Abstract
Tree stems exchange CO2, CH4 and N2O with the atmosphere but the magnitudes, patterns and drivers of these greenhouse gas (GHG) fluxes remain poorly understood. Our understanding mainly comes from static-manual measurements, which provide limited information on the temporal variability and magnitude of these fluxes. We measured hourly CO2, CH4 and N2O fluxes at two stem heights and adjacent soils within an upland temperate forest. We analyzed diurnal and seasonal variability of fluxes and biophysical drivers (i.e., temperature, soil moisture, sap flux). Tree stems were a net source of CO2 (3.80 ± 0.18 µmol m−2 s−1; mean ± 95% CI) and CH4 (0.37 ± 0.18 nmol m−2 s−1), but a sink for N2O (−0.016 ± 0.008 nmol m−2 s−1). Time series analysis showed diurnal temporal correlations between these gases with temperature or sap flux for certain days. CO2 and CH4 showed a clear seasonal pattern explained by temperature, soil water content and sap flux. Relationships between stem, soil fluxes and their drivers suggest that CH4 for stem emissions could be partially produced belowground. High-frequency measurements demonstrate that: a) tree stems exchange GHGs with the atmosphere at multiple time scales; and b) are needed to better estimate fluxes magnitudes and understand underlying mechanisms of GHG stem emissions.
Introduction
Carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O) are the most important greenhouse gases, contributing 60, 20 and 10% to global warming, respectively1. Interactions between soil, vegetation and the atmosphere exert a crucial role controlling the global budget of these gases2. Particularly, forests influence GHG dynamics where their soils and leaves/canopies are active surfaces for GHG exchange3,4. Our current understanding of CH4 and N2O fluxes from forest ecosystems is mainly based on studies of forest soil measurements and canopies2,5–7. However, recent studies have revealed that stem surfaces could play an important role in regulating GHG fluxes8–13. For CO2, it is known that stem emissions are partially produced in the stem itself and partially produced in the rhizosphere and then, dissolved and transported upwards by stem sap flux9. However, much less is known about CH4 and N2O stem fluxes. CH4 in floodplain and wetland ecosystems is produced in soils under anoxic conditions and transported by roots to the stems8,14,15, but this direct relationship between soil and stem emissions is not as clear in upland forests. Several studies in the last 2 years have reported stem CH4 emissions in upland forests where adjacent soils are not a source but a sink of CH412,16–23. Soils are often well aerated (specially in upland forests), and methanotrophic activity results in an uptake of 20 to 45 Tg CH4 y−1 at the global scale24,25. For N2O, the link between stem and soil dynamics is even less clear. Globally, soils are N2O sources (6.6 Tg N2O y−1)26 but in some cases they can act as sinks27–29. In contrast, stems have been described both as N2O sinks or sources, but this information is limited to very few studies20,22,30–32. Arguably, there are three key issues related to CH4 and N2O stem emissions in upland forests that represent a forefront of research33.
First, the magnitudes and patterns of stem emissions of CH4 and N2O in upland forests are poorly known. The few available studies suggest that there is a large variability of emissions between stems within mixed stands12,17, but also a large variability between trees from the same species18,20,21,32. Furthermore, these studies have not identified the main environmental drivers (e.g., temperature, soil moisture/precipitation) and the temporal dynamics of these GHG emissions.
A second issue deals with the origin (i.e., production and transport) of stem CH4 and N2O emissions in upland forests. Are these gases largely produced in the soil and transported upwards through the stem or are they mainly locally produced within the heartwood? Field studies have suggested that CH4 could originate in the soil as they report high CH4 concentrations in deep soil close to the measured stem21, a decrease in CH4 emissions with stem height18, or correlations between stem and soil CH4 fluxes20. In contrast, high CH4 concentrations in the heartwood34,35, correlations between heartwood CH4 concentrations or heartwood water content and stem emissions19, or the lack of relationships between stem emissions and soil variables12,17 suggest that emitted CH4 could also be produced in the heartwood by the activity of methanogenic Archaea and/or heart rot infection. Additionally, wood decomposition by basidiomycete fungi and stress-induced (e.g. UV light, temperature, insect herbivory) degradation of methoxyl groups in pectin or lignin are also possible sources of locally produced CH4 within tree stems36–38. Some studies report results consistent with CH4 production both in the soil and in the heartwood17. The mechanisms underlying stem N2O emissions are even less known, and consequently, the origin of stem CH4 and N2O emissions is still a topic of ongoing research.
A third issue is the interest for upscaling stem CH4 and N2O fluxes to quantify their role in ecosystem GHG balance. Studies reporting upscaled stem CH48,12,20 and N2O emissions2,20,32,39 are subjected to multiple uncertainties that need to be addressed to obtain accurate ecosystem-level GHG fluxes33. The magnitude of GHG emissions vary spatially, within individual tree stems, between stems of different diameters (e.g., stem vs twigs) and among stems within a forest stand. Moreover, the characterization of seasonal variability of stem GHG emissions is usually based on sporadic manual measurements, which misrepresent information about pulses and daily variability40. To our knowledge, only one study reports limited information on sub-daily co-located measurements of CO2 and CH4 stem emissions17 and suggests that stem CH4 emissions could show a daily pattern (with only 3 days of measurements). If a diurnal pattern is consistent across tree stems and throughout the growing season, then manual measurements may bias estimates of daily total emissions33 and therefore the net impact of stem CH4 emissions for the ecosystem carbon balance. To our knowledge, there is no information based on automated measurements of stem N2O fluxes.
Here, we implement an automated system to continuously measure (i.e., 1-hour resolution) CO2, CH4 and N2O fluxes from stems and soils in an upland forested area in order to better describe magnitudes, emissions and origin of CH4 emissions. We measured these three GHGs at two stem heights (75 and 150 cm) in a bitternut hickory (Carya cordiformis (Wangenh.) K.Koch), and at the adjacent soil. We postulate that high temporal frequency measurements provide: (a) unprecedented estimates of magnitudes and patterns of CO2, CH4 and N2O stem fluxes; and (b) insights about temporal correlations and potential sources of GHG stem fluxes. We highlight that technical and scientific advances are needed to better understand the underlying mechanisms for GHG stem emissions, their incorporation in process-based models, and to quantify their role in local-to-global GHG budgets.
Results
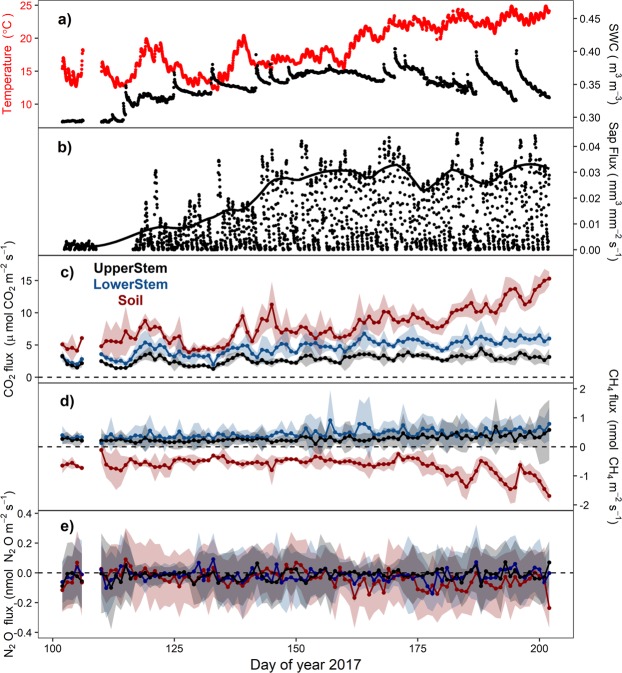
Over the study period, the tree stem acted as a net source of CO2 and CH4 but a sink of N2O. The adjacent soil was a net CO2 source but a CH4 and N2O sink (Table 1). For CO2, emissions were higher in the soil and decreased with height in the tree stem, being 8.24 ± 0.55, 4.76 ± 0.22 and 2.83 ± 0.13 µmol m−2 s−1 on average for Soil, LowerStem and UpperStem, respectively. CH4 emissions also decreased with height along the tree stem (0.46 ± 0.03 and 0.28 ± 0.02 nmol m−2 s−1 for LowerStem and UpperStem, respectively), but the soil was a clear sink of this gas (−0.66 ± 0.06 nmol m−2 s−1). CO2 and CH4 emissions in LowerStem were consistently higher than UpperStem emissions over the study period (Fig. 1). Mean N2O uptake was higher in soil (−0.046 ± 0.011 nmol m−2 s−1) and decreased with stem height (−0.017 ± 0.008 and −0.014 ± 0.006 nmol m−2 s−1 for LowerStem and UpperStem, respectively).
