B) in streak-like domains on the basal surface of satellite cells (Figure 5A). This suggests EGFR localization and activation occur normally in mdx satellite cells.
Figure 6. EGF Stimulation Rescues Polarity Deficits in mdx Satellite Cells.
(A) Signaling status of p-EGFR (green) in Pax7-expressing (red) and DAPI positive (blue) cells on mdx EDL myofibers at 1h culture in vehicle or EGF containing media.
(B) Quantification of p-EGFR staining in mdx satellite cells on EDL myofibers fixed at 1h culture in vehicle or EGF containing media.
(C) Quantification of abnormal, planar, and apicobasal orientated mitotic spindles and (D) Pard3 localization in satellite cells on mdx myofibers at 36h of culture in vehicle or EGF containing media.
(E) Quantification of asymmetric divisions relative to total satellite stem cell divisions in WT and mdx myofibers at 42h of culture in vehicle or EGF containing media.
(F) Number of asymmetric divisions per myofiber in WT and mdx myofibers at 42h of culture in vehicle or EGF containing media.
(G) Quantification of Myog-expressing cells per mdx myofiber and (H) total myogenic cells (Pax7- or Myog-expressing cells) per mdx myofiber at 72h of culture in vehicle or EGF containing media.
(I) Graphic overview of cardiotoxin-induced injury and treatment with recombinant EGF in mdx mice.
(J) Quantification of Pax7-expressing and (K) Myog-expressing cells on sections from mdx TA muscles 10 days post injury treated with saline (vehicle), or recombinant EGF.
(B–E and H–J) Error bars represent means ±SEM; p-values: *=<0.05; **=<0.01; ***=<0.005. (B) n=3 mice; (C, E-F) n=3 WT mice and 7 mdx mice; (D) n=3 mice, (G–H) n=3 WT and 5 mdx mice; (J–K) n=4 mice for each group.
Like WT satellite cells, EGF treatment of mdx satellite cells increased number of apicobasal orientated mitotic centrosomes (Figure 6C) and polarized Pard3 (Figure 6D). However, rate of abnormal division in mdx satellite cells as evidenced by abnormal p-Aurk staining patterns was unaffected by EGF stimulation. This implies cell cycle dysregulation is not completely rescued by polarity signaling alone, however EGF signaling through EGFR/Aurka/Pard3 axis enforces polarity and facilitates productive asymmetric divisions in mdx satellite cells.
To assess if EGFR signaling can stimulate asymmetric division of dystrophin-deficient satellite stem cells, EDL myofibers were isolated from WT and mdx Myf5-Cre/R26R-eYFP mice and cultured 42h with or without EGF. Asymmetric division rate of mdx satellite cells is reduced relative to WT (Figure 6E). Despite an expanded stem cell pool in mdx muscle, absolute numbers of asymmetric satellite stem cell divisions are less than half of WT counterparts (Figure 6F and S6B–C). EGF treatment of WT satellite cells increased asymmetric division by 29% (Figure 6E and F) while EGF stimulation of mdx satellite cells increased the rate of asymmetric division by 67% (Figure 6E). Although EGF stimulation does not completely restore symmetric and asymmetric division rates of mdx satellite stem cells, EGF treatment balances absolute number of asymmetric stem cell divisions similar to numbers in untreated WT samples (Figure 6E–F).
The increased proportion of asymmetric divisions suggests EGF stimulation has the potential to restore the rate of progenitor production in dystrophin-deficient satellite cells (Dumont et al., 2015b). To assess if EGF-driven asymmetric divisions could rescue the reduced generation of myogenic progenitors, single EDL myofibers from mdx mice were cultured for 72h and immunostained for the expression of Myog. Notably, EGF stimulation of mdx satellite cells increased the number of Myog-expressing cells and total number of myogenic cells (Figure 6G and H).
To assess the effect of EGF on dystrophin-deficient satellite cells in vivo, EGF protein was intramuscularly injected at the time of and 2 day after injury (Figure 6G). Notably, EGF-injected mdx muscles contained 26% more Pax7-expressing satellite cells and 50% more Myog-expressing cells (Figure 6J and K). Moreover, regenerating myofibers in EGF-treated muscles exhibited increased Feret diameter compared to vehicle-injected controls (Figure S6H). These results suggest intramuscular supplementation with EGF restores generation of myogenic progenitors and enhances regeneration of mdx muscle.
EGF Treatment Enhances mdx Muscle Function
While acute damage can readout the capacity of muscle stem cells to facilitate regeneration, DMD is a progressive disease that requires long-term maintenance of muscle tissue against chronic myofiber damage. To address effects of long-term EGF treatment, we electroporated an expression plasmid containing human EGF cDNA (Sun et al., 2015) into mdx TA muscles (Figure 7A). Secretion of EGF was validated in vitro through transfection in HEK 293T cells (Figure S7A).
Figure 7. EGF Enhances Regeneration of Dystrophin-Deficient Skeletal Muscle.
(A) Graphic overview of electroporation of EGF vector in mdx mice.
(B) Muscle mass, (C) cross-sectional area, (D) quantification of Myog-expressing cells and (E) quantification of myofibers from TA muscles of mdx mice 30 or 150 days after electroporation with empty vector (Ev) or EGF expression vector (EGFv).
(F) Proportion of branched myofibers isolated from electroporated EDLs of mdx mice 150 days after electroporation with Ev or EGFv.
(G) Representative mask generated from SMASH analysis of cross sections of TA muscles and (H) size distribution of myofibers from TA muscles of mdx mice 30 days after electroporation with Ev or EGFv.
(I) Representative mask generated from SMASH analysis of cross sections of TA muscles and (J) size distribution of myofibers from TA muscles of mdx mice 150 days after electroporation with Ev or EGFv.
(K) Max tetanic force and (L) specific max force of TA muscles of mdx mice 30 or 150 days after electroporation with Ev or EGFv.
Dpi: days post intervention; (B–K) Error bars represent means ±SEM; p-values: *=<0.05; **=<0.01; ***=<0.005. n=4 mice for each group at 30dpi and 3 mice for each group at 150dpi.
TA muscles were electroporated at 4 weeks of age, during onset of muscle degeneration, and collected 30 and 150 days post-intervention (dpi). Strikingly, mdx muscles electroporated with EGF expression vector (EGFv) exhibited an 18% increase in mass by 30dpi (Figure 7B). This increase in muscle mass is reflected in the overall cross-sectional area of muscles electroporated with EGFv compared to the empty-vector (Ev) controls (Figure 7C).
Similar to effects we observed with short-term EGF treatment, electroporation with EGFv boosted the number of Myog-expressing progenitors at 30dpi and maintained their numbers at 150dpi whereas fewer Myog-expressing cells were found in Ev controls (Figure 7D). EGFv increased the total number of myofibers by ~30% consistently at 30 and 150dpi (Figure 7E–J) without a change in the distribution of fiber Feret, suggesting they are not arising from the survival of hypertrophic fibers. Consistent with reduced dystrophic pathology, EGFv reduced the progressive increase in fibrosis and deposition of extracellular matrix proteins as measured by wheat germ agglutinin staining (Figure S7C-D).
To determine if increased myofiber number was due to increased branching, single EDL myofibers from electroporated an non-electroporated muscles at 150dpi were isolated. We observed no change in proportion of non-branched fibers between non-electroporated or electroporated muscles (Figure S7F). However, EGFv muscles exhibited an increase in single branched fibers but decreased numbers of double and triple branched fibers compared to Ev muscles (Figure 7F). Together this supports that EGFv mdx muscle exhibits delayed progression of the dystrophic phenotype.
