Abstract
Aspergillus fumigatus causes a range of diseases in humans, some of which are characterized by fungal persistence. Aspergillus fumigatus, being a generalist saprotroph, may initially establish lung colonization due to its physiological versatility and subsequently adapt through genetic changes to the human lung environment and antifungal treatments. Human lung-adapted genotypes can arise by spontaneous mutation and/or recombination and subsequent selection of the fittest genotypes. Sexual and asexual spores are considered crucial contributors to the genetic diversity and adaptive potential of aspergilli by recombination and mutation supply, respectively. However, in certain Aspergillus diseases, such as cystic fibrosis and chronic pulmonary aspergillosis, A. fumigatus may not sporulate but persist as a network of fungal mycelium. During azole therapy, such mycelia may develop patient-acquired resistance and become heterokaryotic by mutations in one of the nuclei. We investigated the relevance of heterokaryosis for azole-resistance development in A. fumigatus. We found evidence for heterokaryosis of A. fumigatus in patients with chronic Aspergillus diseases. Mycelium from patient-tissue biopsies segregated different homokaryons, from which heterokaryons could be reconstructed. Whereas all variant homokaryons recovered from the same patient were capable of forming a heterokaryon, those from different patients were heterokaryon-incompatible. We furthermore compared heterokaryons and heterozygous diploids constructed from environmental isolates with different levels of azole resistance. When exposed to azole, the heterokaryons revealed remarkable shifts in their nuclear ratio, and the resistance level of heterokaryons exceeded that of the corresponding heterozygous diploids.
Keywords: Aspergillus fumigatus, heterokaryon incompatibility, azole resistance, diploid, flexible nuclear ratio
1. Introduction
The vast majority of invasive mould infections in humans are caused by Aspergillus fumigatus. Azole antifungals are the mainstay of management of Aspergillus diseases, but treatment is hampered by the emergence of multi-azole-resistant A. fumigatus isolates [1–3]. Azole resistance is now reported globally [4–12], with resistance rates varying between 3.9 and 19% in clinical isolates in The Netherlands [13,14] and 20% in the National Aspergillosis Centre of the UK [15–17]. The majority of highly resistant mutants have modifications in the coding and promoter region of the cyp51A-gene [18–20]. This gene encodes the enzyme lanosterol 14-α-demethylase (CYP51A), the target of azoles that is essential to the ergosterol synthesis pathway. These cyp51A mutations are believed to emerge by exposure of A. fumigatus to medical azoles or azole fungicides [21].
Although many aspects of azole-resistance development in A. fumigatus remain to be investigated, the capacity of the fungus to create genetic diversity is crucial. Genetic variation may be generated during the various aspects of the life cycle of A. fumigatus: mycelial growth, asexual sporulation and sexual reproduction. Several studies in A. fumigatus have focused on the consequences of asexual [22] and sexual reproduction for fungal adaptation [23,24], but the role of the mycelium has been largely overlooked. Although patient-acquired azole resistance appears to be associated with the presence of a pulmonary cavity, which is an environment that allows for asexual reproduction, A. fumigatus hyphal biofilms are present in patients with cystic fibrosis (CF) and chronic pulmonary aspergillosis (CPA). A variety of azole-resistance mutations has been observed in patients with CF and CPA, indicating that fungal adaptation takes place [4]. Somatic mutations may occur during mitotic divisions leading to genetic variation within the mycelium. Such heterokaryotic mycelium may subsequently undergo parasexual recombination and segregate with a genetic variety of clones [25,26].
Heterokaryosis (i.e. genetically different nuclei within the same cytoplasm) is common in fungi. Hansen [27] found that in various fungi, more than 50% of natural mycelia were heterokaryons that upon single-spore culturing segregated in cultures of morphologically distinct types with respect to the abundance of aerial hyphae and conidia produced [27]. A heterokaryon can result from mutations in one or more nuclei in a homokaryotic mycelium, or from anastomosis of hyphae from genetically distinct homokaryons. The latter is however restricted by heterokaryon incompatibility, a common fungal allorecognition mechanism limiting successful fusion of hyphae to clonally related strains with the same heterokaryon-compatibility alleles [28–30]. Heterokaryon incompatibility has not been demonstrated yet in A. fumigatus, but if it exists, mutation is the likely initial cause of heterokaryon formation in patients, especially for isolated long-lasting cultures.
Since asexual spores of A. fumigatus are uninucleate, newly formed colonies start as a homokaryon and may produce heterokaryon during mycelial growth. Heterokaryosis is thus a transient characteristic of the mycelium that is lost upon asexual sporulation and dispersal of spores by air. Therefore, heterokaryons may form and persist particularly in long-lived mycelium cultures. This may be the case for chronic A. fumigatus infections, where the fungus has been shown to persist sometimes for many years [1,31–33]. Over time, the emergence of azole resistance in chronically colonized patients has indeed been described in consecutive A. fumigatus cultures that were concluded to be isogenic based on microsatellite genotyping [4,34]. Evidence for a possible role of heterokaryosis in azole-resistance development was also found in evolutionary laboratory experiments where an ancestral strain was allowed to evolve resistance during several weeks of azole exposure [22]. Both from patients and from evolutionary laboratory experiments, different evolved clones of A. fumigatus have been isolated, some of which are poorly sporulating or completely aconidial [35,36]. Different morphotypes from patients have been encountered, especially in chronic infections such as sinusitis, aspergilloma or in CF [6,31,35–37], but the characteristics and significance of such variants have not been studied.
Here, we study the relevance of heterokaryosis and somatic variation in the adaptive development of A. fumigatus. We address the following questions. (i) What are the characteristics of different variants of A. fumigatus isolated from evolved cultures of patients or laboratory experiments? (ii) Are the different successive or coexisting variants from a patient heterokaryon- compatible, and does heterokaryon incompatibility exist in A. fumigatus? (iii) How does azole resistance of heterokaryons relate to that of the individual homokaryons and heterozygous diploids? (iv) Is there plasticity in the nuclear ratio within a heterokaryon in response to changing azole concentrations?
2. Material and methods
(a). Strains used in this study
Fourteen clinical A. fumigatus strains, stored in the Radboud University Medical Centre, were available from five different patients suffering from various Aspergillus diseases (see electronic supplementary material, table S1 for patient characteristics). Four successive isolates were collected from patients P1 (aspergilloma) and P2 (chronic granulomatous disease) during azole treatment, another two strains were isolated from patient P3, a kidney transplant recipient, patient P4 with invasive aspergillosis and patient P5 a CF patient. Furthermore, we used wild-type strain CBS140053 (isolated from soil in Wageningen, The Netherlands, 1992) and five derived strains, that evolved during a seven weeks evolutionary experiment on medium with 1 µg ml−1 difenoconazole [36] (table 1).
Table 1.