Table 1.
CO2, CH4 and N2O mean fluxes and cumulative fluxes over the study period (mean ± 95% CI) for each position (UpperStem, LowerStem and Soil).
| CO2 | CH4 | N2O | ||||
|---|---|---|---|---|---|---|
| mean | cumulative | mean | cumulative | mean | cumulative | |
| µmol CO2 m−2 s−1 | kg CO2 m−2 | nmol CH4 m−2 s−1 | g CH4 m−2 | nmol N2O m−2 s−1 | g N2O m−2 | |
| UpperStem | 2.83 ± 0.13 | 0.39 ± 0.05 | 0.28 ± 0.02 | 0.106 ± 0.008 | −0.014 ± 0.006 | −0.005 ± 0.002 |
| LowerStem | 4.76 ± 0.22 | 0.66 ± 0.08 | 0.46 ± 0.03 | 0.175 ± 0.011 | −0.017 ± 0.008 | −0.006 ± 0.003 |
| Soil | 8.24 ± 0.55 | 1.14 ± 0.21 | −0.66 ± 0.06 | −0.251 ± 0.023 | −0.046 ± 0.011 | −0.017 ± 0.004 |
Figure 1.
Seasonal course of hourly mean soil temperature and soil water content (SWC) (panel (a)), sap flux per unit sapwood area (SF) (panel (b)) and daily means of CO2, CH4 and N2O fluxes (panels (c–e), respectively; mean ± SD) associated to UpperStem (black), LowerStem (blue) and Soil (red) chambers. Line in panel b) depicts smoothed patterns for midday SF values.
Overall, CO2 and CH4 stem emissions increased over the growing season in parallel with soil temperature and SF (Fig. 1c,d). Both GHGs showed similar seasonal patterns between UpperStem and LowerStem. In contrast, stem N2O fluxes did not show any clear seasonal pattern or similarity between UpperStem and LowerStem. Like stem CO2 emissions, soil CO2 emissions increased along the growing season. However, soil CH4 flux showed the opposite pattern than stem CH4, whereby soils showed an increasing CH4 uptake over the growing season. Soil N2O fluxes showed a slight increase in uptake (i.e., fluxes more negative) during the growing season (Fig. 1c). Despite seasonal patterns were evident for CO2 and CH4 fluxes, the high-temporal resolution data revealed a high variability in the magnitude of the fluxes (Fig. 2, SFig. 1).
Figure 2.
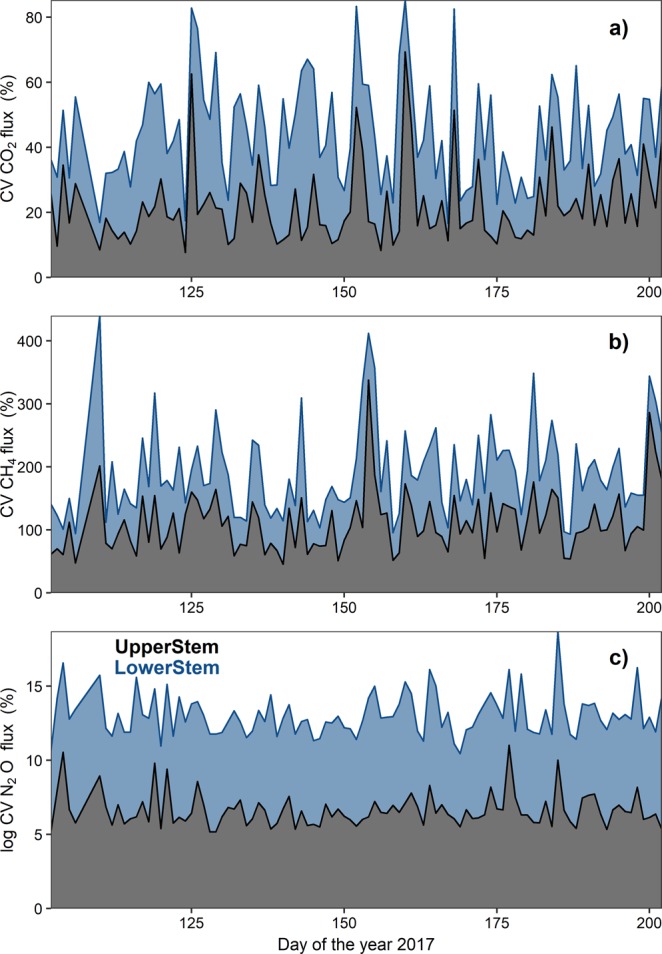
Seasonal course of the daily coefficient of variation (CV) of CO2, CH4 and N2O fluxes (panels (a–c), respectively) associated to UpperStem (black), LowerStem (blue) chambers. CV is reported in absolute values. CV is log transformed for N2O.
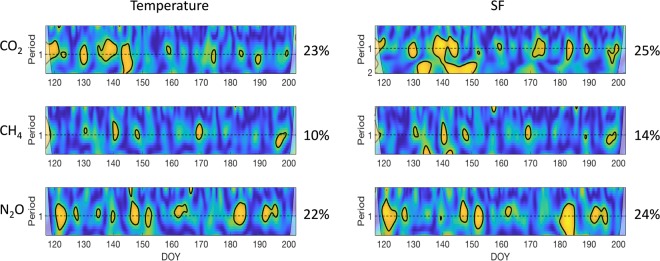
Stem CO2, CH4 and N2O fluxes showed temporal correlations at the 1-day period with temperature or SF (Fig. 3). However, these temporal correlations were not consistent throughout the growing season. Stem CO2 and N2O fluxes showed temporal correlations at the 1-day period with temperature (23% of the days) and SF (25% of the days) (similar percentage for both gases). In contrast, stem CH4 fluxes showed temporal diurnal correlations at the 1-day period with temperature (10% of the days) and SF (14% of the days). Overall, diurnal correlations between GHG fluxes and their drivers did not have a relationship with the temporal progression of the growing season. In other words, there were no differences in temporal correlations at the 1-day period between the early and late growing season.
Figure 3.
Wavelet coherence analyses output and percentage of days with daily significant correlations between CO2, CH4 and N2O measures at LowerStem with Temperature (left panels) and SF (right panels) from hourly data. Yellow color indicates significant temporal correlations (p < 0.05).
