Among patients with relapsed/refractory chronic lymphocytic leukemia, those who have higher stress related to their cancer experience at the start of treatment have worse psychological functioning during the first five months of treatment.
Keywords: Stress, Chronic lymphocytic leukemia, Psychological, Symptoms, Quality of life
Abstract
Background
Chronic lymphocytic leukemia is the most prevalent adult leukemia. The disease is incurable with a cycling of treatment and relapse common. Little is known about the psychological and physical functioning of patients with relapsed/refractory chronic lymphocytic leukemia. Cancer-specific stress is an important individual difference variable that predicts psychological and physical outcomes.
Purpose
To examine cancer-specific stress at treatment initiation as a predictor of psychological and physical functioning trajectories in patients with relapsed/refractory chronic lymphocytic leukemia during the first 5 months of treatment.
Methods
Patients with relapsed/refractory chronic lymphocytic leukemia (N = 152) enrolled in a phase II clinical trial completed self-report measures at treatment initiation (baseline), 1, 2, and 5 months of treatment. Cancer-specific stress at baseline was examined as a predictor of psychological (cognitive-affective depressive symptoms, negative mood, mental health quality of life) and physical functioning (fatigue interference, sleep problems, physical health quality of life), controlling for demographic and treatment variables.
Results
Using multilevel modeling, higher baseline cancer-specific stress was related to worse psychological (cognitive-affective depressive symptoms, negative mood, mental health quality of life) and physical functioning (fatigue interference, sleep problems) at baseline and more rapid improvements during the next 5 months. Despite these improvements, higher baseline cancer-specific stress remained associated with poorer 5-month psychological, though not physical, functioning.
Conclusions
Findings suggest cancer-specific stress at treatment initiation may be a risk factor for poorer psychological functioning during treatment for patients with relapsed/refractory chronic lymphocytic leukemia.
Introduction
Chronic lymphocytic leukemia is the most prevalent adult leukemia in the USA and accounts for about one-quarter of new leukemia cases [1]. Chronic lymphocytic leukemia has a unique disease trajectory. Asymptomatic patients diagnosed with early-stage disease (i.e., Rai stages 0–II) do not receive treatment but are continually monitored until requiring treatment, as treatment at this stage has not been shown to offer a survival advantage [2]. For those patients with initially advanced disease or those who become symptomatic, chemoimmunotherapy provides high rates of response with the majority of patients having significant improvements in disease burden. However, treatments are not curative and all patients eventually relapse requiring further treatment for their disease, with a pattern of relapse and re-treatments for many.
For patients with chronic lymphocytic leukemia, undergoing the multiple cycling of treatments may take a psychological and physical toll. Yet, psychosocial data on patients with chronic lymphocytic leukemia are limited, particularly in comparison to that of solid tumor patients [3, 4]. With all leukemias accounting for less than 4% of the annual number of new cancer cases, accrual of adequate numbers for psychosocial studies is difficult unless done at a large cancer center or with internet surveys [5]. Available psychosocial data rely primarily on quality of life measures, such as the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire. Findings support that patients with treated and untreated chronic lymphocytic leukemia have poorer quality of life compared to populations norms, as might be expected [6–8]. While quality of life measures are useful as global indicators, additional inclusion of specific measures of psychological functioning (e.g., negative mood) administered longitudinally would provide new clarity about the patient experience.
Studies in chronic lymphocytic leukemia also find that patients are burdened with significant physical symptoms. In a clinical trial of patients beginning their first treatment, 81% of patients reported fatigue and 56% reported sleep disturbances at treatment initiation [8]. In a mixed sample of previously treated and untreated patients, fatigue was significantly worse compared to healthy matched controls [6]. Despite the poor quality of life and high prevalence of physical symptoms, little is known about predictors of worse psychological and physical functioning in this population. Empirical identification of predictors of psychological and physical functioning is important for identifying patients at risk for poor long-term outcomes and further, could inform the provision of supportive services for them. This is particularly relevant for patients with chronic lymphocytic leukemia, as they may live with their disease for years.
Traumatic stress, or cancer-specific stress, is one candidate for predicting worse psychological and physical functioning. The news of a cancer diagnosis or recurrence, coupled with the anticipation of treatment, is stressful and akin to a traumatic stressor [9–11]. Exposure to traumatic stressors increases risk for poorer mental and physical health outcomes [12]. Additionally, with individual variability in response to a trauma, those individuals who develop traumatic stress symptoms are at risk for even worse outcomes. In response to a medical diagnosis or other traumatic stressor, greater traumatic stress symptoms are not only related to poorer psychological functioning, but also poorer physical health and worse disease outcomes [12–19].
In the context of cancer, cancer-specific stress is commonly assessed with the Impact of Event Scale [20, 21], and as such is conceptualized as the experience of intrusive thoughts (e.g., other things kept me thinking about it) and avoidant thoughts/behaviors (e.g., I stayed away from reminders of it). The Impact of Event Scale was developed out of studies of traumatic experiences suggesting that the stress response can include periods of intrusive, negative thoughts coinciding or oscillating with periods of avoidant thoughts and behaviors [20, 22]. An excessive or persistent stress response can prolong the experience of the trauma and influence psychological and physical functioning outcomes [22]. When considered in this manner, cancer-specific stress covaries with measures of other important constructs, such as depressive symptoms, negative mood, quality of life, and biologic responses (e.g., downregulated immunity [23–26]). It is also the case that cancer-specific stress is an important individual difference variable that predicts subsequent psychological functioning [27–29]. For example, in newly diagnosed, post-surgery breast cancer patients, cancer-specific stress predicted quality of life 4 and 12 months later [27]. To our knowledge, the relationship of cancer-specific stress and psychological functioning in patients with chronic lymphocytic leukemia has not been previously studied.
Cancer-specific stress has also been shown to covary with physical health. In the only study to date, to our knowledge, examining cancer-specific stress and physical health in patients with chronic lymphocytic leukemia, cancer-specific stress was associated with symptom burden in early-stage patients receiving active surveillance [30]. This is consistent with studies in other cancers finding that cancer-specific stress covaries with sleep difficulties [31], treatment toxicities [24], and symptom burden [25, 32]. The longitudinal relationship of cancer-specific stress and physical functioning in patients with relapsed/refractory chronic lymphocytic leukemia undergoing treatment is unknown.
To address gaps in the literature, a longitudinal design is used to study cancer-specific stress at treatment initiation as a predictor of concurrent and subsequent psychological and physical functioning in patients with relapsed/refractory chronic lymphocytic leukemia from the time of treatment initiation through its continuation in the next 5 months. Psychological functioning is conceptualized as cognitive-affective depressive symptoms, negative mood, and mental health quality of life. Physical functioning was assessed using measures of fatigue interference, sleep problems, and physical health quality of life. Multilevel models test the hypothesis that higher levels of baseline cancer-specific stress would be associated with poorer concurrent psychological functioning and predict worse psychological functioning trajectories. Similarly, models test the hypothesis that cancer-specific stress would be associated with worse physical functioning at baseline and predict worse physical functioning trajectories.