Consecutive and coexisting isolates from five patients and from laboratory evolution experiments with different macroscopic morphology, azole-resistance mutations and resistance phenotype. —, information is not available.
| strains | isolation date | specimen | treatment/exposurea | spore size (µm) | growth rate on MEA (cm d−1) | azole-resistance mutationsd | MIC (resistance level)b | |||
|---|---|---|---|---|---|---|---|---|---|---|
| ITR | VOR | POS | ||||||||
| P1 | V74-61 | 29 Sep 2008 | sputum | ITC | 2.55 ± 0.02 | 9.375 ± 0.03 | CYP51A(A9T) | 0.5 | 1 | 0.063 |
| V77-41 | 17 Dec 2008 | sputum | POS | 2.65 ± 0.01 | 8.125 ± 0.02 | CYP51A(A9T/F291I) | >16 | 1 | 1 | |
| V80-28 | 9 Mar 2009 | sputum | POS | 2.65 ± 0.01 | 6.875 ± 0.01 | CYP51A(A9T/F291I) | >16 | 8 | >16 | |
| V83-14 | 7 Jun 2009 | BALc | L -AMB + CAS | 2.70 ± 0.01 | 6.625 ± 0.01 | CYP51A(A9T/G54E) | >16 | 0.5 | 1 | |
| P2 | V67-35 | 0 week | respiratory | VOR | 2.50 ± 0.02 | 10.25 ± 0.04 | NC | 0.125 | 0.5 | 0.016 |
| V67-36 | 108 weeks | respiratory | CAS + POS | 2.72 ± 0.01 | 8.875 ± 0.02 | NC | 0.25 | 0.5 | 0.031 | |
| V67-37 | 125 weeks | respiratory | CAS + POS | 2.87 ± 0.02 | 8.375 ± 0.03 | HapE(P88 L) | >16 | 4 | 0.25 | |
| V67-38 | 127 weeks | respiratory | CAS + POS | 2.87 ± 0.02 | 6.25 ± 0.01 | HapE(P88 L) | >16 | 4 | 0.25 | |
| P3 | V094-15 | 20 Feb 2010 | subcutaneous abscess | — | 2.77 ± 0.02 | 4.125 ± 0.02 | CYP51A(TR46/Y121F/T289A) | 1 | >16 | 0.25 |
| V094-16 | 20 Feb 2010 | subcutaneous abscess | — | 2.76 ± 0.02 | 7.375 ± 0.01 | CYP51A(TR46/Y121F/T289A) | 0.5 | >16 | 0.25 | |
| P4 | V186-81 | 3 Sep 2015 | sfenoid | — | 2.65 ± 0.01 | 6.625 ± 0.01 | NC | 0.25 | 0.25 | 0.063 |
| V187-02 | 3 Sep 2015 | sfenoid | — | — | 3.75 ± 0.02 | NC | — | — | — | |
| P5 | V148-33 | 6 May 2013 | sputum | no antifungal | 2.52 ± 0.01 | 3.4 ± 0.01 | NC | 0.5 | 0.5 | 0.125 |
| V186-45 | 29 Aug 2015 | sputum | no antifungal | 2.52 ± 0.01 | 3.4 ± 0.01 | NC | 0.5 | 1 | 0.25 | |
| E1 | CBS 140053 | 0 week | agricultural field | — | 2.49 ± 0.02 | 3.125 ± 0.01 | NC | 0.125 | 0.5 | 0.016 |
| CBS 140053 D1-7 | 7 weeks | evolutionary lines | DIF | 2.53 ± 0.01 | 8.625 ± 0.03 | CYP51A(G138S) | 32 | 4 | 0.5 | |
| E3 | CBS 140053 D3-7A | 7 weeks | evolutionary lines | DIF | 2.46 ± 0.02 | 6.25 ± 0.03 | NC | 8 | 2 | 0.5 |
| CBS 140053 D3-7B | 7 weeks | evolutionary lines | DIF | 2.51 ± 0.01 | 6.125 ± 0.01 | NC | 8 | 1 | 0.5 | |
| E6 | CBS 140053 D6-7A | 7 weeks | evolutionary lines | DIF | 2.49 ± 0.01 | 3.125 ± 0.02 | NC | 8(50%)/16(50%) | 2 | 0.25 |
| CBS 140053 D6-7B | 7 weeks | evolutionary lines | DIF | 2.47 ± 0.02 | 6.25 ± 0.01 | NC | 8 | 2 | 0.5 | |
aCAS, caspofungin; DIF, difenoconazole; ITR, itraconazole; L-AMB, liposomal amphotericin B; POS, posaconazole; VOR, voriconazole.
bMIC was determined four times for each isolate, most replicates showed consistence within replicates. There is one exception in E6, half showed 8 and another half showed 16. Resistance is indicated in italics (clinical breakpoints for resistance are MIC greater than 0.25 mg l−1 for POS and greater than 2 mg l−1 for ITR and, VOR).
cBAL, bronchoalveolar lavage.
dAmino acid changes in CYP51A and HapE; NC, no changes both on CYP51A and HapE.
(b). Culture media
Minimal medium (MM) was used for culturing heterokaryon and heterozygous diploid. MM consists of 6.0 g NaNO3, 1.5 g KH2PO4, 0.5 g MgSO4·7H2O, 0.5 g KCl, 10 mg of FeSO4, ZnSO4, MnCl2 and CuSO4 and 15 g agar + 1000 ml H2O (pH 5.8). Malt extract agar (MEA), used for counting spores and measuring the mycelial growth rate, was purchased from Sigma Company (Sigma Aldrich, Germany). The azole fungicide difenoconazole, and the medical azoles itraconazole (ITR), voriconazole (VOR) and posaconazole (POS) were purchased from Sigma Company (Sigma Aldrich, Germany) [22].
(c). Aspergillus fumigatus variants and morphotypes isolated from evolved cultures of patients and experimental evolution experiments
The isolates of A. fumigatus that were derived from the same patient or evolution experiment showed considerable variation. Morphological phenotypes were studied on MEA plates after 4 days incubation at 37°C. The spore size of A. fumigatus was measured with a Coulter counter (Beckman Coulter, The Netherlands). Mycelium growth rate (MGR) assays were performed by averaging the colony diameters (in mm) as measured in two randomly chosen perpendicular directions after 4 days of growth. We performed antifungal susceptibility testing and determined the minimal inhibitory concentration (MIC) for environmental isolates and clinical isolates from patients P3 and P4 according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) reference method. Relevant clinical information from patients P1, P2 and P3 was retrieved from the literature [4,34,38].
(d). Heterokaryon-compatibility testing
The capacity of strains to form heterokaryon (heterokaryon compatibility) was tested following standard methods as explained in figure 1 [39–41]. The complementing recessive nitrate non-using mutations, nia and cnx, were introduced by ultraviolet radiation of conidiospores of the various strains. Heterokaryon compatibility was tested among all clinical isolates from different patients and evolutionary line isolates.
Figure 1.
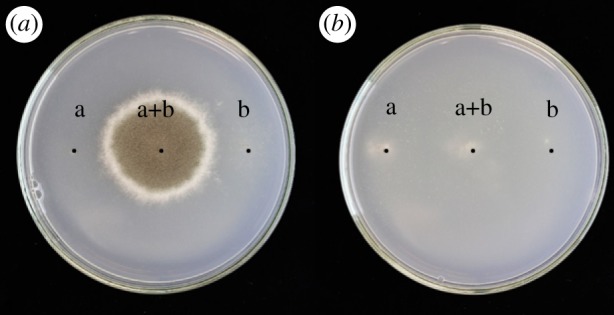
Heterokaryon-compatibility test. Aspergillus fumigatus strains with complementing mutations in different genes required for nitrate utilization (nia and cnx) are inoculated separately on the left- or right-hand side and together in the middle of the agar plate with nitrate as the sole N-source (positions a and b). (a) Heterokaryon formation between co-inoculated heterokaryon-compatible strains results in complementation and vigorous growth on nitrate as sole N-source after 7 days at 37°C. (b) Strains that are heterokaryon-incompatible do not show complementation.