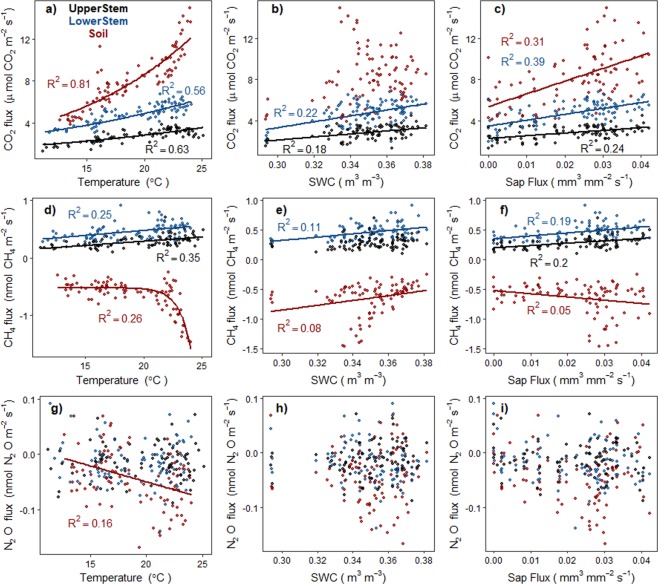
Daily-mean GHG fluxes from the three locations correlated with temperature, SWC and SF, especially CO2 and CH4 (Fig. 4). However, GLS models showed that interactions between temperature, SWC and SF (rather than each independent variable) explain a large fraction of the variability of seasonal GHG emissions (Table 2). For example, soil CH4 fluxes showed a correlation of 0.26 with temperature and 0.08 with SWC, but their interaction could explain 92% of their temporal variability. Best models for CO2 fluxes across different locations were similar. First order interactions between temperature, SWC and SF could explain >90% of the seasonal variability of CO2 emissions (Table 2). Models explaining stem seasonal CH4 fluxes were similar at the two heights. Stem CH4 fluxes were explained by temperature and SF in UpperStem and by temperature and SWC in LowerStem, accounting for 40 and 33% of the seasonal variability, respectively. In the soil, the interaction between temperature and SWC had a much stronger effect on CH4 fluxes than in the tree stem, explaining up to 92% of seasonal variability (Table 2). Stem and soil N2O fluxes were less explained by environmental drivers. For UpperStem, the independent effects of temperature, SWC and SF were included in the best model, but only explained 10% of the variability of N2O fluxes. For soil, only temperature affected N2O fluxes, explaining 22% of the seasonal variability.
Figure 4.
Daily CO2, CH4 and N2O regressions with temperature, soil water content and sap flux for UpperStem, LowerStem and Soil (black, blue and red, respectively). Soil temperature was used for soil emissions comparisons and stem temperature measured at each height was used for stem emissions comparisons. Exponential regression was fitted for CO2 and temperature (panel a), sigmoidal regression was fit for soil CH4 and temperature (panel d) and linear regressions were fitted for the other cases. Regression fit and R2 were placed if significant (p < 0.05).
Table 2.
Summary of the selected models for each greenhouse gas (CO2, CH4 and N2O) and each position (UpperStem, LowerStem and Soil). All variables were scaled to improve the performance and interpretability of the models.
| MODEL | Variables | Estimate | SE | t-value | p-value |
|---|---|---|---|---|---|
| UpperStem logCO2 | (Intercept) | −0.113 | 0.125 | −0.909 | 0.366 |
| adjR2 = 0.93 | Temperature | 0.634 | 0.084 | 7.578 | <0.001 |
| p-value < 0.001 | SWC | 0.566 | 0.106 | 5.351 | <0.001 |
| SF | −0.001 | 0.058 | −0.002 | 0.998 | |
| Temp*SF | −0.200 | 0.045 | −4.456 | <0.001 | |
| SWC*SF | 0.318 | 0.067 | 4.772 | <0.001 | |
| LowerStem logCO2 | (Intercept) | 0.106 | 0.142 | 0.747 | 0.457 |
| adjR2 = 0.92 | Temperature | 0.517 | 0.095 | 5.446 | <0.001 |
| p-value < 0.001 | SWC | 0.192 | 0.104 | 1.841 | 0.069 |
| SF | 0.159 | 0.049 | 3.266 | 0.002 | |
| Temp*SWC | 0.206 | 0.079 | 2.606 | 0.011 | |
| Temp*SF | −0.254 | 0.044 | −5.723 | <0.001 | |
| Soil logCO2 | (Intercept) | 0.162 | 0.254 | 0.639 | 0.525 |
| adjR2 = 0.99 | Temperature | 1.011 | 0.116 | 8.684 | <0.001 |
| p-value < 0.001 | SWC | 0.174 | 0.095 | 1.825 | 0.072 |
| SF | 0.081 | 0.036 | 2.255 | 0.027 | |
| Temp*SWC | −0.240 | 0.072 | −3.315 | 0.001 | |
| UpperStem CH4 | (Intercept) | 0.001 | 0.088 | 0.010 | 0.992 |
| adjR2 = 0.40 | Temperature | 0.451 | 0.098 | 4.488 | <0.001 |
| p-value < 0.001 | SF | 0.254 | 0.097 | 2.620 | 0.01 |
| LowerStem CH4 | (Intercept) | 0.002 | 0.096 | 0.021 | 0.983 |
| adjR2 = 0.33 | Temperature | 0.447 | 0.097 | 4.613 | <0.001 |
| p-value < 0.001 | SWC | 0.279 | 0.096 | 2.903 | 0.005 |
| Soil CH4 | (Intercept) | −0.142 | 0.090 | −1.580 | 0.118 |
| adjR2 = 0.92 | Temperature | −0.666 | 0.085 | −7.822 | <0.001 |
| p-value < 0.001 | SWC | 0.761 | 0.076 | 9.966 | <0.001 |
| Temp*SWC | 0.427 | 0.065 | 6.540 | <0.001 | |
| UpperStem N2O | (Intercept) | 0.003 | 0.114 | 0.026 | 0.980 |
| adjR2 = 0.10 | Temperature | 0.240 | 0.126 | 1.899 | 0.061 |
| p-value = 0.032 | SWC | 0.260 | 0.136 | 1.923 | 0.058 |
| SF | −0.404 | 0.143 | −2.816 | 0.006 | |
| LowerStem N2O | |||||
| p-value = n.s. | |||||
| Soil N2O | (Intercept) | −0.006 | 0.131 | −0.045 | 0.964 |
| adjR2 = 0.22 | Temperature | −0.382 | 0.129 | −2.960 | 0.004 |
| p-value = 0.001 |
Among locations, CO2 emissions were positively correlated, with stronger relationships between UpperStem and LowerStem, intermediate relationships between LowerStem and Soil and the least correlation between UpperStem and Soil (Fig. 5). CH4 fluxes were well correlated among locations, with a positive relationship between UpperStem and LowerStem and negative relationship between stem and soil emissions. We only found a positive correlation between LowerStem and Soil N2O fluxes (Fig. 5).
Figure 5.
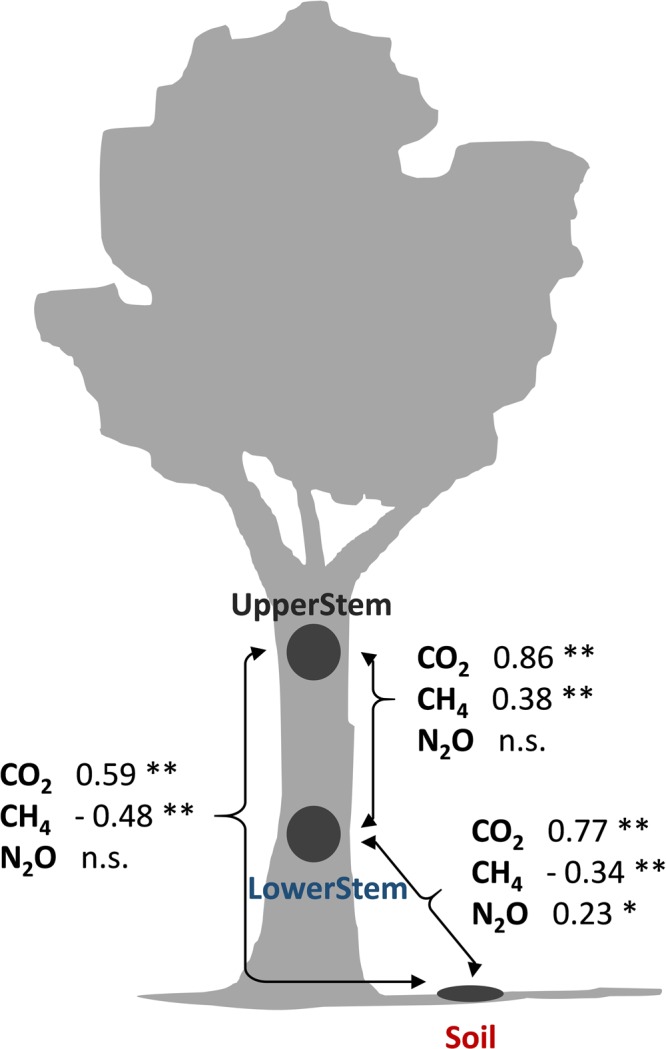
Correlation between daily-mean CO2, CH4 and N2O fluxes between different locations within the tree (Pearson correlation; **p-value < 0.001, *p-value < 0.05).