Methods
Participants
A total of 152 patients with relapsed/refractory chronic lymphocytic leukemia participated. Participants were predominantly male (n = 108; 71%) and Caucasian (n = 147; 97%), with a mean age of 64.1 years (SD = 10.8 years; range = 26–91). The majority reported an education level beyond high school/technical school (68%) and were in a relationship with a significant other (86%). Patients had received a median of 3 (mean = 3.5; SD = 2.6; range = 1–16) prior therapies and 32% of patients had at least one additional comorbidity as assessed with the Charlson Comorbidity Index (described below [33]).
Procedures
The Institutional Review Board associated with a university-affiliated, National Cancer Institute-designated comprehensive cancer center granted ethical approval for a phase II, non-randomized drug (ibrutinib) trial. Eligibility requirements included a current indication of treatment, failure of at least one prior therapy, life expectancy greater than 2 months, Eastern Cooperative Oncology Group performance status of 0–2 (i.e., spending more than 50% of waking hours up and about), and other medical requirements (e.g., normal organ function; #NCT01589302 at clinicaltrials.gov). Informed consent was obtained from all individual participants included in the study.
One hundred seventy-one patients provided consent for the trial from May 2012 to July 2014. Nineteen were found to be ineligible and the remaining participants (N = 152) started treatment on the study. Treatment was 420 mg ibrutinib taken daily by mouth in continuous, 28-day cycles until disease progression or unacceptable toxicity. The current study used questionnaires completed by participants as part of the trial at four timepoints: prior to or on the first day of treatment (day 1 of cycle 1), day 1 of cycles 2 (month 1), 3 (month 2), and 6 (month 5). The majority completed the baseline assessment on day 1 of cycle 1 (n = 144), while eight completed it at a screening day within 14 days prior to day 1.
Measures
Predictor
Cancer-specific stress: The Impact of Event Scale-Revised [20, 21] is a 22-item questionnaire that assesses intrusive thoughts (e.g., any reminders brought back feelings about having chronic lymphocytic leukemia), avoidant thoughts/behaviors (e.g., I tried not to talk about chronic lymphocytic leukemia), and hyperarousal (e.g., I was jumpy and easily startled). Participants rated the intensity of these feelings or events in the past week using a five-point Likert scale ranging from 0 = not at all to 4 = extremely. Commonly used in cancer studies [10, 20, 23], the original Impact of Event Scale did not include the hyperarousal items and, thus, they were not included here. The intrusive thoughts and avoidant behaviors/thoughts items were summed for a total score (range 0–64). The baseline Cronbach’s alpha was .87.
Outcomes: psychological functioning
Cognitive-affective depressive symptoms: The Beck Depression Inventory-2nd edition [34] is a 21-item measure of depressive symptoms. Participants rated their symptoms in the past month on a scale from 0 to 3. Items were summed, with higher scores indicating more depressive symptoms. Scores were calculated representing the cognitive-affective (items 1–14; e.g., sadness, loss of pleasure) and the somatic symptoms associated with depression (items 15–21; e.g., fatigue, insomnia), as has been previously done [35, 36]. As the somatic symptoms are confounded with physical symptoms experienced by cancer patients, analyses were conducted using only the cognitive-affective subscale [37]. The scores on the cognitive-affective subscale could range from 0 to 42. The Cronbach’s alpha ranged from .80 to .88 across assessments.
Negative mood: The Profile of Mood States–Short Form [38] is a 37-item questionnaire used to assess negative mood. Participants rated how much they felt a certain emotion (e.g., tense, angry) in the past week on a five-point Likert scale (0 = not at all, 1 = a little, 2 = moderately, 3 = quite a bit, 4 = extremely). Six mood subscales were obtained: anxiety, depression, anger, vigor, fatigue, and confusion. The total mood disturbance score was calculated from the sum of the subscale scores (with the vigor scale subtracted from the total) with higher scores indicating greater negative mood. Due to researcher error, one confusion subscale item (i.e., unable to concentrate) was incorrectly specified for almost half the assessments and removed from the analyses. Thus, the possible range on the total mood disturbance score was −24 to 120. The Cronbach’s alpha for the total mood disturbance ranged from .95 to .96 across assessments.
Mental health quality of life: The Mental Component Summary score of the Medical Outcomes Study–12-Item Short-Form Health Survey (SF-12 [39, 40]) is a measure of mental health quality of life. The 12-Item Short-Form Health Survey assessed eight aspects of quality of life including physical functioning, role functioning-physical, bodily pain, general health perceptions, vitality, social functioning, role functioning-emotional, and mental health. The eight primary subscales were summarized into two component scores: the Mental Component Summary and the Physical Component Summary. Higher scores reflect better quality of life. The 12-Item Short-Form Health Survey is a widely used measure of quality of life and there is evidence for its reliability and validity in chronic illness populations [40].
Outcomes: physical functioning
Fatigue interference: The seven-item total disruption index subscale of the Fatigue Symptom Inventory [41] was used to measure the degree of interference of fatigue on various aspects of life in the past week (e.g., enjoyment of life, work, relations with other people). Items were rated on an 11-point Likert scale from 0 = no interference to 10 = extreme interference. Total scores could range from 0 to 70, with higher scores indicating greater fatigue interference. Cronbach’s alpha ranged from .94 to .95 across assessments.
Sleep problems: The six-item sleep problems index I of the Medical Outcomes Study–Sleep Scale [42] was used to assess sleep problems. Participants reported how often they experienced six specific difficulties with sleep (e.g., awaken during your sleep and have trouble falling asleep again) on a six-point Likert scale (1 = all of the time to 6 = none of the time). Scores were transformed into a 0–100 scale with higher scores indicating greater sleep problems. Cronbach’s alpha ranged from .73 to .74 across assessments.
Physical health quality of life: As described above, the Medical Outcomes Study–12-Item Short-Form Health Survey measured quality of life. The Physical Component Summary measured physical health quality of life.
Covariates
Data for gender were self-reported, and age and prior number of therapies were obtained from chart review. The Charlson Comorbidity Index, a validated measure of risk of death from comorbid conditions, was used to estimate the prevalence of health comorbidities [33]. The index is comprised of 19 conditions, with each weighted from 1 to 6 based on severity and relationship to mortality. The two points for chronic lymphocytic leukemia were not included in the scoring. Total scores could range from 0 to 30, with scores not adjusted for age.