(e). Heterokaryons and diploids construction
From the evolutionary line, three environmental-compatible isolates (CBS 140053-sensitive (S), CBS 140053-D3-7B-intermediate (I) and CBS 140053-D1-7-resistant (R)) with a different level of resistance to difenoconazole (see electronic supplementary material, table S2) were chosen for further heterokaryon and diploid formation. Heterokaryons (SNG&SCW; ING&SCW; RNG&SCW) were constructed from sensitive (S), intermediate (I) and resistant (R) strains differing in their nia or cnx mutation and conidial colour. For example, SCW is a sensitive stain with a cnx mutation and white spores, whereas RNG is a resistant strain with a nia mutation and green spores. Heterozygous diploids (SNG//SCW; ING//SCW; RNG//SCW) were isolated from heterokaryons by using the sandwich method [42]. MGR assays of heterokaryons and diploids were compared after 4 days of growth. Heterozygous diploids and constructed heterokaryons were inoculated on MM and MM supplemented with 1 µg ml−1 of difenoconazole and 0.6 µg ml−1 of VOR, for individual homokaryons, MM + ureum with 1 µg ml−1 of difenoconazole and 0.6 µg ml−1 of VOR were used.
(f). The nuclear ratio in heterokaryons in different azole environments
Assuming that the nuclear ratio among the conidiospores reflects the nuclear ratio within the mycelium, we tested the effect of azole exposure on the constitution of the heterokaryon. In brief, heterokaryons (SNG&SCW; ING&SCW; RNG&SCW) were grown on MM plates with or without 1 µg ml−1 difenoconazole. After 4 days of growth, spores were harvested from the heterokaryon into 0.5 ml of saline (distilled water with NaCl 0.8 g l−1) supplemented with Tween 80 (0.05% v/v) and dilutions were spread on an MEA agar plate. The number of colonies of either colour was counted and the ratio was calculated. SPSS independent-samples T-test was used for statistical analyses.
3. Results
(a). Different variants and morphotypes occur in successive and coexisting isolates from the same patient, and from an evolutionary experiment
The four consecutive A. fumigatus strains from patient P1 and the four from patient P2 showed an increase in spore size, a decrease in growth rate and an increase in azole resistance over time (table 1 and figure 2). The two isolates of patients P3 and P4, cultured from the same clinical specimen, were morphologically very distinct (figure 2). Also the cultures collected in the 7-week evolution experiment showed different morphology when compared with the ancestor and each other in addition to increasing azole resistance (table 2 and figure 2).
Figure 2.
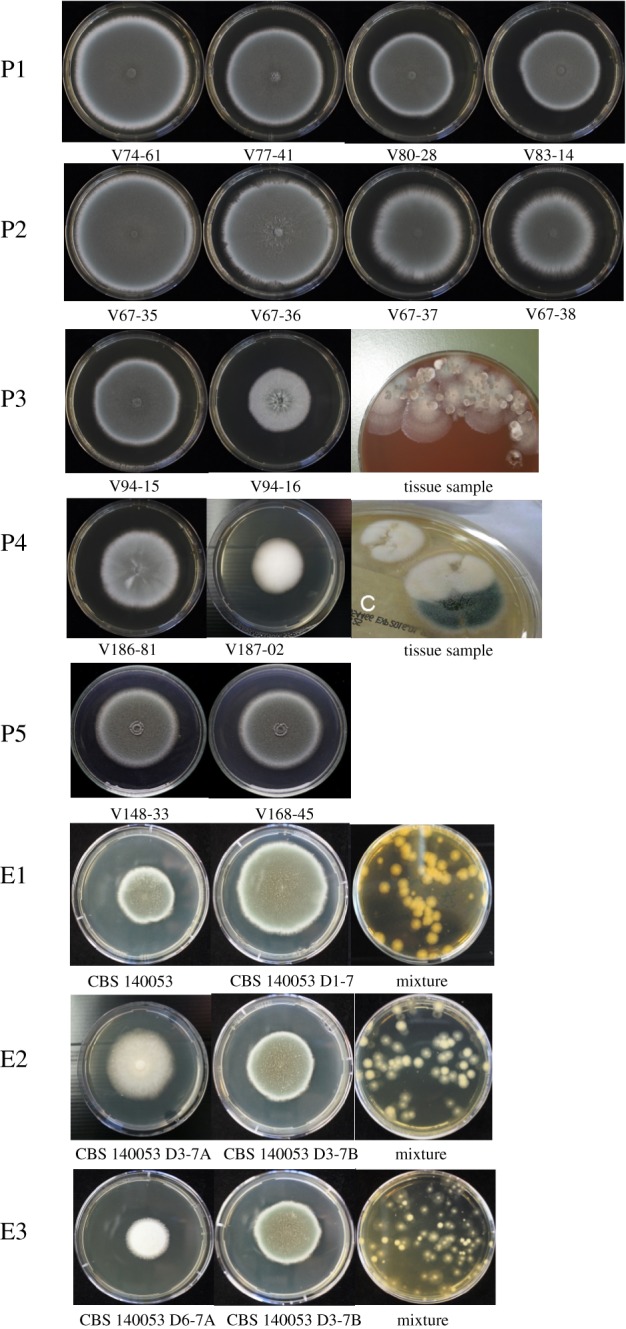
Different morphotypes among consecutive and coexisting isolates from patients (P1–P5) as well as from laboratory evolution experiments (E1–E3).
Table 2.
Compatibility testing of all patient strains and evolutionary evolved strains. P, patient isolate; E, evolutionary line; C, compatible; NC, not compatible. From patient 4, no mutants were derived and therefore no heterokaryon compatibility could be tested.