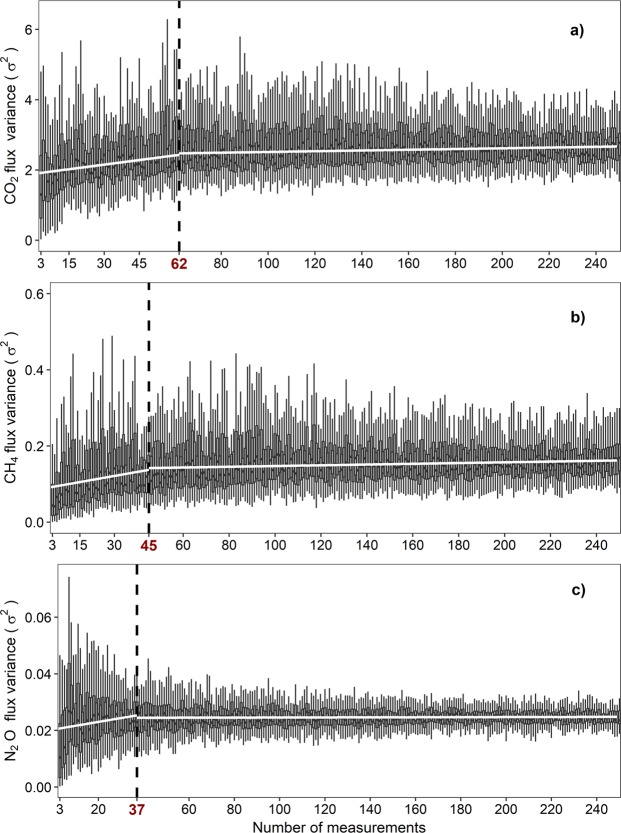
Our results show that that stem GHG fluxes in a tree from an upland forest are highly variable in time (Figs 1, 2). That said, an open question is to know how many measurements are needed for estimating seasonal stem GHG emissions. Our analysis based on random resampling of high-frequency measurements from the total pool of 5000 measurements for each GHG showed that the minimum number of punctual measurements required for estimating seasonal stem GHG emissions (based on variance stabilization) was 62, 45 and 37 for CO2, CH4 and N2O, respectively (Fig. 6). In other words, these are the minimum number of measurements necessary to explain the same amount of variance as using the whole population of measurements.
Figure 6.
Minimum number of measurements for estimating the whole period stem fluxes, represented by the breakpoint between two variance regression trends (white solid lines). Median, quartiles and data range of CO2, CH4 and N2O (panels (a–c), respectively) are represented in box-and-whisker plots as a function of sample size. σ is expressed in µmol m−2 s−1 for CO2 and in nmol m−2 s−1 for CH4 and N2O.
Discussion
Our results show that tree stems and soils were active surfaces for exchange for GHGs with the atmosphere at multiple time scales. Stems were net sources of CO2 and mean efflux values (3.80 ± 0.18 µmol m−2 s−1) where similar41,42 or higher12,21,43 than those reported in previous studies from upland forests. The soil was also a net source of CO2 with a mean flux of 8.24 ± 0.55 µmol m−2 s−1, consistent with the growing season average derived from autochambers at the study site (8.54 µmol m−2 s−1)44, but higher than the efflux reported in 99% of studies from temperate ecosystems worldwide45, indicating that the study site is a hot spot for soil respiration. Soils were net CH4 sinks (−0.66 ± 0.06 nmol m−2 s−1), with values half of those reported for other temperate forests12,16,21. However, despite this soil net uptake, stems were a net source of CH4 (mean emissions from both heights was 0.37 ± 0.18 nmol m−2 s−1). These CH4 stem emissions were within the range reported in nearby temperate forests12,17, but the literature reports a wide range, from close to zero emissions20,23 to 6-fold in our findings19. On average, stems were a N2O sink during the study period (−0.016 ± 0.008 nmol m−2 s−1) and soils were almost three times a stronger sink (−0.045 ± 0.011 nmol m−2 s−1). There is limited information to compare stem N2O fluxes, but a few studies using manual measurements report that stems could act as a N2O source15,20,22,32,39 or sink30. Our results revealed that there is a large temporal variability in stem N2O emissions, so manual measurements could lead to biased daily and seasonal estimates due to the limited sampling rate as has seen in soils40 or for other gases33. Soils were net sinks of N2O, and despite they are commonly considered as net sources in upland forests, there are numerous reports of soil N2O uptake in forests ecosystems28. Our results clearly suggest that tree stem emissions (and not only soils) could play a crucial role for the ecosystem-scale (e.g., a temperate forest) GHG balance.
Stem emissions showed temporal patterns from daily- (within days) (Fig. 3) to seasonal-scales (Fig. 1) for the three GHGs. At daily-scales, emissions showed high variability (Fig. 2, SFig. 1), especially for CH4 and N2O. We did not find temporal clusters in flux variability throughout our study (e.g., dry periods could have lower emissions variability than wet periods), but longer studies of high-frequency measurements might be useful for detecting potential temporal changes in emissions variability. We found temporal correlation at the 1-day time-period for specific days between GHGs and temperature or SF (more frequent for CO2 or N2O than for CH4 fluxes). Several studies have also shown that diurnal patterns of stem CO2 emissions are correlated with temperature or SF41,46. Stem CH4 fluxes showed a temporal correlation at the 1-day time period with temperature and SF over 10 and 14% of the studied period, respectively. Our study demonstrates temporal CH4 emissions were associated with SF at diurnal (Fig. 3) and seasonal scales (Fig. 4, Table 2). One study reporting three days of CH4 measurements from tree stems using autochambers found one tree stem (Liriodendron tulipifera) with daily cycles, but no daily cycles were observed in a second tree stem (Fagus grandifolia)17. This lack of consistency between individual trees suggest that more information derived from automated measurements is needed to clarify patterns among tree species and individuals. Our results show that stem CH4 emissions can present daily cycles but these are not consistent throughout the growing season. In fact, persistent daily cycles may be the exception rather than the norm for CH4 (and N2O) emissions from stems in upland forests. To our knowledge, there are no published high-frequency measurements of tree stem N2O fluxes. Our results show that over 25% of the studied period, stem N2O uptake showed a temporal correlation with temperature and SF at the 1-day time period (Fig. 3). Even if GHGs production had a clear and consistent diurnal patterns, the low radial gas diffusivity in stems47,48 would smooth the potential daily pattern of the emissions at the stem-surface level. This would be consistent with the lack of daily cycles observed for most of the studied period.
Seasonal patterns of stem GHGs were evident for CO2 and CH4 (Fig. 1). Both stem heights and soil showed similar CO2 seasonal patterns, explained by interactions between temperature, SWC and SF (Fig. 4, Table 2). High SWC under warm temperatures could enhance soil activity (both autotrophic and heterotrophic components) resulting in high soil respiration49–51, promoting sap flux and increasing stem respiration41,52. Seasonal CH4 patterns were also similar for both stem heights, with temperature, SF and SWC as controlling drivers. Seasonal stem CH4 emissions with temperature dependency were also found in another temperate forest19, but not detected in other upland forests nearby our study site12,16.
We have demonstrated that automated chamber-based measurements are a powerful tool to properly estimate seasonal or daily stem emissions and to study temporal correlations and drivers of soil-tree GHG fluxes. It is evident that multiple measurements per day are required for studying diurnal patterns of stem CH4 emissions33 given the high diurnal variability of the emissions (Fig. 2), but we also demonstrated that they could be crucial for studying seasonal dynamics and long-term mean magnitudes. Manual measurements of stem emissions cannot capture the high-variability of stem emissions at both diurnal and seasonal scales33, with implications for the estimation of temporal trends and GHG budgets. Using automated measurements, we calculated that the minimum number of random measurements required for properly estimating the whole period stem CH4 emissions was 45 measurements (Fig. 6). Multiple measurements per day could also better detect seasonal patterns than single manual measurements, integrating the large short-term heterogeneity of stem emissions as seen in soils53,54 or integrating diurnal patterns33,55. The ability to detect seasonal patterns in our dataset, but not in datasets with much less frequent sampling (e.g., once every 2–4 weeks)12,16,22, suggests that future studies may need to incorporate more frequent sampling in order to detect temporal patterns against a background of high variability in flux rates. The need for high-frequency measurements could be even more important for stem N2O emissions, since the magnitude of these emissions is low and the short-term variability is high (Fig. 2) (quickly shifting between positive and negative fluxes within hours; SFig. 1).