Analytic strategy
Summary statistics were calculated, and bivariate correlations were conducted between covariates and outcomes and between cancer-specific stress and outcomes at baseline. For the primary analyses, multilevel modeling tested baseline cancer-specific stress as a predictor of trajectories of psychological and physical functioning. Multilevel modeling is advantageous to other repeated-measures analyses because it utilizes all available data, while producing consistent, efficient estimates. Specifically, it uses full information maximum likelihood for estimating parameters in the presence of missing data [43].
Separate models were conducted for each outcome. Each model was constructed in three steps: (i) An unconditional growth model containing only fixed effects using linear and quadratic trajectories as the only predictors was conducted for the outcome of interest, with the quadratic model retained if the quadratic slope was significant (p < .05). (ii) Intercept (baseline), linear change, and quadratic change (if needed) and their covariances were tested as random effects for all models by examining the Akaike Information Criterion and −2 log likelihood to determine if model fit improved with inclusion of the random effects [44]. The variance associated with the random intercept, linear slope, and quadratic slope indicates variability between participants at baseline, in their linear rate of change, and in their quadratic rate of change, respectively. (iii) All possible covariates and baseline cancer-specific stress were then added to the models. All main effects and two-way interactions with linear and quadratic (if needed) functions were entered into the model. Scaled identity (constant variance), diagonal, first order autoregressive, and unstructured error covariance structures were examined and the best fitting was determined using the Akaike Information Criterion and the −2 log likelihood. The following were entered as covariates: age (continuous, mean-centered), gender, number of prior therapies (continuous, mean-centered), and comorbidity index (continuous). Analyses revealed the trajectory of each outcome and the association of baseline cancer-specific stress with this trajectory.
Results
Preliminary Analyses
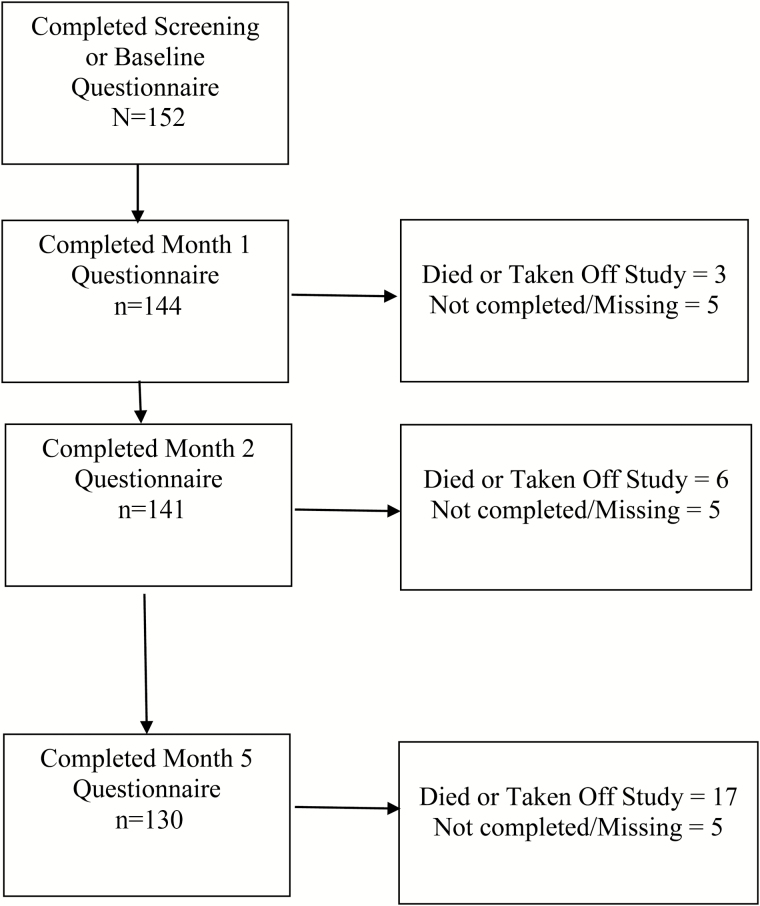
Of the 152 patients, 144 (95%) completed a 1-month assessment, 141 (93%) completed a 2-month assessment, and 130 (86%) completed a 5-month assessment (see Fig. 1). Seventeen participants either died or were taken off study protocol due to disease progression or adverse events by 5 months.
Fig. 1.
Flow diagram of participants.
Descriptive statistics are presented in Table 1. At baseline, participants, on average, reported low levels of psychological distress at treatment initiation, but reported physical health comparable to other cancer populations. For example, the baseline mental health quality of life mean (M = 52.45) was comparable to published norms for the U.S. population (M = 49.37) and greater than that of a general cancer population (M = 47.12 [40]). The baseline physical health quality of life mean was 39.62, comparable to norms for cancer patients (M = 40.76) and worse than the U.S. population average (M = 49.63 [40]).
Table 1.
Descriptive statistics for cancer-specific stress and psychological and physical outcomes at baseline, 1 month, 2 months, and 5 months
| Baseline (N = 152) | Month 1 (n = 144) | Month 2 (n = 141) | Month 5 (n = 130) | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Cancer-specific stress | 9.18 (8.35) | – | – | – |
| n = 152 | ||||
| Cognitive-affective depressive symptoms | 2.82 (3.66) | 1.85 (2.86) | 1.65 (2.48) | 1.88 (3.11) |
| n = 152 | n = 143 | n = 139 | n = 130 | |
| Negative mood | 7.55 (20.17) | −1.67 (15.14) | −1.21 (16.39) | −0.89 (18.12) |
| n = 148 | n = 139 | n = 139 | n = 128 | |
| Mental health quality of lifea | 52.45 (8.89) | 54.30 (8.39) | 54.04 (8.42) | 53.98 (8.72) |
| n = 151 | n = 141 | n = 136 | n = 126 | |
| Fatigue interference | 16.15 (15.59) | 11.92 (15.15) | 10.77 (13.42) | 9.70 (13.11) |
| n = 151 | n = 142 | n = 138 | n = 129 | |
| Sleep problems | 29.53 (18.43) | 23.59 (16.60) | 23.88 (16.89) | 24.08 (17.23) |
| n = 152 | n = 143 | n = 137 | n = 129 | |
| Physical health quality of lifea | 39.62 (11.82) | 43.10 (11.14) | 43.74 (11.08) | 44.23 (11.31) |
| n = 151 | n = 141 | n = 136 | n = 126 |
aHigher scores indicate better functioning.
At baseline, age was significantly correlated with physical health quality of life (r = −.16; p = .05) and prior number of therapies was significantly correlated with fatigue interference (r = .18; p = .03) and physical health quality of life (r = −.28; p < .001). Cancer-specific stress was significantly correlated with cognitive-affective depressive symptoms (r = .50; p < .001), negative mood (r = .60; p < .001), mental health quality of life (r = −.42; p < .001), fatigue interference (r = .33; p < .001), and sleep problems (r = .30; p < .001), but not physical health quality of life (r = −.01; p = .93.).