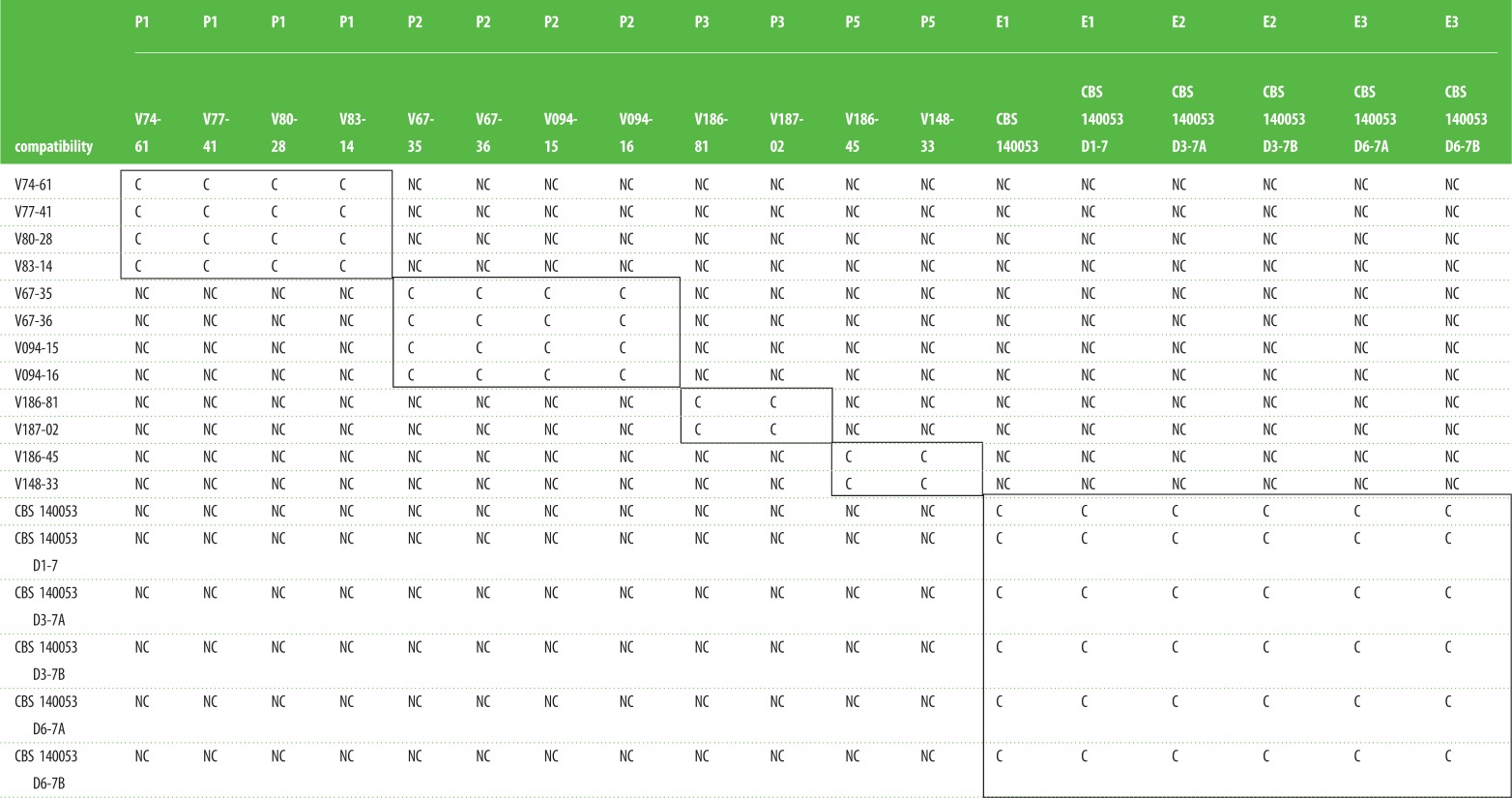 |
(b). Variant strains from the same patient or evolution experiment are heterokaryon-compatible, strains from different patients are heterokaryon-incompatible
The heterokaryon-compatibility test is based on complementation of recessive deficiency markers in a heterokaryon (figure 1). For many fungi, including several Aspergilli, it has been found that only clonally related isolates are heterokaryon-compatible, whereas non-clonal isolates are heterokaryon-incompatible. Isolates from the evolution experiments all share a common ancestor and are therefore expected to be heterokaryon-compatible. We first tested these known isogenic lines from the evolutionary experiment. Indeed, all these isolates, even though morphogically distinct (figure 1), were heterokaryon-compatible. This indicates that heterokaryon compatibility is stable during the evolutionary experiment. We next introduced heterokaryon-forcing markers in the isolates from patients P1, P2, P3 and P5 and tested for heterokaryon compatibility. We found that all within-patient isolates were heterokaryon-compatible while isolates from different patients showed heterokaryon incompatibility (table 2). It was not possible to obtain markers from the non-sporulating isolate from patient P4 and therefore this isolate did not allow for heterokaryon testing.
(c). Aspergillus fumigatus heterokaryons constructed from environmental isolates have higher azole resistance than corresponding heterozygous diploids
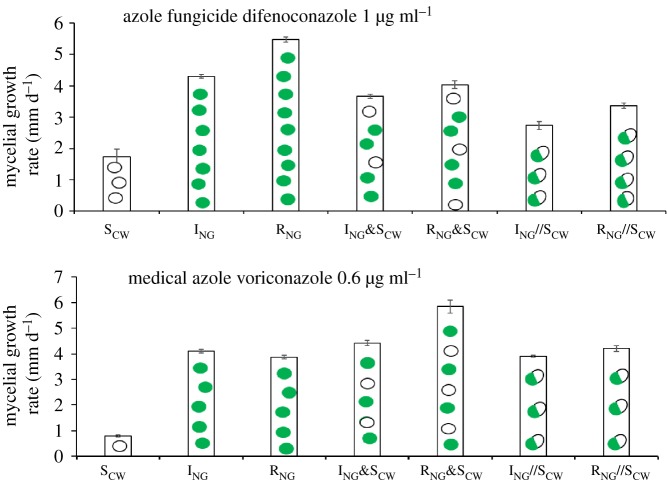
The azole-resistance level, measured as the MGR on difenoconazole-containing medium (1 µg ml−1) or VOR medium (0.6 µg ml−1), of heterozygous diploids constructed from environmental isolates was compared with that of the corresponding heterokaryons and their constituting haploid strains (figure 3). The MGRs of the heterokaryons (ING&SCW; RNG&SCW) were significantly higher (T-test, T6, 2 = −7.348, p < 0.05; T-test, T6,2 = −6.791, p < 0.05) than those of heterozygous diploids (ING//SCW; RNG//SCW). Also on VOR medium (0.6 µg ml−1), heterokaryons showed higher MGRs than the heterozygous diploid (T-test, T6,2 = 4.866, p < 0.05; T-test, T6, 2 = 1.557, p < 0.05).
Figure 3.
The azole resistance of heterokaryons constructed from environmental isolates relative to that of the individual homokaryons and heterozygous diploids was measured as the mycelial growth rate on azole (1 µg ml−1 of difenoconazole and 0.6 µg ml−1 of VOR)-containing media (for diploid and heterokaryon, MM with azole was used; for individual homokaryons, MM + ure with azole was used). R//S and I//S are diploids; R&S and I&S are heterokaryons.