Our results support the general consensus that CO2 emitted by stems could be partially produced belowground9. First, emissions were higher in the soil and decreased with stem height, which is consistent with a belowground origin and subsequent stem degasification. Second, if CO2 is originated belowground, it should be partially dissolved into soil water and transported through the sap; consequently, we found correlations between stem emissions and SWC of SF at diurnal and seasonal scales. Third, stem and soil showed similar seasonal patterns with high correlation between soil and stem emissions, which suggest that both fluxes may depend on soil CO2 production.
There is no scientific consensus regarding the origin of CH4 emitted by the stems13, but our findings suggest a potential belowground origin. First, we found higher CH4 emissions in LowerStem than in UpperStem as observed in other studies17,21, which can be interpreted as stem degasification with height. Belowground processes could regulate the origin of CH4 emitted by stems regardless of soil being a sink of CH4. Soils can be net CH4 sinks at surface level but could produce CH4 at deeper depths21. We postulate that it is possible that tree roots might take up water from deep layers with dissolved CH4 produced in deep anoxic layers or anoxic microsites56,57 and bypass the surface methanotrophic layers in the soil7. Second, across certain days along the growing season we found temporal correlation at the 1-day period between CH4 fluxes and SF, which directly links stem emissions with stem water transport and thus, with water coming from belowground in the transpiration stream. As mentioned before, the lack of correlation at 1-day period for most of the growing season could be explained by the low radial stem diffusivity, but this limitation is likely less important at seasonal scale. Therefore, we found strong correlation between seasonal CH4 stem emissions and SF or SWC, indicating that belowground could be the origin of CH4 during the growing season. However, we also found a negative correlation between CH4 stem emissions and soil uptake. Stem emissions and soil uptake could be related by sharing belowground biochemical pathways between CH4 production and consumption across the soil profile. Under low soil moisture conditions, we would expect low transpiration and thus, less stem CH4 emissions, but also high soil diffusivity resulting in higher diffusion of atmospheric CH4 and O2 into the soil, and consequently more soil CH4 consumption. On the other hand, high SWC would enhance transpiration, resulting in higher sap flux and high stem emissions but it would also cause a reduction of soil diffusivity, then a reduction in soil oxygenation and CH4 diffusion from the atmosphere and consequently a reduction of CH4 soil uptake58,59.
Although our data support a possible soil origin of stem CH4 emissions, we recognize that there is an ongoing debate about underlying mechanisms13,33. Findings from other studies such as internal heartwood CH4 production18,60, high heartwood CH4 concentrations34,35, correlation between CH4 stem emissions and moisture CH4 concentration in heartwood19, or the presence of methanogenic archaea inhabiting the heartwood61 would suggest that emitted CH4 could also be produced in the stems. Observational studies measuring simultaneous high-frequency fluxes of stems and soils coupled with soil and heartwood CH4 concentrations, the analysis of heartwood microbial composition and isotopic experiments tracing the origin of the emitted CH4 would shed some light on this debate33.
N2O stem emissions were low and highly variable, making our results challenging to interpret as have been reported for ecosystem-scale N2O fluxes62. We found a significant correlation between soil and LowerStem N2O, but not with UpperStem, which could mean that soil influence is decreasing with stem height. Other studies have found a correlation between soil and stem emissions in upland forests under natural conditions20 or after soil fertilization32, suggesting that N2O emitted by stems could be originated belowground, but those studies reported emissions and not uptake. In our case, we speculate that stem and soil fluxes may not be directly related to stem water transport as for CH4 but indirectly, sharing drivers that potentially promote N2O consumption in stems and soils. This could be supported by the fact that temperature, SF and SWC explained part of the variability of both, soil and LowerStem N2O uptake. Stem N2O consumption has been described in the presence of cryptogamic cover30, but the apparent absence of this kind of covers in our tree makes this explanation unlikely. An alternative explanation for this N2O consumption could be heartwood decomposition. Net consumption of N2O and production of CH4 could be indicators of anaerobic wood decay as shown for deadwood63, which agrees with the observed CH4 emissions and N2O uptake in stems. Further understanding of these processes would require high-frequency CH4 and N2O emissions coupled with measurements of heartwood decay and presence of denitrifying and methanogenic bacteria. Finally, if sap has lower N2O concentrations than the atmosphere (which could be expected since soils are net sinks of N2O), diffusion of N2O from the atmosphere into the sapwood driven by this concentration gradient might result in a net N2O uptake. The positive correlation between LowerStem and Soil fluxes might be consistent with this explanation.
We highlight that CH4 and N2O emissions from trees are an emerging science frontier for plant physiology with implications for ecosystem processes, ecosystem management and atmospheric sciences. In upland forests, adequate characterization of temporal variability in CH4 and N2O emissions from stems and soils may be even more important than accounting for spatial variability20,33. The high temporal variability of stem GHG emissions highlight the need for measuring tree stem emissions with high temporal frequency and for longer periods in order to understand the multi-temporal dynamics and to better characterize the magnitude of these stem GHG fluxes. Information of temporal variability of stem GHG emissions obtained with automated measurements could therefore be useful for designing spatial experiments using manual measurements. Consequently, these and future studies will continue to provide insights about drivers and will aid to formulate and parameterize process-based ecosystem models that will include CH4 and N2O fluxes from tree stems.
Materials and Methods
Study site
We performed this study in a temperate forested area in the Mid Atlantic of the USA (St Jones Estuarine Reserve, a component of the Delaware National Estuarine Research Reserve [DNERR] [39°5′20′′N, 75°26′21′′W]). Mean annual temperature and precipitation were 13.3 °C and 1119 mm, respectively. Soils are Othello silt loam with a texture of 40% sand, 48% silt and 12% clay, and with 5.82%, 0.39% and 577.05 mg Kg−1 of total C, total N and total P, respectively. The forested site is dominated by bitternut hickory (Carya cordiformis), American holly (Ilex opaca (Ashe)), black gum (Nyssa sylvatica (Marshall)), eastern red cedar (Juniperus virginiana L.) and sweet gum (Liquidambar styraciflua L.). See previous study for more information related to the study area44.
Experimental setup
We continuously measured CO2, CH4 and N2O fluxes (1-hour resolution) from April to July 2017 (100 days) around a hickory tree (diameter at breast height [DBH] of 51 cm and 14 m height) at three different locations: (a) two stem heights represented as UpperStem (150 cm) and LowerStem (75 cm); and (b) one adjacent soil (1.5 m from the stem base). We installed two PVC collars (317.8 cm2) at the respective heights on the stem surface of the tree, and inserted a third collar 5 cm into the soil. Automated chambers (Li-COR 8100-104, Lincoln, Nebraska), controlled by a multiplexer (Li-COR 8150, Lincoln, Nebraska), were installed to measure fluxes at each collar. Since the automated chambers are designed to be placed horizontally to measure soil fluxes, we modified the stem-surface chambers to perform measurements in a vertical position. Automated measurements were taken with a closed-path infrared gas analyzer (Li-8100A, Lincoln, Nebraska) coupled to a cavity ring-down spectrometer (Picarro G2508, Santa Clara, California) as described in previous publications44,64. For each flux observation, gas concentrations were measured every second during 150 and 350 seconds for soil and stem chambers, respectively. Co-located with the chambers, we installed soil temperature and volumetric soil moisture (SWC) sensors at 10 cm depth into the soil and temperature sensors at 5 cm into the stem (EC-5, Decagon Devices, Pullman, WA).