To measure the impact of histological changes on function, we performed in situ measurements of muscle force. Strikingly, TA muscles electroporated with the EGFv generated 32% greater force at tetanus compared to Ev at 30dpi (Figure 7K). Moreover, normalizing to physiological cross-sectional area, TA muscles electroporated with EGFv exhibited 25% higher specific force at 30dpi, which remained 17% higher than Ev muscles at 150dpi (Figure 7L and S7K). Normalizing to maximum force, there was no change to force frequency response of either group at any time point, suggesting EGF treatment does not alter fiber type composition (Figure S7L).
Together, these results indicate EGF treatment provides long-term enhancement of muscle strength in mdx mice by slowing progression of the dystrophic phenotype. Thus, EGF-stimulation of muscle stem cell asymmetric division results in increased generation of progenitors, improved regeneration potential, and amelioration of disease progression in a mouse model of DMD.
DISCUSSION
Extrinsic regulation of tissue-specific stem cell fate is critical for balancing regeneration and stem cell maintenance. Effectors acting on long term-engrafting stem cells are amplified through the proliferation of committed progenitors. Here we identified EGFR-Aurka signaling as a polarity regulator in long term-engrafting Myf5Neg satellite stem cells through an in-niche small molecule screen (Figure 1C).
EGFR down-regulation occurs during mouse and human myoblast differentiation (Leroy et al., 2013; Olwin and Hauschka, 1988), but its role in satellite cells is not well characterized. Our findings support EGFR signaling positively effects the capacity of Myf5Neg satellite stem cells to undergo asymmetric cell divisions and generate committed Myf5Pos progeny. Stimulation with EGF increases asymmetric satellite stem cell divisions by 2.5-fold in vitro (Figures 3G–H), which translated to a 3-fold increase in number of progeny that engrafts as muscle fibers in vivo (Figure 4B–D). SiRNA or pharmacological inhibition of EGFR reduce asymmetric divisions (Figure 2A–C and 3J–L). Importantly, EGF stimulation or inhibition of EGFR did not impact cell cycle re-entry (Figure 2F), rate of mitosis (Figure S3C-D), or total cell numbers in vitro (Figure S3G), which enforces that EGFR is a determinant of stem cell fate and not acting as a mitogen. Moreover, EGF treatment of cultured myoblasts derived from Myf5Neg satellite stem cells does not activate Myf5 (Figure S3H–I), which suggests this effect is stage-specific and/or dependent on polarity mechanisms lacking in 2D culture.
EGFR signaling transiently increases during skeletal muscle regeneration (Figure 3F), localizing to the basal surface of satellite cells 2 days post injury (Figure 3E). Transient EGFR signaling directs satellite cells to self-renew or give rise to committed progeny during regeneration in vivo. Specific deletion of EGFR in satellite cells dysregulates the stem cell pool and results in a reduction in the number of Pax7-expressing cells by 10 days post injury (Figure 4F). Exogenous EGF supplementation enhances the numbers of Pax7-expressing cells and Myog-expressing progenitors, where loss of EGFR expression in satellite cells abrogates the effects of EGF (Figure 4G-H). Despite reduced numbers of Pax7-expressing cells in EGFR cKO mice, we did not observe less Myog-expressing cells during regeneration. These data suggest EGFR cKO satellite stem cells precociously commit to compensate the demand of progenitors necessary for differentiation or directly differentiate like previous reports of EGFR-downregulation in myoblasts (Leroy et al., 2013; Olwin and Hauschka, 1988), resulting in the exhaustion of the stem cell pool.
EGFR is an epithelial polarity regulator (Goodyer et al., 1988), which adds to a body of evidence that suggests quiescent satellite cells share polarity regulators found in epithelial cell types. Unlike polarized epithelial cells, satellite cells do not have a luminal surface. The basal surface of satellite cells attaches to the basal lamina and the apical surface to the myofiber. M-cadherin, a well-known satellite cell marker, and its partner β-catenin are localized to the apical surface and form cell-cell adherence junctions with the myofiber (Goel et al., 2017; Irintchev et al., 1994). Pard3, a member of the Par complex, is localized along the apical surface of satellite cells (Dumont et al., 2015b; Troy et al., 2012). This suggests the apical surface of satellite cells resemble the apical-lateral domain of epithelial cells. Indeed, the polarity effector Scribble is apically distributed in satellite cells and segregates to the committed daughter cell during asymmetric divisions (Ono et al., 2015). This distinction makes satellite cells a unique system to study polarity effector function in asymmetric stem cell division.
Similar to polarized renal epithelial cells (Goodyer et al., 1988) and enterocytes (Playford et al., 1996), EGFR is localized to the basal surface of quiescent satellite cells (Figure 3A). This localization primes activation of the signaling cascade in a polarized manner (Figure 3B–C and S3B). Surprisingly, phosphorylation of EGFR propagates from a localized ‘streak’-like domain in quiescent satellite cells, suggestive of receptor clustering or localized activation (Figure 3B and 6A). This localized activation likely recruits EGF-dependent interactors and drives tyrosine phosphorylation of EGFR substrates at the basal surface. Interactions with integrins present at the basal surface can lead to the specific activity of EGFR. Basolateral EGFR signaling is able to activate unique effectors, such as focal adhesion kinase, compared to apical or generalized activation (Kuwada et al., 1998). In satellite stem cells, Aurka is recruited to p-EGFR and orients mitotic centrosomes along the apicobasal axis (Figure 3G-H and Figure 5). Aurka directly phosphorylates Pard3 resulting in its release from the Par complex (Khavari and Püschel, 2009). Thus, EGFR-Aurka signaling establishes a signaling gradient which polarizes the Par complex (Figure 6D). It will be interesting to examine the mechanism of EGFR localization, whether other canonical EGFR signaling effectors are polarized, and if these play a role in determining cell fate post asymmetric divisions.
Following acute injury, the satellite cell niche undergoes dramatic changes, accumulating infiltrating immune cells and fibroadipogenic progenitors (Bentzinger et al., 2013a). A possible source of EGF is the large number of neutrophils and macrophages that accumulate in damaged muscle to clear necrotic myofibers (Goswami et al., 2005). Specifically, M2 macrophages can secrete high levels of EGF (Xiao et al., 2014) and are closely associated with myogenic progenitors during muscle regeneration (Saclier et al., 2013). However, ligands including transforming growth factor- α (TGF-α), heparin-binding EGF-like growth factor (HB-EGF), amphiregulin, epiregulin also have the potential to activate EGFR signaling (Schneider and Wolf, 2009). Alternatively, amphiregulin is secreted by Foxp3-expressing regulatory T-cells during regeneration, depletion of these cells in mdx mice causes accelerated muscle degeneration and increased fibrosis (Burzyn et al., 2013). Thus, further studies are required to determine the source of EGFR activating ligands in regenerating muscle.
Importantly, EGF treatment rescues asymmetric divisions in dystrophin-deficient satellite stem cells (Figure 6E–F). This suggests redundancies in overlapping polarity signaling can provide compensation toward establishing asymmetric division. Moreover, EGF stimulated the productive generation of myogenic progenitors required to form new myofibers (Figure 7E–J). Unlike mislocalized Par complex proteins in mdx satellite cells (Dumont et al., 2015b), EGFR is properly localized and activated in response to EGF stimulation (Figure 6A). This suggests EGFR-mediated polarity can function without dystrophin-dystroglycan signaling.