Primary Analyses
In the unconditional growth models (cancer-specific stress and covariates not included) with fixed effects, the quadratic rate of change was significant for all outcomes, except mental health quality of life. Excepting the mental health quality of life (which had a nonsignificant linear term), significant curvilinear improvement was found on all measures as evidenced by significant linear and quadratic effects in the unconditional models.
The results of the conditional multilevel models of baseline cancer-specific stress predicting the psychological and physical outcomes with covariates included are presented in Table 2 and described next.
Table 2.
Multilevel model results of cancer-specific stress predicting the psychological and physical functioning outcomes (N = 152)
| Psychological functioning | Physical functioning | |||||
|---|---|---|---|---|---|---|
| Cognitive-affective depressive symptoms |
Negative moodb | Mental health quality of lifec | Fatigue interference | Sleep problems | Physical health quality of lifec | |
| Estimate (SE) | Estimate (SE) | Estimate (SE) | Estimate (SE) | Estimate (SE) | Estimate (SE) | |
| Fixed effects | ||||||
| Intercept | 0.50 (0.44) | −7.06 (2.26)** | 57.65 (1.06)*** | 9.58 (2.05)*** | 20.84 (2.46)*** | 41.70 (1.58)*** |
| Lineara | 0.02 (0.29) | −0.41 (1.70) | −0.43 (0.24) | −0.71 (1.47) | −2.71 (1.78) | 2.22 (1.10)* |
| Quadratic | 0.02 (0.05) | 0.12 (0.30) | – | 0.06 (0.26) | 0.65 (0.33)* | −0.34 (0.19) |
| Cancer-specific stress | 0.22 (0.03)*** | 1.37 (0.16)*** | −0.40 (0.07)*** | 0.67 (0.14)*** | 0.68 (0.17)*** | −0.04 (0.11) |
| Cancer-specific stress × linear | −0.08 (0.02)*** | −0.65 (0.12)*** | 0.06 (0.02)** | −0.38 (0.10)*** | −0.18 (0.12)d | 0.01 (0.08) |
| Cancer-specific stress × quadratic | 0.01 (0.003)** | 0.09 (0.02)*** | – | 0.05 (0.02)** | 0.01 (0.02) | 0.004 (0.01) |
| Age | −0.03 (0.02) | 0.02 (0.12) | 0.06 (0.06) | 0.08 (0.11) | −0.08 (0.13) | −0.13 (0.09) |
| Age × linear | 0.05 (0.02)** | 0.14 (0.09) | −0.01 (0.01) | 0.02 (0.08) | 0.07 (0.10) | −0.06 (0.06) |
| Age × quadratic | −0.01 (0.003)** | −0.03 (0.02) | – | −0.01 (0.01) | −0.02 (0.02) | 0.02 (0.01) |
| Comorbidity | 0.31 (0.27) | 0.96 (1.40) | −1.30 (0.64)* | 1.30 (1.24) | 2.42 (1.48) | −1.33 (0.95) |
| Comorbidity × linear | −0.11 (0.17) | 0.73 (1.07) | 0.20 (0.15) | 0.26 (0.90) | −1.14 (1.09) | 0.35 (0.68) |
| Comorbidity × quadratic | 0.01 (0.03) | −0.09 (0.19) | – | −0.02 (0.16) | 0.18 (0.20) | −0.09 (0.12) |
| Gender (female) | 0.28 (0.57) | 2.12 (2.96) | −0.16 (1.36) | −1.23 (2.64) | 1.87 (3.16) | −2.66 (2.03) |
| Gender × linear | −0.17 (0.37) | −1.14 (2.22) | 0.07 (0.30) | 1.28 (1.89) | 2.72 (2.31) | 1.05 (1.46) |
| Gender × quadratic | 0.03 (0.06) | 0.27 (0.40) | – | −0.19 (0.34) | −0.55 (0.42) | −0.21 (0.25) |
| Number of prior therapies | 0.10 (0.10) | 1.00 (0.50)* | 0.01 (0.23) | 1.28 (0.45)** | 0.99 (0.54) | −1.20 (0.35)** |
| Number of prior therapies × linear | −0.08 (0.06) | −0.21 (0.38) | −0.02 (0.05) | −0.38 (0.32) | −0.16 (0.39) | 0.20 (0.25) |
| Number of prior therapies × quadratic | 0.01 (0.01) | 0.04 (0.07) | – | 0.04 (0.06) | −0.01 (0.07) | −0.04 (0.04) |
| Random effects | ||||||
| Intercept variance | 8.25 (1.10)*** | 217.62 (30.92)*** | 36.21 (5.27)*** | 147.11 (24.61)*** | 231.10 (34.35)*** | 102.13 (14.07)*** |
| Linear variance | 2.27 (0.49)*** | 92.20 (21.80)*** | – | 26.93 (15.11)* | 69.76 (19.91)*** | 33.32 (7.86)*** |
| Quadratic variance | 0.06 (0.01)*** | 2.74 (0.73)*** | – | 0.72 (0.50) | 2.20 (0.66)*** | 0.85 (0.24)*** |
| Intercept-linear covariance | −2.48 (0.60)*** | −62.87 (19.83)** | – | −20.47 (14.24) | −55.51 (20.40)** | −22.67 (8.00)** |
| Intercept-quadratic covariance | 0.41 (0.10)*** | 12.05 (3.47)** | – | 2.30 (2.42) | 8.78 (3.58)* | 2.92 (1.33)* |
| Linear-quadratic covariance | −0.36 (0.08)*** | −15.70 (3.92)*** | – | −4.32 (2.68) | −12.08 (3.55)** | −5.18 (1.34)*** |
| Random effects covariance structure | Unstructured | Unstructured | Intercept only | Unstructured | Unstructured | Unstructured |
| Residual covariance structure | Constant variance | First order autoregressive | Constant variance | Constant variance | Constant variance | Constant variance |
The bold values indicate the parameters of interest related to cancer-specific stress predicting outcomes.
aLinear refers to time (in months).
bFor negative mood analyses, n = 151.
cHigher scores indicate better functioning.
dFor sleep problems, if the cancer-specific stress × quadratic variable is removed, the cancer-specific stress × linear variable becomes significant (cancer-specific stress × linear parameter estimate = −0.15 (0.03); p < .001).
*p < .05, **p < .01, ***p < .001.