(d). The nuclear ratio in heterokaryons is flexible
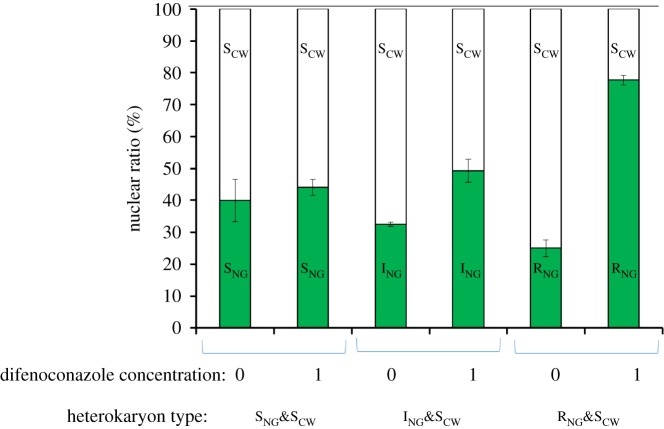
The growth pattern of heterokaryons was strikingly different on medium without azoles compared to that on medium with 1 µg ml−1 difenoconazole. Whereas erratic growth of the forced heterokaryon was typically seen on medium without azoles, a more compact circular colony was formed on azole-containing medium (figure 4a). The use of a white spore-colour mutation in one of the strains in a heterokaryon allowed for direct testing of the nuclear ratio in a heterokaryon on media with or without azoles (figure 4b). The ratios of white to green colonies from spores of heterokaryons SNG&SCW, ING&SCW and RNG&SCW were analysed (figure 5). For the sensitive heterokaryon SCW&SNG, there was no significant difference between the nuclear ratio on medium with and without 1 µg ml−1 difenoconazole (T-test, T6, 2 = −2.151, p > 0.05). For the heterokaryon formed between the sensitive and intermediately resistant nuclei (ING&SCW), the percentage of intermediate resistant nuclear, ING shifted from 32 to 50%: a 1.7-fold increase (T-test, T6, 2 = −9.843, p < 0.01). For the heterokaryon between sensitive and resistant nuclei (RNG&SCW), the percentage of RNG-nuclei shifted from 25 to 78%: a 3.5-fold increase (T-test, T6, 2 = −38.689, p < 0.01). On medium without azole, the fraction RNG in RNG&SCW heterokaryon as well as of ING in ING&SCW heterokaryon was significantly lower than the fraction SNG in SNG&SCW (T- test, T6, 2 = 3.587, p < 0.05), which suggests a cost of resistance of RNG and ING on MM without azole (figure 5).
Figure 4.
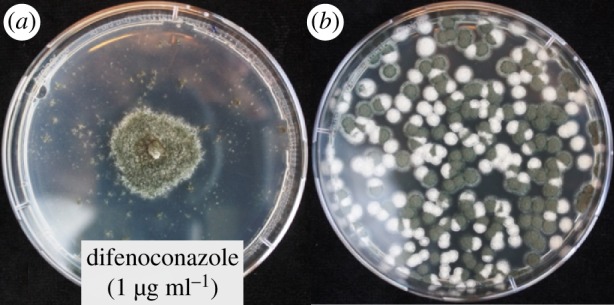
(a) Heterokaryon ScW and RNG (see electronic supplementary material, S1) growing on heterokaryon-selective medium with difenoconazole (1 µg ml−1). (b) Spore sample from the heterokaryon plated on non-selective medium shows segregation into homokaryotic white-spored sensitive and green-spored resistant cultures.
Figure 5.
The nuclear ratio of heterokaryons (SNG&SCW, ING&SCW and RNG&SCW) based on the nuclear ratio among the uninucleate spores from the heterokaryon grown on different concentrations of difenoconazole (0 or 1 µg ml−1).
4. Discussion
In this study, we investigated the relevance of heterokaryosis for A. fumigatus infection and persistence. We provide evidence for heterokaryosis of A. fumigatus isolates recovered from patients with chronic Aspergillus diseases. Furthermore, we demonstrate that heterokaryons constructed from environmental isolates have growth characteristics different from homokaryons and heterozygous diploids, and add to the phenotypical plasticity of the isolate. The higher mycelial growth rate of a heterokaryon than the corresponding heterozygous diploids upon azole exposure is associated with a nuclear ratio shift in the heterokaryon. In addition, we formally demonstrate the existence of heterokaryon incompatibility between A. fumigatus isolates from different patients and an environmental strain.
Aspergillus fumigatus is a generalist saprotrophic fungus that thrives in decaying plant material, and causes, as a side effect of its metabolic versatility, opportunistic and chronic infections in various patient groups. Survival in the human host involves adaptation to the lung environment, often accompanied by development of azole resistance in patients receiving chronic azole therapy. In general, A. fumigatus may adapt to any new environment through genetic and phenotypic plasticity. Genetic variability is created by recombination and mutation. Recombination, at least in the laboratory, can arise during sexual reproduction, followed by selection of progeny most capable to survive [24,43]. Mutation, the ultimate source of genetic variation, was shown to arise, particularly during abundant asexual spore formation [22]. However, in the patient, sexual reproduction of A. fumigatus is not likely, and asexual reproduction may only occur when a pulmonary cavity is present. In addition to genetic changes, somatic variation by heterokaryosis is another mechanism of increasing phenotypic plasticity facilitating adaptation [44].
Like heterozygosity in diploids, heterokaryosis allows genetic complementation of recessive mutations and heterosis effects. Heterokaryons are phenotypically more flexible than diploids, as in a heterokaryon, all possible nuclear ratios can occur from 0 to 100% and ratios can change depending on environmental conditions, whereas in a heterozygous diploid, the allele frequency is fixed at 50%. Natural heterokaryons have been described that enjoy an advantage in growth rate compared with the homokaryons under specific conditions and changing of nuclear ratios [44]. Heterokaryons can occur in many fungal species, but the biological significance remains largely unclear [27,44,45]. A heterokaryon can store and facilitate genetic variation by somatic mutation, parasexual recombination and segregation upon selection pressure. This aspect of fungal growth may be particularly relevant to allow persistence of mycelial forms in the lung, but has so far not received much attention in the research of Aspergillus infections.
Whereas colonies of A. fumigatus in nature that develop and reproduce within days on relatively short-lived substrates are probably ephemeral, a single inhaled uninucleate airborne conidiospore of A. fumigatus may establish an infection or airway colonization that may persist as mycelium for many years in the patient [1]. During this long period, the multinucleate mycelium may enable adaptation to the host stress factors such as antifungal treatment. Several observations are consistent with derived genetic variation in Aspergillus species that persist in the host. When Aspergillus infections from patients are sampled and tested, morphologically distinct variants may be cultured in successive respiratory samples or even from the same clinical sample. These colonies might differ in certain characteristics such as azole-resistance phenotype [4,34,35]. Particularly in CF patients, A. fumigatus is thought to persist by the formation of biofilms and thus as an exclusive mycelial morphotype [46]. Microsatellite genotyping indicates that a single A. fumigatus genotype may persist for many years [31–33]. Phenotypic variation in A. fumigatus that is regularly found in patient cultures is also observed in laboratory evolution experiments initiated with a single ancestral genotype [22], but the underlying genetic background and possible clinical relevance of successive and co-isolated morphotypes is unclear.
We here present the evidence for heterokaryosis in clinical isolates. Mycelium from tissue samples of patients segregated different homokaryons, from which heterokaryons could be reconstructed. All variant isolates recovered from the same patient were capable of forming a heterokaryon, whereas those from different patients were heterokaryon-incompatible. Aspergillus fumigatus heterokaryons, composed of environmental homokaryons of different azole-resistance phenotypes, shifted the nuclear ratio of the heterokaryon towards more of the resistant nucleus in response to azole exposure. This indicates that heterokaryons of A. fumigatus are indeed flexible in their nuclear composition and have phenotypic characteristics different from homokaryons and heterozygous diploids, a process that might occur in the human lung and represent an important adaptation strategy.
