Abstract
In this study, we investigate the binding interactions of two synthetic antiviral peptides (DET2 and DET4) on type II dengue virus (DENV2) envelope protein domain III. These two antiviral peptides are designed based on the domain III of the DENV2 envelope protein, which has shown significant inhibition activity in previous studies and can be potentially modified further to be active against all dengue strains. Molecular docking was performed using AutoDock Vina and the best-ranked peptide-domain III complex was further explored using molecular dynamics simulations. Molecular mechanics-Poisson–Boltzmann surface area (MM-PBSA) was used to calculate the relative binding free energies and to locate the key residues of peptide–protein interactions. The predicted binding affinity correlated well with the previous experimental studies. DET4 outperformed DET2 and is oriented within the binding site through favorable vdW and electrostatic interactions. Pairwise residue decomposition analysis has revealed several key residues that contribute to the binding of these peptides. Residues in DET2 interact relatively lesser with the domain III compared to DET4. Dynamic cross-correlation analysis showed that both the DET2 and DET4 trigger different dynamic patterns on the domain III. Correlated motions were seen between the residue pairs of DET4 and the binding site while binding of DET2 results in anti-correlated motion on the binding site. This work showcases the use of computational study in elucidating and explaining the experiment observation on an atomic level.
Electronic supplementary material
The online version of this article (10.1007/s10867-018-9515-6) contains supplementary material, which is available to authorized users.
Keywords: DENV2, Antiviral peptides, Molecular dynamics simulations, Molecular docking
Introduction
Dengue is a disease caused by an infection from antigenically distinct serotypes of dengue virus (DENV) that generate a unique host immune response to the infection. There are five different serotypes of DENV, DENV1, DENV2, DENV3, DENV4 [1, 2] and DENV5, discovered in 2007 [3]. The first infection with the dengue virus triggers the immune system to generate an antibody against that specific serotype which causes dengue fever, and at the same time, a life-long immunity against that specific serotype is developed. The problem arises when the same patient is infected with a different serotype. The antigens of different dengue serotypes are similar enough for the immune system to get tricked into not developing a new antibody, yet, different enough that the existing antibody is unable to recognize and neutralize the virus. Therefore, developing a cure to inhibit all serotypes is a great challenge in drug development and most of the reported inhibitors are serotype dependent, which limits their application in treating dengue infection. Apart from drug-based treatment, vaccine or antibody must be tetravalent, neutralizing all four serotypes, since the infection caused by a particular serotype will stimulate an adaptive immune response that is highly cross-reactive between serotypes. The subsequent infection will increase the virus replication, which puts the patient at a greater risk of developing more severe complications such as dengue hemorrhagic fever [4].
Structural and non-structural proteins have served as targets for DENV treatment, whether to block cell attachments or to disrupt viral replication. The structural proteins of a matured dengue virus are made up of capsid, membrane, and envelope glycoprotein, and are responsible for viral particle formation. The envelope protein mainly mediates viral entry/attachment to the cell receptor. Upon entry, the envelope protein undergoes rearrangement under acidic pH, exposing the fusion loop to insert into the endosomal membrane. Domain III then pulls the membrane closer when it folds back, which results in forming fusion pores and causes invasion of viral RNA into the cytoplasm [5–7]. Domain III is a potential target for blocking the virus’ entry into the cell. This is supported by evidence such as: (a) mutations on domain III stops the immune system to neutralize the virus [7–9], (b) domain III is the most recognized region for the monoclonal antibodies to block the adsorption on Vero cells [10], (c) infection is significantly reduced when cells are pre-incubated with soluble domain III [11], and (d) immunoglobin-like domain are suggested to have interaction with cellular receptors on target cells [12–14].
Peptides are drawing increasing attention because they possess properties that bridge the gap between small molecule- and protein-based drugs. Their molecular weight ranges between a typical molecular weight of a small molecule to a protein. Peptide-based drugs also have a high specificity, potency, and low toxicity/limited side effects in the biomolecular recognition processes [15, 16]. Furthermore, peptides are part of the components of the defense system found in protozoans, invertebrate, plants, and vertebrate as well as mammals which fight against invaded pathogens such as viruses, bacteria, and fungi by disrupting the orientation of their membrane [17]. A wide range of peptides have been designed and synthesized to inhibit the structural and non-structural proteins of DENV. For example, the binding of tetrapeptides [18] and octapeptides [19] with NS3 protease inhibits the enzymatic activity of NS3 and interferes with its interaction with the NS2B (cofactor) [20, 21] that are indispensable for viral replication and maturation [22, 23].
The development of peptide inhibitors targeting the DENV2 envelope protein is predominantly based on two approaches: high-throughput screening and mimicking peptide generation. High-throughput screening makes use of the structural information of DENV2 envelope protein to screen randomly generated peptides [24–26]. On the other hand, the generation of mimicking peptides was first reported by Hrobowski et al. 2005 [27]. They make use of Wimley–White interfacial hydrophobicity scale in combination with known structural data to determine regions of the DENV envelope proteins that probably play roles in the protein–protein rearrangements or bilayer membrane interactions during the entry and fusion process [27–29]. DN59 derived specifically from the stem region of the envelope protein was shown to inhibit the DENV [27, 30]. The rationale of deriving mimetic peptides from the envelope protein was further supported by a successful remark of using T20, an approved peptide for HIV treatment [31] that mimics part of the C-terminal region of the gp41 glycoprotein of HIV1 and inhibits the fusion of virus to host cells [32–35]. Similarly, peptides that were derived from another region of the envelope protein also inhibited the viral infectivity [26, 27]. Interaction of these peptides and DENV virions led to conformational changes of the glycoproteins, causing viral RNA to be released from the viral particles [30].