Consistent with its effects in myofiber culture, EGF treatment enhances regeneration kinetic of mdx muscles. Loss of polarity signaling from dystrophin-deficiency causes cell cycle defects in mdx satellite cells (Blau et al., 1983; Dumont et al., 2015b; Stuelsatz et al., 2015). Therefore, chronic degeneration of dystrophic myofibers in DMD is not fully repaired. Although methods of re-expressing dystrophin with AAV viral delivery show promise in delaying disease progression (Long et al., 2016; Nelson et al., 2016; Tabebordbar et al., 2016; Zhang et al., 2013), a major step toward regaining muscle function in DMD is to restore satellite stem cell function and enhancing regeneration. In short-term regeneration, EGF treatment boosts numbers of myogenic cells (Figure 6J-K), suggesting satellite stem cells could overcome cell-cycle defects to generate progenitors to facilitate regeneration. This assists recovery of the muscle by producing larger regenerated myofibers compared to the untreated muscles (Figure S6H).
Strikingly, mdx muscles electroporated with an EGF-expressing vector are larger, contain more myogenic progenitors to form new myofibers, and have reduced myofiber branching associated with the mdx phenotype (Figure 7B–J). Nascent myofibers inhibit adipogenic differentiation of fibroadipogenic progenitors in the muscle, thereby tissue fibrosis is directly impacted by the rate of muscle regeneration (Sambasivan et al., 2011; Uezumi et al., 2010). In agreement with this, EGF-electroporated muscles had less fibrosis compared to controls (Figure 7C and D). Importantly, these changes to the muscle architecture are translated to direct enhancements to muscle function, lasting at least 150 days after the treatment (Figure 7K–L). The increase in specific force generation observed with EGF-treated TA muscles indicates additional contractile muscle fibers or increased myofiber cross-sectional area from reduced fibrosis, and attenuates dystrophic progression.
Importantly, balancing symmetric expansion and asymmetric commitment in satellite stem cells is critical to maintain muscle regeneration in DMD. Stimulation of satellite cell expansion with compounds such as Wnt7a (von Maltzahn et al., 2012) can boost regenerative outcomes by promoting a larger pool of satellite cells able to offset deficits in differentiation. Speculatively, prolonged satellite cell expansion may result in proliferative stress and could accelerate telomere shortening leading to stem cell exhaustion (Sacco et al., 2010). Reorienting satellite cell divisions to promote myogenic commitment with EGF tips the balance to promote an increase in absolute number of asymmetric division similar to a wild type context (Figure 6F) and improve regenerative outcomes (Figure 7). The effect of EGF stimulation on human satellite cell function is an exciting prospect as the pathological progression of human DMD is more severe than the mdx mouse model (Duddy et al., 2015; Yucel et al., 2018).
Our findings provide proof-of-principle evidence to support a functional rescue of mdx satellite cells in vivo by stimulating the EGFR-driven polarity pathway. We envision stimulation of muscle stem cell function can be combined with dystrophin restoration to restore muscle function in Duchenne muscular dystrophy. Future studies will further elucidate the role of EGFR signaling in regulation of stem cell polarity and the potential for small molecule activation of EGFR signaling as a therapeutic modality for the treatment of DMD.
STAR★ METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Information and requests for reagents may be directed to the corresponding author Michael A. Rudnicki (mrudnicki@ohri.ca).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Experimental animals
Housing, husbandry and all experimental protocols for mice used in this study were performed in accordance with the guidelines established by the University of Ottawa Animal Care Committee, which is based on the guidelines of the Canadian Council on Animal Care. Mice are housed in ventilated cages with 1/4” corncob bedding changed every two weeks. Mice are supplied food ad libitum with automated acidified RO water or bottles changed every 7 days. Light cycles are 12h-12h cycle with simulated sunrise and sunset. Health and immune status of experimental mice were normal where Sentinels are routinely monitored (fecal, fur and oral swab) for MNV, MHV, Mouse Parvovirus (MPV/MVM), MRV (EDIM), TMEV/GDVII, Helicobacter, P. pneumotropica-Heyl, P. pneumotropica-Jawetz, Entamoeba, Mites, Pneumocystis, Pinworm, and Spironucleus muris. No animals were subjected to previous experimental procedures and are test or treatment naïve.
The following mouse lines were used: B10-Dmd mdx /J (mdx, homozygous DMDmdx females and hemizygous DMDmdx males), NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG), B6.Cg-Pax7tm1(cre/ERT2)Gaka/J (Pax7-CreERT2), B6.129S4-Myf5tm3(cre)Sor/J (Myf5-Cre), B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J (R26R-eYFP), B6.SJL-Pax7tm1.2Tajb (Pax7-nGFP),B6;129S6Gt(ROSA)26Sortm1(CAG-tdTomato*,-EGFP*)Ees/J (R26R-nTnG), B6.129S6-Egfrtm1Dwt/Mmnc (EGFRfl/fl). If not stated differently, 6–8 week old mice were used for all experiments.
Myf5-Cre:ROSA26-eYFP mice (Kuang et al., 2007) were F1 progeny from Myf5-Cre x R26ReYFP breeding pairs. Myf5-Cre:nTnG mice were F1 progeny from Myf5-Cre x R26R-nTnG breeding pairs. Tamoxifen inducible conditional genetic knockout animals were F2 crosses between the offspring of Pax7-CreERT2 (Cre/+) mice (Nishijo et al., 2009) and EGFRfl/fl mice (Lee and Threadgill., 2009) generating Pax7-CreERT2:EGFR(+/+) and Pax7-CreERT2:EGFR(fl/fl) mice.
Cell Lines
HEK293T cells were used for validation of EGF expression following transient transfection. HEK293T cells were purchased from and authenticated by ATCC. These cells were verified to be free from mycoplasma contamination using the MycoSensor PCR Assay Kit (Agilent Technologies). HEK293T cells were cultured at 37°C in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin
METHOD DETAILS
Lineage Tracing with Myf5-Cre/R26R-eYFP and Myf5-Cre/R26R-nTnG
Myf5-Cre/R26R-eYFP transgenic mice, possessing a knock-in of Cre recombinase in the coding-region of the myogenic commitment factor Myf5 (Tallquist et al., 2000) crossed with the knockin of Cre-activated yellow fluorescent protein (eYFP) at the ROSA26 locus (Srinivas et al., 2001), were used as a lineage reporter model to discriminate committed satellite myogenic cells that have expressed Myf5-Cre (eYFPPos) from satellite stem cells that have never expressed Myf5-Cre (eYFPNeg) (Kuang et al., 2007). Myf5-Cre/R26R-nTnG transgenic mice, possess a CMV/b-actin promoter, a loxP flanked nuclear TdTomato (nTdT) and nuclear GFP (nGFP) cassette within the ROSA.26(Sor) locus. When crossed with the Myf5-Cre transgenic line, all cells express nTdT except those cells that have expressed Myf5, which express a nGFP signal. These transgenic models allows for the visualization of de novo Myf5 expression in committed daughter cells during asymmetric divisions (Kawabe et al., 2012).
qPCR Enumeration of eYFPPos and eYFPNeg Cells
Recombination of the R26R-eYFP allele involves the removal of a genomic segment containing a PKG-neomycin resistance (Neo) cassette and three poly-adenylation transcription stop sites flanked by loxP recognition sites (Srinivas et al., 2001). We designed specific primers which amplify either: within the Neo coding region, which will only amplify in eYFPNeg cells; spanning the two loxP sites, which will only amplify in eYFPPos cells; and within the eYFP coding region, which is used to normalize DNA input (Figure S1A). Primer pairs were optimized with purified pBigT plasmid (Addgene) in its native state, or recombined by an in vitro Cre recombinase (New England BioLabs). DNA isolated from cultured eYFPPos and eYFPNeg myoblasts were used to validate the detection efficiency of the primer pairs. Real-time PCR analysis (SSoFast EvaGreen Supermix, Bio-Rad) was performed using the CFX384 real time PCR detection system (BioRad), and results were normalized to eYFP. The Z-factor for detecting eYFPPos cells was 0.935 and eYFPNeg cells was 0.872 (Figure S1B). The primers used for qPCR detection of recombination at the R26R-eYFP allele were as follows. Neo, GGCCGCTTTTCTGGATTCAT and GGCGATACCGTAAAGCACGA; LoxP, TCGCGGTTGAGGACAAACTC and AGCTAGCTTGGGCTGCAGGT; and eYFP, GAACTGTTCGCCAGGCTCAA and CACGGGTAGCCAACGCTATG.
