Abstract
Gut bacteria influence the development of different pathologies caused by bacteria, fungi and parasitoids in insects. Wax moth larvae became more susceptible to fungal infections after envenomation by the ectoparasitoid Habrobracon hebetor. In addition, spontaneous bacterioses occurred more often in envenomated larvae. We analyzed alterations in the midgut microbiota and immunity of the wax moth in response to H. hebetor envenomation and topical fungal infection (Beauveria bassiana) alone or in combination using 16S rRNA sequencing, an analysis of cultivable bacteria and a qPCR analysis of immunity- and stress-related genes. Envenomation led to a predominance shift from enterococci to enterobacteria, an increase in CFUs and the upregulation of AMPs in wax moth midguts. Furthermore, mycosis nonsignificantly increased the abundance of enterobacteria and the expression of AMPs in the midgut. Combined treatment led to a significant increase in the abundance of Serratia and a greater upregulation of gloverin. The oral administration of predominant bacteria (Enterococcus faecalis, Enterobacter sp. and Serratia marcescens) to wax moth larvae synergistically increased fungal susceptibility. Thus, the activation of midgut immunity might prevent the bacterial decomposition of envenomated larvae, thus permitting the development of fungal infections. Moreover, changes in the midgut bacterial community may promote fungal killing.
Introduction
Relationships between parasites and hosts can be mediated by endosymbiotic microorganisms from both the parasite and host sides. Especially, endosymbionts may protect hosts against parasitic invaders, whereas parasites are able to manipulate the hosts microbiota directly or through an alteration of host’s immunity1. In insects, the gut microbiota plays a substantial role in digestion, regulation of the immune system, detoxification of allelochemicals, protection against pathogens, stress responses, and other vital functions2–4. Changes in the structure of the gut microbial community may lead to alterations in the susceptibility to pathogenic microorganisms and to substantial modifications in the development of diseases5–8. Some opportunistic pathogens in the gut can demonstrate pathogenic properties under specific conditions, such as the action of abiotic and biotic stress factors, including different infections5,7–9. The influence of the gut microbiota on the development of pathogenesis was extensively studied for bacterioses caused by Bacillus thuringiensis5–7,9–12; however, the impact of the gut microbiota on the progress of these infections is still debated.
The impact of gut bacteria in the development of mycoses is less studied1. It is well known that gut bacteria can inhibit fungal growth and sporulation13,14. Therefore, germ-free insects can be more susceptible to entomopathogenic ascomycetes after the oral administration of the fungi15. Similarly, the oral secretions of bark beetles contain bacterial complexes that inhibit the growth of opportunistic fungi in galleries16. Kabaluk and coauthors17 showed that bacteria from the genera Pantoea, Pandoraea, Nocardia and Mycobacterium impeded the development of infection by the fungus Metarhizium brunneum in field populations of wireworms. Aphid endosymbionts Spiroplasma and Ricketsia may protect against entomophtoralean fungus Pandora neoahidis18. On the other hand, some authors demonstrated synergy between gut bacteria and fungi. In particular, Wei and coworkers8 showed that topical infection with Beauveria bassiana reduced the taxonomical diversity of the mosquito gut microbiota by decreasing its due to the mass proliferation of Serratia marcescens, which in turn increases susceptibility to the fungus. An additive effect between species of the gut bacteria Pseudomonas and entomopathogenic ascomycetes was demonstrated in locusts19. Moreover, in many cases, fungal infections lead to the bacterial decomposition of the killed insects. These phenomena are usually stipulated by exposure of insects to high conidia doses or changes in production of fungal toxins1,20.
Studies on the relationship between parasitoids and the host insect microbiota have been performed mainly in host-parasite systems involving endoparasitoids and aphids. In most cases, these investigations concentrate on the beneficial effects of endosymbionts21. It was shown that an aphid facultative symbiont, namely, the enterobacteria Hamiltonella defensa, protects aphids against the endoparasitoid Aphidius ervi, leading to the death of the endoparasitoid larvae inside the aphid body22–24. Similarly, the enterobacteria Regiella insecticola provides aphids with protection against the endoparasitic wasps Aphidius colemani and A. ervi25,26. In contrast, a positive correlation between the frequency of infection by bacterial symbionts and parasite pressure was shown for a system including the enterobacteria Arsenophonus, the endoparasitoid Psyllaephagus bliteus and the host Glycaspis brimblecombei27. Relationships between host symbiotic bacteria and ectoparasitoids, as well as the influence of the parasitoids on the structure of the host microbiota have not been studied.
It is important to notice that the way how parasitic organisms affect host’s immunity and gut microbiota was mainly studied on models including one host and one parasite. Influence of co-infections on the physiological parameters was less studied. However, in real conditions insects are exposed to a complex of parasites, commensals, mutualists that lead to complicated interrelation between them and are mediated by host immune reaction8,28,29. Triple host-parasite system, consisting of parasitoid Habrobracon hebetor (Hymenoptera: Braconidae), entomopathogenic ascomycete (e.g., Beauveria) and wax moth Galleria mellonella exhibits one of the intriguing system for parasitological study.
Habrobracon hebetor is a gregarious ectoparasitoid having a wide host range which includes lepidopterans from different families30. The parasitoid females paralyze host larvae through envenomation. The paralysis of hosts is caused by the disruption of glutamatergic receptors on presynaptic membranes31. In addition, envenomation leads to a dramatic decrease in both cellular and humoral immunity in hosts, specifically in the wax moth32,33. Importantly, H. hebetor females paralyze many host individuals, and parasitoid larvae do not completely consume their hosts. Envenomated insects do not survive; they are eventually colonized with different microorganisms, primarily bacteria. However, if envenomated larvae were topically inoculated with the entomopathogenic ascomycetes Beauveria, Isaria, or Metarhizium (even at extremely low doses) they were successfully colonized by the fungi34,35. Interestingly, envenomated wax moth larvae were thousands of folds more susceptible to fungal infection compared to nonenvenomated larvae34, and the susceptibility was increased to pathogenic but not opportunistic fungi36. Importantly, arrest of gut peristalsis occurred under the influence of the venom. We hypothesized that envenomation and the absence of gut peristalsis led to the disruption of the microbiota, changes in the gut immune responses, and the proliferation of some endosymbiont bacteria, while topical fungal infection can prevent the development of bacteria. Additionally, we suggested that changes in the predominant bacterial taxonomic groups could be one of the factors leading to the increase in the susceptibility of wax moth to entomopathogenic fungi.
It is known that changes in the bacterial community are correlated with the immune response in the midgut, e.g.8,11,37,38. The humoral defenses of the wax moth include at least 18 antimicrobial peptides, especially gloverin, gallerimycin and galiomycin, which exhibit antibacterial and/or antifungal activity39,40. The inducible metalloproteinase inhibitor (IMPI) inhibits the activity of metalloproteinases secreted by many pathogenic organisms as virulence factors, thus controlling the degradation of immune-relevant polypeptides in the infected host41,42. The heat shock protein 90 (Hsp90), caspase protein (CASP), glutathione peroxidase (GSH PX) and macrophage migration inhibitory factor (MIF) genes of Galleria mellonella contain conservative domains that are evolutionarily ancient and are found in both vertebrates and invertebrates43–48. These proteins play regulatory and chaperone roles in innate immune, metamorphosis-related and ROS management processes45–48. The expression of these genes may be induced during cell membrane rupture in response to destructive processes, such as the proteinase-mediated lysis caused by fungal and bacterial infections in insects45,46,49. Thus, assays of the expression of these genes could highlight the processes occurring in the wax moth midgut in response to envenomation and fungal infection.
In the present work, we studied changes in the microbiota, humoral immunity and stress response of the wax moth midgut in response to H. hebetor envenomation and B. bassiana-induced mycosis alone and in combination. In addition, we estimated the impact of the predominant cultivable bacteria in the efficacy of B. bassiana infection.
Results
Bioassay
The topical infection of nonenvenomated larvae with B. bassiana led to 12% mortality due to the mycosis over 12 days (Fig. 1), which significantly differed from that of intact (control) larvae (χ2 = 6.4, P = 0.01). Treatment of envenomated larvae with B. bassiana led to 90% mortality due to the fungus (χ2 = 60.1, P < 0.0001, compared to infected nonenvenomated larvae). The development of spontaneous bacterioses was higher in envenomated but noninfected larvae (30%) than in larvae subjected to other treatments (χ2 > 6.3, P < 0.01). Fungal infection caused a significant decrease in the incidence of bacteriosis among envenomated larvae, to 10% (χ2 = 6.25, P = 0.01).