In search of peptide inhibitors targeting DENV2 envelope protein, our collaborators have specifically designed four antiviral peptides (DET1, 2, 3, and 4) based on the external loop of domain III of DENV2 envelope protein using BioMoDroid algorithm, the pairwise score of the sum of hydrophobic and charge compatibility index on receptor or interface against counterpart on the peptides [36]. The external loop of domain III, consisting of ten amino acids (IGVEPGQLKL), plays a major role in the receptor–binding of DENV2 [11, 37]. Hung et al. demonstrated that this external loop (IGVEPGQLKL) of the domain III is involved in the serotype-specific binding to the mosquito c6/36 but not in the mammalian BHK21 cells. In addition, this external loop contains an insertion of four amino acids to form an extended loop between F and G beta strands (FG loop) [38] which is conserved in dengue virus and other mosquito-borne viruses except for tick-borne encephalitis virus [7, 14]. Hence, the peptide that binds to this region would possibly block the viral attachment to the host cell receptor and subsequently inhibit the viral entry into the host cell. Of the four synthetic peptides, DET2 and DET4 were found to inhibit the virus entry of DENV2 (84.6% and 40.6%, respectively), as shown by plaque formation assay, RT-qPCR, and Western-blot analysis. Structural rearrangement of the viral envelope protein was observed through transmission electron microscope images, which explains that these peptides could probably interfere with the virus binding and entry [36]. However, molecular details of the protein–peptide interactions have not been elucidated and discussed further. The binding interactions would reveal the variables that promote the binding and vice versa.
In this study, we use computational approaches to understand the protein–peptide interactions between the designed peptides (DET1 to DET4) with the domain III, by means of molecular docking and molecular dynamics simulations. Using AutoDock Vina, a molecular docking algorithm, we have generated the complexes of the peptides and domain III and computed their binding affinity. Binding free energy calculated by MM-PBSA protocol was used to differentiate the binding strength of DET2 and DET4 after molecular dynamics (MD) simulations and has been further decomposed to reveal contribution differences of the key residues that form the interactions and orient the peptides in the putative receptor binding site on the domain III. Calculated binding affinities from molecular docking and binding free energies obtained from MD simulations correlated well with the experimental findings. Our investigations not only support the previous report, but also provide important details for improving the binding affinity of the peptides. Based on this study, unfavorable residues identified on a peptide can be replaced with other amino acids through computational site-directed mutagenesis to enhance the binding interactions. This information is crucial for future improvement and development of a better and more effective peptide inhibitor.
Methods
Docking studies
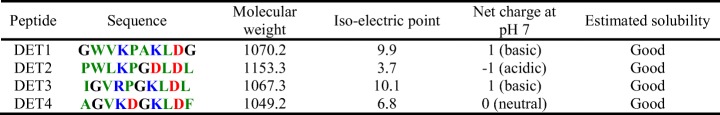
Solution structure of the DENV2 envelope protein domain III was downloaded from Protein Data Bank (PDB) [39] and used as an initial structure for the docking study (PDB code: 2JSF) [40]. After removal of water molecules and non-protein molecules, the initial structure was optimized in Discovery Studio 2.5.5 [41]. The peptides’ three-dimensional structures were generated from peptide tertiary structure prediction server, based on β-turn information together with regular secondary structure states [42] and followed by short minimizations until root mean squared (RMS) gradient tolerance of 0.1000 (kcal mol−1 × Å) is satisfied. AutoDock Vina [43] was used to dock the peptides into the binding site of the target protein. The dimension of the grid box was set to cover residues from 92 to 101 (IGVEPGQLKL), the external loop on the domain III, which was previously identified to play a key role in the viral attachment on the host cell receptor [11, 37]. The peptide properties (molecular weight, extinction coefficient, iso-electric point, net charge, and estimated solubility) were calculated using the peptide property calculator from pepcalc.com [44].
Molecular dynamics simulations and binding free energy calculation
Molecular dynamics (MD) simulation was performed using PMEMD.CUDA [45–47] from AMBER 12 suite of programs [48] on NVIDIA GPU (Quadro 2000D). Structures of peptide–protein complexes from the docking were solvated in a cubic box of TIP3P water. Na+ ions were added to neutralize the complex and the cutoff radius was kept to 15 Å to compute the non-bonded interactions. All simulations were performed under periodic boundary conditions [49] and long-range electrostatic were treated based on the particle mesh Ewald (PME) method [50, 51]. The SHAKE algorithm and Langevin dynamics were applied to constrain bonds that involve hydrogen and to control the temperature. The temperature of each system was gradually increased from 0 to 310.15 Kelvin (K) over a period of 60 ps of NVT dynamics, followed by 300 ps of NPT equilibration at 310.15 K and 1 atm pressure and finally a total 62 ns of the MD run. Trajectory analyses (root mean square deviation and fluctuation, dynamic cross-correlation, hydrogen bond) were carried out using CPPTRAJ module from Amber 12 [52]. The binding free energy of each complex was calculated based on Amber molecular mechanics Poisson–Boltzmann surface area (MM-PBSA) [53] protocol. The structural images were generated using PyMOL 1.6 [54].