Compound Libraries and Small Molecules
The Ontario Institute for Cancer Research kinase inhibitor and toolkit compound libraries (OICR, Toronto, Canada) consists of 400 specific kinase inhibitors and 160 additional small molecule compounds targeting cellular and developmental pathways. The Tocris kinase inhibitor library (Tocris Bioscience) consists of 80 well-characterized kinase inhibitors. Compound libraries were obtained as 1mM solutions dissolved in DMSO.
FDB Myofiber Screening Assay
Single myofibers were isolated from FDB muscles of 6–8 week old Myf5-Cre/R26R-eYFP mice (adapted from EDL myofiber isolations used by Shefer and Yablonka-Reuveni, 2005) following dissection and incubation in DMEM with 2% L-glutamine, 4.5% glucose, and 110 mg/mL sodium pyruvate (Gibco) containing 0.2% collagenase I (Sigma) for 2 hours at 37°C with agitation. Myofibers were isolated using gentle trituration in DMEM+ with 2% L-glutamine, 4.5% glucose, and 110 mg/ml sodium pyruvate (Gibco) containing 20% FBS (Wisent) with a glass pipet. and cultured at 37°C in suspension in 96-well dishes containing DMEM+ with 2% L-glutamine, 4.5% glucose, and 110 mg/ml sodium pyruvate (Gibco) containing 20% FBS (Wisent) and 1% chick embryo extract (CEE, Accurate Chemicals) and supplemented with either 1:1000-dilution of DMSO (Sigma), DMSO +Wnt7a (50ng/mL; R&D Systems), or 1μM of a small molecule compound. Fibers were collected after 42h of culture and genomic DNA was isolated and purified using the Dneasy 96-well Blood and Tissue kit (Qiagen). qPCR enumeration of eYFPPos and eYFPNeg cells was performed as described above. Primary screening was performed in biological duplicates, each with technical duplicate qPCR reactions. Results were normalized to DMSO-only controls and averaged between replicates.
Gene Expression Analysis
Previously published GEO dataset (GSE59272; Bentzinger et al., 2013b) was used. Expression clustering analysis was performed on normalized gene expression fold change with respect to the median expression value using Cluster 3.0 (http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm).
EDL fiber Culture and siRNA Transfection
Myofiber culture was performed as described previously (Dumont et al., 2015b). Briefly, EDL were carefully dissected and incubated at 37°C in DMEM with 2% L-glutamine, 4.5% glucose, and 110 mg/mL sodium pyruvate (Gibco) containing 0.2% collagenase I (Sigma) for 45 min. Myofibers were isolated using gentle trituration in DMEM+ with 2% L-glutamine, 4.5% glucose, and 110 mg/ml sodium pyruvate (Gibco) containing 20% FBS (Wisent) with a glass pipet. Myofibers were cultured at 37°C for 36, 42, or 72h in DMEM+ with 2% L-glutamine, 4.5% glucose, and 110 mg/ml sodium pyruvate (Gibco) containing 20% FBS (Wisent), 1% chick embryo extract (MP Biomedicals), and 2.5ng/ml bFGF (Cedarlane). For pharmacological inhibition, Lapatinib ditosylate (1mM in DMSO; Santa Cruz Biotechnology), TC-A2317 hydrochloride (1mM in DMSO; Tocris Bioscience) was added to the culture medium for a final concentration of 1μM, equal dilution of DMSO was used as vehicle control. For EGF treatment, human recombinant EGF (100 ng/l in PBS with 0.1% BSA, Life Technologies) was added to the culture medium at 100ng/mL where 1%BSA in PBS served as vehicle control. For EdU labelling, EdU was supplemented into the media for the first 20h of culture as per manufactures protocol, followed by extensive washing and replacement of fresh growth media.
To assess the signaling status of EGFR in quiescent satellite cells and 2 days post injury, EDL muscles were fixed in 4% PFA immediately post-dissection. Myofiber bundles were teased from the fixed muscles by tweezers. For studies pertaining to the activation of EGFR, myofibers were isolated in serum-free conditions with DMEM with 2% L-glutamine, 4.5% glucose, and 110 mg/mL sodium pyruvate (Gibco) and then treated with human recombinant EGF (100 ng/μl in PBS with 0.1% BSA Life Technologies) at 100ng/mL for 1h, equal dilution of 0.1% BSA in PBS was used as vehicle control.
Transfection of satellite cells on myofibers was performed using lipofectamine RNAimax (Life Technologies) and validated Smartpool siRNAs for EGFR, Aurka, or scramble (SCR) (Dharmacon). To ensure maximal efficiency, two transfections were performed at 4h and 16h after isolation of the myofibers as described previously (Le Grand et al., 2009). Knockdown efficiencies of the siRNA were validated in myoblasts by western blot and qRT-PCR.
To assess myofiber branching in electroporated muscles, EDL muscles were isolated and fixed in 4% immediately post-dissection and washed extensively. Single myofibers were isolated by enzymatic digestion in 0.4% collagenase I (Sigma) for 30 min with trituration. Fibers were incubated in DAPI and imaged in phase. 75 fibers from each mouse per condition were analyzed as per representative images in Figure S7E.
Characterization of Satellite Cell Divisions
The orientation of mitotic spindles in satellite cells was measured as described previously (Dumont et al., 2015b). Single myofibers were fixed after 36h of in vitro culture, as described above. Satellite cells were identified by Pax7 expression. Pax7 staining becomes cytoplasmic in mitotic satellite cells after the dissociation of the nuclear envelope, but is still discernable. Mitotic satellite cells were identified by positive p-Aurk staining, which labels cells from prometaphase to cytokinesis. Mitotic satellite cells with p-Aurk staining patterns that were not observed in WT cells, including monopolar, multipolar (>2), and abscission defects were quantified as abnormal. Mitotic orientations were manually counted according to the angle between the mitotic spindle and the tangential plane of the satellite cell’s attachment point to the myofiber. Unless stated, >50 fibers were analyzed per condition.
Cell Culture and Co-Immunoprecipitation
Satellite cells isolated from WT or Pax7-nGFP mice by fluorescence activated cell sorting and cultured as primary myoblasts on collagen-coated plates at 37°C in primary myoblast growth media (Ham’s F10 media (Gibco) with 20% FBS (Wisent), 1% penicillin-streptomycin (Gibco) and 5 ng/mL bFGF (Cedarlane)). For EGF stimulation, myoblasts were serum starved for 1h in Ham’s F10 media and then refed in primary growth media with or without recombinant human EGF (100ng/mL) for an additional hour. Co-immunoprecipitation experiments were performed as described previously (Marcon et al., 2015), with slight modifications. In short, sub-confluent myoblasts were collected and lysed in lysis buffer (1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 50mM tris-HCL pH7.4) in the presence of protease inhibitors (cOmplete mini, Roche) and phosphatase inhibitors (Nacalai). 2.5uL of primary antibodies were incubated with cell lysates overnight at 4°C. Immunoprecipitations were performed using Dynabeads Protein G (Novex) at 10:1 (v/v) beads to antibody ratio. Beads were then washed 3 times with washing buffer (0.1% NP-40, 150 mM KCl, 25 mM tris-HCl pH 7.9, 5mM MgCl2, 10% glycerol, 0.3 mM DTT) and bound proteins were eluted by boiling at 100°C in 50μL of 2X Laemmli buffer for 5 min. Primary antibodies used: mouse IgG (cat# sc-2025, Santa Cruz Biotechnology), rabbit IgG (cat# sc-2027, Santa Cruz Biotechnology), rabbit anti-phospho-EGFR Y1068 (cat# 3777S, Cell Signaling technology), mouse anti-Aurka (35C1, cat# ab13824, Abcam).