Figure 1.
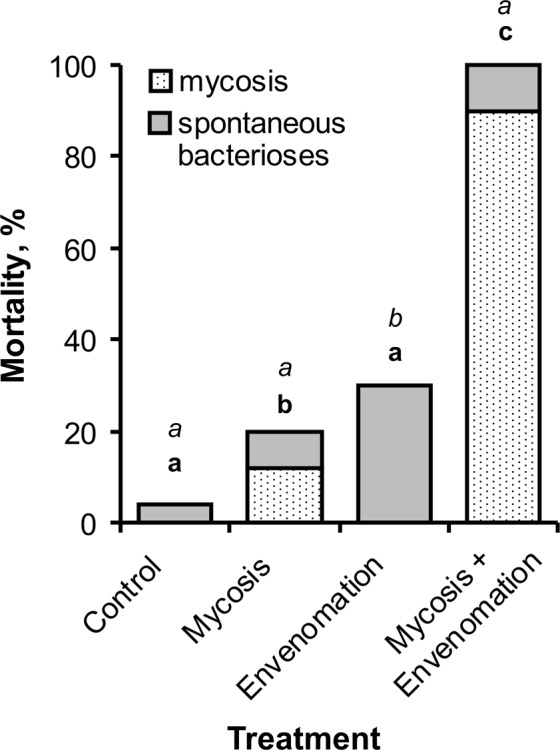
Mortality rate of wax moth larvae for 12 days post envenomation by Habrobracon hebetor or infection with Beauveria bassiana (106 conidia/ml) alone or in combination. Fifty larvae were used for each treatment. Identical italicized letters indicate nonsignificant differences in the bacteriosis incidence between treatments, and identical bold letters indicate nonsignificant differences in death due to B. bassiana infection between treatments (χ2 > 2.3; P > 0.08).
Changes in midgut bacterial communities
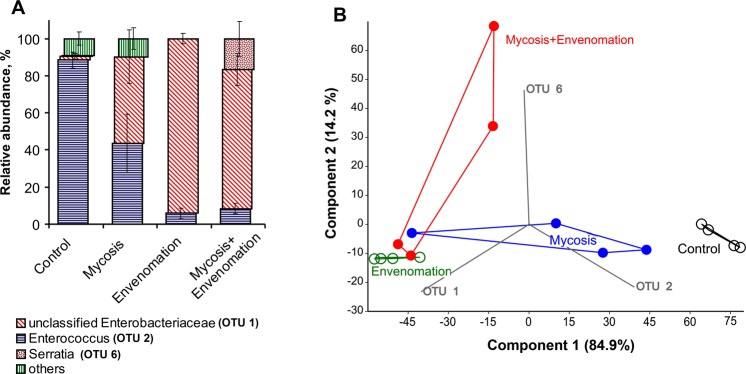
Classification based on 16S rRNA gene sequencing revealed 176 operational taxonomical units OTUs grouped in 117 taxa at the genus level, 72 taxa at the family level and 11 taxa at the phylum level (see Appendix A). Over all treatment groups, the main phyla were Proteobacteria (62.9%), with a predominance of Enterobacteriaceae, and Firmicutes (35.7%), with a prevalence of Enterococcaceae. The envenomation of the wax moth by the parasitoid dramatically decreased the diversity of the bacterial community (Table 1). In particular, a large decrease in the number of OTUs, genera and families (H1,15 > 11.3, P < 0.001), along with a decreasing tendency in the Shannon index (H1,15 = 2.8, P = 0.09) were noted in response to the venom. The effects of the fungus on the taxonomic diversity were not significant (H1,15 < 0.3, P > 0.6). However, an increasing tendency in the Shannon index was observed in response to mycosis (H1,15 = 3.2, P = 0.07) due to the leveling of the abundances between the predominant groups of bacteria (see below).
Table 1.
Diversity characteristics of the microbiota in wax moth midguts at 72 hours post envenomation by Habrobracon hebetor or infection with Beauveria bassiana alone or in combination.
| OTUs | Genera | Families | Shannon Index* | |
|---|---|---|---|---|
| Control | 54 ± 2.9a | 43 ± 2.4a | 31 ± 1.4a | 0.9 ± 0.32ab |
| Mycosis | 45 ± 4.6ac | 37 ± 3.9ac | 28 ± 2.9a | 1.4 ± 0.44a |
| Envenomation | 13 ± 4.6b | 11 ± 3.7b | 10 ± 2.8b | 0.3 ± 0.11b |
| Mycosis + Envenomation | 15 ± 2.3bc | 14 ± 1.9bc | 11 ± 1.0b | 0.9 ± 0.21ab |
*The Shannon index was calculated for the genus level.
The standard error (±SE) was calculated for four replicates. Identical letters indicate nonsignificant differences among treatments (Dunn’s post hoc test: P > 0.05).
Three OTUs dominated in the investigated samples: OTU 1 – unclassified Enterobacteriaceae, OTU 2 − Enterococcus and OTU 6 – Serratia (Fig. 2A). Control (untreated) larvae were characterized by the prevalence of Enterococcus (88 ± 4.7%) and the low relative abundance of enterobacteria (<8.2%) in the midgut (Fig. 2A). Envenomation led to significant alterations in the microbiome structure caused by an increase in the proportion of Enterobacteriaceae and a decrease in the proportion of Enterococcus (H1,15 = 7.45, P = 0.006). A trend of enhancement in the abundance of enterobacteria was noted after treatment with the fungus alone, but the difference were not significant relative to that of the control group (Dunn’s test, P = 0.075). However, an increase in the Serratia abundance was observed during acute mycosis development in envenomated larvae (Dunn’s test, P < 0.04, compared to other treatments). The principal component analysis of the microbial communities showed that the first component explained 84.9% of the variation in the data, which was due to changes in the relationship between enterobacteria (OTU 1) and enterococci (OTU 2) (Fig. 2B). Clear clustering is observed between the envenomated and nonenvenomated (control) larvae. Samples from infected larvae occupy intermediate locations in the plot. The second principal component explained only 14.2% the variation, which was caused by an increase in Otu 6 (Serratia) in envenomated and infected larvae. Notably, a large reduction in the relative abundance of the other taxa was observed in envenomated larvae (Fig. 2A, Appendix A). We hypothesized that this phenomenon was caused by the mass proliferation of enterobacteria; therefore, we estimated the CFUs of the predominant midgut bacteria using selective media.
Figure 2.
Alteration of the midgut microbiota composition in wax moth larvae at 72 hours post envenomation by Habrobracon hebetor or infection with Beauveria bassiana alone or in combination. (A) Histogram showing changes at the genus level. Vertical bars show the standard error (±SE) calculated from four biological replicates. (B) Principal component analysis of microbial communities for the OTU level.
Abundance, identification and antagonistic properties of cultivable bacteria
The plating of midguts on selective media showed an increase in the CFUs of all dominant bacteria (Enterococcus, Enterobacter and Serratia) in response to envenomation (Fig. 3). Importantly, the CFU count of Enterococcus was enhanced 10- to 12-fold, but the CFU count of Enterobacter was enhanced 42- to 45-fold in envenomated (or both infected and envenomated) insects compared to the CFU counts of those bacteria in larvae that were untreated or only infected with the fungus (envenomation effects: H1,27 = 8.1, P = 0.004 for Enterococcus and H1,35 = 19.9, P < 0.0001 for Enterobacter). Fungal infection had no significant effects on the CFUs of Enterococcus and Enterobacter (H < 1.6, P > 0.26); however, it did impact the Serratia abundance. Specifically, the development of mycosis led to an increase in the Serratia CFU count in envenomated and nonenvenomated larvae (H1,11 = 5.8, P = 0.016).
Figure 3.
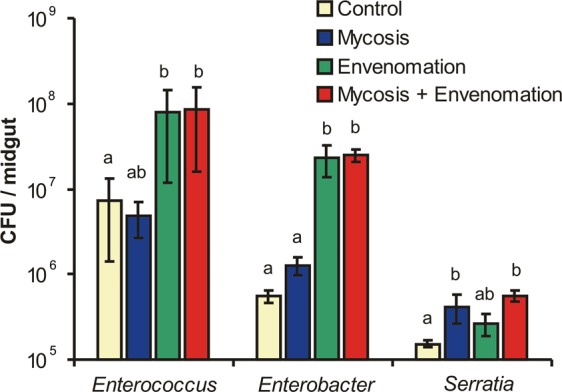
Changes in the CFU counts of cultivable bacteria from the midgut of wax moth larvae at 72 hours post envenomation by Habrobracon hebetor or infection with Beauveria bassiana alone or in combination. Selective media for Enterococcus (modified bile esculin azide agar), Enterobacteriaceae (Endo agar) and Serratia (Serratia differential medium) were used. Vertical bars show the standard error (±SE) calculated from seven replicates (Enterococcus), nine replicates (Enterobacter) or three replicates (Serratia). Identical letters indicate nonsignificant differences among treatments (Dunn’s post hoc test: P > 0.05).