Heterokaryon can formally also arise by anastomosis of hyphae from different strains, such as following multiple infections, but this is restricted by heterokaryon incompatibility which is widespread in fungi [28,30]. As a result of high polymorphism for heterokaryon-incompatibility genes (het-genes) in many fungal populations, heterokaryon compatibility is mostly restricted to clonally related isolates and, therefore, two randomly picked fungal isolates from nature are most probably heterokaryon-incompatible [47–49]. Heterokaryon incompatibility was not known in A. fumigatus previously, but here we clearly find evidence for its existence. We found that A. fumigatus isolates from different patients, and a field isolate, were heterokaryon-incompatible, indicating that they were all of different clonal origin. On the other hand, variant isolates recovered from the same patient that differed in growth characteristics, spore size and/or azole resistance could form a heterokaryon, indicating a single clonal origin of the infection. Also, the isogenic variant isolates derived from a common ancestor during a 7-week experimental evolution showed heterokaryon compatibility.
(a). Relevance of heterokaryon formation for azole-resistance development and in-host adaptation
Our finding that heterokaryon-compatible A. fumigatus variant isolates can develop in patients indicates a potential role for heterokaryosis in azole-resistance development and in-host adaptation. Patient-acquired resistance can be concluded when a phenotype switch takes place from azole-sensitive to azole-resistant in consecutive isolates recovered from individual patients. This has been observed in isogenic isolates recovered from patients treated with azoles and who have a pulmonary cavity in which asexual sporulation can occur [34].
However, azole-resistant A. fumigatus has also been recovered from patients without pulmonary cavities, notably patients with CF or aspergillosis [50]. In this patient group, heterokaryon formation might be a strategy to develop azole resistance. Adaptive nuclear variation in a heterokaryotic Aspergillus mycelium network may involve component mutant nuclei that vary in level of in-host adaptation and azole resistance but together may contribute to a flexible and resilient heterokaryotic fungal infection. Such heterokaryon will break up in single genotypes when grown on laboratory plates and may exhibit low fitness under these conditions, which can be recognized as pleiomorphic growth.
(b). Implications of heterokaryosis in Aspergillus infections and future outlook
We provide evidence for heterokaryosis in clinical A. fumigatus isolates from patients with chronic or persistent Aspergillus diseases, the segregation of different homokaryons and heterokaryon compatibility. Furthermore, we show that constructed heterokaryons exhibit different growth characteristics compared with homokaryons and, at least for the environmental isolates in which it could be tested, a higher azole resistance than the corresponding heterozygous diploid. These changes were associated with a nuclear ratio change, implying a potential role for heterokaryosis in somatic variation and adaptation in patients with chronic Aspergillus infection and colonization. In clinical microbiology, single Aspergillus colonies are used to determine species identification, but little attention is paid to variability in colony morphology. In the best practice guidelines of the British Society of Medical Mycology, no mention is made of variations of colony phenotypes and the implications thereof [51]. With the emergence of azole resistance, more interest was gained to analyse multiple colonies for azole-resistance testing, as mixed infection involving different azole phenotypes have been reported [52]. Also, atypical colony morphology or pleiomorphic growth under laboratory conditions may be an indicator of in-host adaptation of the fungus, and this might have clinical relevance. In-host adaptation might reduce our therapeutic options to eradicate the fungus from the lung [53].
Further research should shed more light on the relevance of heterokaryosis in Aspergillus infections. How common is our finding of heterokaryon-compatible concomitant Aspergillus variants from different types of patients? What is the extent of heterokaryon incompatibility both in patient isolates and in the field? Can evidence be obtained of flexible heterokaryotic growth in patients? If so, does this contribute to azole resistance and fungal persistence in the human host? Answers to these questions may change our view on how to diagnose and manage Aspergillus infection and colonisation.
5. Conclusion
We provide evidence for heterokaryosis of A. fumigatus in patients with chronic Aspergillus diseases. Our results indicate that somatic variation and nuclear flexibility of heterokaryotic mycelium may be a thus far overlooked strategy for A. fumigatus to adapt to the lung environment and to overcome azole exposure. These results indicate that chronic Aspergillus infections are probably heterokaryotic rather than of single genotype. Implications for diagnosis and management of A. fumigatus infections are considered.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Bertha Koopmanschap and Marijke Slakhorst from the Genetics Laboratory of Wageningen University and Ton Rijs from the Department of Medical Microbiology, Radboud University Medical Centre, for technical assistance.
Data accessibility
All data are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.fr3kt2d [54].
Authors' contributions
B.J.Z., P.E.V., J.Z., A.J.M.D. and S.E.S. initiated the project; J.Z., A.J.M.D. and P.E.V. designed the methodology; E.J.K., P.E.V. and M.C.A. provided materials; J.Z. carried out experiments and formal analyses. J.Z., E.E.S. and A.J.M.D. prepared the draft of the manuscript. All authors contributed to the commenting and editing of subsequent versions of the manuscript.
Competing interests
The authors declare no competing interests.
Funding
We gratefully acknowledge funding from the China Scholarship Council to J.Z.
References
- 1.Hamprecht A, Morio F, Bader O, Le Pape P, Steinmann J, Dannaoui E. 2018. Azole resistance in Aspergillus fumigatus in patients with cystic fibrosis: a matter of concern? Mycopathologia 183, 151–160. ( 10.1007/s11046-017-0162-4) [DOI] [PubMed] [Google Scholar]
- 2.Dauchy C, et al. 2018. Emergence of Aspergillus fumigatus azole resistance in azole-naïve patients with chronic obstructive pulmonary disease and their homes. Indoor Air 28, 298–306. ( 10.1111/ina.12436) [DOI] [PubMed] [Google Scholar]
- 3.Fisher MC, Hawkins NJ, Sanglard D, Gurr SJ. 2018. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 360, 739–742. ( 10.1126/science.aap7999) [DOI] [PubMed] [Google Scholar]