We measured sap flow density (SF, mm s−1) every 15 min from April to July 2017 (at 150 cm height; 100 days), using constant heat dissipation sensors65 manufactured in our laboratory. Probe length was 1 cm to minimize the effect of high-radial gradient of sap flow density, which is common for species with ring-porous xylem anatomy such as hickory66. We inserted probe pairs into the xylem with a vertical separation of 12 cm and covered with reflective bubble wrap to minimize natural temperature gradients. Sap flow density was calculated using the original calibration65, considering zero flow conditions only during nights with low evaporative demand and stable sensor readings67. Meteorological and soil variables were recorded at 15-minutes intervals (measured every second during a 1-min period and subsequently averaged to 15-minutes) using a digital data logger (Em50, METER Group, Pullman, WA). The variables were SWC, soil temperature (5TE, METER Group, Pullman, WA), air relative humidity, air temperature, atmospheric pressure (VP-4 Sensor (Temp/RH/Barometer), METER Group, Pullman, WA) and wind speed and direction (DS-2, METER Group, Pullman, WA). There was a gap for GHG measurements between April 16th and 20th and for SF measurements between April 19th and 27th due to power failure.
Greenhouse gas fluxes
We calculated CO2, CH4 and N2O fluxes from the raw data collected by the Picarro G2508 using Soil Flux Pro Software (v4.0; Li-COR, Lincoln, Nebraska). For CO2 and CH4, both linear and exponential fits were adjusted to the measurements of concentrations of each gas and the fit with higher R2 was kept to calculate the fluxes44. Then, we applied a quality assurance/quality control (QA/QC) based on R2 values of measurements. When the R2 of the calculated CO2 efflux was lower than 0.9 (297 of 7265 measurements; 4% of the data) we considered that the physical conditions inside the chamber were not appropriate to calculate accurate fluxes (likely due to an improper chamber closure). Consequently, calculations were removed for CO2, CH4 and N2O fluxes for that particular time-stamp and replaced as not-a-number (i.e, NaN). When conditions inside the chamber were appropriate (R2 for CO2 > 0.9), we kept the fluxes for the three gases regardless of R2 of CH4 and N2O. For N2O measurements, we only calculated fluxes using a linear fit to avoid bias induced by applying an exponential fit at near-zero or negative fluxes68; we highlight that most N2O fluxes were close to zero.
Statistical analyses
For studying temporal correlations at diurnal time-scale (i.e., 1-day period) between stem GHG emissions and temperature or SF, we applied wavelet coherence analyses (WCA) for each GHG using hourly LowerStem emissions. We highlight that emissions from both LowerStem and UpperStem showed similar temporal patterns and consequently provide similar results in a time series analysis using the frequency domain. WCA measures transient signals or signals whose amplitude varies with time between two time series69,70. This technique has been applied for analyzing ecosystem-scale fluxes71,72, for soil respiration73,74, and for studying the relation between soil CO2 and SF fluxes75. We assessed the statistical significance of common power between the two time series (0.05 significance level) applying 10,000 Monte Carlo simulations of white noise time69. Finally, for each pair of variables we calculated the percent of days when WCA showed significant temporal correlations at the 1-day period. WCA was not applied with SWC since no diurnal pattern was expected for SWC75. Additionally, we calculated the daily coefficient of variation for each gas throughout the experiment.
We calculated linear correlations between CO2, CH4 and N2O fluxes and temperature, SWC and SF, controlling for leverage points and Cook’s distance. We used exponential and sigmoidal regressions for CO2 and temperature, and soil CH4 and temperature, respectively, to achieve better regression fit.
Additionally, we applied generalized least squares linear models (GLS) for studying seasonal relationship between stem emissions and stem temperature, SWC and SF. We fitted one model for each GHG using daily means for temperature and SWC, and midday measurements of SF (averaged between 11 and 13 h), as an indicator of maximum stem water transport rates within a day. We log-transformed CO2 efflux values in order to linearize its relation with temperature, and scaled all variables to improve the performance and interpretability of the output models76. We tested the variance inflation factor (VIF) to assess predictor collinearity between temperature, SWC and SF, but in all cases VIF was lower than two, indicating low collinearity77. Assessing for VIC also controls for potential confounding factors associated with the hierarchical controls of temperature and SWC on SF. Additionally, we included the correlation structure in the models in order to avoid temporal autocorrelation between measurements. For the model selection, we evaluated all possible models combining temperature, SWC, SF and their first order interactions or each GHG and location (i.e., UpperStem, LowerStem and Soil) in order to achieve the minimum adequate model according to corrected Akaike information criterion (AICc). When two models were not statistically different (p > 0.05 when performing a likelihood ratio tests between models differing less than two AICc units), we kept the more complex one in terms of variables and number of interactions, since our aim was to understand the relation between gas emissions and their potential drivers rather than create a predictive model. Summaries of all models differing less than two AICc units from the selected model are presented in S.Table 1. Once we had the minimum adequate models, we calculated the adjusted R2.
We calculated the minimum number of stem measurements required for estimating the whole-period average per each gas using a resampling routine with an increasing number of measurements (n) (from n = 3 to n = 250 measurements, with replacement)78. For each n, 40 replicates were obtained by randomly selecting n of our measurements. Then, we calculated the median of the variances of the replicates for each n and plotted this median against sample size, revealing a breakpoint in this relationship. This breakpoint indicates the minimum number of measurements (n) necessary to explain the same amount of variance as using the whole population of measurements. Breakpoints, trends and their statistical significance were estimated using a sequential Mann-Kendall analysis. For these analyses, stem emissions from UpperStem and LowerStem were pooled together for each gas.
Additionally, for each GHG flux we calculated linear correlations between tree locations in order to provide insights on potential sources of stem-gas emissions and/or potential transport paths. WCA were performed using MATLAB R2010b (The MathWorks Inc.) and all the other analyses were carried out using R 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria). GLSs models were performed using the R nlme package79, model selection and comparison was done with the R MuMIn package80, and breakpoint analysis of flux variance trends with sample size was carried out with the R greenbrown package81.
Supplementary information
Acknowledgements
The authors thank the Delaware National Estuarine Research Reserve [DNERR] and the personnel from the St Jones Reserve for support throughout this study. We thank M. Capooci, N. Kowalska, S. Villarreal and M. Guevara for assistance in this project. RV acknowledges support from the National Science Foundation (Grant #1652594). RP was supported by grant CGL2014-55883-JIN from the Spanish MINECO.
Author Contributions
J.B., R.P. and R.V. conceived and designed the experiment; J.B. performed the experiment; J.B., R.P. and R.V. analyzed the data; J.B. wrote the first draft and all authors edited the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-39663-8.
References
- 1.Dalal RC, Allen DE. TURNER REVIEW No. 18. Greenhouse gas fluxes from natural ecosystems. Aust. J. Bot. 2008;56:369. [Google Scholar]
- 2.Smith KA, et al. Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. Eur. J. Soil Sci. 2003;54:779–791. [Google Scholar]
- 3.Bouwman AF, Van der Hoek KW, Olivier JGJ. Uncertainties in the global source distribution of nitrous oxide. J. Geophys. Res. 1995;100:2785. [Google Scholar]
- 4.Anderson, B. et al. Methane and Nitrous Oxide Emissions from Natural Sources. Office of Atmospheric Programs, US EPA, EPA 430-R-10-001, (Washington DC, 2010).