Sequencing of the 16S rRNA region showed that the sequences of seven isolates of Enterococcus were identical to each other and were characterized by 100% sequence identity to the majority of E. faecalis strains, especially the type strain of JSM5803T (Appendix B, Fig. S1). Furthermore, five isolates of Enterobacter also had a molecular haplotype (Appendix B, Fig. S2) identical to that of strain M. pstv. 10.4 (KM108494) E. hormaechei (E. cloacae complex), which was isolated from water containing Aedes aegypti larvae50. The sequences of the investigated isolates differed by one single nucleotide position (SNP) from that of E. hormaechei 69–907 R (KX828280), which was isolated from the gut of the fish Oreochromis niloticus, and by one SNP from that of E. xiangfangensis strain 10–17 T (HF679035), which was isolated from Chinese traditional sourdough51. Meanwhile, the sequence of the type strain of E. hormaechei CIP 103441 (AJ508302) differed from those of our isolates by four SNPs. Thus, due to the ambiguous phylogenetic placement of our Enterobacter isolates and the sequence similarity to both species, we identified the isolates as Enterobacter sp. The sequence was deposited in GenBank under the accession number MH685399. The sequences of two Serratia isolates were identical to each other (Appendix B, Fig. S2) and differed by one SNP from that of the type strain S. marcescens subsp. marcescens DSM 30121 T (AJ233431), which was isolated from pond water52 and from the type strain of S. marcescens subsp. sakuensis KREDT (AB061685), which was isolated from waste water53. The sequence was deposited in GenBank under the accession number MH685393. The E. faecalis and S. marcescens sequences were 100% identical to those of OTU 2 (Enterococcus) and OTU 6 (Serratia), respectively. The sequences of Enterobacter sp. demonstrated 99.4% identity with that of OTU 1 (unclassified Enterobacteriaceae).
The analysis of antagonistic interactions between the investigated bacteria and B. bassiana showed that the fungus did not inhibit the growth of the bacteria. Similarly, Enterobacter sp. had no effect on fungal growth. E. faecalis and S. marcescens inhibited B. bassiana mycelial growth by 4 ± 0.4 mm and 8 ± 2.0 mm, respectively (Appendix B, Fig. S3).
Gene expression after envenomation and fungal infection
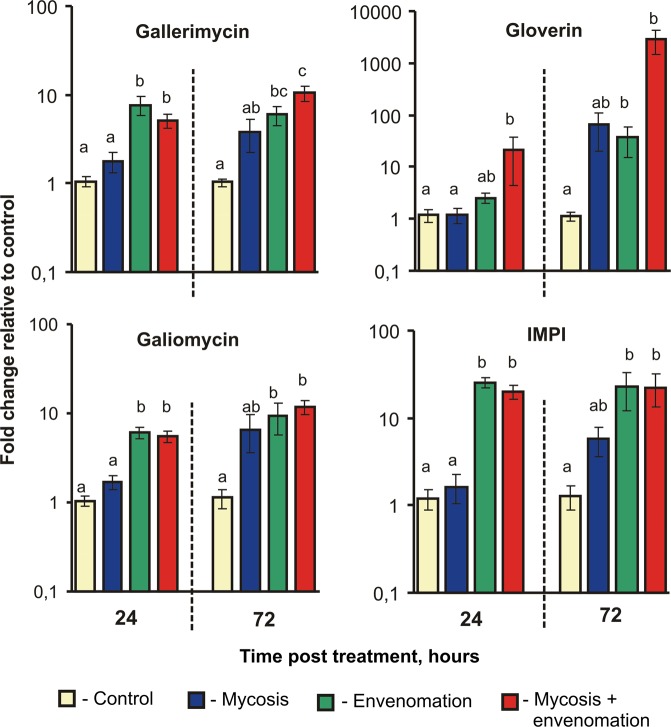
There were similar trends in the expression of AMPs and IMPI in the midguts in response to parasitoid envenomation and mycosis development (Fig. 4). On the first day post treatment, an upregulation in the expression of all investigated AMPs as well as the IMPI gene was observed in response to envenomation (H1,23 = 15.0, P = 0.0001 for galiomycin; H1,23 = 17.2, P < 0.0001 for gallerimycin; H1,23 = 8.3, P = 0.003 for gloverin; and H1,23 = 17.3, P < 0.0001 for IMPI gene expression). Fungal infection did not lead to significant changes in the expression of these genes on the first day (H1,23 < 0.26, P > 0.60). On the third day post treatment, we observed an increase in the expression of all AMPs and IMPI (H1,23 > 6.8, P < 0.01) in response to envenomation. Mycosis caused a significant increase in gloverin gene expression (H1,23 = 4.6, P = 0.03), along with similar effects on galiomycin and gallerimycin expression, at this time point, but the differences in the expression of these two genes were not significant (H1,23 = 3.0, P = 0.08 and H1,23 = 2.8, P = 0.09, respectively). A greater degree of upregulation was noted for gloverin than for the other genes in response to the combination of fungus and venom (2,900-fold compared to the control, Dunn’s test, P < 0.001).
Figure 4.
Relative expression of antimicrobial peptide genes (gallerimycin, gloverin, and galiomycin) and IMPI in response to Habrobracon hebetor envenomation and Beauveria bassiana infection, normalized to the expression of reference genes, at two time points – 24 and 72 hours. The results are expressed as the mean fold change in relative expression compared to that of the control at each time point. The bars show the standard error (±SE) calculated from six biological replicates. Identical letters indicate nonsignificant differences among treatments at each time point (Dunn’s post hoc test: P > 0.05).
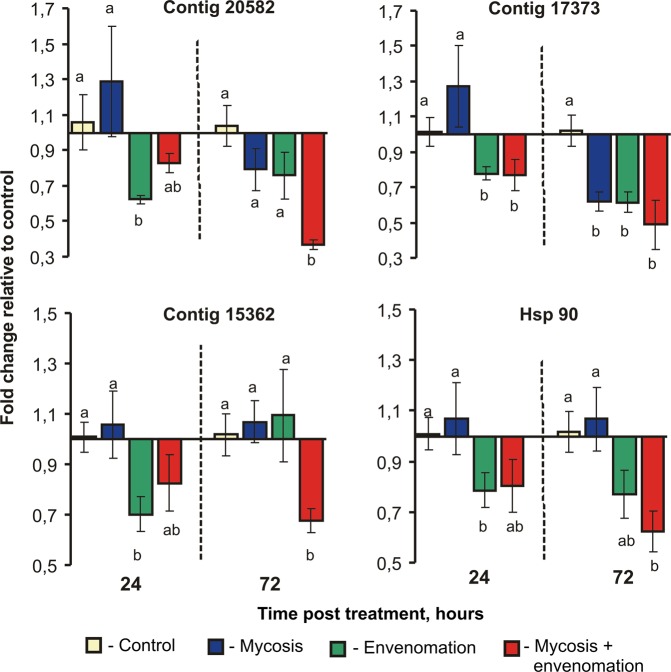
We observed small (no greater than 3-fold) changes in the expression of ROS-, inflammation- and stress-related genes in wax moth midguts; however, the effects of envenomation were significant (Fig. 5). At both time points, envenomation led to the downregulation of Contig 15362 – CASP (H1,23 > 3.9, P < 0.04), Contig 20582 – MIF (H1,23 > 6.5, P < 0.01), Contig 17373 – GSH PX (H1,23 > 3.5, P < 0.06) and Hsp 90 (H1,23 > 4.56, P < 0.03). Fungal infection led to the downregulation of MIF (H1,23 = 6.8, P = 0.009) and GSH PX (H1,23 = 4.2, P = 0.04) in envenomated and nonenvenomated larvae on the third day post treatment. A slight trend towards a decreased expression of the genes was observed on the third day of the experiment in response to the combined treatment, compared to envenomation or mycosis alone. However, significant factor interactions were not observed (H1,23 < 2.0, P > 0.16 for MIF, GSH PX and Hsp 90, and H1,23 < 3.5, P > 0.06 for CASP).