Psychological Functioning
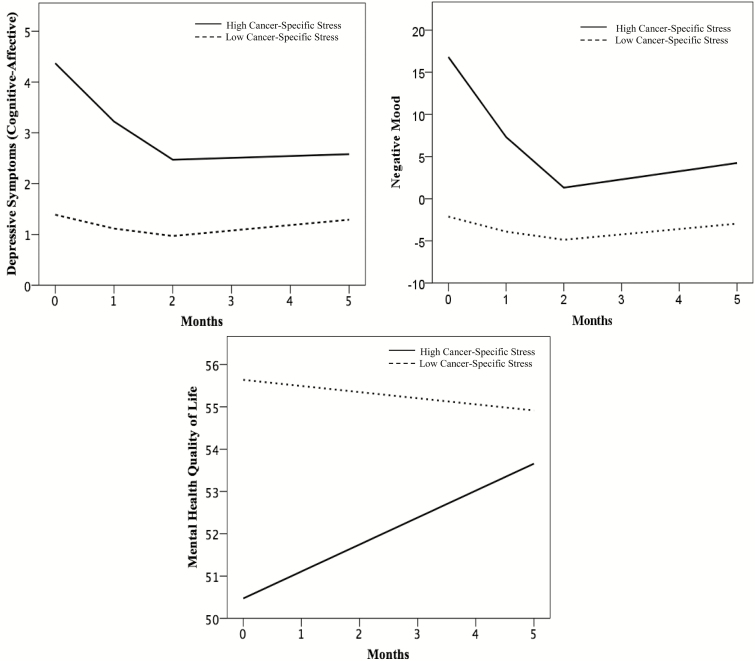
As hypothesized, cancer-specific stress was significantly related to all psychological outcomes at baseline in the expected directions. Higher baseline levels of cancer-specific stress were significantly associated with higher baseline cognitive-affective depressive symptoms and negative mood and lower mental health quality of life. Next, examining the relationship of baseline cancer-specific stress with trajectories, cancer-specific stress was significantly related to both the linear and quadratic slopes of cognitive-affective depressive symptoms and negative mood. Higher baseline cancer-specific stress was related to a faster decline, indicated by a more negative linear slope, in cognitive-affective depressive symptoms and negative mood. Higher cancer-specific stress at baseline was also related to a more positive quadratic slope, indicating a faster deceleration of cognitive-affective depressive symptoms and negative mood. With regards to mental health quality of life, cancer-specific stress at baseline was significantly related to the linear slope. The quadratic slope was not included in analyses, as it was not significant for mental health quality of life. The findings indicate that higher baseline cancer-specific stress was related to a steeper improvement in mental health quality of life, as indicated by a more positive linear slope. In summary, higher cancer-specific stress at baseline was related to poorer psychological functioning at baseline and a more rapid decline of cognitive-affective depressive symptoms and negative mood and more rapid improvement of mental health quality of life.
The pattern of results for the psychological functioning outcomes is presented graphically in Fig. 2 using predicted scores from the multilevel models. In all models, cancer-specific stress was retained as a continuous variable, but for ease of understanding an illustration is provided in which the figures show “low” and “high” groups using a median split (Impact of Event Scale = 8) at baseline. Consistent with the models, the “high” group has higher cognitive-affective depressive symptoms and negative mood and lower mental health quality of life at baseline. The trajectories also show that the “high” group has a more rapid decline of cognitive-affective depressive symptoms and negative mood, and a more rapid improvement of mental health quality of life compared to the “low” group, which appears to have little change over the 5 months on these outcomes.
Fig. 2.
Trajectories of predicted scores of psychological outcomes (cognitive-affective depressive symptoms, negative mood, mental health quality of life) using multilevel modeling. Baseline cancer-specific stress scores are dichotomized using a median split for graphical purposes only. For mental health quality of life, higher scores indicate better functioning.
Physical Functioning
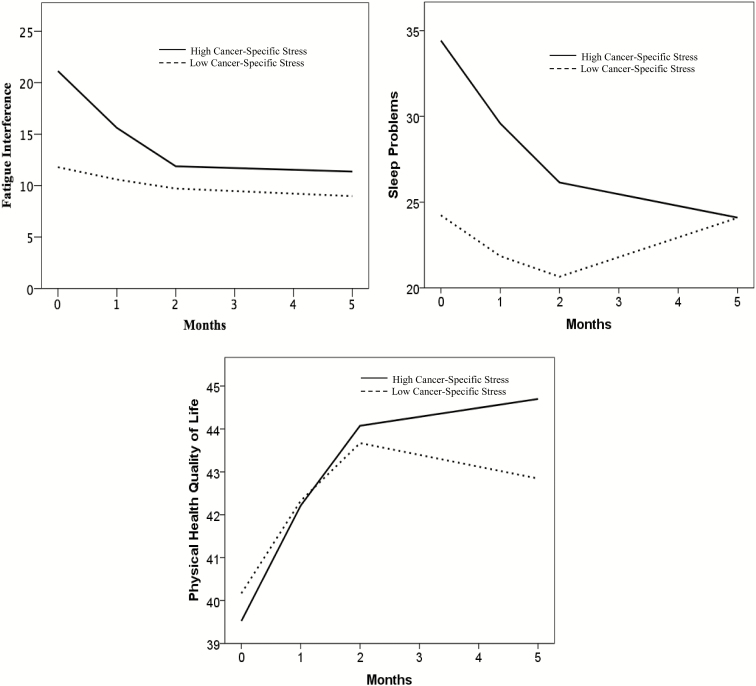
The hypothesis that higher baseline cancer-specific stress would be related to worse baseline levels of physical functioning was supported for fatigue interference and sleep problems. For fatigue interference, higher baseline cancer-specific stress was associated with a more negative linear slope, indicating a faster decline, and a more positive quadratic slope, indicating a faster deceleration. In the case of sleep problems, cancer-specific stress was not related to the linear or quadratic rate of change. However, after examination of the graphical representation of the results, post hoc analyses were conducted to examine whether removing the nonsignificant cancer-specific stress × quadratic term would affect the model results. When the nonsignificant cancer-specific stress × quadratic term was removed, cancer-specific stress was significantly associated with linear slope, indicating that higher cancer-specific stress at baseline was related to faster rates of linear improvement in sleep problems. Cancer-specific stress was not a significant predictor of linear or quadratic slope for physical health quality of life, even when the nonsignificant cancer-specific stress × quadratic term was removed. In summary, higher cancer-specific stress at baseline was related to greater fatigue interference and sleep problems at baseline and more rapid decline in these outcomes. Cancer-specific stress was not related to physical health quality of life.
The pattern of results for the physical functioning outcomes is illustrated in Fig. 3 using predicted scores from the multilevel models. Again, using a median split (Impact of Event Scale = 8), the figures show “low” and “high” cancer-specific stress groups. Consistent with the models, the “high” group has higher fatigue interference and sleep problems, but no difference in physical health quality of life, at baseline. The trajectories also show that the “high” group has a rapid decline of fatigue interference and sleep problems, while the “low” group has little change on these outcomes. For physical health quality of life, the trajectories do not differ between the “high” and “low” groups.