Results and discussion
Dockings of designed peptides at domain III
In the previous study, DET1, DET2, DET3, and DET4 were designed based on the calculation of the total sum of hydrophobic and charge compatibility index of domain III binding site using the BioMoDroid algorithm [36]. The algorithm computes the combination score for each sequence based on iterative pairwise by comparing each amino acid on the protein or interface against its counterpart on the peptides resulting in auto generation of around ten fixed amino acid sequences [36]. These peptides have been evaluated in in vitro experiments. Among the four designed peptides, DET4 reduces the virus’ infection in a dose-dependent manner with an IC50 against DENV2 of 35 μM. Furthermore, DET4 and DET2 showed 84.6% and 40.6% plaque formation reduction, respectively. The RT-qPCR analysis has confirmed that DET2 and DET4 are able to significantly reduce the level of viral RNA load as they block the virus entry into the cell. In addition, less count of envelope protein is observed by Western blot in the cells treated with DET2 and DET4. Transmission electron microscopy images illustrate that the untreated viral control results in a smooth outer surface, which resembles mature flaviviruses morphology, but those treated with DET2 and DET4 have rough edges, suggesting a possible structural rearrangement of the viral envelope protein. DET1 and DET3, however, have demonstrated a complete reversal of inhibition activity as they have failed to stop the dengue virus from infecting the cells [36].
Docking was performed to predict the binding modes and affinity of these peptides on the DENV2 envelope protein domain III, specifically the external loop as the binding site. The tertiary structure of all the peptides (Fig. 1) were predicted using peptide tertiary structure prediction server [42] and were further minimized. This external loop from the domain III of the envelope protein is the receptor attachment site during the entry of the virus into the host cell [11]. All four peptides are bound to the surface of domain III, at the immediate vicinity of the binding site, with DET4 being the nearest to the binding site (Fig. 2). The estimated binding free energy obtained from AutoDock Vina for DET4, DET2, DET1, and DET3, range from − 9.6 kcal/mol to − 8.0 kcal/mol (see Table 1). A more negative estimated binding affinity indicates stronger interactions between the peptides and the protein. Docking calculations were consistent with the previously reported experimental findings, where DET4 outperformed other peptides and appeared to be the most active peptide that binds to the domain III. Subtle differences in the estimated free energy of binding from the docking studies have lead us to further investigate this using molecular dynamics simulations, where dynamics of the protein–peptide complexes in a hydrated environment are taken into account.
Fig. 1.
Tertiary structure of the peptides (initial and minimized structures). The initial peptide structures from the prediction server are shown in grey line representation and the optimized peptide structures are shown in cartoon representation
Fig. 2.

Docked conformations of DET1 to DET4 at the binding site of DENV2 envelope protein domain III (violet). All peptides are bound on the surface of domain III, at the immediate vicinity of the binding site (yellow), with DET4 being the nearest to the binding site
Table 1.
Molecular docking results from AutoDock Vina
| Target | Peptide | Sequence | Inhibition (% μM) | Binding affinity (kcal/mol) |
|---|---|---|---|---|
| 2JSF | DET1 | GWVKPAKLDG | 0 | − 8.3 – (− 7.2) |
| DET2 | PWLKPGDLDL | 40.6 | − 9.0 – (− 7.6) | |
| DET3 | IGVRPGKLDL | 0 | − 8.0 – (− 7.2) | |
| DET4 | AGVKDGKLDF | 84.6 | − 9.6 – (− 8.1) |
Note: Inhibition percentage was obtained from reference [36]
Table 2 shows the properties of DET1 to DET4, as calculated from pepcalc.com [44]. All four peptides have charged (both positive and negative) and hydrophobic residues (polar residues are not found) and have good solubility in water. DET1 and DET3 are basic, while DET2 and DET4 are acidic and neutral, respectively. Although all the peptides are similar to one another (with similar portion of hydrophobic and charged residues), DET4 is the only neutral peptide without proline, while DET2 is the only peptide with one glycine and two prolines. Proline is known to rigidify the polypeptide chain by imposing certain torsion angles on the segment of the structure and glycine contributes high flexibility of a polypeptide chain. The attributes of the peptides probably influence the binding ability of the peptide at the binding site. As observed, DET4 with no proline (no rigid/constraint structure) and extra charged residues compared to other peptides bind better to the binding site. DET2 with two prolines and only one glycine probably has very rigid structure that affects the binding.
Table 2.
Peptides properties
Red: acidic residues, blue: basic residues, green: hydrophobic uncharged residues, black: other residues. Molecular weight is in the unit of g/mol
Molecular dynamics simulations
System stability and flexibility
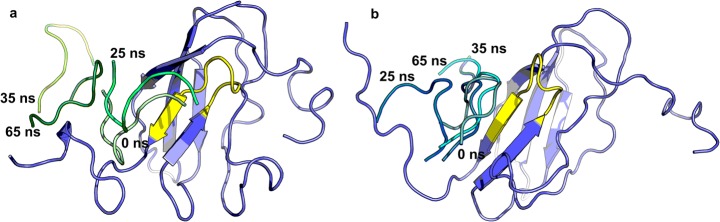
The structural stability of the systems is monitored using root mean square deviations (RMSD) of all Cα-atoms with respect to their minimized starting structure. RMSD is commonly used to determine the equilibrium state of a system [55]. Steady oscillation and less fluctuation of RMSD was observed in DET4-bound complex compared to DET2-bound complex, indicating that the previous complex was more stable and endured lesser conformational changes within the simulated timescale (Fig. 3) [56]. In addition, the analysis of root mean square fluctuations (RMSF) was also computed and plotted (Fig. 4). RMSF is useful to identify or locate the flexible/disordered region as well as the heterogeneity of a system [57–59]. DET2-bound complex shows a greater overall RMSF compared to DET4-bound complex, especially for the peptide – DET2 (residues 118 to 127, shaded in red), indicating that DET2 fluctuates more and tries to search for a conformation that can fit onto the binding site or is probably unstable on the binding site (Fig. 4), while binding of DET4 stabilizes the complex as a whole and results in a reduced fluctuation for both the peptide and protein. It is observed that the fluctuations at the binding site (shaded in yellow) were reduced upon the binding of DET4, indicating that DET4 has a stabilizing effect compared to DET2. Snapshots of the complex conformation at different timescales clearly illustrated that DET4 bound closer to the binding site and remained at the site throughout the simulations, as compared to DET2 (Fig. 5). This is also in agreement with the RMSD calculation, where smaller deviations for domain III in complex with DET4 were observed (Fig. 3).