Figure 5.
Relative expression of ROS-, inflammation- and stress-related genes (Contig 15362 – CASP, Contig 20582 – MIF, Contig 17373 – GSH PX and HSP 90) in response to Habrobracon hebetor envenomation and Beauveria bassiana infection, normalized to the expression of reference genes, at two time points – 24 and 72 hours. The results are expressed as the mean fold change in relative expression compared to that of the control at each time point. The bars show the standard error (±SE) calculated from six biological replicates. Identical letters indicate nonsignificant differences among treatments at each time point (Dunn’s post hoc test: P > 0.05).
Bioassay of the cultivable bacteria and the fungus
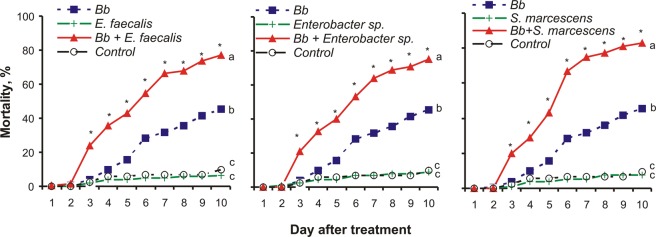
The oral administration of cultivable midgut bacteria (E. faecalis, Enterobacter sp. and S. marcescens) did not result in wax moth larvae mortality (log-rank test: χ2 < 0.7, P > 0.38, compared to the uninfected control). However, synergy between these bacteria and the fungus was observed after combination treatment (Fig. 6). The synergy was observed from 3 to 10 days after infection (χ2 > 25.3, P < 0.001). Though the effects in response to combined treatment with B. bassiana and tested bacteria were similar (a 30–38% increase in the total mortality and a 2-fold decrease in the survival time (ST75) after mixed infection compared to the mortality dynamics of fungal infection alone), the greatest synergy was observed between B. bassiana and S. marcescens (Appendix B, Table S1). All larvae that were treated with the combination of fungus and bacteria, were overgrown with B. bassiana if they died on or after day 3 post treatment.
Figure 6.
Dynamics of wax moth larvae mortality after the oral administration of midgut bacteria (108 cells/3 g of food) and topical infection with the fungus Beauveria bassiana (Bb) (4 × 106 conidia/ml). Data are pooled from three experiments. Total n = 73 larvae for control, 177 larvae for Bb, 140 larvae for E. faecalis, 137 larvae for Enterobacter sp., 135 larvae for S. marcescens, 155 larvae for Bb + E. faecalis, 172 larvae for Bb + Enterobacter sp. and 178 larvae for Bb + S. marcescens. Different letters show significant differences in mortality dynamics between treatments (log-rank test, χ2 > 55.1, P < 0.0001). *synergy between fungi and bacteria, calculated as described by Robertson & Preisler78 (Appendix B, Table S1).
Discussion
The performed study showed that the envenomation of wax moth larvae by the ectoparasitoid H. hebetor leads to the uncontrolled proliferation of midgut bacteria and to a significant predominance shift from gram-positive enterococci to gram-negative enterobacteria. However, activation of midgut immunity (AMPs and IMPI) occurred in envenomated insects. We propose that the activation of midgut immunity can prevent the bacterial decomposition of envenomated larvae for several days, thus permitting the development of fungi and/or parasitoid larvae. Moreover, changes in the midgut bacterial community lead to an increase in the susceptibility to the fungus B. bassiana after topical infection.
The lepidopteran larvae gut is characterized by the high pH, simple structure, and rapid transit time54. The continuous flow of the gut contents causes the regular renewal of the microbiome content and the cells of the peritrophic membrane. Thus, the paralysis of gut peristalsis should disrupt the bacterial balance, pH and absorption functions of the gut cells. Under normal conditions, the gut microbiome of G. mellonella laboratory lines consists mainly of gram-positive bacteria of the genus Enterococcus11,37,38, which agrees with the results of our study. The inhibition of peristalsis can induce competition among bacteria. The analysis of 16S rRNA showed a decrease in the taxonomical diversity and the replacement of Enterococcus by gram-negative Enterobacteriaceae in the midgut in response to parasitoid envenomation. Interestingly, this shift in the community structure from gram-positive to gram-negative bacteria has been observed during other toxicoses. In particular, the replacement of Enterococcus by Enterobacter in wax moth midguts during B. thuringiensis infection was demonstrated by Dubovskiy and coauthors11. The depletion of community diversity and the increase in the abundance of gram-negative bacteria (Stella sp.) in Lymantria dispar after B. thuringiensis infection was also reported by investigators55. Similar results were obtained in response to the topical infection of adult Anopheles stephensi mosquitoes with B. bassiana. In this case, mycosis led to a significant decrease in the bacterial diversity and a shift of the dominant bacteria from Firmicutes to Proteobacteria8. In particular, acute mycosis led to an increase in S. marcescens abundance, which promotes fungal infection in mosquitoes8. In the present work we also found an increase in Serratia abundance during the development of acute fungal infection in envenomated larvae.
In general, changes in the CFU count of the dominant bacteria are correlated with the 16S RNA data: the CFU count of Enterobacter sp. was increased more than that of E. faecalis in response to envenomation. Moreover, fungal infection led to an increase in S. marcescens CFUs. On the basis of these data, we suggest that of the decrease in the diversity of the wax moth microbiome in response to envenomation occurs due to the mass proliferation of enterobacteria. Interestingly, we did not find Enterococcus mundtii, though several researchers noted that E. mundtii is one of the predominant bacteria in the G. mellonella midgut37,38. Most likely, the presence of the bacteria is dependent on the origin of the wax moth strain or on the diet.
It is known that an imbalance in the gut microbial components triggers local AMP expression, which provides an immune response against opportunistic infections and controls the balance of gut symbionts56,57. Enterobacter are classified as facultative anaerobes that can act as opportunistic pathogens in immunosuppressed hosts54,58. Therefore, any disruptions in this balanced system activated defense systems and the synthesis of AMPs. Importantly, envenomated insects respond by expressing AMPs and IMPI upon changes in the bacterial community. The highest activation has been observed for gloverin, which possess antibacterial activity exclusively against gram-negative bacteria59. IMPI is able to inactivate bacterial metalloproteases60, and an increase in its expression after envenomation was also confirmed in studies on bacterioses11,61. In addition, we found an enhanced expression of gallerimycin and galiomycin after envenomation. These proteins are mainly antifungal components40, but an increase in the expression of their genes in the wax moth was revealed in response to various bacterial treatments11,61,62. Furthermore, the level of expression of these genes in the G. mellonella midgut was positively correlated with the diversity of the diet and the CFU count of intestinal bacteria38. Moreover, it was shown that the antifungal component is able to act synergistically with other AMPs against bacteria63. Previously, Wei and coworkers8 suggested that alterations in the bacterial community in response to mycosis development are caused by the downregulation of the expression of AMPs and dual oxidase in the mosquito midgut. We did not observe the downregulation of AMPs in response to fungal infection. In contrast, a significant increase in gloverin gene expression and increasing trends in galiomycin and gallerimycin expression were found. Thus, the change in AMP expression in our system is the effect (symptom) but not the cause of dysbiosis.
We revealed weak changes and downregulation in the expression of ROS-, inflammation- and stress-related genes compared to the changes in AMP expression in response to envenomation and mycosis. This result may indicate the absence of destructive processes caused by oxidative/inflammatory damage during the investigated period (1–3 days post envenomation and infection). It is known that macrophage migration inhibitory factor (Contig 20582) and glutathione peroxidase (Contig 17373) are members of the antioxidant system43,44. The expression of the genes encoding Hsp 90 and caspase-like protein (Contig 15362) were indicative of destructive processes in the gut cells64. The active synthesis of Hsp 90 often occurs in tandem with caspase-like protein and indicates cell disruption by apoptotic or necrotic pathways in insects64. The downregulation of genes after envenomation alone or after envenomation and fungal infection together can be caused by general attenuation and starvation in insects.
We suggest that the activation of AMPs and the absence of midgut damage can prevent septicemia and bacterial decomposition of larvae for at least several days after envenomation. This time period could allow parasitoid larvae to finish feeding, since the developmental time from egg to pupa is only 5–7 days. Furthermore, the successful development of B. bassiana in envenomated larvae requires 3–7 days, even after infection with low doses34. Midgut bacteria can inhibit B. bassiana growth, but fungal colonization forestalls the development of systemic bacteriosis. It seems that the gut immune reaction of the host is beneficial for both parasitoids and fungi in the investigated system.