Fig. 3.
Trajectories of predicted scores of physical outcomes (fatigue interference, sleep problems, physical health quality of life) using multilevel modeling. Baseline cancer-specific stress scores are dichotomized using a median split for graphical purposes only. For physical health quality of life, higher scores indicate better functioning.
Follow-up Analyses
Follow-up analyses were conducted to determine whether the differences in trajectories resulted in differences at 5 months by analyzing associations between baseline cancer-specific stress and outcomes at month 5. Bivariate correlations were conducted between the predicted values from each multilevel model at month 5 and cancer-specific stress scores at baseline. Baseline cancer-specific stress was significantly associated with higher 5-month predicted cognitive-affective depressive symptoms (r = .28, p = .001) and negative mood scores (r = .22, p = .01) and lower mental health quality of life (r = −.17, p = .03). Baseline cancer-specific stress was not associated with predicted 5-month fatigue interference (r = .11, p = .19), sleep problems (r = −.03, p = .70), or physical health quality of life (r = .14, p = .08). In summary, higher cancer-specific stress at baseline was related to poorer psychological outcomes at 5 months, but not physical outcomes.
Discussion
Chronic lymphocytic leukemia has a unique disease trajectory with a cycling of treatment periods, recovery, and relapse. The present study provides insight into the experience of patients with this chronic, incurable disease by examining the association between cancer-specific stress and psychological and physical functioning trajectories in patients with relapsed/refractory disease. At treatment initiation, cancer-specific stress was associated with higher levels of cognitive-affective depressive symptoms, negative mood, fatigue interference, and sleep problems, and lower mental health quality of life. While patients with higher cancer-specific stress at baseline improved more rapidly on these outcomes, follow-up analyses indicated that higher cancer-specific stress at baseline was still associated with poorer psychological outcomes, but not physical outcomes, at 5 months.
When considered psychologically, the experience of patients with relapsed/refractory chronic lymphocytic leukemia may be analogous to repeated trials of stressors, with relapse after relapse and therapy cycle after therapy cycle, with as many as 16 such episodes for one patient in this sample. This phenomenon is unique compared to most other cancers. Overall baseline psychological functioning was high in this group, but baseline cancer-specific stress was still related to higher cognitive-affective depressive symptoms, negative mood, and worse mental health quality of life. The data confirm that for these cancer patients and others, cancer-specific stress is consistently related to poorer psychological functioning [23–25, 27–29].
Interestingly, higher cancer-specific stress at baseline predicted faster improvements in all psychological outcomes, whereas lower cancer-specific stress at baseline seemed to be related to constant, low levels of cognitive-affective depressive symptoms and negative mood, and high mental health quality of life. As patients with higher cancer-specific stress had more room for improvement in their psychological outcomes, this may have accounted for the more rapid rate of improvement. Nevertheless, follow-up analyses demonstrated that greater baseline cancer-specific stress remained important as evidenced by its association with poorer 5-month psychological functioning. A similar pattern was seen in a study of breast cancer survivors, in which higher intrusive thoughts at treatment completion were related to a faster improvement in psychological outcomes (i.e., depressive symptoms and negative affect), but still predicted worse psychological functioning at 12 months following treatment completion [45]. The overall robust relationship with concurrent and subsequent psychological functioning is consistent with studies of traumatic stress in other populations, as well [14–16].
There are studies in chronic lymphocytic leukemia describing patients’ poor physical health [6, 8], though none, to our knowledge, have examined predictors of physical health longitudinally outside of sociodemographic and clinical variables. In the only other study of cancer-specific stress in chronic lymphocytic leukemia, a cross-sectional study with patients in active surveillance, cancer-specific stress covaried with a composite measure of symptoms (i.e., chronic lymphocytic leukemia symptoms, fatigue, pain, and functional status [30]). The latter findings are replicated and extended in several ways. First, the baseline analyses show cancer-specific stress to again covary with fatigue, but in this case, sleep problems as well, with the latter findings consistent with cross-sectional data from other cancer samples [31, 32]. The nonsignificant relationship with concurrent physical health quality of life is consistent with findings in newly diagnosed breast cancer patients [27]. Secondly, the longitudinal data offered study of cancer-specific stress as a predictor, with analyses revealing that higher cancer-specific stress at baseline was related to faster improvements in fatigue and sleep disturbance. However, unlike psychological functioning, follow-up analyses found that due to the rapid improvement of physical outcomes in those with higher baseline cancer-specific stress, baseline cancer-specific stress was not associated with physical functioning outcomes at 5 months. This is also in contrast to studies of other traumatic stressors, and other cancers, which find that traumatic stress symptoms are related to poorer subsequent physical health [12, 17, 18, 27, 45]. The symptom improvement with higher baseline cancer-specific stress may seem paradoxical but potentially suggests the action of ibrutinib “overpowering” the effect of heightened cancer-specific stress. That is, ibrutinib was investigational at the time (i.e., not yet approved by the U.S. Food and Drug Administration), and physicians had minimal information and expectations to share with patients at enrollment about toxicities. Now published trials of ibrutinib find that a large subset of patients have durable remissions with modest side effect profiles [46–48]. Thus, the trajectory of significantly improving physical health accurately reflects the toxicity profile now found in other trials with ibrutinib, and cancer-specific stress, at least at the levels reported by these patients, did not confer additional risk for worse physical outcomes. In addition to the efficacy of the drug, another possible explanation for baseline cancer-specific stress not being associated with 5-month physical outcomes might be a result of the unique experience of relapsed/refractory chronic lymphocytic leukemia. In contrast to other types of traumas, patients may have prior experiences with the stressor (i.e., starting treatment) that then led to successful outcomes (i.e., treatment response). The habituation to the cycling of treatment/response/relapse/treatment and potentially positive past experiences may have significantly weakened the relationship between cancer-specific stress at treatment initiation and subsequent physical health at 5 months. Future studies should examine cancer-specific stress and longitudinal trajectories of functioning in patients with chronic lymphocytic leukemia undergoing active surveillance or treatment for the first time to examine the role of treatment habituation on outcomes.