Fig. 3.
Root mean square deviations (RMSD) of all Cα-atoms of DET2- and DET4-bound systems over 65-ns simulation time. The DET4-bound complex demonstrated a steadier oscillation of RMSD
Fig. 4.
Root mean square fluctuations (RMSF) of DET2- and DET4- bound systems, calculated for every residue of the domain III (residue 1-117) and the peptides (residue 118-127). The binding site of the domain III (residue 92 to 101) is shaded in yellow and the peptides are shaded in red. Binding of DET4 reduces the overall fluctuations of the complex
Fig. 5.
a DET2- and b DET4- bound complexes at different timescales. The binding site is shown in yellow (residues 92-101)
Figure 4 indicates that residues at the N- and C- terminus of the peptides shows higher fluctuations, i.e., PRO and ALA at position 118 and LEU and PHE at position 127. The binding site of the domain III (residue 92 to 101) shows a relatively low RMSF (see Fig. 4, yellow shaded region), except for GLU95 and PRO96, for DET2- and DET4-bound system, respectively. These residues contributed insignificantly to the binding of DET2 and DET4, as shown by the decomposition analysis (see section below).
Binding free energy calculation
Relative binding free energy obtained from MM-PBSA calculation suggests that DET4 binds to the protein better than DET2 (− 39.85 vs. – 19.51 kcal/mol) (Table 3). This is in a good agreement with the experimental findings, where the inhibition activity of DET4 is higher than DET2 by a factor of 2 [36]. The van der Waals (vdW) interactions and non-polar parts of the solvation free energy contributes favorably to the binding, as opposed to the unfavorable total electrostatic contributions (EEL+EPB) (Table 3). Each energy term (vdW, ENPOLAR, EEL+EPB) was found to be at least two times more favorable upon the binding of DET4, compared to that for DET2, e.g., – 55.97 vs. – 22.46 kcal/mol for the vdW interactions.
Table 3.
Relative binding free energies of complexes estimated using MM-PBSA
| Complex | EEL | vdW | EPB | ENPOLAR | ∆Ebinding |
|---|---|---|---|---|---|
| DET2 + domain III | − 108.53 ± 42.12 | − 22.46 ± 6.00 | 115.48 ± 41.35 | − 3.99 ± 0.99 | − 19.51 ± 6.61 |
| DET4 + domain III | − 204.63 ± 33.74 | −55.97 ± 5.86 | 230.60 ± 29.28 | − 9.85 ± 0.49 | − 39.85 ± 8.97 |
Note: The EEL and vdW represent the electrostatic and van der Waals contributions from MM, respectively. EPB stands for PB electrostatic contribution to the solvation free energy, and ENPOLAR is the nonpolar contribution to the solvation free energy. ∆Ebinding (in kcal/mol, binding energy neglecting the contribution of entropy) is the final estimated binding free energy calculated from the terms above
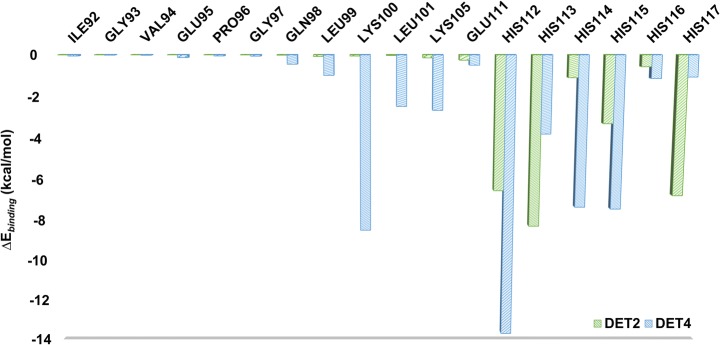
To determine the important amino acids that contribute to the binding affinity, pairwise energy decomposition was calculated for DET2- and DET4-bound complexes. Figures 6 and 7 illustrate the decomposed energies on a per residue basis for the peptides (DET2 and DET4) and the key binding residues in the domain III. The positive and negative values indicate the unfavorable and favorable contributions, respectively. PRO118 in DET2 contributed the most to the binding follow by LEU120. Most of the residues in DET4 involved in the binding and possessed favorable interaction energy than the DET2, except for PHE127 (Fig. 6). This implicating that DET4 has more residues involved in holding the peptide in place at the binding site via favorable peptide–protein interactions. Figure 7 shows the decomposition binding energy upon the binding of DET2 and DET4 on the domain III. DET4 has more favorable contact with the residues in the binding site of domain III compared to DET2. Both peptides have minimal contacts with residues 92-97 from the binding site but making additional contacts with the C-terminal where favorable interactions were formed between the peptides and HIS112-117 (see Fig. 8 in the next section).