Interestingly, the oral administration of three cultivable bacteria, namely, E. faecalis, Enterobacter sp. and S. marcescens, did not cause larval mortality but significantly increased larval susceptibility to B. bassiana. Thus, despite the antagonistic properties of the bacteria towards B. bassiana, they are able to increase larval mortality after topical fungal infection. The obtained data confirm that the gut microbiota can strongly promote fungal killing activity8. The mechanism of the observed synergy is unclear. Previously, we showed that one of the main antifungal reactions – encapsulation – was decreased in wax moth hemolymph after envenomation by H. hebetor32,35. The decrease in this parameter can be caused by either the direct action of the venom on hemocytes33 or through the influence of midgut bacteria. Krams and coworkers38 revealed that the decrease in the encapsulation rate in wax moth larvae correlates with the increase in gut bacteria abundance. These researchers38 hypothesized that there is antagonistic cross-regulation between different components of the immune system – AMP expression in the midgut and encapsulation in the hemolymph. Possibly, the disturbance of the symbiotic balance under a high load of bacteria leads either to changes in the prioritization of immune responses between the midgut, integument and hemocoel, or to the suppression of antifungal immune reactions by bacterial metabolites, as shown by Park & Kim28 in the Spodoptera exigua – Xenorhabdus nematophila – B. bassiana system. Reasons for the synergy between the midgut bacteria and B. bassiana should be revealed in future studies.
Thus, the work has shown a substantial impact of the host gut microbiota and gut immunity on interaction in three-component system host - parasitoid - entomopathogenic fungus. Though both parasites do not affect the gut directly, they cause significant alterations in gut physiology. Parasitoid envenomation significantly decreases the midgut bacterial diversity due to the mass proliferation of enterobacteria. In addition, fungal infection increases abundance of opportunistic pathogen Serratia, particularly in envenomated insects. However, envenomated and infected insects respond by enhancing AMP expression, which can confine bacteria within the gut lumen and permit the development of fungus. Moreover, the additional load of cultivable bacteria in the gut promotes the development of fungal infection. Previously, many researchers considered mechanisms of synergy between entomopathogenic fungi and various intestinal or systemic toxicants (including parasitoid venoms) based on different changes in humoral and cellular immune reactions35,65–70. However, the impact of the toxicants on microbial communities was insufficiently understood. Our study shows that changes in the gut microbiota can be an important reason for the synergy. Further investigations could focus on the crosstalk between gut bacteria and fungi penetrating through the integument as well as on the interaction between fungi, toxicants and gut bacteria. Understanding these interactions may lead to new strategies for the biological control of insect pests.
Material and Methods
Insects and fungal material
Insects and fungi from the Siberian Zoological Museum of the Institute of Systematics and Ecology of Animals SB RAS (Novosibirsk) were used for the experiments. A laboratory population of G. mellonella (Siberian line) was maintained at 28 °C and 60% RH on artificial medium containing 22.5% corn meal, 12.5% beeswax, 12.5% honey, 12.5% glycerol, 12.5% milk solids, 10% wheat flour, 5% yeast and 12.5% water. H. hebetor was grown on the later instars of G. mellonella larvae32. Adult parasitoids were maintained at 28 °C with a photoperiod of 14:10 h (light:dark) on a diet of a 12% water-honey solution. The Sar-31 Beauveria bassiana s.s. isolate was used in the study. The conidia for infection were grown on Sabouraud dextrose agar (SDA) at 26 °C for 10 days, harvested by scraping, air-dried at 25 °C for 1 week and stored at 4 °C. For infections, conidia were suspended in sterile 0.003% Tween 20 and vortexed for 1 min. Concentrations of conidia were determined using a Neubauer hemocytometer.
Envenomation and inoculation procedures
Two H. hebetor females were placed for 10 h in 90 mm Petri dishes with ten fifth-instar larvae of G. mellonella for envenomation. Then, eggs of the parasitoid were washed off of paralyzed larvae with distilled water. Envenomated and nonenvenomated larvae were dipped in either a water-Tween suspension of B. bassiana (1.0 × 106 conidia/mL), or in a conidia-free water-Tween solution for 10 s. Then, the larvae were placed in 90 mm Petri dishes (10 larvae per dish) with 80 mm discs of filter paper moistened by sterile water (1 mL per disk). Petri dishes with insects were maintained in a climatic chamber (KBWF 240, Binder, Germany) at 28 °C and 75% RH. Larval mortality was estimated over 12 days. Cadavers were placed in moisture chambers to confirm mortality due to B. bassiana.
DNA extraction and Illumina sequencing of wax moth midguts
Seventy-two hours after fungal inoculation and/or envenomation, wax moth larvae were surface-sterilized with 70% ethanol and were dissected. Midguts with content were extracted and frozen in liquid nitrogen (5 midguts = one sample). Four samples of each treatment were used for analysis. DNA was isolated by a PowerSoil DNA Isolation Kit (Mo Bio). The V3-V4 region of the 16S rRNA genes was amplified with the primer pair 343 F (5′-CTCCTACGGRRSGCAGCAG-3′) and 806 R (5′-GGACTACNVGGGTWTCTAAT-3′) combined with Illumina adapter sequences71. The PCR conditions were as described previously72. A total of 200 ng of PCR product from each sample was pooled and purified with a MinElute Gel Extraction Kit (Qiagen). The 16S libraries were sequenced with 2 × 300 bp paired-end reagents on a MiSeq (Illumina) platform in the SB RAS Genomics Core Facility (ICBFM SB RAS, Novosibirsk, Russia). Raw sequences were analyzed with the UPARSE pipeline73 using USEARCH v8.1.1861. The UPARSE pipeline included paired read merging, read quality filtering, length trimming, identical read merging (dereplication), singleton read discarding, chimera removal and OTU clustering using the UPARSE-OTU algorithm. The OTU sequences were assigned a taxonomy using SINTAX74. Additionally, OTUs classified as chloroplasts were removed. The final data set contained 176 OTUs (308819 reads). All the rarefaction curves had a tendency to approach the saturation plateau, which indicated a reasonable volume of the sequenced reads (Appendix B, Fig. S4). Raw MiSeq reads were deposited in GenBank under the project accession number PRJNA479958 and the sequence read archive (SRA) accession number SRP152548.
Abundance and molecular identification of cultivable bacteria
Seventy-two hours post treatment, midguts were isolated as described in the previous section and homogenized in 1 mL of 0.1 M phosphate buffer (one sample = 3 midguts). Then, the suspension was diluted to 10−2, 10−3, 10−4 and 10−5. One-hundred-microliter aliquots of the 10−3 to 10−6 midgut dilutions were inoculated onto the surface of media selective for Enterococcus, Enterobacteriaceae and Serratia, namely, modified bile esculin azide agar (HiMedia, India), Endo agar (HiMedia, India), and Serratia differential medium (HiMedia, India), respectively. Petri dishes were incubated at 28 °C for 72 h. Then, enumeration of the colonies on each medium was performed. Representative colonies were selected and passaged three times under the same conditions to select pure strains. Colonies were suspended in sterile water and heated to 95 °С for 5 min, and cellular debris was precipitated by centrifugation. Then, supernatants were used as templates for further PCR and sequencing. Bacterial strains were identified by sequencing a 1308 bp fragment of the 16S rRNA gene using primers 8 F 5′-AGRGTTTGATCCTGGCTCA-3′ and 1350 R 5′-GACGGGCGGTGTGTACAAG-3′, as described previously75. The sequences of the 16S rRNA genes of the selected strains were deposited in the GenBank database under accession numbers MH685393 and MH685399. The newly obtained and GenBank-available reference sequences (http://www.ncbi.nlm.nih.gov) were aligned in BioEdit, resulting in an alignment of the genes. To perform phylogenetic analysis, MEGA 7.0 software76 was used.
Antagonism between the fungus and the cultivable bacteria
The antagonistic interaction between B. bassiana and cultivable gut bacteria was studied on artificial media by the dual culture method. Plugs (8 mm in diameter) of tryptose agar (Ferak, Germany) with 2-day-old bacterial cultures were placed on fungal lawns freshly plated on SDA medium in 90 mm Petri dishes. The effect of fungi on bacterial growth was assessed in a similar manner: plugs of SDA medium with 4-day-old fungal cultures were placed on tryptose agar medium with freshly plated bacteria. The inhibition zone formed between the fungus and the bacteria was measured on day 4 of dual cultivation.