The strengths and limitations of the current study are considered. These data were part of an important, early investigational trial and are a significant contribution to medical oncology’s understanding of patient-reported outcomes for patients with relapsed/refractory chronic lymphocytic leukemia, and more specifically, for patients on targeted therapies. With approval by the Food and Drug Administration now established, ibrutinib is now frequently utilized in patients with relapsed/refractory chronic lymphocytic leukemia and in elderly patients as first-line therapy, making these data especially relevant. More broadly, this is the first study of cancer-specific stress and trajectories of psychological and physical functioning in this important patient group. The longitudinal design beginning at treatment initiation, inclusion of all patients in the trial, and trajectory analyses are methodological strengths. The homogeneity of treatment and full participation enhanced the internal validity of cancer-specific stress as a predictor of trajectories. However, external validity may be limited, as similar to other clinical trials, there was an underrepresentation of minorities [49] and older adults [50]. This study accrued only 3% of minorities and the mean age of 64 years was less than that of the general chronic lymphocytic leukemia population (mean = 71 years [1]). As such, the level of the physical functioning trajectory is likely overestimated, but the effect on the level of stress or any other psychological outcome is unknown. Furthermore, aspects of clinical trial participation, such as close, active monitoring of participants, may have also impacted trajectories, as it is more likely that all levels of toxicities are quickly identified and treated. Finally, although longitudinal, the data are observational and do not allow for causal inferences to be made on the relationship between cancer-specific stress and psychological and physical functioning.
These data chronicle how the psychological and physical functioning trajectories for patients with chronic lymphocytic leukemia are altered when cancer-specific stress is high during the early months of a then new but now standard targeted therapy. On average, patients reported minimal psychological distress at treatment initiation, indicating that most patients are doing well. However, the data suggest patients experiencing greater levels of cancer-specific stress to be at risk for persistently worse psychological functioning. There has been a call to screen cancer patients [51] to determine those who may be at risk for poor outcomes, and assessment of cancer-specific stress may have clinical utility as an individual difference predictor of psychological responses. Even with an oral targeted therapy with comparatively less toxicity, the findings also illustrate the importance of repeated assessment of psychological responses during treatment as has been recommended [51]. These findings suggest that integration of psychological intervention for patients who have high cancer-specific stress at baseline might be appropriate for this population. Future studies should examine whether targeting patients with high cancer-specific stress at treatment initiation for psychosocial intervention are conferred any benefits to participation.
Compliance with Ethical Standards
Conflict of Interest: K.J. Maddocks has received research funding from Pharmacyclics and has served as a consultant for Pharmacyclics and Janssen. J.C. Byrd has received research funding from Pharmacyclics, Acerta, Genentech, and Janssen. The remaining authors declare no relevant conflicts of interest.
Ethical Approval: All research procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee (institutional and national) and the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author contributions: All authors contributed to the conception and design of the study. N.G. Goyal conducted the analysis of data, with all other authors providing additional interpretation of the data. N.G. Goyal wrote the original draft. All authors revised and edited the manuscript and approved the final version.
Acknowledgments
This research was supported by the National Cancer Institute (K05 CA098133, R25 CA122061, R35 CA197734, R01 CA177292), Pharmacyclics, The D. Warren Brown Foundation, Mr. and Mrs. Michael Thomas, Four Winds Foundation, and a Pelotonia Idea Award from the Ohio State University Comprehensive Cancer Center and Solove Research Institute.
References
- 1. American Cancer Society. Chronic Lymphocytic Leukemia 2017. Available at https://www.cancer.org/cancer/chronic-lymphocytic-leukemia.html. Accessibility verified March 1, 2017.
- 2. Hallek M, Cheson BD, Catovsky D et al. ; International Workshop on Chronic Lymphocytic Leukemia Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008; 111(12):5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Molica S. Quality of life in chronic lymphocytic leukemia: a neglected issue. Leuk Lymphoma. 2005;46(12):1709–1714. [DOI] [PubMed] [Google Scholar]
- 4. Levin TT, Li Y, Riskind J, Rai K. Depression, anxiety and quality of life in a chronic lymphocytic leukemia cohort. Gen Hosp Psychiatry. 2007;29(3):251–256. [DOI] [PubMed] [Google Scholar]
- 5. Howlader N, Noone A, Krapcho M et al. SEER Cancer Statistics Review, 1975–2013. April 2016 ed. Bethesda, MD: National Cancer Institute; 2016. [based on November 2015 SEER data submission]. Available at http://seer.cancer.gov/csr/1975_2013/. Accessibility verified March 15, 2017. [Google Scholar]
- 6. Holzner B, Kemmler G, Kopp M, Nguyen-Van-Tam D, Sperner-Unterweger B, Greil R. Quality of life of patients with chronic lymphocytic leukemia: results of a longitudinal investigation over 1 yr. Eur J Haematol. 2004;72(6): 381–389. [DOI] [PubMed] [Google Scholar]
- 7. Eichhorst BF, Busch R, Obwandner T, Kuhn-Hallek I, Herschbach P, Hallek M; German CLL Study Group Health-related quality of life in younger patients with chronic lymphocytic leukemia treated with fludarabine plus cyclophosphamide or fludarabine alone for first-line therapy: a study by the German CLL Study Group. J Clin Oncol. 2007; 25(13):1722–1731. [DOI] [PubMed] [Google Scholar]
- 8. Else M, Smith AG, Cocks K et al. Patients’ experience of chronic lymphocytic leukaemia: baseline health-related quality of life results from the LRF CLL4 trial. Br J Haematol. 2008;143(5):690–697. [DOI] [PubMed] [Google Scholar]
- 9. Andersen BL, Kiecolt-Glaser JK, Glaser R. A biobehavioral model of cancer stress and disease course. Am Psychol. 1994;49(5):389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cella DF, Mahon SM, Donovan MI. Cancer recurrence as a traumatic event. Behav Med. 1990;16(1):15–22. [DOI] [PubMed] [Google Scholar]
- 11. Kelly B, Raphael B, Smithers M et al. Psychological responses to malignant melanoma. An investigation of traumatic stress reactions to life-threatening illness. Gen Hosp Psychiatry. 1995;17(2):126–134. [DOI] [PubMed] [Google Scholar]
- 12. D’Andrea W, Sharma R, Zelechoski AD, Spinazzola J. Physical health problems after single trauma exposure: when stress takes root in the body. J Am Psychiatr Nurses Assoc. 2011;17(6):378–392. [DOI] [PubMed] [Google Scholar]
- 13. Schnurr PP, Spiro A 3rd. Combat exposure, posttraumatic stress disorder symptoms, and health behaviors as predictors of self-reported physical health in older veterans. J Nerv Ment Dis. 1999;187(6):353–359. [DOI] [PubMed] [Google Scholar]