Fig. 6.
Decomposed binding free energy (kcal/mol) of DET2 and DET4 on a per-residue basis. All the residues in DET4 contribute significantly to the binding
Fig. 7.
Decomposed binding free energy (kcal/mol) on the key binding site residues of domain III. DET4 making more contacts with the domain III
Fig. 8.
Conformations of a DET2-bound and b DET4-bound complexes at 65 ns. For the purpose of clarity, only hydrogen bond forming residues are shown in stick representation and are labeled. For the peptides, only the first and the last residues are labeled in italics small letter. The binding site is shown in yellow and the residues within 4 Å from the peptides are shown in pale pink (additional contacts). DET4 was oriented in the binding site through more extensive hydrogen bonds network as compared to DET2
Hydrogen Bond analysis
Hydrogen bonds between the peptides and domain III with occupancy of more than 10% are shown in Table 4. DET4 forms more hydrogen bonds compared to DET2, where only four hydrogen bonds were observed between DET2 and the domain III. PRO118 that was shown to contribute the most in the decomposition analysis was the only residue in DET2 that is making hydrogen bond with the domain III (Fig. 6). In addition, ALA118, GLY119, and VAL120 of DET4 that showed favorable binding interactions in the decomposition analysis are found to form a hydrogen bond with the domain III at a high occupancy (Table 4). The number of hydrogen bonds between the peptides and domain III alone is able to explain the reason why DET4 interacts with the domain III distinctively from the other peptides. Figure 8 shows the final conformations of DET2- and DET4- bound complexes at 65 ns simulations, with hydrogen bond forming residues shown in stick representation. DET4 is oriented in the binding pocket through extensive favorable hydrogen bonding with the domain III residues, including ARG57, TYR89, HIS112, HIS114, HIS115, LYS100, ASN102, and LEU110 with distance range of 2.70–2.93 Å. The C-terminal loop of the domain III is seen folding up and wrapping the DET4 and making good contacts with DET4, in contrast with DET2 (Fig. 8).
Table 4.
Hydrogen bond formation of DET2 and DET4 complexes throughout the MD simulations
Note: residues from DET2 and DET4 are colored green and blue, respectively
Dynamic cross-correlation
To observe the impact of DET2 and DET4 binding to the dynamics of domain III, the dynamics cross-correlation was calculated. Figure 9 shows the dynamic cross-correlation map of the backbone atoms of the domain III and the bound DET2 and DET4. In correlated motion, the movement towards the same direction between the residue pairs has positive values (green-low, yellow-medium, and red-high correlated motion), while in anti-correlated motion, the movement of opposite direction has negative values (grey-low, cyan-medium, and blue-high anti-correlated motion). The diagonal square corresponds to the relationship of a residue with itself, i.e., the only region observed to show highly positive values (red). Domain III shows different dynamic patterns and correlations upon the binding of DET2 and DET4, with DET4-bound domain III dominated primarily by correlated motion (lesser cyan region). The red arrows point to the crossed region of the peptides and the binding site of the domain III (residues 92-101). The biggest differences observed from the dynamic correlation maps are that DET2 triggered an anti-correlated motion on the binding site as opposed to DET4 (Fig. 9). Both the DET2 and DET4 have established additional contacts with the C-terminal of the domain III apart from the binding site. A correlation motion is observed for this region (residues 105-117, red arrows in Fig. 9). This also explains the less decomposition binding free energy for the binding site observed during the MM-PBSA calculation and how they were compensated by the additional contacts from the C-terminal region.
Fig. 9.

Dynamic cross-correlation map for DET2-bound (left) and DET4-bound (right) complexes. Positive and negative values are represented by red, yellow, green, andgray, cyan, blue, respectively. Black dotted lines separate the region between the domain III and the peptide. The crossed region of the peptide and the binding site are noted with red arrows. The binding of DET2 and DET4 have induced different dynamics patterns on the domain III
Conclusions
Binding modes and binding free energy of the four designed peptides (DET1 to DET4) on the DENV2 envelope protein domain III have been evaluated using computational approaches. Results from docking and molecular dynamics simulations are in good agreement with the experimental finding. The dynamic cross-correlation is used to infer the dynamics pattern induced by DET2 and DET4 as well as the correlation between the binding site and the peptides. Relative binding free energy calculations has distinguished the binding of DET2 and DET4, where DET4 bound to the domain III with far more favorable interactions. Decomposition analysis has identified major contributors to the binding for both the DET2 and DET4. These residues are also associated with the high occupancy of hydrogen bonding between the peptide and domain III. Residues at the position of 124, 125, and 127 are shown to contribute lesser to the binding and hence require modifications to improve the binding. In our lab, continuous effort is undergoing by means of single-point mutation and MM-PBSA calculation. Since these peptides were originally designed specifically based on the external loop of the domain III, we believe that these peptides could successfully block the receptor attachment site on the envelope protein and subsequently stop the virus from attaching to the host cell. These peptides are also probably active against all the serotypes of dengue, as the external loop is a conserved region across all the serotypes. They are indeed good potential leads to begin with for the design and development of more potent analogous peptides to fight against dengue infection. DET4 can be the standard peptide for the subsequent binding energy calculations or interaction study of those newly improved or designed peptide. Our works showcase the use of computational approaches in elucidating the experimental observation and provide crucial information for future improvement and development of better and more effective peptide inhibitors.