Gene expression in the midgut
Wax moth midguts were extracted at 24 and 72 h post treatment, dissected in PBS and cleaned and washed to remove the contents of the gut lumen. After cleaning, midguts were immediately frozen in liquid nitrogen and stored at −80 °C. Before extraction, samples were lyophilized at −65 °C and 500 mTorr for 12 h and homogenized with micropestles in liquid nitrogen. Ten midguts (larvae from one Petri dish) were pooled in one sample, and this sample was used as a biological replicate. Six biological replicates per treatment and per time point were used and the whole experiment was conducted one-time. Total RNA was extracted by TRI Reagent® for DNA, RNA and protein isolation (Sigma-Aldrich) according to the manufacturer’s instructions. RNA concentration and purity were detected spectrophotometrically (8453, Agilent, USA). DNase treatment was performed by RQ1 RNase-free DNase (Promega). RNA was converted to cDNA using 6 mg of DNA-free RNA, 3 µL of 100 nm random nanomers and 4 µL of RevertedAidTM M-MuLV reverse transcriptase (Fermentas, Lithuania).
qPCR was conducted with HS-qPCR SYBR Blue (2×) mix (BioLabMix, Russia) in a CFX96 Touch system (Bio-Rad, USA). qPCR was performed in three technical replicates under the following conditions: 95 °C for 3 min; followed by 40 cycles of 94 °C for 15 s and annealing and elongation at 62/60/57 °C, depending on the primers (Appendix B, Table S2), for 30 s; and generation of melting curves (70–90 °C). The quality of the PCR products was verified by gel electrophoresis. The melting curves for each sample were analyzed after each run to check the specificity of amplification. Gene expression was calculated by the ΔΔCq protocol with Bio-Rad CFX Manager software (Bio-Rad). Two genes of Galleria mellonella were used as reference genes: translation elongation factor 1-alpha (EF1a) and 11 subunit of eukaryotic DNA-dependent RNA polymerase II (RBP11). Eight genes of interest (gloverin, gallerimycin, galiomycin, IMPI, Hsp 90, Contig 15362, Contig 17373, and Contig 20582) were investigated. Primer sequences are provided in Appendix B, Table S2. Primer properties were estimated by IDT OligoAnalyzer 3.1 (http://eu.idtdna.com/calc/analyzer).
Bioassay of culturable bacteria
Three-day-old colonies of Enterococcus faecalis (isolate GC1), Enterobacter sp. (isolate GB23) and Serratia marcescens (isolate GS46) were suspended in sterile 0.1 M phosphate buffer, and the concentrations were diluted to 1.0 × 108 propagules/mL. The suspensions were mixed with wax moth food and placed in Petri dishes (1 mL of suspension per 3 g of food per Petri dish). For the control variants, the food was treated with 1 M phosphate buffer. Fourth-instar larvae were dipped in a water-Tween suspension of B. bassiana (4.0 × 106 conidia/mL) for 10 s and placed in Petri dishes (10 larvae per dish) with treated food and moistened disks of filter paper. Control larvae were treated with the water-Tween solution. Petri dishes were maintained at 28 °C and 75% RH. Mortality was monitored for 10 days. Mummified insects were placed in moisture chambers to establish the cause of mortality. At least three replicates of each treatment were used, and the whole experiment was repeated three times.
Statistics
Data analyses were performed using SigmaStat 3.1 (Systat Software Inc., USA), Statistica 8 (StatSoft Inc., USA), PAST 377 and AtteStat 12.578. As the distribution of the studied parameters deviated from normal (Shapiro–Wilk test, P < 0.05), we used the nonparametric equivalent of a two-way ANOVA, namely, the Scheirer-Ray-Hare test79, followed by Dunn’s post hoc test. Differences in the mortality of wax moth larvae after various treatments were analyzed by the χ2 test. Differences in survival durations were estimated by the log-rank test followed by the Holm-Sidak adjustment. The synergistic and additive effects after combined treatments were differentiated by comparing the expected and observed mortality rates using the χ2 test, as suggested by Robertson and Preisler80.
Supplementary information
Acknowledgements
The study was supported by Russian Foundation for Basic Research (18-04-00335, 18-34-20060) and the Federal Fundamental Scientific Research Program for 2013–2020 (АААА-А16-116121410123-1). Strains of bacteria were deposited in The Collection of Extremophile Microorganisms and Type Cultures of ICBFM SB RAS, which is financed by the Federal Agency for Scientific Organizations program to support bioresource collections, under project 0309-2017-0008. Sequencing was conducted in the SB RAS Genomics Core Facility (ICBFM SB RAS, Novosibirsk, Russia). We are also grateful to Mikhail Beketov and Maria Buiskaya for English correction.
Author Contributions
V.Y.K., O.V.P. and V.V.G. designed the experiments. O.V.P., M.V.T., U.N.R., V.V.M., K.M. and T.A. performed the experiments. M.R.K., V.Y.K., O.V.P., A.V.K. and U.N.R. analyzed the data. V.Y.K., M.R.K. and N.A.K. wrote the paper. V.V.G. conceived the project.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-40301-6.
References
- 1.Boucias DG, Zhou Y, Huang S, Keyhani NO. Microbiota in insect fungal pathology. Appl. Microbiol. Biotechnol. 2018;102:5873–5888. doi: 10.1007/s00253-018-9089-z. [DOI] [PubMed] [Google Scholar]
- 2.Engel P, Moran NA. The gut microbiota of insects – diversity in structure and function. FEMS Microbiol. Rev. 2013;37:699–735. doi: 10.1111/1574-6976.12025. [DOI] [PubMed] [Google Scholar]
- 3.Douglas AE. Multiorganismal insects: diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015;60:17–34. doi: 10.1146/annurev-ento-010814-020822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gressel J. Microbiome facilitated pest resistance: potential problems and uses. Pest Manag. Sci. 2018;74:511–515. doi: 10.1002/ps.4777. [DOI] [PubMed] [Google Scholar]
- 5.Broderick NA, Raffa KF, Handelsman J. Midgut bacteria required for Bacillus thuringiensis insecticidal activity. PNAS. 2006;103:15196–15199. doi: 10.1073/pnas.0604865103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broderick NA, et al. Contributions of gut bacteria to Bacillus thuringiensis induced mortality vary across a range of Lepidoptera. BMC Biology. 2009;7:11. doi: 10.1186/1741-7007-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mason KL, et al. From commensal to pathogen: translocation of Enterococcus faecalis from the midgut to the hemocoel of Manduca sexta. mBio. 2011;2:e00065–11. doi: 10.1128/mBio.00065-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei G, et al. Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. PNAS. 2017;114:5994–5999. doi: 10.1073/pnas.1703546114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caccia S, et al. Midgut microbiota and host immunocompetence underlie Bacillus thuringiensis killing mechanism. PNAS. 2016;113:9486–91. doi: 10.1073/pnas.1521741113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston PR, Crickmore N. Gut bacteria are not required for the insecticidal activity of Bacillus thuringiensis toward the tobacco hornworm, Manduca sexta. Appl. Environ. Microbiol. 2009;75:5094–5099. doi: 10.1128/AEM.00966-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubovskiy I, et al. Immuno-physiological adaptations confer wax moth Galleria mellonella resistance to Bacillus thuringiensis. Virulence. 2016;7:860–870. doi: 10.1080/21505594.2016.1164367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grau T, Vilcinskas A, Joop G. Probiotic Enterococcus mundtii isolate protects the model insect Tribolium castaneum against Bacillus thuringiensis. Front. Microbiol. 2017;8:1261. doi: 10.3389/fmicb.2017.01261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blackburn MB, Gundersen-Rindal DE, Weber DC, Martin PAW, Farrar RR., Jr. Enteric bacteria of field-collected Colorado potato beetle larvae inhibit growth of the entomopathogens Photorhabdus temperata and Beauveria bassiana. Biol. Control. 2008;46:434–441. doi: 10.1016/j.biocontrol.2008.05.005. [DOI] [Google Scholar]