Immunoblotting
Proteins were separated on 10% SDS-PAGE and transferred to Immobilon-P PVDF membrane (EMD Millipore). Membranes were probed with primary antibodies, followed by light chain specific HRP-conjugated secondary antibodies at 1:5000 (Bio-Rad) and developed using Immobilon Western HRP substrate (EMD Millipore). EGF levels were detected by dot plots. Conditioned media from transfected 293T cells were concentrated 15x using 3kDa ultrafiltration tubes (Amicon, EMD Millipore). Serial dilution of recombinant EGF protein was used as a standard curve. 2uL of non-denatured protein preparation was loaded directly onto 0.2μm nitrocellulose membrane (GE Life Sciences) and allowed to dry. Membranes were blocked in 5% BSA in TBST for 1h and probed with rabbit anti-EGF (cat# ab9695, Abcam), followed by HRP-conjugated secondary antibody at 1:5000 (Bio-Rad) and developed using Immobilon Western HRP substrate (EMD Millipore). Membranes were visualized using FluorChem HD2 (Alpha Innotech) or exposed to BIOMAX film (Eastman Kodak).
Electroporation and Cardiotoxin Injury
I.M. cardiotoxin injections (Latoxan, 50ul of 10μM solution in saline) were injected directly into the right TA muscle through the skin under general anesthesia. For pharmacological inhibition, Lapatinib ditosylate (1mM in DMSO; Santa Cruz Biotechnology), TC-A2317 hydrochloride (1mM in DMSO; Tocris Bioscience) was mixed into the cardiotoxin solution for a final concentration of 1μM, equal dilution of DMSO was used as vehicle control. Supplemental injection of inhibitors (20uL of 2.5μM of inhibitors in saline) was performed 2 days after cardiotoxin injection. For recombinant EGF injections, 10ng of human recombinant EGF (100 ng/μl in saline, Life Technologies) was mixed into 50uL of cardiotoxin solution or 20uL of saline, equal volumes of saline were used as vehicle control. Muscles were harvested 10 days post injury as a mid-point of regeneration where the number of Myog-expressing cells are above baseline and myofibers are generally reformed. Extended timepoints of 30 days and 150 days post injury represent full repair and onset of DMD disease progression respectively.
Electroporations were performed as described previously (Bentzinger et al., 2013b). 30ug of purified endotoxin-free pTT3-CD4d3+4-bio empty vector control (cat #32402, Addgene) (Bushell et al., 2008) or pTT3-EGF-CD4d3+4-bio-His expression plasmid (cat# 53340, Addgene) (Sun et al., 2015) expression plasmid in saline was injected into the right hind TA muscle of 4 week old mdx mice through the skin under general anesthesia. Immediately after injection, electric stimulation was applied to the TA by a pulse generator (ECM 830, BTX) of 100–150 volts for 6 pulses, with a fixed duration of 20ms and an interval of 200ms using 5mm needle electrodes (BTX).
Immunostaining, PLA and Antibodies
EDL myofibers were fixed for 10 min in 2% PFA and washed with PBS. Fibers were blocked and permeablized in horse serum blocking buffer (5% horse serum, 1% BSA (Sigma), and 0.5% Triton X-100 (Sigma) in PBS) for 1h at room temperature or at 4°C overnight. Primary antibodies were applied in blocking solution for 2h at room temperature or at 4°C overnight. Samples were subsequently washed with PBS and stained with appropriate fluorescently labeled secondary antibodies (Alexa fluor 488, 546, or 647) for 1h at room temperature. After washing with PBS, samples were mounted with Permafluor (Fisher).
Muscle samples were embedded in OCT and frozen in liquid nitrogen cooled isopentane and cryosectioned in 12-μm slices. Cross-sections were washed once with PBS and fixed in 2% PFA 10min, permeabilized with 0.1% Triton X-100/0.15M Glycine/PBS 10min and extensively washed with PBS. Samples were blocked using M.O.M. Blocking reagent (Vector) 2h followed by additional blocking in 5% NGS/2%BSA at 4°C overnight. Primary antibodies were applied in blocking at 4°C overnight. Samples were washed extensively in PBS and secondary antibodies were in applied in PBS (Alexa Flour 546, 647) for 1h at room temperature. Following PBS washes, cross-sections were counterstained with DAPI 10min and mounted with Permafluor (Fisher).
Antibodies were as follows: mouse anti-Pax7 (DSHB), chicken anti-GFP (cat# ab13970, Abcam), rabbit anti-EGFR (cat# 4267S, Cell Signaling technology), rabbit anti-phospho-EGFR Y1068 (cat# 3777S, Cell Signaling technology), mouse anti-Aurka (35C1, cat# ab13824, Abcam), rabbit anti-phospho-Aurk (cat# 2914S, Cell Signaling technology), rabbit anti-Pard3 (cat# 07–330, Millipore), rat anti-laminin (cat# L0663, Sigma), rabbit anti-myogenin (M225, cat# sc-576, Santa Cruz).
Proximity ligation assays (PLA) were performed as described previously using Duolink (Sigma) PLA probes mouse and rabbit (Dumont et al., 2015b). Images of immunostainings were taken on an Axio Observer.Z1 microscope equipped with a LSM510 META confocal laser scanner and a plan-Apochromat 63×/1.40 Oil DIC M27 objective or an Axioplan 2 microscope equipped with a plan-Neofluar 40×/1.30 Oil DIC and a plan-Neofluar 100×/1.30 Oil DIC objective. Images were processed and analyzed with Axiovision, Zen, and FIJI software. 3D z-stack images were projected by maximum intensity using Fiji software (http://fiji.sc/Fiji).
For grey value measurements, images of cells were collected with fixed exposures or confocal z-stacks as stated. Fiji software was used to generate masks of satellite cells based on Pax7 staining on raw images. Due to the highly localized staining for p-EGFR and variable epifluorescent background intensity based on the plane of focus, grey values for p-EGFR staining were calculated as “staining intensity = max intensity – average intensity”. Three-dimensional analysis of p-EGFR staining was performed on confocal z-stacks taken at 1μm resolution on a Zeiss 510 confocal microscope. Z-stacks were analyzed for pixel intensity in the apical vs basal side of the cell following measured bi-section along the apical-basal axis. Satellite cells from EGFR cKO mice treated with EGF were used as a biological negative control.
Histological Analysis of Muscle Sections
For Fiber type analysis, non-fixed samples were washed with PBS and blocked in 10% NGS for 1hr at room temperature. Primary antibodies were applied in 10% NGS for 2h at room temperature. Sections were washed extensively with PBS and secondary antibodies were applied in PBS for 1h at room temperature. Following PBS washes, sections were counterstained with DAPI 10min and mounted with Permafluor. Antibodies were as follows: mouse anti-MyH3 (clone F1.652, DSHB), mouse anti-MyH4 (clone BF-F3, DSHB).