Electronic supplementary material
(MPG 4165 kb)
(MPG 3655 kb)
Acknowledgements
The authors thank Hadieh Monajemi for her diligent proofreading of this paper. This research supported financially by Faculty Research Grant, University Malaya (GPF062B-2018).
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Seema, Jain SK. Molecular mechanism of pathogenesis of dengue virus: entry and fusion with target cell. Indian J. Clin. Biochem. 2005;20:92–103. doi: 10.1007/BF02867407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parikesit AA, Kinanty, Tambunan USF. Screening of commercial cyclic peptides as inhibitor envelope protein dengue virus (DENV) through molecular docking and molecular dynamics. Pak. J. Biol. Sci. 2013;16:1836–1848. doi: 10.3923/pjbs.2013.1836.1848. [DOI] [PubMed] [Google Scholar]
- 3.Mustafa LCMS, Rasotgi CV, Jain CS, Gupta LCV. Discovery of fifth serotype of dengue virus (DENV-5): a new public health dilemma in dengue control. Med. J. Armed Forces India. 2015;71:67–70. doi: 10.1016/j.mjafi.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leitmeyer KC, Vaughn DW, Watts DM, Salas R, Chacon IVD, Ramos C, Rico-Hesse R. Dengue virus structural differences that correlate with pathogenesis. J. Virol. 1999;89:4738–4747. doi: 10.1128/jvi.73.6.4738-4747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Modis Y, Ogata S, Clements D, Harrison SC. Structure of the dengue virus envelope protein after membrane fusion. Nature. 2004;427:313–319. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- 6.van der Schaar, H.M., Rust, M.J., Waarts, B.-L., Van der Ende-Metselaar, H., Kuhn, R.J., Wilschut, J., Zhuang, X., Smit, J.M.: Characterization of the early events in dengue virus cell entry by biochemical assays and single-virus tracking. J. Virol. 81, 12019–12028 (2007) [DOI] [PMC free article] [PubMed]
- 7.Zhang Y, Zhang W, Ogata S, Clements D, Strauss JH, Baker TS, Kuhn RJ, Rossmann MG. Conformational changes of the flavivirus E glycoprotein. Structure. 2004;12:1607–1618. doi: 10.1016/j.str.2004.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modis Y, Ogata S, Clements D, Harisson SC. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J. Virol. 2005;79(2):1223–1231. doi: 10.1128/JVI.79.2.1223-1231.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierson TC, Diamond MS. Molecular mechanisms of antibody-mediated neutralisation of flavivirus infection. Expert Rev. Mol. Med. 2008;10:e12. doi: 10.1017/S1462399408000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crill WD, Roehrig JT. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J. Virol. 2001;75(16):7769–7773. doi: 10.1128/JVI.75.16.7769-7773.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung JJ, Hsieh MT, Young MJ, Kao CL, King CC, Chang W. An external loop region of domain III of dengue virus type 2 envelope protein is involved in serotype-specific binding to mosquito but not mammalian cells. J. Virol. 2004;78:378–388. doi: 10.1128/JVI.78.1.378-388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Maguire T, Hileman RE, Fromm JR, Esko JD, Linhardt RJ, Marks RM. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 1997;3:866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 13.Chiu MW, Yang YL. Blocking the dengue virus 2 infections on BHK-21 cells with purified recombinant dengue virus 2 E protein expressed in Escherichia coli. Biochem. Biophys. Res. Commun. 2003;309:672–678. doi: 10.1016/j.bbrc.2003.08.053. [DOI] [PubMed] [Google Scholar]
- 14.Rey FA, Heinz FX, Mandl C, Kunz C, Harrison SC. The envelope glycoprotein from tick-borne encephalitis virus at 2 Å resolution. Nature. 1995;375:291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 15.Otvos L, Wade JD. Current challenges in peptide-based drug discovery. Front. Chem. 2014;2:62. doi: 10.3389/fchem.2014.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Röckendorf N, Borschbach M, Frey A. Molecular evolution of peptide ligands with custom-tailored characteristics for targeting of glycostructures. PLoS Comput. Biol. 2012;8:1–10. doi: 10.1371/journal.pcbi.1002800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hancock REW, Lehrer R. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 1998;16:82–88. doi: 10.1016/S0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 18.Yin Z, Patel SJ, Wang W-L, Wang G, Chan W-L, Rao KRR, Alam J, Jeyaraj DA, Ngew X, Patel V, Beer D, Lim SP, Vasudevan SG, Keller TH. Peptide inhibitors of dengue virus NS3 protease. Part 1: warhead. Bioorganic med. Chem. Lett. 2006;16:36–39. doi: 10.1016/j.bmcl.2005.09.062. [DOI] [PubMed] [Google Scholar]
- 19.Prusis P, Junaid M, Petrovska R, Yahorava S, Yahorau A, Katzenmeier G, Lapins M, Wikberg JES. Design and evaluation of substrate-based octapeptide and non substrate-based tetrapeptide inhibitors of dengue virus NS2B–NS3 proteases. Biochem. Biophys. Res. Commun. 2013;434:767–772. doi: 10.1016/j.bbrc.2013.03.139. [DOI] [PubMed] [Google Scholar]