- 5.Schlesinger WH, Andrews JA. Soil respiration and the global carbon cycle. Biogeochemistry. 2000;48:7–20. [Google Scholar]
- 6.Le Mer J, Roger P. Production, oxidation, emission and consumption of methane by soils: A review. Eur. J. Soil Biol. 2001;37:25–50. [Google Scholar]
- 7.Megonigal JP, Guenther AB. Methane emissions from upland forest soils and vegetation. Tree Physiol. 2008;28:491–498. doi: 10.1093/treephys/28.4.491. [DOI] [PubMed] [Google Scholar]
- 8.Pangala SR, et al. Large emissions from floodplain trees close the Amazon methane budget. Nature. 2017;552:230. doi: 10.1038/nature24639. [DOI] [PubMed] [Google Scholar]
- 9.Teskey RO, Saveyn A, Steppe K, McGuire MA. Origin, fate and significance of CO2 in tree stems. New Phytol. 2008;177:17–32. doi: 10.1111/j.1469-8137.2007.02286.x. [DOI] [PubMed] [Google Scholar]
- 10.Bruhn D, Møller IM, Mikkelsen TN, Ambus P. Terrestrial plant methane production and emission. Physiol. Plant. 2012;144:201–209. doi: 10.1111/j.1399-3054.2011.01551.x. [DOI] [PubMed] [Google Scholar]
- 11.Nisbet RER, et al. Emission of methane from plants. Proc. R. Soc. 2009;276:1347–1354. doi: 10.1098/rspb.2008.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warner DL, Villarreal S, McWilliams K, Inamdar S, Vargas R. Carbon dioxide and methane fluxes from tree stems, coarse woody debris, and soils in an upland temperate forest. Ecosystems. 2017;20:1205–1216. [Google Scholar]
- 13.Covey, K. R. & Megonigal, J. P. Methane Production and Emissions in Trees and Forests. New Phytol. in press (2018). [DOI] [PubMed]
- 14.Pangala SR, Moore S, Hornibrook ERC, Gauci V. Trees are major conduits for methane egress from tropical forested wetlands. New Phytol. 2013;197:524–531. doi: 10.1111/nph.12031. [DOI] [PubMed] [Google Scholar]
- 15.Rusch H, Rennenberg H. Black alder (Alnus Glutinosa (L.) Gaertn.) trees mediate methane and nitrous oxide emission from the soil to the atmosphere. Plant Soil. 1998;201:1–7. [Google Scholar]
- 16.Pitz SL, Megonigal JP, Chang C-H, Szlavecz K. Methane fluxes from tree stems and soils along a habitat gradient. Biogeochemistry. 2018;137:307–320. [Google Scholar]
- 17.Pitz S, Megonigal JP. Temperate forest methane sink diminished by tree emissions. New Phytol. 2017;214:1432–1439. doi: 10.1111/nph.14559. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z-P, et al. Methane emissions from the trunks of living trees on upland soils. New Phytol. 2016;211:429–439. doi: 10.1111/nph.13909. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z-P, et al. Methane production explained largely by water content in the heartwood of living trees in upland forests. J. Geophys. Res. Biogeosciences. 2017;122:2479–2489. [Google Scholar]
- 20.Machacova K, et al. Pinus sylvestris as a missing source of nitrous oxide and methane in boreal forest. Sci. Rep. 2016;6:23410. doi: 10.1038/srep23410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maier M, Machacova K, Lang F, Svobodova K, Urban O. Combining soil and tree-stem flux measurements and soil gas profiles to understand CH4 pathways in Fagus sylvatica forests. J. Plant Nutr. Soil Sci. 2018;181:31–35. [Google Scholar]
- 22.Welch, B., Gauci, V. & Sayer, E. J. Tree stem bases are sources of CH4 and N2O in a tropical forest on upland soil during the dry to wet season transition. Glob. Chang. Biol., 10.1111/gcb.14498 (2018). [DOI] [PubMed]
- 23.Plain, C., Ndiaye, F.-K., Bonnaud, P., Ranger, J. & Epron, D. Impact of vegetation on the methane budget of a temperate forest. New Phytol., 10.1111/nph.15452 (2018). [DOI] [PubMed]
- 24.Kirschke S, et al. Three decades of global methane sources and sinks. Nat. Geosci. 2013;6:813–823. [Google Scholar]
- 25.Schlesinger, W. H. & Bernhardt, E. S. Biogeochemistry: An Analysis of Global Change, 3rd Edition (Academic Press 2013).
- 26.Ussiri, D. & Lal, R. In Soil Emission of Nitrous Oxide and its Mitigation 1–28, 10.1007/978-94-007-5364-8_1 (Springer Netherlands, 2013).
- 27.Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S. Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2013;368:20130122. doi: 10.1098/rstb.2013.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapuis-Lardy L, Wrage N, Metay A, Chotte J-L, Bernoux M. Soils, a sink for N2O? A review. Glob. Chang. Biol. 2007;13:1–17. [Google Scholar]
- 29.Wen Y, et al. Disentangling gross N2O production and consumption in soil. Sci. Rep. 2016;6:36517. doi: 10.1038/srep36517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Machacova K, Maier M, Svobodova K, Lang F, Urban O. Cryptogamic stem covers may contribute to nitrous oxide consumption by mature beech trees. Sci. Rep. 2017;7:13243. doi: 10.1038/s41598-017-13781-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenhart K, et al. Nitrous oxide and methane emissions from cryptogamic covers. Glob. Chang. Biol. 2015;21:3889–3900. doi: 10.1111/gcb.12995. [DOI] [PubMed] [Google Scholar]
- 32.Díaz-Pinés E, et al. Nitrous oxide emissions from stems of ash (Fraxinus angustifolia Vahl) and European beech (Fagus sylvatica L.) Plant Soil. 2016;398:35–45. [Google Scholar]
- 33.Barba, J. et al. Methane emissions from tree stems: a new frontier in the global carbon cycle. New Phytol., 10.1111/nph.15582 (2018). [DOI] [PubMed]
- 34.Covey KR, Wood SA, Warren RJ, Lee X, Bradford MA. Elevated methane concentrations in trees of an upland forest. Geophys. Res. Lett. 2012;39:1–6. [Google Scholar]
- 35.Zeikus JG, Ward JC. Methane formation in living trees: a microbial origin. Science (80-.). 1974;184:1181–1183. doi: 10.1126/science.184.4142.1181. [DOI] [PubMed] [Google Scholar]
- 36.Lenhart K, et al. Evidence for methane production by saprotrophic fungi. Nat. Commun. 2012;3:1–8. doi: 10.1038/ncomms2049. [DOI] [PubMed] [Google Scholar]
- 37.Messenger D, McLeod AR, Fry SC. The role of ultraviolet radiation, photosensitizers, reactive oxygen species and ester groups in mechanisms of methane formation from pectin. Plant. Cell Environ. 2009;32:1–9. doi: 10.1111/j.1365-3040.2008.01892.x. [DOI] [PubMed] [Google Scholar]
- 38.McLeod AR, et al. Ultraviolet radiation drives methane emissions from terrestrial plant pectins. New Phytol. 2008;180:124–132. doi: 10.1111/j.1469-8137.2008.02571.x. [DOI] [PubMed] [Google Scholar]
- 39.Wen Y, Corre MD, Rachow C, Chen L, Veldkamp E. Nitrous oxide emissions from stems of alder, beech and spruce in a temperate forest. Plant Soil. 2017;420:423–434. [Google Scholar]
- 40.Vargas R, Carbone MS, Reichstein M, Baldocchi DD. Frontiers and challenges in soil respiration research: from measurements to model-data integration. Biogeochemistry. 2011;102:1–13. [Google Scholar]
- 41.Gansert D, Burgdorf M. Effects of xylem sap flow on carbon dioxide efflux from stems of birch (Betula pendula Roth) Flora - Morphol. Distrib. Funct. Ecol. Plants. 2005;200:444–455. [Google Scholar]
- 42.Wen-Jie W, et al. Stem Respiration of a Larch (Larix gmelini) Plantation in Northeast China. Acta Bot. Sin. 2003;45:1387–1397. [Google Scholar]
- 43.Cernusak LA, Hutley LB, Beringer J, Tapper NJ. Stem and leaf gas exchange and their responses to fire in a north Australian tropical savanna. Plant, Cell Environ. 2006;29:632–646. doi: 10.1111/j.1365-3040.2005.01442.x. [DOI] [PubMed] [Google Scholar]
- 44.Petrakis S, Barba J, Bond-Lamberty B, Vargas R. Using greenhouse gas fluxes to define soil functional types. Plant Soil. 2018;423:285–294. [Google Scholar]
- 45.Bond-Lamberty B, Thomson A. A global database of soil respiration data. Biogeosciences. 2010;7:1915–1926. [Google Scholar]
- 46.Hölttä T, Kolari P. Interpretation of stem CO2 efflux measurements. Tree Physiol. 2009;29:1447–1456. doi: 10.1093/treephys/tpp073. [DOI] [PubMed] [Google Scholar]
- 47.Steppe K, Saveyn A, McGuire MA, Lemeur R, Teskey RO. Resistance to radial CO2 diffusion contributes to between-tree variation in CO2 efflux of Populus deltoides stems. Funct. Plant Biol. 2007;34:785. doi: 10.1071/FP07077. [DOI] [PubMed] [Google Scholar]
- 48.Sorz J, Hietz P. Gas diffusion through wood: implications for oxygen supply. Trees. 2006;20:34–41. [Google Scholar]
- 49.Rey A, et al. Annual variation in soil respiration and its components in a coppice oak forest in Central Italy. Glob. Chang. Biol. 2002;8:851–866. [Google Scholar]
- 50.Barba J, Curiel Yuste J, Poyatos R, Janssens IA, Lloret F. Strong resilience of soil respiration components to drought-induced die-off resulting in forest secondary succession. Oecologia. 2016;182:27–41. doi: 10.1007/s00442-016-3567-8. [DOI] [PubMed] [Google Scholar]
- 51.Ruehr NK, Buchmann N. Soil respiration fluxes in a temperate mixed forest: seasonality and temperature sensitivities differ among microbial and root-rhizosphere respiration. Tree Physiol. 2010;30:165–176. doi: 10.1093/treephys/tpp106. [DOI] [PubMed] [Google Scholar]
- 52.Ceschia E, Damesin C, Lebaube S, Pontailler J-Y, Dufrene E. Spatial and seasonal variations in stem respiration of beech trees (Fagus sylvatica) Ann. For. Sci. 2002;59:801–812. [Google Scholar]
- 53.Vargas, R. et al. Hot-moments of soil CO2 efflux in a water-limited grassland. Soil Systems. 2, 47, 10.3390/soilsystems2030047 (2018).