- 14.Sivakumar G, et al. Characterization and role of gut bacterium Bacillus pumilus on nutrition and defense of leafhopper (Amrasca biguttula biguttula) of cotton. INDIAN J. Agr. Sci. 2017;87:534–539. [Google Scholar]
- 15.Zhang F, et al. The interactions between gut microbiota and entomopathogenic fungi: a potential approach for biological control of Blattella germanica (L.) Pest Manag. Sci. 2018;74:538–447. doi: 10.1002/ps.4726. [DOI] [PubMed] [Google Scholar]
- 16.Cardoza YJ, Klepzig KD, Raffa KF. Bacteria in oral secretions of an endophytic insect inhibit antagonistic fungi. Ecol. Entomol. 2006;31:636–645. doi: 10.1111/j.1365-2311.2006.00829.x. [DOI] [Google Scholar]
- 17.Kabaluk T, Li-Leger E, Nam S. Metarhizium brunneum - An enzootic wireworm disease and evidence for its suppression by bacterial symbionts. J. Invertebr. Pathol. 2017;150:82–87. doi: 10.1016/j.jip.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Lukasik P, Guo H, van Asch M, Ferrari J, Godfray HCJ. Protection against a fungal pathogen conferred by the aphid facultative endosymbionts Rickettsia and Spiroplasma is expressed in multiple host genotypes and species and is not influenced by co-infection with another symbiont. J. Evol. Biol. 2013;26:2654–2661. doi: 10.1111/jeb.12260. [DOI] [PubMed] [Google Scholar]
- 19.Lednev GR, et al. Dynamics of mortality of the migratory locust under synchronous infection with entomopathogenic fungi (Beauveria bassiana, Metarhizium anisopliae) and bacteria Pseudomonas sp. Contemp. Probl. Ecol. 2008;1:210–213. doi: 10.1134/S1995425508020069. [DOI] [Google Scholar]
- 20.Kershaw MJ, Moorhouse ER, Bateman R, Reynolds SE, Charnley AK. The role of destruxins in the pathogenicity of Metarhizium anisopliae for three species of insect. J. Invertebr. Pathol. 1999;74:213–223. doi: 10.1006/jipa.1999.4884. [DOI] [PubMed] [Google Scholar]
- 21.Kaltenpoth M, Engl T. Defensive microbial symbionts in Hymenoptera. Funct. Ecol. 2013;28:315–327. doi: 10.1111/1365-2435.12089. [DOI] [Google Scholar]
- 22.Oliver, K. M., Moran, N. A. & Hunter, M. S. Variation in resistance to parasitism in aphids is due to symbionts not host genotype. PNAS. 102, 12795–12800, 10/1073/pnas.0506131102 (2005). [DOI] [PMC free article] [PubMed]
- 23.Oliver KM, Degnan PH, Hunter MS, Moran NA. Bacteriophages encode factors required for protection in a symbiotic mutualism. Science. 2009;325:992–994. doi: 10.1126/science.1174463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guay J-F, Boudreault S, Michaud D, Cloutier C. Impact of environmental stress on aphid clonal resistance to parasitoids: Role of Hamiltonella defens a bacterial symbiosis in association with a new facultative symbiont of the pea aphid. J. Insect Physiol. 2009;55:919–926. doi: 10.1016/j.jinsphys.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Vorburger C, Gehrer L, Rodriguez P. A strain of the bacterial symbiont Regiella insecticola protects aphids against parasitoids. Biol. Lett. 2010;6:109–111. doi: 10.1098/rsbl.2009.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen AK, Vorburger C, Moran NA. Genomic basis of endosymbiont-conferred protection against an insect parasitoid. Genome Res. 2012;22:106–114. doi: 10.1101/gr.125351.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen AK, Jeong G, Paine TD, Stouthamer R. Frequency of secondary symbiont infection in an invasive psyllid relates to parasitism pressure on a geographic scale in California. Appl. Environ. Microbiol. 2007;73:7531–7535. doi: 10.1128/AEM.01672-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park J, Kim Y. Benzylideneacetone suppresses both cellular and humoral immune responses of Spodoptera exigua and enhances fungal pathogenicity. J. Asia Pac. Entomol. 2011;14:423–427. doi: 10.1016/j.ibmb.2015.09.012. [DOI] [Google Scholar]
- 29.Yaroslavtseva ON, et al. Immunological mechanisms of synergy between fungus Metarhizium robertsii and bacteria Bacillus thuringiensis ssp. morrisoni on Colorado potato beetle larvae. J. Insect Physiol. 2017;96:14–20. doi: 10.1016/j.jinsphys.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Ghimire MN, Phillips TW. Suitability of different lepidopteran host species for development of Bracon hebetor (Hymenoptera: Braconidae) Environ. Entomol. 2010;39:449–458. doi: 10.1603/EN09213. [DOI] [PubMed] [Google Scholar]
- 31.Walther C, Rathmayer W. The Effect of Habrobracon venom on excitatory neuromuscular transmission in insects. J. Comp. Physiol. 1974;89:23–38. doi: 10.1007/BF00696160. [DOI] [Google Scholar]
- 32.Kryukova NA, et al. The effect of Habrobracon hebetor venom on the activity of the prophenoloxidase system. the generation of reactive oxygen species and encapsulation in the haemolymph of Galleria mellonella larvae. J. Insect Physiol. 2011;57:796–800. doi: 10.1016/j.jinsphys.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Kryukova NA, et al. Venom from the ectoparasitic wasp Habrobracon hebetor activates calcium dependent degradation of Galleria mellonella larval hemocytes. Arch. Insect Biochem. Physiol. 2015;90:117–130. doi: 10.1002/arch.21247. [DOI] [PubMed] [Google Scholar]
- 34.Kryukov VY, Kryukova NA, Glupov VV. Susceptibility of Galleria mellonella larvae to anamorphic entomopathogenic ascomycetes under envenomation and parasitization by Habrobracon hebetor. Russ. J. Ecol. 2013;44:89–92. doi: 10.1134/S1067413613010074. [DOI] [Google Scholar]
- 35.Kryukov VY, Kryukova NA, Tyurin MV, Yaroslavtseva ON, Glupov VV. Passive vectoring of entomopathogenic fungus Beauveria bassiana among the wax moth Galleria mellonella larvae by the ectoparasitoid Habrobracon hebetor females. Insect Sci. 2018;25:643–654. doi: 10.1111/1744-7917.12457. [DOI] [PubMed] [Google Scholar]
- 36.Kryukov VY, et al. Immunosuppression of insects by the venom of Habrobracon hebetor increases the sensitivity of bait method for the isolation of entomopathogenic fungi from soils. Biol. Bull. 2017;44:401–405. doi: 10.1134/S1062359017030050. [DOI] [Google Scholar]
- 37.Johnston PR, Rolff J. Host and symbiont jointly control gut microbiota during complete metamorphosis. PLOS Pathog. 2015;11:e1005246. doi: 10.1371/journal.ppat.1005246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krams IA, et al. Microbiome symbionts and diet diversity incur costs on the immune system of insect larvae. J. Exp. Biol. 2017;220:4204–4212. doi: 10.1242/jeb.169227. [DOI] [PubMed] [Google Scholar]
- 39.Mak P, Chmiel D, Gacek GJ. Antibacterial peptides of the moth Galleria mellonella. Acta. Biochim. Pol. 2001;48:1191–1205. [PubMed] [Google Scholar]
- 40.Wojda I. Immunity of the greater wax moth Galleria mellonella. Insect Sci. 2017;24:342–357. doi: 10.1111/1744-7917.12325. [DOI] [PubMed] [Google Scholar]
- 41.Wedde M, Weise C, Nuck R, Altincicek B, Vilcinskas A. The insect metalloproteinase inhibitor gene of the lepidopteran Galleria mellonella encodes two distinct inhibitors. Biol. Chem. 2007;388:119–127. doi: 10.1515/BC.2007.013. [DOI] [PubMed] [Google Scholar]
- 42.Vertyporokh L, Wojda I. Expression of the insect metalloproteinase inhibitor IMPI in the fat body of Galleria mellonella exposed to infection with Beauveria bassiana. Acta Biochim. Pol. 2017;64:273–278. doi: 10.18388/abp.2016_1376. [DOI] [PubMed] [Google Scholar]
- 43.Huang W-S, et al. Macrophage migration inhibitory factor (MIF) family in arthropods: cloning and expression analysis of two MIF and one D-dopachrome tautomerase (DDT) homologues in Mud crabs. Scylla paramamosain. Fish Shellfish Immunol. 2016;50:142–149. doi: 10.1016/j.fsi.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 44.Vogel H, Altincicek B, Glöckner G, Vilcinskas A. A comprehensive transcriptome and immune-gene repertoire of the lepidopteran model host Galleria mellonella. BMC Genomic. 2011;12:308. doi: 10.1186/1471-2164-12-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khoa DB, Trang LT, Takeda M. Expression analyses of caspase-1 and related activities in the midgut of Galleria mellonella during metamorphosis. Insect Mol. Biol. 2012;21:247–256. doi: 10.1111/j.1365-2583.2011.01131.x. [DOI] [PubMed] [Google Scholar]