- 14. Roth RS, Geisser ME, Bates R. The relation of post-traumatic stress symptoms to depression and pain in patients with accident-related chronic pain. J Pain. 2008;9(7):588–596. [DOI] [PubMed] [Google Scholar]
- 15. Joseph S, Dalgleish T, Thrasher S, Yule W, Williams R, Hodgkinson P. Chronic emotional processing in survivors of the Herald of Free Enterprise disaster: the relationship of intrusion and avoidance at 3 years to distress at 5 years. Behav Res Ther. 1996;34(4):357–360. [DOI] [PubMed] [Google Scholar]
- 16. Johansen VA, Wahl AK, Eilertsen DE, Weisaeth L, Hanestad BR. The predictive value of post-traumatic stress disorder symptoms for quality of life: a longitudinal study of physically injured victims of non-domestic violence. Health Qual Life Outcomes. 2007;5(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andersen TE, Karstoft KI, Brink O, Elklit A. Pain-catastrophizing and fear-avoidance beliefs as mediators between post-traumatic stress symptoms and pain following whiplash injury - a prospective cohort study. Eur J Pain. 2016; 20(8):1241–1252. [DOI] [PubMed] [Google Scholar]
- 18. Shemesh E, Yehuda R, Milo O et al. Posttraumatic stress, nonadherence, and adverse outcome in survivors of a myocardial infarction. Psychosom Med. 2004;66(4):521–526. [DOI] [PubMed] [Google Scholar]
- 19. Cavalcanti-Ribeiro P, Andrade-Nascimento M, Morais-de-Jesus M et al. Post-traumatic stress disorder as a comorbidity: impact on disease outcomes. Expert Rev Neurother. 2012; 12(8):1023–1037. [DOI] [PubMed] [Google Scholar]
- 20. Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41(3): 209–218. [DOI] [PubMed] [Google Scholar]
- 21. Weiss DS, Marmar CR. The impact of Events Scale - revised. In: Wilson JP, Keane TM, eds. Assessing Psychological Trauma and PTSD. New York, NY: The Guilford Press; 1997: 399–411. [Google Scholar]
- 22. Horowitz MJ. Stress-response syndromes: a review of posttraumatic and adjustment disorders. Hosp Community Psychiatry. 1986;37(3):241–249. [DOI] [PubMed] [Google Scholar]
- 23. Golden-Kreutz DM, Andersen BL. Depressive symptoms after breast cancer surgery: relationships with global, cancer-related, and life event stress. Psychooncology. 2004;13(3): 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mykletun A, Dahl AA, Haaland CF et al. Side effects and cancer-related stress determine quality of life in long-term survivors of testicular cancer. J Clin Oncol. 2005;23(13): 3061–3068. [DOI] [PubMed] [Google Scholar]
- 25. Kang DH, Park NJ, McArdle T. Cancer-specific stress and mood disturbance: implications for symptom perception, quality of life, and immune response in women shortly after diagnosis of breast cancer. ISRN Nurs. 2012;2012:608039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andersen BL, Farrar WB, Golden-Kreutz D et al. Stress and immune responses after surgical treatment for regional breast cancer. J Natl Cancer Inst. 1998;90(1):30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Golden-Kreutz DM, Thornton LM, Wells-Di Gregorio S et al. Traumatic stress, perceived global stress, and life events: prospectively predicting quality of life in breast cancer patients. Health Psychol. 2005;24(3):288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang HC, Brothers BM, Andersen BL. Stress and quality of life in breast cancer recurrence: moderation or mediation of coping?Ann Behav Med. 2008;35(2):188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Primo K, Compas BE, Oppedisano G, Howell DC, Epping-Jordan JE, Krag DN. Intrusive thoughts and avoidance in breast cancer: individual differences and association with psychological distress. Psychol Health. 2000;14(6):1141–1153. [DOI] [PubMed] [Google Scholar]
- 30. Morrison EJ, Flynn JM, Jones J, Byrd JC, Andersen BL. Individual differences in physical symptom burden and psychological responses in individuals with chronic lymphocytic leukemia. Ann Hematol. 2016;95(12):1989–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mystakidou K, Parpa E, Tsilika E, Gennatas C, Galanos A, Vlahos L. How is sleep quality affected by the psychological and symptom distress of advanced cancer patients?Palliat Med. 2009;23(1):46–53. [DOI] [PubMed] [Google Scholar]
- 32. Thekdi SM, Milbury K, Spelman A et al. Posttraumatic stress and depressive symptoms in renal cell carcinoma: association with quality of life and utility of single-item distress screening. Psychooncology. 2015;24(11):1477–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 34. Beck AT, Steer RA, Brown BK.. Manual for Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 35. Ganz PA, Kwan L, Castellon SA et al. Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. J Natl Cancer Inst. 2013;105(11):791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thombs BD, Ziegelstein RC, Pilote L et al. Somatic symptom overlap in Beck Depression Inventory-II scores following myocardial infarction. Br J Psychiatry. 2010;197(1):61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wedding U, Koch A, Röhrig B et al. Requestioning depression in patients with cancer: contribution of somatic and affective symptoms to Beck’s Depression Inventory. Ann Oncol. 2007;18(11):1875–1881. [DOI] [PubMed] [Google Scholar]
- 38. Shacham S. A shortened version of the Profile of Mood States. J Pers Assess. 1983;47(3):305–306. [DOI] [PubMed] [Google Scholar]
- 39. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 40. Ware JE, Kosinski M, Turner-Bowker DM, Gandek B.. SF-12v2: How to Score Version 2 of the SF-12 Health Survey. Lincoln, RI: QualityMetric Inc; 2002. [Google Scholar]
- 41. Hann DM, Jacobsen PB, Azzarello LM et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7(4): 301–310. [DOI] [PubMed] [Google Scholar]
- 42. Spitzer KL, Hays RD.. MOS Sleep Scale: A Manual for Use and Scoring, Version 1.0. Los Angeles, CA; 2003. [Google Scholar]
- 43. Raudenbush SW, Bryk AS.. Hierarchical Linear Models. Newbury Park, CA: Sage; 2002. [Google Scholar]
- 44. Singer JD, Willett JB.. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 45. Dupont A, Bower JE, Stanton AL, Ganz PA. Cancer-related intrusive thoughts predict behavioral symptoms following breast cancer treatment. Health Psychol. 2014;33(2):155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Byrd JC, Furman RR, Coutre SE et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015; 125(16):2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Burger JA, Tedeschi A, Barr PM et al. ; RESONATE-2 Investigators Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25): 2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maddocks K, Jones JA. Bruton tyrosine kinase inhibition in chronic lymphocytic leukemia. Semin Oncol. 2016;43(2): 251–259. [DOI] [PubMed] [Google Scholar]
- 49. Heller C, Balls-Berry JE, Nery JD et al. Strategies addressing barriers to clinical trial enrollment of underrepresented populations: a systematic review. Contemp Clin Trials. 2014; 39(2):169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Eichhorst BF, Busch R, Stilgenbauer S et al. ; German CLL Study Group (GCLLSG) First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114(16):3382–3391. [DOI] [PubMed] [Google Scholar]
- 51. Andersen BL, Rowland JH, Somerfield MR. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology Guideline adaptation. J Oncol Pract. 2015;11(2):133–134. [DOI] [PubMed] [Google Scholar]