- 20.Clum S, Ebner K, Padmanabhan R. Cotranslational membrane insertion of the serine proteinase precursor NS2B-NS3 (pro) of dengue virus. J. Biol. Chem. 1997;272:30715–30723. doi: 10.1074/jbc.272.49.30715. [DOI] [PubMed] [Google Scholar]
- 21.Falgout B, Miller RH, Lai CJ. Deletion analysis of dengue virus type 4 nonstructural protein NS2B: identification of a domain required for NS2B-NS3 protease activity. J. Virol. 1993;67:2034–2042. doi: 10.1128/jvi.67.4.2034-2042.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falgout B, Pethel M, Zhang YM, Lai CJ. Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J. Virol. 1991;65:2467–2475. doi: 10.1128/jvi.65.5.2467-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Mohan PM, Padmanabhan R. Processing and localization of dengue virus type 2 polyprotein precursor NS3-NS4A-NS4B-NS5. J. Virol. 1992;66:7549–7554. doi: 10.1128/jvi.66.12.7549-7554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt AG, Yang PL, Harrison SC. Peptide inhibitors of dengue-virus entry target a late-stage fusion intermediate. PLoS Pathog. 2010;6:e1000851. doi: 10.1371/journal.ppat.1000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt AG, Yang PL, Harrison SC. Peptide inhibitors of flavivirus entry derived from the E protein stem. J. Virol. 2010;84:12549–12554. doi: 10.1128/JVI.01440-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costin JM, Jenwitheesuk E, Lok S-M, Hunsperger E, Conrads KA, Fontaine KA, Rees CR, Rossmann MG, Isern S, Samudrala R, Michael SF. Structural optimization and de novo design of dengue virus entry inhibitory peptides. PLoS Negl. Trop. Dis. 2010;4:e721. doi: 10.1371/journal.pntd.0000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hrobowski YM, Garry RF, Michael SF. Peptide inhibitors of dengue virus and West Nile virus infectivity. J. Virol. 2005;2:49. doi: 10.1186/1743-422X-2-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y, Rahman NA, Othman R, Hu P, Huang M. Computational identification of self-inhibitory peptides from envelope proteins. Proteins. 2012;80:2154–2168. doi: 10.1002/prot.24105. [DOI] [PubMed] [Google Scholar]
- 29.Panya A, Sawasdee N, Junking M, Srisawat C, Choowongkomon K, Yenchitsomanus PT. A peptide inhibitor derived from the conserved ectodomain region of DENV membrane (M) protein with activity against dengue virus infection. Chem. Biol. Drug Des. 2015;86:1093–1104. doi: 10.1111/cbdd.12576. [DOI] [PubMed] [Google Scholar]
- 30.Lok SM, Costin JM, Hrobowski YM, Hoffmann AR, Rowe DK, Kukkaro P, Holdaway H, Chipman P, Fontaine KA, Holbrook MR, Garry RF, Kostyuchenko V, Wimley WC, Isern S, Rossmann MG, Michael SF. Release of dengue virus genome induced by a peptide inhibitor. PLoS One. 2012;7:e50995. doi: 10.1371/journal.pone.0050995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lalezari JP, Henry K, O’Hearn M, Montaner JS, Piliero PJ, Trottier B, Walmsley S, Cohen C, Kuritzkes DR, Eron JJ, Jr, Chung J, DeMasi R, Donatacci L, Drobnes C, Delehanty J, Salgo M. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N. Engl. J. Med. 2003;348:2175–2185. doi: 10.1056/NEJMoa035026. [DOI] [PubMed] [Google Scholar]
- 32.Champagne K, Shishido A, Root MJ. Interactions of HIV-1 inhibitory peptide T20 with the gp41 N-HR coiled coil. J. Biol. Chem. 2009;284:3619–3627. doi: 10.1074/jbc.M809269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wild C, Oas T, McDanal C, Bolognesi D, Matthews T. A synthetic peptide inhibitor of human immunodeficiency virus replication: correlation between solution structure and viral inhibition. Proc. Natl. Acad. Sci. U. S. A. 1992;89:10537–10541. doi: 10.1073/pnas.89.21.10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kilby JM, Hopkins S, Venetta TM, DiMassimo B, Cloud GA, Lee JY, Alldredge L, Hunter E, Lambert D, Bolognesi D, Matthews T, Johnson MR, Nowak MA, Shaw GM, Saag MS. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. J. Nat. Med. 1998;4:1302–1307. doi: 10.1038/3293. [DOI] [PubMed] [Google Scholar]
- 35.Chan DC, Kim PS. HIV entry and its inhibition. Cell. 1998;93:681–684. doi: 10.1016/S0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 36.Alhoot MA, Rathinam AK, Wang SM, Manikam R, Sekaran SD. Inhibition of dengue virus entry into target cells using synthetic antiviral peptides. Int. J. Med. Sci. 2013;10:719–729. doi: 10.7150/ijms.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazumder R, Hu ZZ, Vinayaka CR, Sagripanti JL, Frost SDW, Kosakovsky Pond SL, Wu CH. Computational analysis and identification of amino acid sites in dengue E proteins relevant to development of diagnostics and vaccines. Virus Genes. 2007;35:175–186. doi: 10.1007/s11262-007-0103-2. [DOI] [PubMed] [Google Scholar]