For analysis of myofiber Feret’s diameter and CNF, non-fixed samples were washed with PBS and stained with Wheat Germ Agglutinin Alexa 647 conjugate (Fisher) for 1h at room temperature. Samples were washed once with PBS and counterstained with DAPI. Samples were washed with PBS and mounted with Permafluor. Images were taken immediately following staining. Minimum fiber Feret measurement was performed using the SMASH software in MATLAB 2015a as described previously (Smith and Barton, 2014). Samples with significant staining artifacts were excluded from automated analyses. Total number of myofibers in each tissue was verified manually. >95% of myofibers were quantified in across each section in its entirety.
Satellite cell transplantation and analysis
Satellite cells from Myf5-Cre/R26R-nTnG transgenic mice were FACS isolated based on FSC/SSC, CD31/CD11b/CD45/SCA1 (V450), a7-Integrin (APC) and nGFP/nTdT (Figure S4A). Mice were processed independently to allow one donor per experimental replicate. Satellite cells were isolated, spun down and fractioned into 10,000 cell aliquots. Satellite cells are treated with EGF or saline on ice for 3h, washed twice and resuspended. 10,000 cells from the same donor (nTdT control or nTdT + EGF) were injected into the left and right TA muscle of 8–12 week NOD.scid.gamma mice injured with cardiotoxin one day prior to transplantation. Mice were allowed to recover for 10 days post injury followed by perfusion fixation. Isolated muscles were frozen and sectioned at 20μm with ~200μm spacing between consecutive sections. Quantification of transplanted cells are averages from five consecutive sections. 100,000 cultured nGFP myoblast (passage 4) from the same initial donors were transplanted under the same experimental plan to determine the role of EGF on committed myogenic cells.
In situ Force Measurements
Force measurements were performed on a custom setup in accordance to experimental protocols outlined previously (Hakim et al., 2013). Briefly, mice were anesthetized with 2–5% vaporized Isoflurane mixed with O2. Mice were position on top of a heated surface in order to maintain the body and muscle temperature to 30°C. Knees were secured to a fixed steel post using surgical suture and their feet were pinned to a platform to prevent movement from the contraction of other muscle groups. The distal tendon of both TA muscles, electroporated and contralateral, was attached to separate FT03 force transducers connected to a 79E physiograph (Grass Technologies, Warwick, U.S.A.), which in turn was connected to a KCP13104 data acquisition system (Keithley, U.S.A.). Data were sampled at recorded at 5 kHz. Exposed muscles were kept from drying with physiological saline solution (118.5 mM NaCl, 4.7 mM KCl, 2.4 mM CaCl2, 3.1 mM MgCl2, 25 mM NaHCO3, 2 mM NaH2PO4, and 5.5 mM D-glucose). Electrical stimulations were applied across two needle electrodes, placed through the skin just above and below the knee to stimulate the tibial nerve. The electrodes were connected to a Grass S88 stimulator and a Grass SIU5 isolation unit (Grass Technologies). Tetanic contractions were elicited every 100 s with 200 ms trains of 0.3 ms, 5 V (supramaximal voltage) pulses at frequencies varying between 1 and 200 Hz. Tetanic force was defined as the force generated upon stimulation and calculated as the difference in force at the maximum height of contraction and the force just prior to the stimulation. Tetanic force was normalized as follows:
where FNormalized was the normalized force in N/cm2; FMeasured, the measured force in g; CF, the converting factor 0.00980665 N/g; CSA, the cross-sectional area in cm2; Le, the experimental muscle length in cm; We, the muscle weight in g; and MDensity, the muscle density taken as 1.06 g/cm3.
Real-Time PCR
Total RNA was isolated (NucleoSpin RNA II, Macherey-Nagel). Reverse transcription was carried out using a mixture of oligodT and random hexamer primers (iScript cDNA Synthesis Kit, Bio-Rad). Sybr Green, real-time PCR analysis (SSoFast EvaGreen Supermix, Bio-Rad) was performed using the CFX384 real time PCR detection system (Bio-Rad), and results were normalized to Gapdh and Tbp or PPIA and 18s expression and analyzed by Bio-Rad CFX Manager software. See Table S2 for a detailed list of primers used in this study.
QUANTIFICATION AND STATISTICAL ANALYSIS
Compiled data are expressed as mean ± standard error of the mean (SEM). Experiments were performed with a minimum of three biological replicates. For statistical comparisons of two conditions, the Student’s t-test was used. Paired tests were used for biologically matched samples. Unpaired tests were used to compare unrelated samples. Feret’s size and force measurements were analyzed using two-way ANOVA with two-stage setup to control for false discovery rate. No data was removed as outliers, with data point representation graphically where appropriate. Experimental design incorporated user blinding when possible. Statistical analysis was performed in GraphPad Prism or Microsoft Excel. The level of significance is indicated as follows: * p < 0.05, ** p < 0.01, *** p < 0.005.
Supplementary Material
Supplemental Movie S1 : 3D reconstruction of a satellite cell expressing polarized p-EGFR 2 days post injury related to Figure 3. Myofibers were stained for DAPI (blue), Pax7 (red) and p-EGFR (green). Series of pictures was taken by confocal microscopy and reconstructed into a 3D image using ImageJ.
Supplemental Table S1 : FDB screening hits with annotated primary targets related to Figure 1.
Supplemental Table S2 : Oligonucleotides used in this study related to STAR methods.
HIGHLIGHTS.
EGFR expression and activation is polarized in satellite cells
EGF stimulates asymmetric satellite stem cell division
Polarized EGFR activation orients mitotic centrosomes through Aurka
EGF stimulation rescues mdx satellite cell function in vitro and in vivo
ACKNOWLEDGEMENTS
OICR Kinase Inhibitor, Toolkit libraries and Tocris Kinase Inhibitor library were kindly provided by Dr. Mick Bhatia and Dr. Rima Al-Awar. EGF plasmids were gifts from Gavin Wright (Bushell et al., 2008; Sun et al., 2015). We thank Dr. F Bentzinger for support and guidance during development our in-niche screen. We thank Dr. W Lin for training with in situ force measurement. Y.X.W. is supported by fellowships from QEII-GSST and the CIHR. P.F. is supported by a fellowship from CIHR. C.E.B. is supported by Postdoctoral Fellowships from the OIRM and AFM-Téléthon. N.A.D. is supported by a Postdoctoral Fellowship from the CIHR. M.A.R. holds the Canada Research Chair in Molecular Genetics. These studies were carried out with support of grants to M.A.R. from the US National Institutes for Health [R01AR044031], Canadian Institutes for Health Research [FDN-148387], E-Rare-2: Canadian Institutes of Health Research/Muscular Dystrophy Canada [ERA-132935], Muscular Dystrophy Association, Ontario Institute for Regenerative Medicine, and the Stem Cell Network.