- 46.Wojda I, Kowalski P. Galleria mellonella infected with Bacillus thuringiensis involves Hsp90. Cent. Eur. J. Biol. 2013;8:561–569. doi: 10.2478/s11535-013-0162-9. [DOI] [Google Scholar]
- 47.Tarhan L, Kayali HA, Karacali S. The glutathione-related detoxication responses to juvenile and ecdysone hormones in Galleria mellonella. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 2013;158:117–121. doi: 10.1016/j.cbpc.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Cote’ NM, Jaworski DC, Wasala NB, Morgan MS, Arlian LG. Identification and expression of macrophage migration inhibitory factor in Sarcoptes scabiei. Exp. Parasitol. 2013;135:175–181. doi: 10.1016/j.exppara.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Porta H, Muñoz-Minutti C, Soberón M, Bravo A. Induction of Manduca sexta larvae caspases expression in midgut cells by Bacillus thuringiensis Cry1Ab toxin. Psyche J. Entom. 2011;2011:938249. doi: 10.1155/2011/938249. [DOI] [Google Scholar]
- 50.Dada N, et al. Comparative assessment of the bacterial communities associated with Aedes aegypti larvae and water from domestic water storage containers. Parasit. Vectors. 2014;7:391. doi: 10.1186/1756-3305-7-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gu CT, et al. 2013 as Kosakonia sacchari comb. nov. Int. J. Syst. Evol. Microbiol. 2014;64:2650–2656. doi: 10.1099/ijs.0.064709-0. [DOI] [PubMed] [Google Scholar]
- 52.Spröer C, Mendrock U, Swiderski J, Lang E, Stackebrandt E. The phylogenetic position of Serratia, Buttiauxella and some other genera of the family Enterobacteriaceae. Int. J. Syst. Bacteriol. 1999;49:1433–1438. doi: 10.1099/00207713-49-4-1433. [DOI] [PubMed] [Google Scholar]
- 53.Ajithkumar B, Ajithkumar VP, Iriye R, Doi Y, Sakai T. Spore-forming Serratia marcescens subsp. sakuensis subsp. nov., isolated from a domestic wastewater treatment tank. Int. J. Syst. Evol. Microbiol. 2003;53:253–258. doi: 10.1099/ijs.0.02158-0. [DOI] [PubMed] [Google Scholar]
- 54.Hammer TJ, Janzen DH, Hallwachs W, Jaffe SP, Fierer N. Caterpillars lack a resident gut microbiome. PNAS. 2017;114:9641–9646. doi: 10.1073/pnas.1707186114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martemyanov VV, et al. Phenological asynchrony between host plant and gypsy moth reduces insect gut microbiota and susceptibility to Bacillus thuringiensis. Ecol. Evol. 2016;6:7298–7310. doi: 10.1002/ece3.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buchon, N., Silverman, N. & Cherry, S. Immunity in Drosophila melanogaster-from microbial recognition to whole-organism physiology. Nat. Rev. Immunol.14, 796–810, 10.1038/nri3763 (2014). [DOI] [PMC free article] [PubMed]
- 57.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 58.Jiang, H., Vilcinskas, A., Kanost, M. R. Immunity in lepidopteran insects. Söderhäll K. (ed.) Invertebrate immunity, 181–204. (Springer, 2004). [DOI] [PMC free article] [PubMed]
- 59.Kaneko Y, Furukawa S, Tanaka H, Yamakawa M. Expression of antimicrobial peptide genes encoding enbocin and gloverin isoforms in the silkworm. Bombyx mori. Biosci. Biotechnol. Biochem. 2007;71:2233–2241. doi: 10.1271/bbb.70212. [DOI] [PubMed] [Google Scholar]
- 60.Lovgren A, Zhang M, Engstrom A, Dalhammar G, Landen R. Molecular characterization of immune inhibitor A, a secreted virulence protease from Bacillus thuringiensis. Mol. Microbiol. 1990;4:2137–2146. doi: 10.1111/j.1365-2958.1990.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 61.Wojda I, Taszlow P. Heat shock affects host–pathogen interaction in Galleria mellonella infected with Bacillus thuringiensis. J. Insect Physiol. 2013;59:894–905. doi: 10.1016/j.jinsphys.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 62.Harding CR, et al. Legionella pneumophila pathogenesis in the Galleria mellonella infection model. Infect. Immun. 2012;80:2780–2790. doi: 10.1128/IAI.00510-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moghaddam BMR, et al. The potential of the Galleria mellonella innate immune system is maximized by the co-presentation of diverse antimicrobial peptides. Biol. Chem. 2016;397:939–945. doi: 10.1515/hsz-2016-0157. [DOI] [PubMed] [Google Scholar]
- 64.Arya R, Mallik M, Lakhotia SC. Heat shock genes - integrating cell survival and death. J. Biosci. 2007;32:595–610. doi: 10.1007/s12038-007-0059-3. [DOI] [PubMed] [Google Scholar]
- 65.Krukov VY, et al. Effects of fluorine-containing usnic acid and fungus Beauveria bassiana on the survival and immune-physiological reactions of Colorado potato beetle larvae. Pest Manag. Sci. 2018;74:598–-606. doi: 10.1002/ps.4741. [DOI] [PubMed] [Google Scholar]
- 66.Zibaee A, Bandani AR, Malagoli D. Methoxyfenozide and pyriproxifen alter the cellular immune reactions of Eurygaster integriceps Puton (Hemiptera: Scutelleridae) against Beauveria bassiana. Pest. Biochem. Physiol. 2012;102:30–37. doi: 10.1016/j.pestbp.2011.10.006. [DOI] [Google Scholar]
- 67.Jia M, et al. Biochemical basis of synergism between pathogenic fungus Metarhizium anisopliae and insecticide chlorantraniliprole in Locusta migratoria (Meyen) Sci. Rep. 2016;6:28424. doi: 10.1038/srep28424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tomilova OG, et al. Immune-physiological aspects of synergy between avermectins and the entomopathogenic fungus Metarhizium robertsii in Colorado potato beetle larvae. J. Invert. Pathol. 2016;140:8–15. doi: 10.1016/j.jip.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 69.Fisher JJ, Castrillo LA, Donzelli BGG, Hajek AE. Starvation and imidacloprid exposure influence immune response by Anoplophora glabripennis (Coleoptera: Cerambycidae) to a fungal pathogen. J. Econ. Entomol. 2017;110:1451–1459. doi: 10.1093/jee/tox124. [DOI] [PubMed] [Google Scholar]
- 70.Farooq M, Steenberg T, Højland DH, Freed S, Kristensen M. Impact of sequential exposure of Beauveria bassiana and imidacloprid against susceptible and resistant strains of Musca domestica. BioControl. 2018;63:707–718. doi: 10.1007/s10526-018-9892-6. [DOI] [Google Scholar]
- 71.Fadrosh DW, et al. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome. 2014;2(1):6. doi: 10.1186/2049-2618-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brouchkov A, et al. Bacterial community in ancient permafrost alluvium at the Mammoth Mountain (Eastern Siberia) Gene. 2017;636:48–53. doi: 10.1016/j.gene.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 73.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 74.Edgar RC. SINTAX, a simple non-bayesian taxonomy classifier for 16S and ITS sequences. bioRxiv. 2016 doi: 10.1101/074161. [DOI] [Google Scholar]
- 75.Bryanskaya AV, Rozanov AS, Slynko NM, Shekhovtsov SV, Peltek SE. Geobacillus icigianus sp. nov., a thermophilic bacterium isolated from a hot spring. Int. J. Syst. Evol. Microbiol. 2015;65:864–869. doi: 10.1099/ijs.0.000029. [DOI] [PubMed] [Google Scholar]
- 76.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001;4:1–9. [Google Scholar]
- 78.Gaidyshev, I. P. Solving scientific and engineering problems by means of Excel. VBA. and C++. 512 p. (in Russian) (BKhV-Peterburg, 2004).
- 79.Scheirer CJ, Ray WS, Hare N. The analysis of ranked data derived from completely randomized factorial designs. Biometrics. 1976;32:429–434. doi: 10.2307/2529511. [DOI] [PubMed] [Google Scholar]
- 80.Robertson, J. L. & Preisler, H. K. Pesticide bioassays with arthropods. 127 p (CRC Press, 1992).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.