- 4.Arendrup MC, Mavridou E, Mortensen KL, Snelders E, Frimodt-Moller N, Khan H, Melchers WJ, Verweij P.E. 2010. Development of azole resistance in Aspergillus fumigatus during azole therapy associated with change in virulence. PLoS ONE 5, e10080 ( 10.1371/journal.pone.0010080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lockhart SR, Frade JP, Etienne KA, Pfaller MA, Diekema DJ, Balajee SA. 2011. Azole resistance in Aspergillus fumigatus isolates from the ARTEMIS global surveillance study is primarily due to the TR/L98H mutation in the cyp51A Gene. Antimicrob. Agents Chemother. 55, 4465–4468. ( 10.1128/Aac.00185-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mortensen KL, Jensen RH, Johansen HK, Skov M, Pressler T, Howard SJ, Leatherbarrow H, Mellado E, Arendrup MC. 2011. Aspergillus species and other molds in respiratory samples from patients with cystic fibrosis: a laboratory-based study with focus on Aspergillus fumigatus azole resistance. J. Clin Microbiol. 49, 2243–2251. ( 10.1128/JCM.00213-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfaller M, Boyken L, Hollis R, Kroeger J, Messer S, Tendolkar S, Diekema D. 2011. Use of epidemiological cutoff values to examine 9-year trends in susceptibility of Aspergillus species to the triazoles. J. Chin. Microbiol. 49, 586–590. ( 10.1128/JCM.02136-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowdhary A, Kathuria S, Randhawa HS, Gaur SN, Klaassen CH, Meis JF. 2012. Isolation of multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR/L98H mutations in the cyp51A gene in India. J. Antimicrob. Chemother. 67, 362–366. ( 10.1093/jac/dkr443) [DOI] [PubMed] [Google Scholar]
- 9.Morio F, Aubin GG, Danner-Boucher I, Haloun A, Sacchetto E, Garcia-Hermoso D, Bretagne S, Miegeville M, Le Pape P.. 2012. High prevalence of triazole resistance in Aspergillus fumigatus, especially mediated by TR/L98H, in a French cohort of patients with cystic fibrosis. J. Antimicrob. Chemother. 67, 1870–1873. ( 10.1093/jac/dks160) [DOI] [PubMed] [Google Scholar]
- 10.Gisi U. 2013. Assessment of selection and resistance risk for demethylation inhibitor fungicides in Aspergillus fumigatus in agriculture and medicine: a critical review. Pest Manag. Sci. 70, 352–364. ( 10.1002/ps.3664) [DOI] [PubMed] [Google Scholar]
- 11.Bignell E. 2014. 2 Aspergillus fumigatus: saprotroph to pathogen. In The Mycota: a comprehensive treatise on fungi as experimental systems for basic and applied research (ed. O. Kurzai), pp. 19–43. Berlin, Germany: Springer. [Google Scholar]
- 12.Chowdhary A, Sharma C, Hagen F, Meis JF. 2014. Exploring azole antifungal drug resistance in Aspergillus fumigatus with special reference to resistance mechanisms. Future Microbiol. 9, 697–711. ( 10.2217/fmb.14.27) [DOI] [PubMed] [Google Scholar]
- 13.van der Linden JW, et al. 2011. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007–2009. Emerg. Infect. Dis. 17, 1846–1854. ( 10.3201/eid1710.110226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snelders E, et al. 2008. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 5, e219 ( 10.1371/journal.pmed.0050219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard SJ, Webster I, Moore CB, Gardiner RE, Park S, Perlin DS, Denning DW. 2006. Multi-azole resistance in Aspergillus fumigatus. Int. J. Antimicrob. Agents 28, 450–453. ( 10.1016/j.ijantimicag.2006.08.017) [DOI] [PubMed] [Google Scholar]
- 16.Howard SJ, et al. 2009. Frequency and evolution of Azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg. Infect. Dis. 15, 1068–1076. ( 10.3201/eid1507.090043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bueid A, Howard SJ, Moore CB, Richardson MD, Harrison E, Bowyer P, Denning DW. 2010. Azole antifungal resistance in Aspergillus fumigatus: 2008 and 2009. J. Antimicrob. Chemother. 65, 2116–2118. ( 10.1093/jac/dkq279) [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, et al. 2017. A novel environmental azole resistance mutation in Aspergillus fumigatus and a possible role of sexual reproduction in its emergence. MBio 8, e00791-17 ( 10.1128/mBio.00791-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riat A, Plojoux J, Gindro K, Schrenzel J, Sanglard D. 2018. Azole resistance of environmental and clinical Aspergillus fumigatus isolates from Switzerland. Antimicrob. Agents Chemother. 62, e02088-e02017 ( 10.1128/AAC.02088-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdolrasouli A, Rhodes J, Beale MA, Hagen F, Rogers TR, Chowdhary A, Meis JF, Armstrong-James D, Fisher MC. 2015. Genomic context of azole resistance mutations in Aspergillus fumigatus determined using whole-genome sequencing. MBio 6, e00536-00515 ( 10.1128/mBio.00536-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verweij PE, Kema GH, Zwaan B, Melchers WJ. 2013. Triazole fungicides and the selection of resistance to medical triazoles in the opportunistic mould Aspergillus fumigatus. Pest Manag. Sci. 69, 165–170. ( 10.1002/ps.3390) [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Debets AJ, Verweij PE, Melchers WJ, Zwaan BJ, Schoustra SE. 2015. Asexual sporulation facilitates adaptation: the emergence of azole resistance in Aspergillus fumigatus. Evolution 69, 2573–2586. ( 10.1111/evo.12763) [DOI] [PubMed] [Google Scholar]
- 23.Losada L, et al. 2015. Genetic analysis using an isogenic mating pair of Aspergillus fumigatus identifies azole resistance genes and lack of MAT Locus's role in virulence. PLoS Pathog. 11, e1004834 ( 10.1371/journal.ppat.1004834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon-Chung KJ, Sugui JA. 2009. Sexual reproduction in Aspergillus species of medical or economical importance: why so fastidious? Trends Microbiol. 17, 481–487. ( 10.1016/j.tim.2009.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pontecorvo G, Roper J, Chemmons L, MacDonald K, Bufton A. 1953. The genetics of Aspergillus nidulans. Adv. Genet. 5, 141–238. ( 10.1016/S0065-2660(08)60408-3) [DOI] [PubMed] [Google Scholar]
- 26.Debets AJM. 1998. Parasexuality in fungi: mechanisms and significance in wild populations. In Molecular variability of fungal pathogens (eds Bridge P, Couteaudier Y, Clarkson J), pp. 41–52. Wallingford, UK: CAB International. [Google Scholar]
- 27.Hansen H. 1938. The dual phenomenon in imperfect fungi. Mycologia 30, 442–455. ( 10.2307/3754469) [DOI] [Google Scholar]
- 28.Glass NL, Kuldau GA.. 1992. Mating type and vegetative incompatibility in filamentous ascomycetes. Annu. Rev. Phytopathol. 30, 201–224. ( 10.1146/annurev.py.30.090192.001221) [DOI] [PubMed] [Google Scholar]
- 29.Saupe SJ. 2000. Molecular genetics of heterokaryon incompatibility in filamentous ascomycetes. Microbiol. Mol. Biol. Rev. 64, 489–502. ( 10.1128/mmbr.64.3.489-502.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aanen D, Debets AJM, Glass NL, Saupe SJ. 2010. Biology and genetics of vegetative incompatibility in fungi. In Cellular and molecular biology of filamentous fungi (eds Borkovich K, Ebbole D), pp. 274–288. Washington, DC: American Society of Microbiology. [Google Scholar]