Footnotes
CONFLICTS OF INTEREST
M.A.R. is a Founding Scientist of Satellos Bioscience Inc. The other authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson MS, and Kunkel LM (1992). The molecular and biochemical basis of Duchenne muscular dystrophy. Trends Biochem. Sci 17, 289–292. [DOI] [PubMed] [Google Scholar]
- Ando R, Ikegami H, Sakiyama M, Ooike S, Hayashi M, Fujino Y, Abe D, Nakamura H, Mishina T, Kato H, et al. (2010). 3-Cyano-6-(5-methyl-3-pyrazoloamino)pyridines: selective Aurora A kinase inhibitors. Bioorg. Med. Chem. Lett 20, 4709–4711. [DOI] [PubMed] [Google Scholar]
- Arsenio J, Metz PJ, and Chang JT (2015). Asymmetric Cell Division in T Lymphocyte Fate Diversification. Trends Immunol. 36, 670–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bañón‐Rodríguez I, Gálvez‐Santisteban M, Vergarajauregui S, Bosch M, Borreguero Pascual A, and Martín‐Belmonte F (2014). EGFR controls IQGAP basolateral membrane localization and mitotic spindle orientation during epithelial morphogenesis. EMBO J. 33, 129–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CD, and Conen PE (1968). Histopathological changes in Duchenne muscular dystrophy. J. Neurol. Sci 7, 529–544. [DOI] [PubMed] [Google Scholar]
- Bell GP, Fletcher GC, Brain R, and Thompson BJ (2015). Aurora kinases phosphorylate Lgl to induce mitotic spindle orientation in Drosophila epithelia. Curr. Biol. CB 25, 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, Dumont NA, and Rudnicki MA (2013a). Cellular dynamics in the muscle satellite cell niche. EMBO Rep. 14, 1062–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H, and Rudnicki MA (2013b). Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernet JD, Doles JD, Hall JK, Kelly Tanaka K, Carter TA, and Olwin BB (2014). p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat. Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau HM, Webster C, and Pavlath GK (1983). Defective myoblasts identified in Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. U. S. A 80, 4856–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GR, Bamford AM, Bowyer J, James DS, Rankine N, Tang E, Torr V, and Culbert EJ (2000). Naphthyl ketones: a new class of Janus kinase 3 inhibitors. Bioorg. Med. Chem. Lett 10, 575–579. [DOI] [PubMed] [Google Scholar]
- Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, et al. (2013). A Special Population of Regulatory T Cells Potentiates Muscle Repair. Cell 155, 1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushell KM, Söllner C, Schuster-Boeckler B, Bateman A, and Wright GJ (2008). Largescale screening for novel low-affinity extracellular protein interactions. Genome Res. 18, 622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charville GW, Cheung TH, Yoo B, Santos PJ, Lee GK, Shrager JB, and Rando TA (2015). Ex Vivo Expansion and In Vivo Self-Renewal of Human Muscle Stem Cells. Stem Cell Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T-C, Liu Y-W, Huang Y-H, Yeh Y-C, Chou T-Y, Wu Y-C, Wu C-C, Chen Y-R, Cheng H-C, Lu P-J, et al. (2013). Protein phosphorylation profiling using an in situ proximity ligation assay: phosphorylation of AURKA-elicited EGFR-Thr654 and EGFRSer1046 in lung cancer cells. PloS One 8, e55657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn RD, and Campbell KP (2000). Molecular basis of muscular dystrophies. Muscle Nerve 23, 1456–1471. [DOI] [PubMed] [Google Scholar]
- Cohn RD, Henry MD, Michele DE, Barresi R, Saito F, Moore SA, Flanagan JD, Skwarchuk MW, Robbins ME, Mendell JR, et al. (2002). Disruption of Dag1 in Differentiated Skeletal Muscle Reveals a Role for Dystroglycan in Muscle Regeneration. Cell 110, 639–648. [DOI] [PubMed] [Google Scholar]
- Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, Llewellyn ME, Delp SL, and Blau HM (2014). Rejuvenation of the aged muscle stem cell population restores strength to injured aged muscles. Nat. Med 20, 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley DO, Rivera-Pérez JA, Schliekelman M, He YJ, Oliver TG, Lu L, O’Quinn R, Salmon ED, Magnuson T, and Van Dyke T (2009). Aurora-A Kinase Is Essential for Bipolar Spindle Formation and Early Development. Mol. Cell. Biol 29, 1059–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duddy W, Duguez S, Johnston H, Cohen TV, Phadke A, Gordish-Dressman H, Nagaraju K, Gnocchi V, Low S, and Partridge T (2015). Muscular dystrophy in the mdx mouse is a severe myopathy compounded by hypotrophy, hypertrophy and hyperplasia. Skelet. Muscle 5, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont NA, Bentzinger CF, Sincennes M-C, and Rudnicki MA (2015a). Satellite Cells and Skeletal Muscle Regeneration. Compr. Physiol 5, 1027–1059. [DOI] [PubMed] [Google Scholar]
- Dumont NA, Wang YX, von Maltzahn J, Pasut A, Bentzinger CF, Brun CE, and Rudnicki MA (2015b). Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat. Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel AJ, Rieder M-K, Arnold H-H, Radice GL, and Krauss RS (2017). Niche Cadherins Control the Quiescence-to-Activation Transition in Muscle Stem Cells. Cell Rep. 21, 2236–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyer PR, Kachra Z, Bell C, and Rozen R (1988). Renal tubular cells are potential targets for epidermal growth factor. Am. J. Physiol 255, F1191–1196. [DOI] [PubMed] [Google Scholar]
- Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, Stanley ER, Segall JE, and Condeelis JS (2005). Macrophages Promote the Invasion of Breast Carcinoma Cells via a Colony-Stimulating Factor-1/Epidermal Growth Factor Paracrine Loop. Cancer Res. 65, 5278–5283. [DOI] [PubMed] [Google Scholar]
- Gurevich DB, Nguyen PD, Siegel AL, Ehrlich OV, Sonntag C, Phan JMN, Berger S, Ratnayake D, Hersey L, Berger J, et al. (2016). Asymmetric division of clonal muscle stem cells coordinates muscle regeneration in vivo. Science. [DOI] [PubMed] [Google Scholar]
- Hakim CH, Wasala NB, and Duan D (2013). Evaluation of muscle function of the extensor digitorum longus muscle ex vivo and tibialis anterior muscle in situ in mice. J. Vis. Exp. JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irintchev A, Zeschnigk M, Starzinski-Powitz A, and Wernig A (1994). Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev. Dyn. Off. Publ. Am. Assoc. Anat 199, 326–337. [DOI] [PubMed] [Google Scholar]
- Irintchev A, Zweyer M, and Wernig A (1997). Impaired functional and structural recovery after muscle injury in dystrophic mdx mice. Neuromuscul. Disord. NMD 7, 117–125. [DOI] [PubMed] [Google Scholar]
- Johnston CA, Hirono K, Prehoda KE, and Doe CQ (2009). Identification of an AuroraA/PinsLINKER/Dlg spindle orientation pathway using induced cell polarity in S2 cells. Cell 138, 1150–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe Y, Wang YX, McKinnell IW, Bedford MT, and Rudnicki MA (2012). Carm1 Regulates Pax7 Transcriptional Activity through MLL1/2 Recruitment during Asymmetric Satellite Stem Cell Divisions. Cell Stem Cell 11, 333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazaei MR, and Püschel AW (2009). Phosphorylation of the par polarity complex protein Par3 at serine 962 is mediated by aurora a and regulates its function in neuronal polarity. J. Biol. Chem 284, 33571–33579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA (2008). Mechanisms of Asymmetric Stem Cell Division. Cell 132, 583–597. [DOI] [PubMed] [Google Scholar]
- Kollareddy M, Zheleva D, Dzubak P, Brahmkshatriya PS, Lepsik M, and Hajduch M (2012). Aurora kinase inhibitors: Progress towards the clinic. Invest. New Drugs 30, 2411–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Le Grand F, and Rudnicki MA (2007). Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129, 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwada SK, Lund KA, Li XF, Cliften P, Amsler K, Opresko LK, and Wiley HS (1998). Differential signaling and regulation of apical vs. basolateral EGFR in polarized epithelial cells. Am. J. Physiol 275, C1419–1428. [DOI] [PubMed] [Google Scholar]