- 54.Cueva A, Bullock SH, López-Reyes E, Vargas R. Potential bias of daily soil CO2 efflux estimates due to sampling time. Sci. Rep. 2017;7:11925. doi: 10.1038/s41598-017-11849-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keane JB, Ineson P. Technical note: Differences in the diurnal pattern of soil respiration under adjacent Miscanthus × giganteus and barley crops reveal potential flaws in accepted sampling strategies. Biogeosciences. 2017;14:1181–1187. [Google Scholar]
- 56.von Fischer JC, Hedin LO. Controls on soil methane fluxes: Tests of biophysical mechanisms using stable isotope tracers. Global Biogeochem. Cycles. 2007;21:1–9. [Google Scholar]
- 57.Brewer PE, Calderón F, Vigil M, von Fischer JC. Impacts of moisture, soil respiration, and agricultural practices on methanogenesis in upland soils as measured with stable isotope pool dilution. Soil Biol. Biochem. 2018;127:239–251. [Google Scholar]
- 58.Maier M, Paulus S, Nicolai C, Stutz K, Nauer P. Drivers of Plot-Scale Variability of CH4 Consumption in a Well-Aerated Pine Forest Soil. Forests. 2017;8:193. [Google Scholar]
- 59.von Fischer JC, Butters G, Duchateau PC, Thelwell RJ, Siller R. In situ measures of methanotroph activity in upland soils: A reaction-diffusion model and field observation of water stress. J. Geophys. Res. 2009;114:G01015. [Google Scholar]
- 60.Mukhin VA, Voronin PY. Methanogenic activity of woody plants. Russ. J. Plant Physiol. 2009;56:138–140. [Google Scholar]
- 61.Yip, D. Z., Veach, A. M., Yang, Z. K., Cregger, M. A. & Schadt, C. W. Methanogenic Archaea dominate mature heartwood habitats of Eastern Cottonwood (Populus deltoides). New Phytol., 10.1111/nph.15346 (2018). [DOI] [PubMed]
- 62.Mammarella I, et al. A case study of eddy covariance flux of N2O measured within forest ecosystems: quality control and flux error analysis. Biogeosciences. 2010;7:427–440. [Google Scholar]
- 63.Covey KR, et al. Greenhouse trace gases in deadwood. Biogeochemistry. 2016;130:215–226. [Google Scholar]
- 64.Petrakis S, Seyfferth A, Kan J, Inamdar S, Vargas R. Influence of experimental extreme water pulses on greenhouse gas emissions from soils. Biogeochemistry. 2017;133:147–164. [Google Scholar]
- 65.Granier A. Une nouvelle methode pour la measure du flux de seve brute dans le tronc des arbres. Ann. des Sci. For. 1985;42(2):193–200. [Google Scholar]
- 66.Clearwater MJ, Meinzer FC, Andrade JL, Goldstein G, Holbrook NM. Potential errors in measurement of nonuniform sap flow using heat dissipation probes. Tree Physiol. 1999;19:681–687. doi: 10.1093/treephys/19.10.681. [DOI] [PubMed] [Google Scholar]
- 67.Poyatos R, Aguadé D, Galiano L, Mencuccini M, Martínez-Vilalta J. Drought-induced defoliation and long periods of near-zero gas exchange play a key role in accentuating metabolic decline of Scots pine. New Phytol. 2013;200:388–401. doi: 10.1111/nph.12278. [DOI] [PubMed] [Google Scholar]
- 68.Barba J, et al. Comparing ecosystem and soil respiration: Review and key challenges of tower-based and soil measurements. Agric. For. Meteorol. 2018;249:434–443. [Google Scholar]
- 69.Torrence C, Compo GP. A Practical Guide to WaveletAnalysis. Bull. Am. Meteorol. Soc. 1998;79:61–78. [Google Scholar]
- 70.Grinsted A, Moore JC, Jevrejeva S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Processes in Geophysics. 2004;11:561–566. [Google Scholar]
- 71.Vargas R, et al. Precipitation variability and fire influence the temporal dynamics of soil CO2 efflux in an arid grassland. Glob. Chang. Biol. 2012;18:1401–1411. [Google Scholar]
- 72.Ding R, Kang S, Vargas R, Zhang Y, Hao X. Multiscale spectral analysis of temporal variability in evapotranspiration over irrigated cropland in an arid region. Agric. Water Manag. 2013;130:79–89. [Google Scholar]
- 73.Vargas R, et al. On the multi-temporal correlation between photosynthesis and soil CO(2) efflux: reconciling lags and observations. New Phytol. 2011;191:1006–1017. doi: 10.1111/j.1469-8137.2011.03771.x. [DOI] [PubMed] [Google Scholar]
- 74.Vargas R, Detto M, Baldocchi DD, Allen MF. Multiscale analysis of temporal variability of soil CO2 production as influenced by weather and vegetation. Glob. Chang. Biol. 2010;16:1589–1605. [Google Scholar]
- 75.Barba J, Lloret F, Poyatos R, Molowny-Horas R, Yuste JC. Multi-temporal influence of vegetation on soil respiration in a drought-affected forest. iForest. 2018;11:189–198. [Google Scholar]
- 76.Gelman, A. & Hill, J. Data analysis using regression and multilevel/hierarchical models. (Cambridge University Press 2007).
- 77.Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010;1:3–14. [Google Scholar]
- 78.Barba J, Curiel Yuste J, Martínez-Vilalta J, Lloret F. Drought-induced tree species replacement is reflected in the spatial variability of soil respiration in a mixed Mediterranean forest. For. Ecol. Manage. 2013;306:79–87. [Google Scholar]
- 79.Pinheiro, J., Bates, D., DebRoy, S. & Sarkar, D. nlme: Linear and Nonlinear Mixed Effects Models. R package (2013).
- 80.Barton K. MuMIn: Multi-model inference. R package version. 2014;3:1–96. [Google Scholar]
- 81.Forkel M, et al. Trend Change Detection in NDVI Time Series: Effects of Inter-Annual Variability and Methodology. Remote Sens. 2013;5:2113–2144. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.