- 31.de Valk HA, Klaassen CH, Yntema JB, Hebestreit A, Seidler M, Haase G, Müller FM, Meis JF.. 2009. Molecular typing and colonization patterns of Aspergillus fumigatus in patients with cystic fibrosis. J. Cyst. Fibros. 8, 110–114. ( 10.1016/j.jcf.2008.10.003) [DOI] [PubMed] [Google Scholar]
- 32.Neuvéglise C, Sarfati J, Debeaupuis JP, Thien HV, Just J, Tournier G, Latgé JP. 1997. Longitudinal study of Aspergillus fumigatus strains isolated from cystic fibrosis patients. Eur. J. Clin. Microbiol. Infect. Dis. 16, 747–750. ( 10.1007/bf01709257) [DOI] [PubMed] [Google Scholar]
- 33.Symoens F, Bertout S, Piens MA, Burnod J, Renaud F, Nolard N, Chapuis F, Grillot R. 2001. A longitudinal study of lung transplant recipients infected with Aspergillus: genetic polymorphism of Aspergillus fumigatus. J. Heart Lung Transplant. 20, 970–978. ( 10.1016/s1053-2498(01)00287-x) [DOI] [PubMed] [Google Scholar]
- 34.Camps SM, van der Linden JW, Li Y, Kuijper EJ, van Dissel JT, Verweij PE, Melchers WJ.. 2012. Rapid induction of multiple resistance mechanisms in Aspergillus fumigatus during azole therapy: a case study and review of the literature. Antimicrob. Agents Chemother 56, 10–16. ( 10.1128/AAC.05088-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad S, Joseph L, Hagen F, Meis JF, Khan Z. 2015. Concomitant occurrence of itraconazole-resistant and -susceptible strains of Aspergillus fumigatus in routine cultures. J. Antimicrob. Chemother. 70, 412–415. ( 10.1093/jac/dku410) [DOI] [PubMed] [Google Scholar]
- 36.Zhang C, Kong Q, Cai Z, Liu F, Chen P, Song J, Lu L, Sang H. 2015. The newly nonsporulated characterization of an Aspergillus fumigatus isolate from an immunocompetent patient and its clinic indication. Fungal Genet. Biol. 81, 250–260. ( 10.1016/j.fgb.2015.03.001) [DOI] [PubMed] [Google Scholar]
- 37.De Valk HA, Meis JF, De Pauw BE, Donnelly PJ, Klaassen CH.. 2007. Comparison of two highly discriminatory molecular fingerprinting assays for analysis of multiple Aspergillus fumigatus isolates from patients with invasive aspergillosis. J. Clin. Microbiol. 45, 1415–1419. ( 10.1128/JCM.02423-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuipers S, Brüggemann RJM, de Sévaux RGL, Heesakkers JPFA, Melchers WJG, Mouton JW, Verweij PE.. 2011. Failure of posaconazole therapy in a renal transplant patient with invasive Aspergillosis due to Aspergillus fumigatus with attenuated susceptibility to posaconazole. Antimicrob. Agents Chemother. 55, 3564–3566. ( 10.1128/aac.01544-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cove D. 1976. Chlorate toxicity in Aspergillus nidulans: the selection and characterization of chlorate resistant mutants. Heredity 36, 191–203. ( 10.1038/hdy.1976.24) [DOI] [PubMed] [Google Scholar]
- 40.Debets AJM, Swart K, Bos CJ. 1990. Genetic analysis of Aspergillus niger: isolation of chlorate resistance mutants, their use in mitotic mapping and evidence for an eighth linkage group. Mol. Gen. Genet. 221, 453–458. ( 10.1007/BF00259411) [DOI] [PubMed] [Google Scholar]
- 41.Todd RB, Davis MA, Hynes MJ. 2007. Genetic manipulation of Aspergillus nidulans: heterokaryons and diploids for dominance, complementation and haploidization analyses. Nat. Protoc. 2, 822–830. ( 10.1038/nprot.2007.113) [DOI] [PubMed] [Google Scholar]
- 42.Bos C, Debets AJM, Swart K, Huybers A, Kobus G, Slakhorst S. 1988. Genetic analysis and the construction of master strains for assignment of genes to six linkage groups in Aspergillus niger. Curr. Genet. 14, 437–443. ( 10.1007/BF00521266) [DOI] [PubMed] [Google Scholar]
- 43.Dyer PS, O'Gorman CM. 2012. Sexual development and cryptic sexuality in fungi: insights from Aspergillus species. FEMS Microbiol. Rev. 36, 165–192. ( 10.1111/j.1574-6976.2011.00308.x) [DOI] [PubMed] [Google Scholar]
- 44.Jinks JL. 1952. Heterokaryosis: a system of adaptation in wild fungi. Proc. R. Soc. Lond. B 140, 83–99. ( 10.1098/rspb.1952.0046) [DOI] [PubMed] [Google Scholar]
- 45.Leslie JF. 1993. Fungal vegetative compatibility. Annu. Rev. Phytopathol. 31, 127–150. ( 10.1146/annurev.py.31.090193.001015) [DOI] [PubMed] [Google Scholar]
- 46.Seidler MJ, Salvenmoser S, Müller FMC. 2008. Aspergillus fumigatus forms biofilms with reduced antifungal drug susceptibility on bronchial epithelial cells. Antimicrob. Agents Chemother. 52, 4130–4136. ( 10.1128/aac.00234-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bastiaans E, Debets AJM, Aanen DK, van Diepeningen AD, Saupe SJ, Paoletti M.. 2014. Natural variation of heterokaryon incompatibility gene het-c in Podospora anserina reveals diversifying selection. Mol. Biol. Evol. 31, 962–974. ( 10.1093/molbev/msu047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Debets AJM, Griffiths AJF. 1998. Polymorphism of het-genes prevents resource plundering in Neurospora crassa. Mycol. Res. 102, 1343–1349. ( 10.1017/S095375629800639X) [DOI] [Google Scholar]
- 49.van Diepeningen AD, Debets AJM, Hoekstra RF.. 1997. Heterokaryon incompatibility blocks virus transfer among natural isolates of black Aspergilli. Curr. Genet. 32, 209–217. ( 10.1007/s002940050268) [DOI] [PubMed] [Google Scholar]
- 50.Burgel PR, Paugam A, Hubert D, Martin C. 2016. Aspergillus fumigatus in the cystic fibrosis lung: pros and cons of azole therapy. Infect. Drug Resist. 9, 229–238. ( 10.2147/idr.s63621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schelenz S, Barnes RA, Barton RC, Cleverley JR, Lucas SB, Kibbler CC, Denning DW. 2015. British Society for Medical Mycology best practice recommendations for the diagnosis of serious fungal diseases. Lancet Infect. Dis. 15, 461–474. ( 10.1016/S1473-3099(15)70006-X) [DOI] [PubMed] [Google Scholar]
- 52.Astvad KMT, Jensen RH, Hassan TM, Mathiasen EG, Thomsen GM, Pedersen UG, Christensen M, Hilberg O, Arendrup MC. 2014. First detection of TR(46)/Y121F/T289A and TR(34)/L98H alterations in Aspergillus fumigatus isolates from azole-naive patients in Denmark despite negative findings in the environment. Antimicrob. Agents Chemother. 58, 5096–5101. ( 10.1128/aac.02855-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verweij PE, Zhang J, Debets AJM, Meis JF, van de Veerdonk FL, Schoustra SE, Zwaan BJ, Melchers WJ.. 2016. In-host adaptation and acquired triazole resistance in Aspergillus fumigatus: a dilemma for clinical management. Lancet Infect. Dis. 16, e251–e260. ( 10.1016/S1473-3099(16)30138-4) [DOI] [PubMed] [Google Scholar]
- 54.Zhang J, Snelders EE, Zwaan BJ, Schoustra SE, Kuijper EJ, Arendrup MC, Melchers WJG, Verweij PE, Debets AJM. 2019. Data from: Relevance of heterokaryosis for adaptation and azole-resistance development in Aspergillus fumigatus Dryad Digital Repository. ( 10.5061/dryad.fr3kt2d) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Zhang J, Snelders EE, Zwaan BJ, Schoustra SE, Kuijper EJ, Arendrup MC, Melchers WJG, Verweij PE, Debets AJM. 2019. Data from: Relevance of heterokaryosis for adaptation and azole-resistance development in Aspergillus fumigatus Dryad Digital Repository. ( 10.5061/dryad.fr3kt2d) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.fr3kt2d [54].