- Le Grand F, Jones AE, Seale V, Scimè A, and Rudnicki MA (2009). Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell 4, 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T-C, and Threadgill DW (2009). Generation and validation of mice carrying a conditional allele of the epidermal growth factor receptor. Genes. N. Y. N 2000 47, 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy MC, Perroud J, Darbellay B, Bernheim L, and Konig S (2013). Epidermal growth factor receptor down-regulation triggers human myoblast differentiation. PloS One 8, e71770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C, Amoasii L, Mireault AA, McAnally JR, Li H, Sanchez-Ortiz E, Bhattacharyya S, Shelton JM, Bassel-Duby R, and Olson EN (2016). Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 351, 400–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Maltzahn J, Renaud J-M, Parise G, and Rudnicki MA (2012). Wnt7a treatment ameliorates muscular dystrophy. Proc. Natl. Acad. Sci 109, 20614–20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcon E, Jain H, Bhattacharya A, Guo H, Phanse S, Pu S, Byram G, Collins BC, Dowdell E, Fenner M, et al. (2015). Assessment of a method to characterize antibody selectivity and specificity for use in immunoprecipitation. Nat. Methods 12, 725–731. [DOI] [PubMed] [Google Scholar]
- Matsuzaki F, and Shitamukai A (2015). Cell Division Modes and Cleavage Planes of Neural Progenitors during Mammalian Cortical Development. Cold Spring Harb. Perspect. Biol 7, a015719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi P, Honda R, and Nigg EA (2004). Aurora kinases link chromosome segregation and cell division to cancer susceptibility. Curr. Opin. Genet. Dev 14, 29–36. [DOI] [PubMed] [Google Scholar]
- Morin X, and Bellaïche Y (2011). Mitotic Spindle Orientation in Asymmetric and Symmetric Cell Divisions during Animal Development. Dev. Cell 21, 102–119. [DOI] [PubMed] [Google Scholar]
- Nelson CE, Hakim CH, Ousterout DG, Thakore PI, Moreb EA, Rivera RMC, Madhavan S, Pan X, Ran FA, Yan WX, et al. (2016). In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351, 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olwin BB, and Hauschka SD (1988). Cell surface fibroblast growth factor and epidermal growth factor receptors are permanently lost during skeletal muscle terminal differentiation in culture. J. Cell Biol 107, 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y, Urata Y, Goto S, Nakagawa S, Humbert PO, Li T-S, and Zammit PS (2015). Muscle Stem Cell Fate Is Controlled by the Cell-Polarity Protein Scrib. Cell Rep. 10, 1135–1148. [DOI] [PubMed] [Google Scholar]
- Playford RJ, Hanby AM, Gschmeissner S, Peiffer LP, Wright NA, and McGarrity T (1996). The epidermal growth factor receptor (EGF-R) is present on the basolateral, but not the apical, surface of enterocytes in the human gastrointestinal tract. Gut 39, 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price FD, von Maltzahn J, Bentzinger CF, Dumont NA, Yin H, Chang NC, Wilson DH, Frenette J, and Rudnicki MA (2014). Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat. Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge JR, Wiley JA, Talago EA, Young EM, Johns LL, Kundert JA, Sonsteng KM, Halford WP, Capecchi MR, and Schmidt EE (2013). Nuclear double-fluorescent reporter for in vivo and ex vivo analyses of biological transitions in mouse nuclei. Mamm. Genome Off. J. Int. Mamm. Genome Soc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, Blasco MA, and Tajbakhsh S (2012). A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell 148, 112–125. [DOI] [PubMed] [Google Scholar]
- Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P, Shkreli M, Delp S, Pomerantz JH, Artandi SE, et al. (2010). Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell 143, 1059–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, et al. (2013). Differentially Activated Macrophages Orchestrate Myogenic Precursor Cell Fate During Human Skeletal Muscle Regeneration. STEM CELLS 31, 384–396. [DOI] [PubMed] [Google Scholar]
- Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, and Galy A (2011). Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Dev. Camb. Engl 138, 3647–3656. [DOI] [PubMed] [Google Scholar]
- Schneider MR, and Wolf E (2009). The epidermal growth factor receptor ligands at a glance. J. Cell. Physiol 218, 460–466. [DOI] [PubMed] [Google Scholar]
- Shefer G, and Yablonka-Reuveni Z (2005). Isolation and Culture of Skeletal Muscle Myofibers as a Means to Analyze Satellite Cells. Methods Mol. Biol Clifton NJ 290, 281–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LR, and Barton ER (2014). SMASH - semi-automatic muscle analysis using segmentation of histology: a MATLAB application. Skelet. Muscle 4, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, and Costantini F (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuelsatz P, Shearer A, Li Y, Muir LA, Ieronimakis N, Shen QW, Kirillova I, and Yablonka-Reuveni Z (2015). Extraocular muscle satellite cells are high performance myoengines retaining efficient regenerative capacity in dystrophin deficiency. Dev. Biol 397, 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Vandenbriele C, Kauskot A, Verhamme P, Hoylaerts MF, and Wright GJ (2015). A Human Platelet Receptor Protein Microarray Identifies the High Affinity Immunoglobulin E Receptor Subunit α (FcεR1α) as an Activating Platelet Endothelium Aggregation Receptor 1 (PEAR1) Ligand. Mol. Cell. Proteomics MCP 14, 1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabebordbar M, Zhu K, Cheng JKW, Chew WL, Widrick JJ, Yan WX, Maesner C, Wu EY, Xiao R, Ran FA, et al. (2016). In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 351, 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallquist MD, Weismann KE, Hellström M, and Soriano P (2000). Early myotome specification regulates PDGFA expression and axial skeleton development. Dev. Camb. Engl 127, 5059–5070. [DOI] [PubMed] [Google Scholar]
- Tierney MT, Aydogdu T, Sala D, Malecova B, Gatto S, Puri PL, Latella L, and Sacco A (2014). STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat. Med 20, 1182–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy A, Cadwallader AB, Fedorov Y, Tyner K, Tanaka KK, and Olwin BB (2012). Coordination of Satellite Cell Activation and Self-Renewal by Par-Complex-Dependent Asymmetric Activation of p38α/β MAPK. Cell Stem Cell 11, 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A, Fukada S, Yamamoto N, Takeda S, and Tsuchida K (2010). Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol 12, 143–152. [DOI] [PubMed] [Google Scholar]
- Wirtz-Peitz F, Nishimura T, and Knoblich JA (2008). Linking cell cycle to asymmetric division: Aurora-A phosphorylates the Par complex to regulate Numb localization. Cell 135, 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Gaffar I, Guo P, Wiersch J, Fischbach S, Peirish L, Song Z, El-Gohary Y, Prasadan K, Shiota C, et al. (2014). M2 macrophages promote beta-cell proliferation by upregulation of SMAD7. Proc. Natl. Acad. Sci 111, E1211–E1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel N, Chang AC, Day JW, Rosenthal N, and Blau HM (2018). Humanizing the mdx mouse model of DMD: the long and the short of it. NPJ Regen. Med 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yue Y, Li L, Hakim CH, Zhang K, Thomas GD, and Duan D (2013). Dual AAV therapy ameliorates exercise-induced muscle injury and functional ischemia in murine models of Duchenne muscular dystrophy. Hum. Mol. Genet 22, 3720–3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Movie S1 : 3D reconstruction of a satellite cell expressing polarized p-EGFR 2 days post injury related to Figure 3. Myofibers were stained for DAPI (blue), Pax7 (red) and p-EGFR (green). Series of pictures was taken by confocal microscopy and reconstructed into a 3D image using ImageJ.
Supplemental Table S1 : FDB screening hits with annotated primary targets related to Figure 1.
Supplemental Table S2 : Oligonucleotides used in this study related to STAR methods.