- 38.Erb SM, Butrapet S, Moss KJ, Luy BE, Childers T, Calvert AE, Silengo SJ, Roehrig JT, Huang CY, Blair CD. Domain-III FG loop of the dengue virus type 2 envelope protein is important for infection of mammalian cells and Aedes aegypti mosquitoes. J. Virol. 2010;406:328–335. doi: 10.1016/j.virol.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 39.Protein Data Bank (PDB). http://www.rcsb.org/pdb/ (2014). Accessed 20 January 2014
- 40.Volk DE, Lee Y-C, Li X, Thiviyanathan V, Gromowski GD, Li L, Lamb AR, Beasley DWC, Barrett AD, Gorenstein DG. Solution structure of the envelope protein domain III of dengue-4 virus. J. Virol. 2007;364:147–154. doi: 10.1016/j.virol.2007.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Accelrys. Discovery Studio (version 2.5.5). San Diego, California. (2009)
- 42.Kaur H, Garg A, Raghava GPS. PEPstr: a de novo method for tertiary structure prediction of small bioactive peptides. Protein Pept. Lett. 2007;14:626–630. doi: 10.2174/092986607781483859. [DOI] [PubMed] [Google Scholar]
- 43.Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.PepCalc.com. http://pepcalc.com/ (2015). Accessed 15 May 2015
- 45.Götz, A.W., Williamson, M.J., Xu, D., Poole, D., Grand, S.L., Walker, R.C.: Routine microsecond molecular dynamics simulations with AMBER - part I: generalized Born. J. Chem. Theory Comput. 8, 1542–1555 (2012) [DOI] [PMC free article] [PubMed]
- 46.Grand SL, Götz AW, Walker RC. SPFP: speed without compromise—a mixed precision model for GPU accelerated molecular dynamics simulations. Comput. Phys. Commun. 2013;184:374–380. doi: 10.1016/j.cpc.2012.09.022. [DOI] [Google Scholar]
- 47.Salomon-Ferrer R, Götz AW, Poole D, Grand SL, Walker RC. Routine microsecond molecular dynamics simulations with AMBER on GPUs. 2. Explicit solvent particle mesh Ewald. J. Chem. Theory Comput. 2013;9:3878–3888. doi: 10.1021/ct400314y. [DOI] [PubMed] [Google Scholar]
- 48.Case DA, Darden TA, Cheatham TEIII, Simmerling CL, Wang J, Duke RE, Luo R, Walker RC, Zhang W, Merz KM, Roberts B, Hayik S, Roitberg A, Seabra G, Swails J, Goetz AW, Kolossváry I, Wong KF, Paesani F, Vanicek J, Wolf RM, Liu J, Wu X, Brozell SR, Steinbrecher T, Gohlke H, Cai Q, Ye X, Wang J, Hsieh MJ, Cui G, Roe DR, Mathews DH, Seetin MG, Salomon-Ferrer R, Sagui C, Babin V, Luchko T, Gusarov S, Kovalenko A, Kollman PA. Amber 12. San Francisco: University of California; 2012. [Google Scholar]
- 49.Weber W, Hünenberger P, McCammon J. Molecular dynamics simulations of a polyalanine octapeptide under Ewald boundary conditions: influence of artificial periodicity on peptide conformation. J. Phys. Chem. 2000;B104:3668–4575. doi: 10.1021/jp9937757. [DOI] [Google Scholar]
- 50.Darden T, York D, Pedersen L. Particle mesh Ewald: an N log (N) method for Ewald sums in large systems. J. Chem. Phys. 1993;98:10089–10092. doi: 10.1063/1.464397. [DOI] [Google Scholar]
- 51.Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen L. A smooth particle meshes Ewald potential. J. Chem. Phys. 1995;103:8577–8592. doi: 10.1063/1.470117. [DOI] [Google Scholar]
- 52.Roe DR, Cheatham TE. PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013;9:3084–3095. doi: 10.1021/ct400341p. [DOI] [PubMed] [Google Scholar]
- 53.Kollman PA, Massova I, Reyes C, Kuhn B, Huo S, Chong L, Lee M, Lee T, Duan Y, Wang W, Donini O, Cieplak P, Srinivasan J, Case DA, Cheatham TE. Calculating structures and free energies of complex molecules: combining molecular mechanics and continuum models. Acc. Chem. Res. 2000;33:889–897. doi: 10.1021/ar000033j. [DOI] [PubMed] [Google Scholar]
- 54.Schrödinger . The PyMOL Molecular Graphics System (Version 1.6) New York: LLC; 2015. [Google Scholar]
- 55.Law, R.J., Capener, C., Baaden, M., Bond, P.J., Campbell, J., Patargias, G., Arinaminpathy, Y., Sansom, M.S.P.: Membrane protein structure quality in molecular dynamics simulations. J. Mol. Graph. Model. 157–165 (2005) [DOI] [PubMed]
- 56.Knapp B, Frantal S, Cibena M, Schreiner W, Bauer P. Is an intuitive convergence definition of molecular dynamics simulations solely based on the root mean square deviation possible? J. Comput. Biol. 2011;18:997–1005. doi: 10.1089/cmb.2010.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Król M, Roterman I, Spólnik P. Analysis of correlated domain motions in IgG light chain reveals possible mechanisms of immunological signal transduction. Proteins. 2005;59:545–554. doi: 10.1002/prot.20434. [DOI] [PubMed] [Google Scholar]
- 58.Sousa SF, Fernandes PA, Ramos MJ. Molecular dynamics simulations on the critical states of the farnesyltransferase enzyme. Bioorganic Med. Chem. 2009;17:3369–3378. doi: 10.1016/j.bmc.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 59.Yin J, Bowen D, Southerland WM. Barnase thermal titration via molecular dynamics simulations: detection of early denaturation sites. J. Mol. Graph. Model. 2006;24:233–243. doi: 10.1016/j.jmgm.2005.08.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(MPG 4165 kb)
(MPG 3655 kb)